Abstract
Background
Equine neuroaxonal dystrophy/equine degenerative myeloencephalopathy (eNAD/EDM) is a neurodegenerative disorder affecting genetically predisposed foals maintained on an α‐tocopherol (α‐TOH) deficient diet. Currently no antemortem diagnostic test for eNAD/EDM is available.
Hypothesis
Because α‐TOH deficiency is associated with increased lipid peroxidation, it was hypothesized that F2‐isoprostanes (F2IsoP), F4‐neuroprostanes (F4NP) and oxysterols derived from free radical oxidation would be increased in the cerebrospinal fluid (CSF) and neural tissue of eNAD/EDM affected horses and could serve as potential biomarkers for disease.
Animals
Isoprostane Study A: 14 Quarter horse foals (10 healthy foals and 4 eNAD/EDM affected foals) at 1 and 6 months of age. Isoprostane Study B: 17 eNAD/EDM affected and 10 unaffected horses ≥ 1‐4 years of age. Oxysterol study: eNAD/EDM affected (n = 14, serum; n = 11, CSF; n = 10, spinal cord [SC]) and unaffected horses 1‐4 years of age (n = 12, serum; n = 10, CSF; n = 7, SC).
Procedures
Cerebrospinal fluid [F2IsoP] and [F4NP] were assessed using gas chromatography‐negative ion chemical ionization mass spectrometry. Serum, CSF, and cervical SC [oxysterols] were quantified using high performance liquid chromatography mass spectrometry. Results were compared with respective α‐TOH concentrations.
Results
Spinal cord [7‐ketocholesterol], [7‐hydroxycholesterol], and [7‐keto‐27‐hydrocholesterol] were higher in eNAD/EDM horses whereas [24‐ketocholesterol] was lower. No significant difference was found in CSF [F2IsoP] and [F4NP], serum [oxysterols] and CSF [oxysterols] between eNAD/EDM affected and unaffected horses. No correlation was found between [F2IsoP], [F4NP], or [oxysterols] and respective [α‐TOH].
Conclusions and Clinical Importance
In the SC, targeted markers of cholesterol oxidation were significantly increased in horses with eNAD/EDM.
Keywords: ataxia, equine, genetics, vitamin E
Abbreviations
- α‐TOH
α‐tocopherol
- CI
confidence interval
- CSF
cerebrospinal fluid
- CYP
cytochrome P450
- EDM
equine degenerative myeloencephalopathy
- eNAD
equine neuroaxonal dystrophy
- EtOH
ethanol
- F2IsoP
F2‐isoprostanes
- F4NP
F4‐neuroprostanes
- HPLC–APCI–MS/MS
high‐performance liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry
- HPODE
hydroperoxy octadecadionoates
- LC/MS
liquid chromatography/mass spectrometry
- LXR
liver X receptor
- MDA
malondialdehyde
- PUFA
polyunsaturated fatty acid
- QH
Quarter Horse
- RRR‐α‐TP
natural (or ‐d) α‐tocopherol
- SC
spinal cord
- SRM
selective reaction monitoring
- TBAR
thiobarbituric acid reactivity
- UHPLC–MS/MS
ultra‐high performance liquid chromatography tandem mass spectrometry
- VitE
vitamin E
1. INTRODUCTION
Equine neuroaxonal dystrophy/equine degenerative myeloencephalopathy (eNAD/EDM) is a neurologic condition that develops in genetically predisposed foals maintained on an α‐tocopherol (α‐TOH) deficient diet.1, 2 The disease appears to develop during the first year of life3 and, in humans, there is strong evidence that the developing nervous system is particularly at risk from α‐TOH deficiency.4 The underlying genetic cause remains unknown. Although α‐TOH deficiency in the cerebrospinal fluid (CSF) may be supportive of the diagnosis in younger horses (ie, <6 months of age),5 no definitive antemortem diagnostic test currently is available. Decreased CSF α‐TOH concentrations in horses > 6 months of age may not be specific for eNAD/EDM.
