Abstract
Background
Pharmacological treatment of atrial fibrillation (AF) in horses can be challenging because of low efficacy and adverse effects. Flecainide has been tested with variable efficacy.
Objective
To test whether the efficacy of flecainide is dependent on AF duration.
Animals
Nine Standardbred mares.
Methods
Factorial study design. All horses were instrumented with a pacemaker and assigned to a control or an AF group. On day 0, all horses were in sinus rhythm and received 2 mg/kg flecainide IV. Atrial fibrillation subsequently was induced in the AF group by pacemaker stimulation. On days 3, 9, 27, and 55, flecainide was administered to all horses, regardless of heart rhythm.
Results
All horses in AF cardioverted to sinus rhythm on days 3 and 9. On day 27, 5/6 horses cardioverted, whereas only 2/6 cardioverted on day 55. The time from the start of flecainide infusion to cardioversion (range, 3–185 min, log transformed) showed linear correlation with the cumulative duration of AF (r 2 = .80, P < .0001). Flecainide induced abnormal QRS complexes in 4/6 AF horses and 1/3 controls. A positive correlation was found between heart rate before flecainide infusion and number of abnormal QRS complexes (0.14, P < .05). One horse suffered from cardiac arrest and died after flecainide infusion.
Conclusions and Clinical Importance
Flecainide is effective for cardioversion of short‐term induced AF, but the effect decreases with AF duration. Controlling heart rate may minimize adverse effects caused by flecainide, but the drug should be used with great caution.
Keywords: arrhythmia, atria, cardioversion, electrocardiography, heart, wide QRS tachycardia
Abbreviations
- AF
atrial fibrillation
- AFL
atrial flutter
- bpm
beats per minute
- BW
body weight
- HR
heart rate
- HRrest
resting heart rate
- JTc
corrected JT interval
- QTc
corrected QT interval
1. INTRODUCTION
Atrial fibrillation (AF) is the most common pathological arrhythmia in horses. The arrhythmia may lead to decreased performance and cause safety issues for the rider or driver.1 Atrial fibrillation leads to electrical, contractile, and structural remodeling of the atria, making successful cardioversion more difficult to achieve with increased AF duration.2 It is therefore important to know the duration of AF when choosing a treatment strategy for the horse.3 Cardioversion of AF can be performed pharmacologically with quinidine, which has an efficacy of about 85% in horses with no underlying cardiac disease.3 However, this treatment frequently results in adverse cardiac or noncardiac reactions, and treatment may need to be terminated before cardioversion. The search for alternative solutions is therefore ongoing, and antiarrhythmic drugs tested in horses include flecainide,4, 5, 6 amiodarone,7, 8 propafenone,9 sotalol,10 NS8593,11 dofetilide, and ranolazine.12 Some of these drugs have only been tested in an experimental setting,11, 12 whereas others also have shown some effect in naturally occurring AF.7, 8
Flecainide is a class Ic antiarrhythmic drug that blocks sodium currents and thereby slows intramyocardial conduction. Electrophysiological studies in horses have shown an increase in the AF cycle length preceding cardioversion, but no effect on the atrial effective refractory period.4, 6 Studies on flecainide for cardioversion of AF have provided conflicting results. High efficacy was seen in horses with experimentally induced AF,4, 5 but flecainide was only effective in 1 of 10 horses with naturally occurring AF and in 41% of horses with recent‐onset AF.6, 13 Reported adverse effects include agitation, restlessness, neck sweating, decreased borborygmi5, 14 and several cases of ventricular proarrhythmia and sudden death.6, 15, 16, 17 Flecainide resulted in widening of the QRS complex4, 6, 15 and 1 study also reported prolongation of the QT interval.4 Flecainide is widely used for cardioversion and the maintenance of sinus rhythm in humans suffering from AF. Guidelines for selecting treatments in human AF patients indicate that flecainide can be used for those with recent‐onset AF and no other major cardiac abnormalities such as hypertension, ischemia, or impaired left ventricular function.18, 19
We hypothesized that the antiarrhythmic effects of flecainide in horses are dependent on the duration of AF. Information on the maximum duration of AF allowing for cardioversion with flecainide would help to guide clinicians considering flecainide as a treatment option. Therefore, the aim of our study was to test flecainide treatment in horses with induced AF at selected time points and different AF durations.