The term vitamin E (vitE) encompasses a closely related family of 8 fat soluble naturally occurring compounds. Of these forms, α‐TOH most consistently prevents lipid peroxidation in vitro 6 and in vivo.7, 8 Biomarkers of lipid peroxidation often are assessed as indicators of oxidative damage. Potential biomarkers include F2‐isoprostanes (F2IsoP), formed by oxidation of arachidonic acid and F4‐neuroprostanes (F4NP) from docosahexaenoic acid.9 The measurement of lipid peroxidation products is more reliable than the detection of reactive oxygen species, reactive nitrogen species, and other active oxidants.10
Lipid peroxidation products have various biological functions in vivo such as regulating gene expression and cell signaling.11, 12 In eNAD/EDM affected horses, we recently identified upregulation of specific genes targeted by a nuclear transcription factor, the liver X receptor (LXR).13 The natural ligands for LXR activation are oxysterols.14, 15 Preliminary evaluation of eNAD/EDM affected horses identified a trend toward increased spinal cord (SC) 7‐ketocholesterol and 7‐hydroxycholesterol concentrations.13 These 2 oxysterols result from autooxidation of cholesterol and oxidation of 7‐dehydrocholesterol by cytochrome P450 7A1 (CYP7A1; 7‐alpha‐hydroxylase).16
We hypothesized that measures of lipid peroxidation, including F2IsoP, F4NP, and oxysterols would be significantly increased in the CSF of eNAD/EDM affected horses. Additionally, we hypothesized that the same oxysterol markers would be increased in SC tissue of eNAD/EDM affected horses. The purpose of our study was to determine the concentrations of these biomarkers in eNAD/EDM affected horses to potentially provide an antemortem diagnostic tool while further investigating the etiology of the disease.
2. MATERIALS AND METHODS
2.1. Animals
2.1.1. Isoprostane study A: Foal samples
For repeat measurements of F2IsoP and F4NP at 1 and 6 months of age, 14 Quarter horse foals were used as previously described (Table 1).5 These included 4 foals that developed eNAD/EDM during the first year of life, which was confirmed on postmortem examination at 1.5 years, and 10 healthy control foals that were maintained in the same environment and fed an identical α‐TOH deficient diet. Postmortem evaluation of the brain and SC tissue from eNAD animals indicated histologic lesions typical of eNAD, namely chromatolytic neurons and spheroid development that were especially prominent in the lateral accessory cuneate, medial cuneate, and gracile nuclei. Serum and CSF samples were collected as previously described.5 All protocols were approved by the UCD Institutional Animal Care and Use Committee (Protocol # 15866).
Table 1.
Sample sizes used for assays in this study
| Analysis | Age | Analytes | Sample | Horses | |
|---|---|---|---|---|---|
| #Affected | #Unaffected | ||||
| Isoprostane Study A | Repeated samples 1 and 6 mo | F2‐isoprostane and F4‐neuroprostanes | CSF | 4 | 10 |
| Isoprostane Study B | 1–4 y | F2‐isoprostane and F4‐neuroprostanes | CSF | 17 | 10 |
| Oxysterol | 1–4 y | Cholesterol and oxysterols | Serum | 14 | 12 |
| Oxysterol | 1–4 y | Cholesterol and oxysterols | CSF | 11 | 10 |
| Oxysterol | 1–4 y | Cholesterol and oxysterols | SC | 10 | 7 |
For a full list of individual horses, refer to Supporting Information Table S1.
Abbreviations: CSF, cerebrospinal fluid; SC, spinal cord; mo, months; y, years.
2.1.2. Isoprostane study B: Adult samples
Over a period of 8 years, samples from 19 postmortem‐confirmed eNAD/EDM and 16 unaffected horses were collected (Supporting Information Table S1). All horses were donated by owners for the purposes of the study and were between 1 and 4 years of age. Biologic samples (ie, serum, CSF, and SC tissue) were available on subsets of eNAD/EDM affected and unaffected horses (Table 1 and Supporting Information Table S1) and collected immediately before (serum) or after (CSF, SC tissue) euthanasia. A moderate phenotype (eNAD) or severe phenotype (eNAD/EDM) was diagnosed using histologic lesions as previously described.2 Unaffected horses were euthanized for reasons other than neurologic disease and subclinical eNAD was excluded by histologic evaluation of the central nervous system.
2.1.3. Oxysterol study
Samples from n = 14 (serum), n = 11 (CSF), and n = 10 (SC) eNAD/EDM affected and n = 12 (serum), n = 10 (CSF), and n = 7 (SC) unaffected horses ages 1–4 years were available for oxysterol quantification (Table 1 and Supporting Information Table S1). Spinal cord sections were collected at the level of C1 as previously described.13
2.2. Sample collection and storage
Cerebrospinal fluid (10–12 mL) was aliquoted into plastic light‐protected vials on ice and centrifuged at 4°C (2000g for 15 minutes) within 1 hour of collection. Supernatant was aliquoted 1 mL at a time into precooled cryovials, immediately flash frozen in liquid nitrogen and maintained at −80°C until analysis. Serum samples were collected from an IV catheter after removal of 6 mL of heparinized blood. Serum samples were processed in an identical manner as CSF and frozen in 1 mL aliquots. For F2IsoP, F4NP, and oxysterol quantification, samples were shipped to the respective laboratories overnight on dry ice.