2. MATERIALS AND METHODS
2.1. Horses
Nine Standardbred mares were studied; 6 in an AF group (horses 1–6) and 3 in a group of sham‐operated time‐matched controls (horses 7–9). The AF group had a mean age of 10 ± 3 years (range, 5‐14 years) and mean body weight (BW) of 479 ± 49 kg (range, 408‐572 kg) at inclusion in the study, and the control group had a mean age of 12 ± 6 years (range, 4‐17 years) and mean BW of 466 ± 32 kg (range, 423‐498 kg). Baseline, routine venous blood analyses including hematology and serum biochemistry profiles were performed for all horses and all results were within normal limits. The horses included in the study had no signs of cardiovascular disease after clinical examination, thorough cardiac auscultation, 12‐hour Holter ECG (KRUTECH Televet, Kruuse A/S, Maarslev, Denmark) and routine echocardiographic examination (2D, M‐mode and color flow Doppler)20 with a portable ultrasound unit with a phased array transducer (Vivid I and 3S Phased Array transducer, GE Healthcare, Horten, Norway). The present study was part of a larger study on chronic AF development in horses, and the same horses were therefore included in other studies.
The study was approved by the local ethical committee at the Department of Veterinary Clinical Sciences, University of Copenhagen and The Danish Animal Experiments Inspectorate (license number 2015‐15‐0201‐00693), and was performed in accordance with the European Commission Directive 86/609/EEC.
2.2. Pacemaker implantation
All horses (both controls and AF group) had a dual‐chamber pacemaker implanted (Assurity MRI 2272, St. Jude Medical, St. Paul, MN) with 2 bipolar leads (Tendril STS Pacing Leads 100 cm, St. Jude Medical, St. Paul, MN) in the right atrium, by a modified technique described previously.43 In brief, the horses were restrained in a stock, sedated with 0.01 mg/kg BW detomidine (Domosedan, Orion Pharma Animal Health, Copenhagen, Denmark) and 0.01 mg/kg BW butorphanol (Torbugesic, Orion Pharma Animal Health, Copenhagen, Denmark) followed by a constant‐rate infusion of 180–360 mL/h of 1.0 mg/mL xylazine (Xysol, ScanVet Animal Health A/S, Fredensborg, Denmark). A modified base‐apex ECG was recorded throughout the procedure. The area of the right lateral pectoral groove was locally anesthetized and an incision of 6–8 cm was made followed by blunt preparation of the cephalic vein. The pacemaker leads were introduced to the right atrium through a venotomy in the cephalic vein, and their placement was guided by stimulation via the programmer (Merlin Patient Care System Programmer, St. Jude Medical, St. Paul, MN) while advancing the leads until atrial capture. Once the leads had been fixed with a screw‐in technique at a position where atrial capture was consistent, the stimulation threshold and lead impedance were determined by the programmer. The leads were secured with a ligature around the cephalic vein and connected to the pacemaker. The pacemaker was fixed to the underlying tissue in a SC pocket distal to the incision, and the incision was closed in 3 layers. The operation time was between 60 and 150 min. All horses were treated with antibiotic and nonsteroidal anti‐inflammatory therapy for the next 3 days. The animals were tied to prohibit lying down or rolling for 1 week after the procedure, and the experiments began after a recovery period of 3 weeks.
2.3. Study protocol
An overview of the study protocol is presented in Figure 1. The study was performed over a period of 55 days. All horses (both the AF and control groups) had flecainide (Tambocor, Meda A/S, Allerød, Denmark; 2 mg/kg, rate 0.2 mg/kg/min) administered IV while in sinus rhythm, which allowed performance of ECG analyses (see below) after flecainide infusion in the AF group before induction of AF. Induction of AF with burst‐ and high‐rate pacing was initiated in the AF group 6‐ 8 hours after baseline flecainide administration, and continued until AF was self‐sustained or the next flecainide administration on days 3, 9, 27, and 55 by the same protocol as described above. No AF induction was performed in the control group. Pacing was stopped 30 minutes before flecainide infusion for horses in the AF group without self‐sustained AF. If cardioversion was successful, AF induction was restarted approximately 6 hours later and continued until self‐sustained AF was reached or the next flecainide testing was scheduled. Flecainide administration was performed between 9 AM and 12 PM on all procedure days. In addition, cardioversion of AF was attempted in horse 1 on day 59 with 2 doses of flecainide. The second dose of flecainide was identical to the first and was infused 10 min after the first infusion ended. Because of an episode of colic, flecainide was not administered on day 3 in 1 control horse (horse 9).
Figure 1.