2.3. α‐TOH concentrations
For isoprostane Study A, α‐TOH concentrations were assessed in serum and CSF from foals at 1 and 6 months of age as previously described.5 In Isoprostane Study B and oxysterol studies, α‐TOH concentrations were assessed in serum (n = 19, eNAD/EDM affected; n = 8, unaffected), CSF (n = 15, eNAD/EDM affected; n = 7, unaffected), and SC tissue (n = 5, eNAD/EDM affected; n = 6, unaffected) as previously described (Supporting Information Table S1).13
2.4. F2‐isoprostane and F4‐neuroprostane concentrations
F2‐isoprostane (F2‐IsoP, also referred to as 8‐iso‐PGF2α) and F4‐NP concentrations were assessed in CSF from horses in Study A at 1 and 6 months of age and in Study B at the time of euthanasia (1–4 years of age). Quantification of F2IsoP and F4NP was performed using a gas chromatography‐negative ion chemical ionization‐mass spectrometry (GC‐NICI‐MS) approach employing stable isotope dilution as previously described.17 The limit of detection for both assays was 1 pg.
2.5. Cholesterol and oxysterol concentrations
Selected oxysterol concentrations were assessed based on preliminary data indicating detectable concentrations in biologic samples.13 Serum 7‐ketocholesterol, 7‐hydroxycholesterol, 24‐hydroxycholesterol, and 4β‐hydroxycholesterol; CSF 7‐ketocholesterol and 24‐epoxycholesterol; and SC tissue 7‐ketocholesterol, 7‐hydroxycholesterol, 24‐hydroxycholesterol, 24‐ketocholesterol, 7‐keto‐27‐hydrocholesterol, and 7–27‐dihydroxycholesterol concentrations were quantified.
2.5.1. Materials
Liquid chromatography/mass spectrometry (LC/MS) grade solvents (methylene chloride, chloroform, methanol, water, formic acid) were purchased from Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, Massachusetts). The d7‐7‐ketocholesterol was prepared as reported previously.18 Two‐hundred proof ethanol (USP Specification; Decon Laboratories, King of Prussia, Pennsylvania), potassium hydroxide (ACS certified; Thermo Fisher Scientific, Waltham, Massachusetts), and sodium chloride (ACS certified; Thermo Fisher Scientific, Waltham, Massachusetts) were used.
2.5.2. Lipid extraction from tissues and fluids
Before lipid extraction, the internal standards, d7‐7‐ketocholesterol (500 ng), d6‐24‐epoxycholesterol (500 ng), and d7‐cholesterol (2.5 µg) were added to each sample. For SC tissue, the tissues were homogenized in Folch solution (4 mL, chloroform : methanol = 2 : 1) by a blade homogenizer. For serum (100 µL), 4 mL of Folch solution was added directly to each sample. Sodium chloride aqueous solution (0.9%, 1 mL) then was added, and the resulting mixture was briefly vortexed and centrifuged for 5 minutes. The lower organic phase was recovered and dried at room temperature using a SpeedVac (Thermo Fisher Scientific, Waltham, Massachusetts), and then re‐dissolved in methylene chloride (1 mL for tissue samples and 500 µL for CSF samples). For serum samples, extracts were dried under SpeedVac and the dried samples then were reconstituted in 2 mL 2.5% KOH in 90% ethanol (EtOH) and incubated in a water bath at 55°C for 55 minutes. Another lipid extraction was performed on the solution from the base hydrolysis and the extracts were dried under speed vacuum (Savant SpeedVac, Thermo Fischer Scientific, Waltham, Massachusetts). Each sample was reconstituted in methylene chloride (500 µL).
2.5.3. High‐performance liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry (HPLC–APCI–MS/MS) analyses of sterols and oxysterols
Analysis of cholesterol and oxysterols was performed by ultra‐HPLC tandem mass spectrometry (UHPLC–MS/MS) using a triple quadrupole mass spectrometer (API 4000, Sciex, Framingham, Massachusetts) equipped with APCI. For analysis, an appropriate amount of sample was transferred to a LC vial, dried under a stream of argon, and reconstituted in 90% methanol with 0.1% formic acid (for oxysterol analysis of SC tissue, 50 µL was reconstituted in 200 µL; for serum, 100 µL to 50 µL and for CSF, 100 µL to 50 µL). For cholesterol analysis, 20 µL was reconstituted in 200 µL for SC tissue, serum, and CSF. Reverse‐phase chromatography was performed under the following conditions: C18 column (1.7 µm, 100 mm by 2.1 mm; Kinetex, Phenomenex, Torrance, California); flow rate, 0.4 mL/min and elution solvent, 90% methanol with 0.1% formic acid. Mass spectroscopy conditions were nebulizer current, 3 mA; curtain gas, 10 psi; collision gas, high; ion source gas, 20 pounds per square inch; entrance potential, 10 V; collision energy, 25 V; declustering potential, 80 V; collision cell exit potential, 20 V and temperature, 300°C. For MS analysis, selective reaction monitoring (SRM) was employed to monitor the dehydration process of the ion [M + H]+ or [M + H‐H2O]+ as described previously.13 The internal standard, d7‐7‐ketocholesterol, was used to quantify the analytes 7‐ketocholesterol, 4β‐hydroxycholesterol, and 7‐hydroxycholesterol. d6‐24‐epoxycholesterol was used to quantify 24‐epoxycholesterol, 24‐ketocholesterol, and 24‐hydroxycholesterol; and, d7‐cholesterol was used to quantify cholesterol. Selective reaction monitoring mass transitions were d7‐7‐ketocholesterol m/z 408.3 → 390.3; d6‐24‐epoxycholesterol m/z 389.3 → 371.3; 7‐ketocholesterol, m/z 401.3 → 383.3; 7‐hydroxycholesterol, 4β‐hydroxycholesterol, and 24‐hydroxycholesterol, m/z 385.3 → 367.3; 24‐epoxycholesterol and 24‐ketocholesterol m/z 383.3 → 365.3; d7‐cholesterol m/z 376.3 → 376.3; cholesterol m/z 369.3 → 369.3. All concentrations were normalized to cholesterol concentrations for quantification.