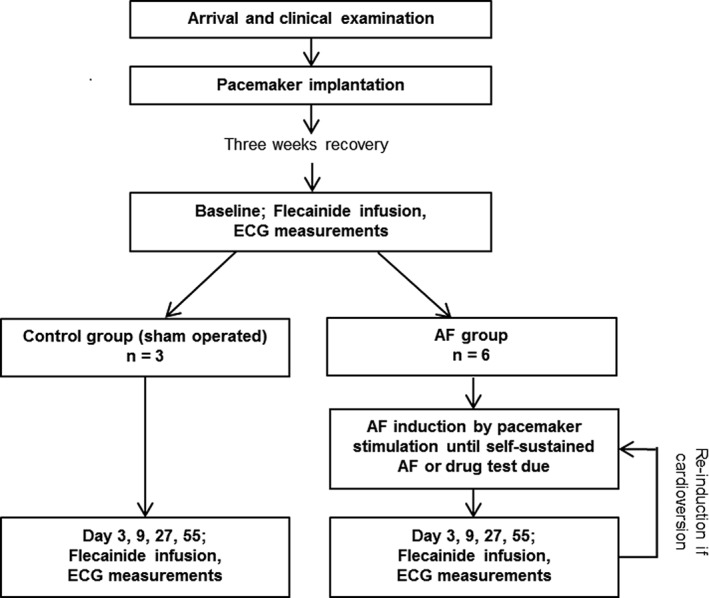
Overview of the study protocol. The flowchart shows the order of interventions for the horses in the two groups. AF = atrial fibrillation
2.4. AF induction
A pacemaker was used to induce AF by burst‐pacing (20 seconds, 10 Hz, pulse amplitude 7.5 mV, pulse width 1.5 ms) in the right atrium. Burst‐pacing was limited to strains of 20 seconds by the pacemaker. These strains were delivered on a daily basis (1‐8 hours per horse) until AF was self‐sustained. In between burst‐pacing sessions, the pacemaker was set to stimulate in an inhibitory mode with an atrial rate of 170 per minute (pulse amplitude 2.5 mV, pulse width 0.4 ms) from each lead, leaving the horse with an atrial paced rhythm of 340 per minute. This practice ensured that the atria were not left in sinus rhythm but constantly were subjected to a fast rate throughout the study. Self‐sustained AF was defined as AF of >10 minutes duration without stimulation from the pacemaker. When self‐sustained AF had reached a duration of more than 24 hours, the pacemaker was switched off. The horses were subsequently auscultated daily to confirm AF.
2.5. ECG recordings
Electrocardiograms were recorded throughout each procedure day with a Holter unit with 2 separate channels and bipolar leads as previously described.4 Additionally ECGs were recorded between procedure days to assess heart rhythm if auscultation was not feasible. During AF induction, ECG was continuously recorded. If a horse was in self‐sustained AF after a burst‐pacing session, the Holter unit remained on to assess if and when cardioversion occurred, but if AF was not self‐sustained and the horse was left with an atrial paced rhythm over night, the Holter unit was removed until burst‐pacing was initiated again. The cumulative duration of AF (including all periods of self‐sustained AF) for each horse was assessed by analyzing the ECG recordings and documented auscultations. Burst‐pacing, pacemaker activity, and time periods of unknown activity in the atria were not included.
Heart rate at rest (HRrest) was measured for all horses before day 0 on surface ECGs obtained between 8 PM and 6 AM, because the horses were undisturbed in their stables during this period. Three time‐points at which the heart rate (HR) was low and stable were chosen from the heart rate view (Televet) and HR was measured as an average of 10 beats for each time point. The HRrest was calculated again in the AF group once AF was self‐sustained, and on day 27 or 55 of the study (minimum, 11 hours after flecainide infusion) for the control group. The mean HR before flecainide infusion was calculated for all horses by averaging all RR intervals for 30 minutes before the start of infusion.
The QRS durations and QT intervals were manually analyzed in lead II at 4 different time points: before, 1 minute after, 30 minutes after, and 60 minutes after the end of flecainide infusion. Five repeated measurements were performed at each time point. The QT measurements were corrected according to HR with a piecewise linear regression model,21 and JTc intervals were calculated as QTc minus QRS. To identify abnormal QRS complexes, ECGs were manually analyzed 30 minutes before, during and 30 minutes after the end of flecainide infusion. The QRS complexes were classified as abnormal if they had bizarre morphology or the R‐on‐T phenomenon was present. Occurrence was characterized as either single or coupled abnormal QRS complexes or episodes of consecutive abnormal QRS complexes (≥3 abnormal complexes sequentially).
2.6. Statistical analysis
Data are presented as mean ± SD when normally distributed and without SD when the distribution was not normal. The cumulative duration of AF for responders (cardioversion to sinus rhythm after flecainide infusion) and nonresponders (no cardioversion) was compared using an unpaired t test. The time (min) to cardioversion was log transformed before performing linear regression of the time to cardioversion as a function of the cumulative AF duration (in days). We compared the mean HRrest before and after the start of the study in both the AF group and the controls by paired t tests (GraphPad Prism 5 software, GraphPad Software, San Diego, CA). A linear mixed model including heart rhythm (AF or sinus rhythm) and study day was used to test the correlation between number of abnormal QRS complexes after flecainide infusion and mean HR before infusion. Analyses of QRS duration, JTc and QTc also were performed by a linear mixed model (R 3.3.1 software, The R Foundation for Statistical Computing, Vienna, Austria). The QRS duration and QTc after flecainide infusion were compared for the AF group and the controls by an unpaired t test. P ≤ .05 was considered to be significant.
3. RESULTS
On day 3, only 1 horse had self‐sustained AF and this horse cardioverted 3.0 minutes after flecainide infusion began. The cumulative duration of AF up to day 3 was 0.4 ± 0.5 days (range, 0.1‐1.3 days). On day 9, 5 of 6 horses had self‐sustained AF and all 5 horses cardioverted to sinus rhythm within 8.8 minutes (range, 6.0‐12.0 minutes) of the start of flecainide infusion. The cumulative duration of AF up to day 9 was 1.4 ± 0.9 days (range, 0.5‐2.8 days). All horses were in self‐sustained AF on day 27 and day 55. Five of 6 horses cardioverted on day 27 within 25.0 minutes (range, 4.0‐62.0 minutes), whereas 2 of 6 cardioverted on day 55 at 96.0 and 185.0 minutes, respectively. The cumulative duration of AF up to day 27 was 11.25 ± 6.8 days (range, 0.8‐17.3 days) for the responders, with the nonresponder exhibiting the longest AF duration of 18.3 days. Before day 55, the cumulative duration of AF was 37.4 ± 11.8 days (range, 15.2‐46.3 days) for all paced horses. The AF duration for the nonresponders and responders up to day 55 was 37.9 ± 15.1 and 36.4 ± 2.0 days, respectively (P = .90). Linear regression with log transformed time to cardioversion as the response showed an effect size (slope) of 0.04 (95% confidence interval [CI] 0.02, 0.05) for cumulative AF duration, and an overall coefficient of determination (r 2) of .80 (P < .0001, Figure 2).
Figure 2.
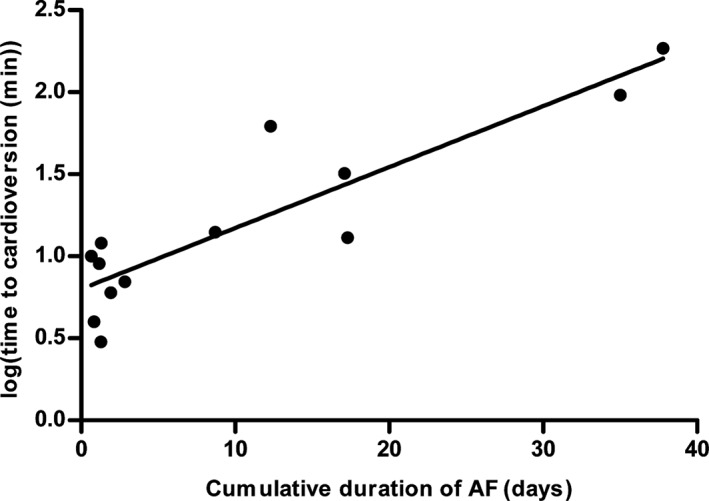
Time to cardioversion as a function of the cumulative duration of atrial fibrillation. Time to cardioversion (in min) was log transformed and plotted against the cumulative duration of self‐sustained atrial fibrillation (AF) for all flecainide infusions in horses with AF. Linear regression revealed a slope of 0.04 (95% CI 0.02, 0.05) with a coefficient of determination of 0.80
The ECG showed that AF slowed during treatment into more distinct, coarse fibrillatory waves in the time preceding cardioversion in the majority of horses. In horse 2 and horse 5, AF was terminated by flecainide on day 55, and this coarse AF state lasted >1 hour and 2.5 hours before cardioversion, respectively. More than 30 minutes of coarse AF also preceded cardioversion on day 27 in horse 5. The transition from AF to coarse AF is illustrated in Figure 3. Coarse AF was not seen in procedures where AF was not cardioverted.
Figure 3.