2.6. Statistical analyses
2.6.1. Isoprostane study A
A repeated measures 2‐way ANOVA with Sidak's multiple comparisons was performed on F2IsoP concentrations. Because all F4NP results were below the limit of detection at 6 months of age, a Mann‐Whitney test was used to compare values at 1 months of age.
2.6.2. Isoprostane study B and oxysterol analyses
Data for each metabolite were analyzed for normality using a Shapiro‐Wilk test. Normally distributed data were analyzed using an unpaired T‐test with Welch's correction and non‐normally distributed data with a Mann‐Whitney test. Correction for multiple testing was performed at a false discovery rate of 0.1 within each sample subset.
2.6.3. Correlation with age and α‐TOH concentrations
Correlations of each metabolite with age and corresponding α‐TOH concentrations (serum, CSF and SC tissue) were performed using a Pearson correlation for normally distributed data and a Spearman's rank correlation for non‐normally distributed data. Analyses were performed using commercial software (GraphPad Prism7, La Jolla, California). Values are reported as mean ± SD and P < .05 was considered significant.
3. RESULTS
3.1. F2‐isoprostane and F4‐neuroprostane concentrations
3.1.1. Isoprostane study A
In the foal population (1–6 months of age), no effect of disease (P = .42) or age (P = .92) was found on CSF F2IsoP. The F4NP were below the limit of detection in n = 2 eNAD/EDM affected and n = 3 unaffected foals at 1 months of age. No significant effect of disease was found on CSF F4NP (P = .91).
3.1.2. Isoprostane study B
A total of n = 17 eNAD/EDM affected (6 geldings, 2 stallions, 9 mares) and n = 10 unaffected (2 geldings and 8 mares) samples were available for analysis of CSF F2IsoP and F4NP from adult horses (Table 1 and Supporting Information Table S1). No correlation of CSF F2IsoP concentrations with age was found (R2 = 0.09) and no significant difference in age between experimental groups was found (eNAD/EDM = 2.09 ± 1.03 years, unaffected = 2.77 ± 1.13 years; P = .12). No significant effect of disease on CSF F2IsoP in horses aged ≥ 1–4 years (P = .93) was identified. All CSF F4NP were below the limit of detection.
3.1.3. Correlation with CSF α‐TOH
For both Studies A and B, no significant correlation was found between CSF F2IsoP and CSF α‐TOH concentrations (R2 = 0.12, P = .22, and R 2 = 0.003, P = .82, respectively).
3.2. Cholesterol and oxysterol concentrations
3.2.1. Serum
Serum samples were available from n = 14 eNAD/EDM affected (6 geldings, 2 stallions, 6 mares) and n = 12 unaffected (3 geldings, 1 stallion, 8 mares) horses. No correlation of serum 7‐ketocholesterol, 7‐hydroxycholesterol, 24‐hydroxycholesterol, and 4β‐hydroxycholesterol concentrations with age was found and no significant difference in age between experimental groups (eNAD/EDM = 1.93 ± 0.96 years, unaffected = 2.37 ± 1.43 years; P = .37) was identified. In serum, no effect of disease on serum 7‐ketocholesterol (P = .23), 7‐hydroxcholesterol (P = .32), 24‐hydroxycholesterol (P = .53), or 4β‐hydrocholesterol (P = .16) concentrations was found.
3.2.2. Correlation with serum α‐TOH
Serum α‐TOH concentrations were available from n = 17 of the 26 samples (n = 12 eNAD/EDM and n = 5 unaffected horses, Supporting Information Table S1). No correlation was identified between any serum oxysterol assessed and serum α‐TOH concentrations.