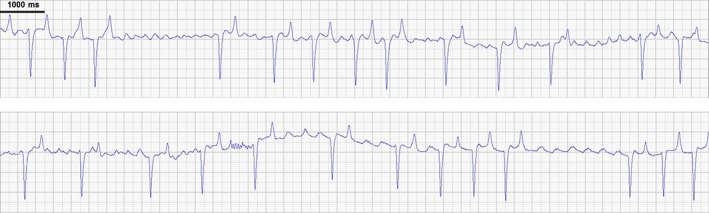
Transition from fine to coarse AF. The ECG sections (lead II) should be viewed in succession. The ECG was recorded 12 min after the end of flecainide infusion on day 27. It is evident that the atrial fibrillation becomes coarse, with distinct fibrillatory waves. The atrial rate is 170/min (352 ms interval) in the last part of the ECG. The horse cardioverts to sinus rhythm 40 min later
The mean HRrest was significantly increased after AF induction compared to before induction in the AF group: ΔHRrest = 12 ± 9 beats per minute (bpm); range, 4‐26 bpm, P < .001 (Figure 4). The increase was largely attributed to 2 horses (horses 1 and 4) with a very high ΔHRrest: 17 and 26 bpm, respectively. An ECG was not available for horse 3 in AF in the specified time period (8 PM‐6 AM) and this horse was not included in the analysis.
Figure 4.
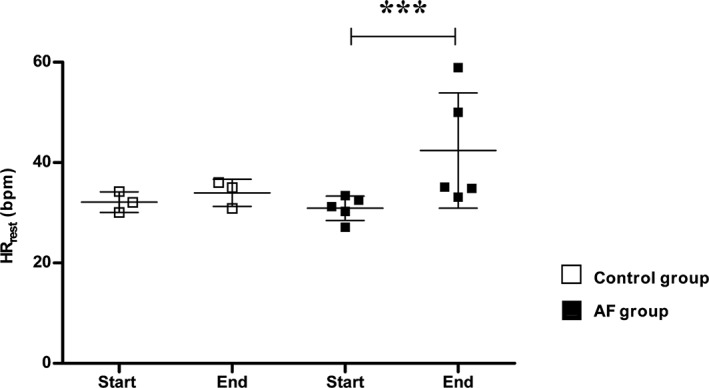
Mean heart rate at rest. Mean heart rate at rest (HRrest) for the individual horses measured on surface baseline ECG “start” and after pacing “end” in control and AF groups. The line and error bars reflect the mean ± SD. *** = P < 0.001
The ECG parameters QRS duration and QTc were significantly prolonged by flecainide infusion compared to baseline measurements (P < .001) in the first hour after infusion, with the largest prolongation measured immediately after the end of flecainide infusion (“0 min”, Figure 5). The difference between QTc and QRS, represented by JTc, also was prolonged at all time points after flecainide infusion (P < .001). The QRS duration after flecainide infusion was 125 ± 18 ms in the AF group and 114 ± 11 ms in the control group (P < .001). The QTc was 477 ±38 ms and 481 ±32 ms in the AF and control groups, respectively (P = .223).
Figure 5.
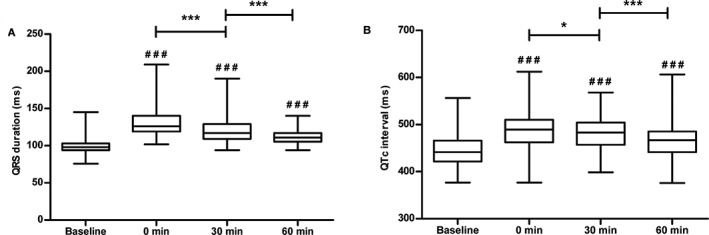
Ventricular effects of flecainide administration. QRS duration (A) and corrected QT intervals (QTc) (B) before and after flecainide treatment in all horses (n = 9) presented in box plots with whiskers from minimum to maximum values. # Represents the significance level of the comparison between the time points after flecainide infusion and the baseline (### = P < 0.001). * = P < 0.05, *** = P < 0.001
None of the horses experienced any noncardiac adverse reactions to flecainide, but adverse cardiac reactions were seen in several horses and 1 horse suffered from sudden cardiac death after 2 consecutive flecainide infusions. Table 1 summarizes the number of animals that experienced abnormal QRS complexes 30 minutes before, during and 30 minutes after flecainide infusion. Abnormal QRS complexes were observed in 4 of the horses in the AF group (horses 1‐4), with horses 1 and 4 also suffering episodes of consecutive abnormal QRS complexes. All 4 horses experienced abnormal QRS complexes both with and without the influence of flecainide. Only 1 abnormal complex was found after flecainide infusion in the control group. Examples of both single abnormal QRS complexes and an episode of consecutive abnormal QRS complexes after infusion of flecainide are shown in Figure 6. Detomidine (5 mg) was administered to 2 of the horses with consecutive abnormal QRS complexes (Table 1) in an attempt to stop the tachycardia. No further subsequent abnormal QRS complexes were identified. A correlation was found between the mean HR for the 30 minutes preceding the start of flecainide infusion and the number of abnormal QRS complexes after the start of infusion (effect size = 0.14; 95% CI, 0.0047, 0.2766; P < .05).
Table 1.
Prevalence of abnormal QRS complexes and ventricular tachycardia before and after flecainide infusion
| AF group | Control group | ||||
|---|---|---|---|---|---|
| Abnormal QRS complex | Consecutive abnormal QRS complexes | Abnormal QRS complex | Consecutive abnormal QRS complexes | ||
| Day 0 | Before treatment | 1 (1) | – | – | – |
| Flecainide | – | – | – | – | |
| Day 3 | Before treatment | 1 (20) | – | – | – |
| Flecainide | – | – | – | – | |
| Day 9 | Before treatment | 2 (6/12*) | – | – | – |
| Flecainide | 3 (2/3/6) | – | 1 (1) | – | |
| Day 27 | Before treatment | 1 (1) | – | – | – |
| Flecainide | 4 (1/3/7*/12*) | 2 (1/4#) | – | – | |
| Day 55 | Before treatment | 2 (2/8*) | 1 (1) | – | – |
| Flecainide | 2 (10*/16*) | 2 (1/1#) | – | – | |
Number of horses experiencing single or coupled abnormal QRS complexes and number of horses experiencing episodes of consecutive abnormal QRS complexes in separate columns. The number of abnormal complexes and episodes of consecutive abnormal complexes are specified in brackets with the number for each horse separated by “/”. * indicates that some of the abnormal QRS complexes are coupled. # indicates that detomidine was administered. AF = atrial fibrillation; Before = 30 min before flecainide infusion; Flecainide = during and 30 min after flecainide infusion.
Figure 6.
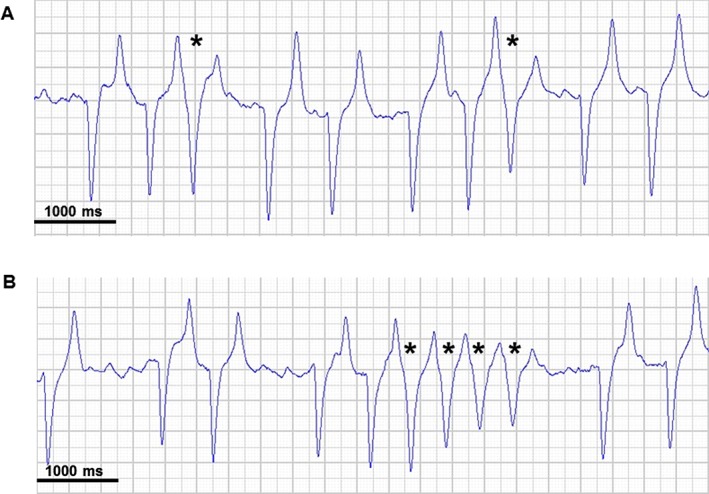
Abnormal ECG findings after flecainide infusion. Single abnormal QRS complexes (A) and an episode of consecutive abnormal QRS complexes (B). The QRS complexes were classified as abnormal because they display the R‐on‐T phenomenon. All ECG examples are lead II. * indicates abnormal complexes
One dose of flecainide was unable to cardiovert horse 1 on day 55, and thus 2 doses were used on day 59 (10 minutes between doses). No abnormal QRS complexes were encountered during infusion of the first dose or in the 10 minutes after infusion. However, the horse developed several runs of torsades‐like ventricular tachycardia with preceding short‐long‐short coupling initiation before developing ventricular fibrillation and eventually cardiac arrest during administration of the second dose. Flecainide infusion was stopped when the ventricular tachycardia developed but no other drugs were administered to this horse because of concerns about personnel safety in establishing IV access. Figure 7A displays the last 6 minutes of ECG recorded from this horse, and shows that the QRS complex gradually became wider. Simultaneously, AF became coarser, as illustrated by the appearance of distinct fibrillatory waves, similar to those observed in the horses before cardioversion to sinus rhythm (Figure 7B).
Figure 7.