3.2.3. CSF
A total of n = 11 eNAD/EDM affected (4 geldings, 2 stallions, 5 mares) and n = 10 unaffected (3 geldings, 7 mares) horses were available for analysis of CSF oxysterols. No correlation of CSF 7‐ketocholesterol or 24‐epoxycholesterol concentrations was found with age and no significant difference in age between experimental groups (eNAD/EDM = 1.73 ± 0.72 years, unaffected = 2.4 ± 1.05 years; P = .11) was identified. In CSF, nonsignificantly (P = .07) increased 7‐ketocholesterol concentrations were observed in eNAD/EDM affected horses (Supporting Information Fig. S1). No effect of disease on CSF 24‐epoxycholesterol concentrations was found (P = .99).
3.2.4. Correlation with CSF α‐TOH
Cerebrospinal fluid α‐TOH concentrations were available from n = 16 of the 21 samples (n = 11 eNAD/EDM and n = 5 unaffected horses). No correlation was found between any CSF oxysterol and CSF α‐TOH concentrations.
3.2.5. Spinal cord
Spinal cord samples were available from n = 10 eNAD/EDM affected (4 geldings, 1 stallion, 5 mares) and n = 7 unaffected (2 geldings, 2 stallions, 3 mares). No correlation of SC 7‐ketocholesterol, 7‐hydroxycholesterol, 24‐hydroxycholesterol, 24‐ketocholesterol, and 7,27‐dihydroxycholesterol concentrations was found with age. A significant negative correlation was observed between age and 7‐keto‐27‐hydroxycholesterol concentrations (Spearman r = −0.02 (confidence interval −0.73‐0.04, P = .03). No significant difference in age was found between experimental groups (eNAD/EDM = 1.35 ± 0.34 years, unaffected = 1.18 ± 0.25 years; P = .48).
Spinal cord 7‐ketocholesterol (P = .002), 7‐hydroxycholesterol (P = .0007), and 7‐keto‐27‐hydroxycholesterol (P = .03) concentrations were significantly higher in eNAD/EDM affected horses (Figure 1A‐C) whereas 24‐ketocholesterol was significantly lower (P = .02; Figure 1D), even after accounting for multiple testing. No effect of disease on SC 24‐hydroxycholesterol (Figure 1E) and 7,27‐dihydroxycholesterol concentrations was identified. The majority (16/17) of SC tissue 7,27‐dihydroxycholesterol concentrations were undetectable at the limit of detection across the sample set.
Figure 1.
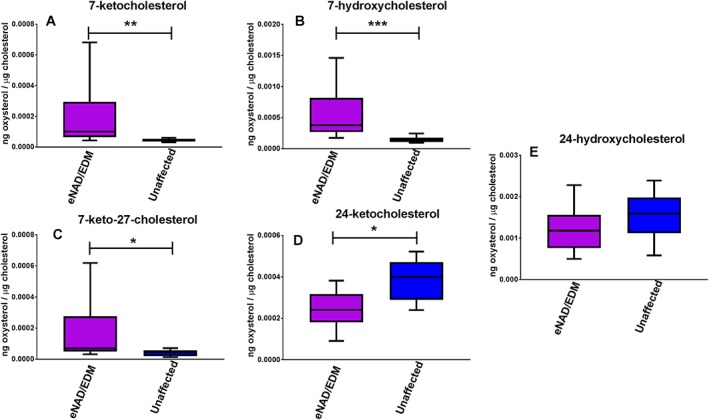
Spinal cord (A) 7‐ketocholesterol, (B) 7‐hydroxycholesterol, (C) 7‐keto‐27‐hydroxycholesterol, (D) 24‐ketocholesterol, and (E) 24‐hydroxycholesterol concentrations from eNAD/EDM affected and unaffected horses. Spinal cord 7‐ketocholesterol, 7‐hydroxycholesterol, and 7‐keto‐27‐hydroxycholesterol concentrations were significantly higher in eNAD/EDM affected horses while 24‐ketocholesterol concentrations were significantly lower. Data as min to max, with box extending from 25th to 75th percentiles and line representing the median. * P < .05, ** P < .01, ***P < .001
3.2.6. Correlation with CSF α‐TOH
Cerebrospinal fluid α‐TOH concentrations were available from n = 9 of the 17 samples (n = 3 eNAD/EDM and n = 6 unaffected horses). No correlation was found between any SC oxysterol and SC α‐TOH concentrations.