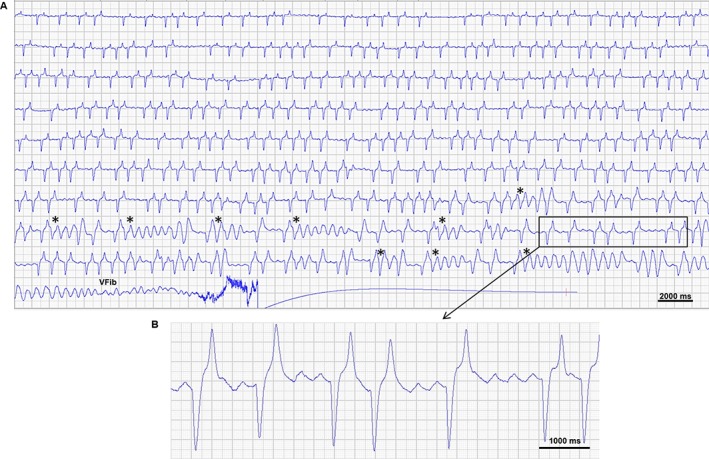
Electrocardiogram obtained during flecainide infusion resulting in cardiac arrest. The ECG (lead II) is from a horse receiving a second dose of flecainide in an attempt to cardiovert atrial fibrillation with a duration of 59 days. After several episodes of torsades‐like ventricular tachycardia with preceding short‐long‐short coupling initiation (*) the horse develops ventricular fibrillation (VFib). A) Is an overview of the last 6 min leading up to cardiac arrest and B) is a section of the ECG where distinct fibrillatory waves appear at a rate of 163/min (368 ms interval)
4. DISCUSSION
Ours is the first systematic study to investigate the time‐dependent ability of flecainide to terminate AF in horses. The results indicate that flecainide could be considered for the cardioversion of short‐duration AF, but also that great caution should be used because the potential adverse effects include sudden death. The HRrest during AF was markedly increased for some horses. A correlation was found between HR and the number of abnormal QRS complexes in the ventricles after flecainide infusion. Furthermore, the horses experiencing episodes of consecutive abnormal QRS complexes were those with the highest HRrest. Abnormal QRS complexes were observed in the AF group before flecainide infusion, and to a greater extent after flecainide infusion, confirming the ventricular proarrhythmicity of flecainide.
With flecainide use, all horses in AF cardioverted on days 3 and 9, and 5 of 6 horses cardioverted on day 27, whereas only 2 of 6 horses cardioverted on day 55 after the start of AF induction. These results correlate with those of previous studies, which found flecainide to be effective in induced AF of very short duration (15 minutes),4, 5 but not in naturally occurring chronic AF.6, 14 In 1 study, naturally occurring AF with a duration of 12 days could be terminated by flecainide infusion,6 and in another study, flecainide was effective in 2 cases of naturally occurring AF.5 Furthermore, flecainide was reported to cardiovert 12 of 29 horses with recent‐onset AF.13 These findings suggest that the effects seen with flecainide are not restricted to experimentally induced AF. Because the horses in our study did not develop AF naturally, they may lack some of the cardiovascular factors leading to AF. A certain degree of remodeling is necessary for AF to be self‐sustained, creating the substrate for the maintenance of AF.22 The cumulative duration of AF in this protocol referred only to self‐sustained AF, implying that remodeling must have been present, mimicking naturally occurring AF. The remodeling we expect to be present is electrical and contractile remodeling which was seen in horses after 4 and 12 hours of induced AF, respectively.15 Furthermore, structural remodeling has been found in goats after 1 week of pacing‐induced AF, which likely also occurred in these horses.23 The time from the start of flecainide infusion to cardioversion was correlated with the cumulative duration of AF. This finding demonstrates the process of remodeling and the importance of knowing AF duration when selecting a therapeutic regimen.