4. DISCUSSION
Markers of lipid peroxidation, including F2IsoP, F4NP, and oxysterols, did not significantly differ in serum and CSF of eNAD/EDM affected and unaffected horses. However, in SC tissue, targeted markers of cholesterol oxidation, including 7‐ketocholesterol, 7‐hydroxycholesterol, and 7‐keto‐27‐hydroxycholesterol were significantly increased with eNAD/EDM. These particular oxysterols are formed by reactive oxygen species‐mediated oxidation of cholesterol (Figure 2).16 7‐Hydroxycholesterol consists of both 7α‐hydroxycholesterol, which is produced by CYP7A1 and free radical oxidation, and 7β‐hydroxycholesterol, formed by free radical oxidation. Although CYP7A1 is not typically expressed in mammalian SC tissue, we recently identified significant upregulation of this transcript in eNAD/EDM affected horses.13 It is therefore possible that the increased SC 7‐hydroxycholesterol concentrations are because of a combination of increased 7α‐hydroxycholesterol from free radical oxidation and CYP7A1 enzymatic activity and that increased 7β‐hydroxycholesterol is a consequence of free radical oxidation. Increases in 7‐ketocholesterol and its metabolite, 7‐keto‐27‐hydroxycholesterol, support further free radical oxidation of cholesterol in SC tissue of eNAD/EDM affected horses (Figure 2).
Figure 2.
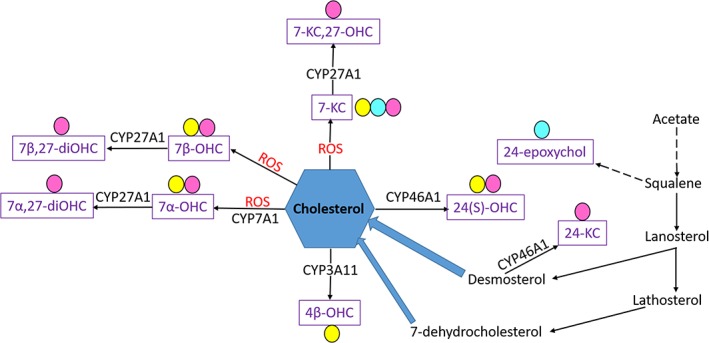
Mechanisms of cholesterol production and generation of oxysterols (purple font) via enzymatic and nonenzymatic oxidation. Specific oxysterols were detectable in equine serum (yellow circle), CSF (teal circle) and SC tissue (pink circle). Cyp, cytochrome P450; ROS, reactive oxygen species; 7‐KC, 7‐ketocholesterol; 7‐KC,27‐OHC, 7‐keto‐27‐hydroxycholesterol; 7β‐OHC, 7β‐hydroxycholesterol; 7β,27‐diOHC, 7β,27‐dihydroxycholesterol; 7α‐OHC, 7α‐hydroxycholesterol; 7,27‐diOHC, 7α,27‐dihydroxycholesterol; 4β‐OHC, 4β‐hydroxycholesterol; 24(S)‐OHC, 24(S)‐hydroxycholesterol; 24‐KC, 24‐ketocholesterol; 24‐epoxychol, 24‐epoxycholesterol
The main mechanism for removing cholesterol from the brain is enzymatic conversion of cholesterol to 24(S)‐hydroxycholesterol through CYP46A1 (Figure 2). A shift toward non‐enzymatic, free‐radical mediated oxidation of cholesterol in the SC tissue of eNAD/EDM affected horses (Figure 1E) indicates that oxidative stress may contribute to disease pathogenesis. Alpha‐TOH prevents lipid peroxidation6 and therefore the associated α‐TOH deficiency observed with eNAD/EDM may directly lead to increased cholesterol oxidation in the central nervous system. However, no direct correlation was observed between SC α‐TOH and oxysterol concentrations. We previously found that although serum, CSF and hepatic α‐TOH concentrations are significantly lower in eNAD/EDM affected horses and highly correlated, SC tissue α‐TOH concentrations did not differ and were not correlated with serum concentrations.13 Therefore, although the pathology associated with eNAD/EDM is confined to the central nervous system and CSF appears to be an appropriate sample to use for assessment of α‐TOH deficiency, SC tissue α‐TOH concentrations may not reflect the deficiency to the same extent. This may be because of variable lipid concentrations in SC tissue, because lipid has the highest concentration of α‐TOH.19 In addition, in the horse, cholesterol may be oxidized to oxidation products not included in our analysis.
The finding of increases in SC 7‐ketocholesterol and 7‐hydroxycholesterol concentrations in eNAD/EDM affected horses agrees with results reported previously. Our previous study identified increased expression of LXR‐responsive transcripts, including CYP7A1 and APOE, in the SC tissue of eNAD/EDM affected horses.13 The liver X receptor is a nuclear transcription factor that requires activation by binding of an oxysterol ligand. The main ligands of LXR include 24(S)‐hydroxycholesterol, 24,25‐epoxycholesterol, 22‐hydroxycholesterol, 20‐hydroxycholesterol and 27‐hydroxycholesterol.20 Of these, only 24‐hydroxycholesterol was quantified in the current study and found to not be increased in the SC tissue of eNAD/EDM affected horses. Therefore, our findings may represent downstream consequences after LXR activation rather than evidence of the initial ligand activation.
24‐Ketocholesterol was significantly decreased in the SC tissues of eNAD/EDM affected horses. Although a negative correlation was observed with age and 24‐ketocholesterol, similar to reports in other species,21 no significant difference in age was found between diseased and healthy groups. 24‐ketocholesterol is produced by CYP46A1 from desmosterol (Figure 2).22 The enzyme CYP46A1 also is responsible for converting cholesterol to 24(S)‐hydroxycholesterol, which was lower, but not significantly, in eNAD/EDM affected horses (Figure 1E), potentially providing further support of decreased CYP46A1 activity. A similar phenomenon was observed by our group in mice deficient in tocopherol (alpha) transfer protein (Ttpa –/–). These mice develop a postnatal sensory neurodegeneration like that observed in eNAD/EDM.21 In wild‐type mice, concentrations of 24(S)‐hydroxycholesterol significantly increase in the SC tissue with age, suggesting adequate CYP46A1 activity. In contrast, concentrations of 24(S)‐hydroxycholesterol do not increase with age in Ttpa –/– mice. Therefore, the enzymatic removal of cholesterol in SC tissue may be altered both in Ttpa –/– mice and eNAD/EDM affected horses during postnatal development. Because various cholesterol products differ in lipophilicity and therefore are eliminated differentially across the blood brain barrier; this situation may contribute to the pathophysiology of α‐TOH deficient neurodegeneration.
Paradoxically, despite higher SC 7‐ketocholesterol concentrations in eNAD/EDM affected horses, CSF 7‐ketocholesterol concentrations were non‐significantly lower (Supporting Information Fig. S1). Like cholesterol, oxysterols can only be eliminated directly from cells to lipophilic acceptors, mediated by specific membrane lipid transporters, including ATP‐binding cassette, subfamily A, member 1 (ACBA1); ATP‐binding cassette, subfamily G, member 1 (ABCG1); and, scavenger receptor BI (SRBI).23 Alterations in these proteins could interfere with oxysterol elimination from cells. Therefore, these are strong candidate genes for eNAD/EDM. Alternatively, altered oxysterol concentrations in neuronal plasma membranes may alter basic membrane properties.24 Although lower CSF 7‐ketocholesterol concentrations in eNAD/EDM affected horses’ warrants further evaluation in a larger sample set, this finding may suggest an altered transport mechanism for 7‐ketocholesterol removal in eNAD/EDM.
F2IsoP, F4NP, and oxysterols are only a subset of markers used to assess lipid peroxidation in biologic systems. Additional markers, including malondialdehyde, which is one of the products formed from decomposition of certain primary and secondary lipid peroxidation products, and thiobarbituric‐acid‐reactivity have been used in other disease processes as markers of lipid peroxidation.9 However, these markers are not recommended for use in biologic systems without correlative data from other indices of fatty peroxide formation and decomposition.25 Therefore, isoprostanes and oxysterols were selected for evaluation in our study. Limitations of our study include inability to perform all assays on all sample types and the lack of matched serum, CSF, and SC tissue results by individual. Additionally, because of limited sample availability, F2IsoP and F4NP were not evaluated in SC tissue. It is therefore possible that, similar to targeted oxysterols, F2IsoP and F4NP may be increased in SC tissue of eNAD/EDM affected horses without increased concentrations in the CSF.
In conclusion, targeted markers of cholesterol oxidation were significantly increased in the SC tissue of eNAD/EDM affected horses. Although serum and CSF biomarkers of eNAD/EDM were not identified, the mechanism of neurodegeneration was further elucidated. Enzymatic removal of cholesterol in SC tissue may be altered in eNAD/EDM affected horses during postnatal development, which could interfere with neuronal membrane signaling mechanisms, resulting in subsequent neurodegeneration.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest
Off‐Label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Institutional Animal Care and Use Committee (IACUC) or Other Approval Declaration
All animal procedures were approved by the University of California‐Davis IACUC and owner consent was obtained for all horses.
Supporting information
Figure S1.
Table S1.
ACKNOWLEDGMENTS
The authors thank the large animal internal medicine residents, veterinary students, and staff at the Center for Equine Health who assisted with this project. This project was supported, in part, by the Center for Equine Health with funds provided by the State of California pari‐mutuel fund and contributions by private donors. Support for this work was provided by the National Institutes of Health (NIH) to CJF. (1K01OD015134 and L40 TR001136) and to LX (R00 HD073270 and R01HD092659). Additional postdoctoral fellowship support was provided by the Morris Animal Foundation (D14EQ‐021) to CJF. All work was performed at the Center for Equine Health, University of California, Davis.
Finno CJ, Estell KE, Winfield L, et al. Lipid peroxidation biomarkers for evaluating oxidative stress in equine neuroaxonal dystrophy. J Vet Intern Med. 2018;32:1740–1747. 10.1111/jvim.15241
Funding information UC Davis Center for Equine Health; National Institutes of Health (NIH), Grant/Award Numbers: 1K01OD015134, L40 TR001136; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R00 HD073270, R01HD092659; Morris Animal Foundation, Grant/Award Number: D14EQ‐021
REFERENCES
- 1. Mayhew IG, Brown CM, Stowe HD, et al. Equine degenerative myeloencephalopathy: A vitamin E deficiency that may be familial. JVet Intern Med. 1987;1:45–50. [DOI] [PubMed] [Google Scholar]
- 2. Aleman M, Finno CJ, Higgins RJ, et al. Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in Quarter Horses. JAm Vet Med Assoc. 2011;239:823–833. [DOI] [PubMed] [Google Scholar]
- 3. Mayhew IG, deLahunta A, Whitlock RH, et al. Spinal cord disease in the horse. Cornell Vet. 1978;68 Suppl 6:1–207. [PubMed] [Google Scholar]
- 4. Muller DP, Goss‐Sampson MA. Neurochemical, neurophysiological, and neuropathological studies in vitamin E deficiency. Crit Rev Neurobiol. 1990;5:239–263. [PubMed] [Google Scholar]
- 5. Finno CJ, Estell KE, Katzman S, et al. Blood and cerebrospinal fluid alpha‐tocopherol and selenium concentrations in neonatal foals with neuroaxonal dystrophy. JVet Intern Med. 2015;29:1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niki E. Role of vitamin E as a lipid‐soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med. 2014;66:3–12. [DOI] [PubMed] [Google Scholar]
- 7. Choi J, Leonard SW, Kasper K, et al. Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. JLipid Res. 2015;56:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDougall M, Choi J, Truong L, et al. Vitamin E deficiency during embryogenesis in zebrafish causes lasting metabolic and cognitive impairments despite refeeding adequate diets. Free Rad Mol Biol. 2017;110:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–5972. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida Y, Umeno A, Shichiri M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. JClin Biochem Nutr. 2013;52:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forman HJ, Fukuto JM, Miller T, et al. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4‐hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zmijewski JW, Landar A, Watanabe N, et al. Cell signalling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem Soc Trans. 2005;33:1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finno CJ, Bordbari MH, Valberg SJ, et al. Transcriptome profiling of equine vitamin E deficient neuroaxonal dystrophy identifies upregulation of liver X receptor target genes. Free Rad Mol Biol. 2016;101:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janowski BA, Grogan MJ, Jones SA, et al. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci USA. 1999;96:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janowski BA, Willy PJ, Devi TR, et al. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996;383:728–731. [DOI] [PubMed] [Google Scholar]
- 16. Guillemot‐Legris O, Mutemberezi V, Muccioli GG. Oxysterols in metabolic syndrome: From bystander molecules to bioactive lipids. Trends Mol Med. 2016;22:594–614. [DOI] [PubMed] [Google Scholar]
- 17. Milne GL, Sanchez SC, Musiek ES, et al. Quantification of F2‐isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. [DOI] [PubMed] [Google Scholar]
- 18. Xu L, Korade Z, Rosado DA Jr, et al. An oxysterol biomarker for 7‐dehydrocholesterol oxidation in cell/mouse models for Smith‐Lemli‐Opitz syndrome. JLipid Res. 2011;52:1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roneus BO, Hakkarainen RV, Lindholm CA, et al. Vitamin E requirements of adult Standardbred horses evaluated by tissue depletion and repletion. Equine Vet J. 1986;18:50–58. [DOI] [PubMed] [Google Scholar]
- 20. Olkkonen VM, Beaslas O, Nissila E. Oxysterols and their cellular effectors. Biomolecules. 2012;2:76–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finno CJ, Bordbari MH, Gianino G, et al. An innate immune response and altered nuclear receptor activation defines the spinal cord transcriptome during alpha‐tocopherol deficiency. Free Rad Mol Biol. 2018;120:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goyal S, Xiao Y, Porter NA, et al. Oxidation of 7‐dehydrocholesterol and desmosterol by human cytochrome P450 46A1. JLipid Res. 2014;55:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jessup W, Gelissen IC, Gaus K, et al. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr Opin Lipidol. 2006;17:247–257. [DOI] [PubMed] [Google Scholar]
- 24. Brown AJ, Jessup W. Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med. 2009;30:111–122. [DOI] [PubMed] [Google Scholar]
- 25. Janero DR. Malondialdehyde and thiobarbituric acid‐reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad Mol Biol. 1990;9:515–540. [DOI] [PubMed] [Google Scholar]
- 26. Lunn DP, Mayhew IG. The neurologic evaluation of horses. Equine Vet Educ. 1989;1:94–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.