Comparing cardioversion and the cumulative duration of self‐sustained AF, flecainide appears to be a plausible treatment option in AF with a duration <2 weeks, provided great caution is taken because the drug may cause severe adverse effects, including sudden death. The horse that did not cardiovert on day 27 also had the longest duration of self‐sustained AF (18.3 days). In human medicine, flecainide primarily is used for acute onset AF (<48 hours), and its reported efficacy is between 52 and 95%.24 A consensus statement indicated that in horses with AF, cardioversion should not be attempted before 24‐48 hours of documented AF because of the chance of spontaneous cardioversion.1 In humans, lack of early intervention may, however, cause recurrence of AF in the long term, and early cardioversion may lower the risk of relapse.25 This can be explained by the electrophysiological remodeling that occurs within a few hours of AF, which facilitates persistence of the arrhythmia.15, 22
Both horses that responded to flecainide on day 55 showed a change from fine to coarse AF before cardioversion to sinus rhythm. The coarse AF likely represents an increase in the atrial fibrillation cycle length as seen previously after flecainide infusion in horses.4, 26 In humans, conversion from AF to atrial flutter (AFL) with class Ic antiarrhythmic compounds is a known complication. By slowing down conduction velocity, flecainide can transform the unorganized multiple microreentry circuits in the atria during AF into a single organized macroreentry circuit that is seen as AFL on the ECG.27 Because class Ic antiarrhythmic drugs do not slow atrioventricular conduction, a risk of 1:1 atrioventricular conduction with wide QRS tachycardia exists.28, 29 In these horses, the coarse AF terminated without further treatment and no 1:1 conduction through the atrioventricular node was observed. Pharmacological cardioversion of AFL tends to be difficult30, 31 and AFL is not well‐characterized in horses. These considerations led us to avoid defining coarse AF as AFL.
Whether abnormal QRS complexes such as those encountered in our study are caused by re‐entry phenomena in the ventricle or rapid atrioventricular conduction must be elucidated further. In humans, ventricular tachycardia often is caused by re‐entry involving some degree of damage in the cardiac tissue, or triggered activity after delayed repolarization.32 Because both single and consecutive abnormal QRS complexes occurred in the AF group without the influence of flecainide and ventricular damage because of AF seems improbable, aberrant conduction appears to be the most likely cause. However, because we were unable to determine the origin of the abnormal complexes, no classification was made.
Flecainide infusion resulted in more abnormal QRS complexes than were encountered before drug infusion, and the drug appears to pose a risk. The number of abnormal QRS complexes observed after the start of flecainide infusion was weakly correlated with HR before infusion. Resting tachycardia in AF usually occurs because of sympathetic nervous stimulation or underlying cardiac disease.1 All horses had healthy hearts upon inclusion in the study, and no horse showed signs of heart failure during the experiments. Sympathetic stimulation because of stress or pain from the experimental setup could be a possible explanation for the increase in HRrest, but the sham‐operated control animals did not experience an increase in HRrest, despite being subjected to the same procedures as the AF horses. The increase in sympathetic stimulation could have had a larger effect on the atrioventricular node compared to the sinoatrial node and consequently only increased HRrest in the AF group. Alternatively, the increase in sympathetic nervous stimulation may be unrelated to the experimental design and caused by stress or pain because of the presence of AF.
Fast atrial rhythm (eg, AF with a slow cycle length or AFL) in combination with an atrioventricular node capable of conducting these high rates into the ventricles poses a risk for severe, life‐threatening arrhythmias.33 The incidence consequently is increased by the presence of adrenergic stimulation,34 and the general advice is to administer atrioventricular nodal blocking drugs such as β‐blockers or nondihydropyridine calcium channel antagonists along with class Ic antiarrhythmic drugs in humans.19 Flecainide has a high affinity for sodium channels in the open state and the effect is therefore strongly use dependent.35 Flecainide also has some blocking effects on potassium channels, but this effect is decreased at higher HRs (reverse use dependency).36 Consequently, increased HR will result in a greater decrease in ventricular conduction velocity by flecainide and could lead to increased risk of re‐entry arrhythmias.37 This substantiates the recommendation of concurrent rate control during flecainide treatment.
A wide QRS tachycardia after flecainide administration previously has been reported in horses, and a slowing of the ventricular rate with the alpha 2‐adrenoreceptor agonist detomidine was able to terminate the dysrhythmia.6 Detomidine administration causes bradycardia by inhibiting the sympathetic nervous system and increasing vagal tone as a response from the baroreceptors to hypertension induced by initial alpha‐2β adrenoreceptor stimulation.38, 39 In accordance with this effect, no further abnormal QRS complexes were seen in the 2 horses in which detomidine was infused in our study. No rate‐controlling drugs were administered with flecainide to the horse that received the double dose and we therefore do not know whether slowing the atrioventricular conduction would have abrogated the occurrence of ventricular fibrillation. Flecainide also can cause monomorphic ventricular tachycardia in humans and it may be challenging to distinguish between this rhythm and 1:1 conduction of AFL.33 However, based on the successful use of detomidine in preventing further consecutive abnormal QRS complexes in these horses, it seems likely that what we observed was 1:1 conduction of the supraventricular tachycardia. These findings strongly indicate that flecainide should be used in conjunction with a rate‐controlling drug in horses, but additional investigations are warranted.
Flecainide infusion prolonged the QRS duration and QTc interval. The QRS duration reflects ventricular conduction velocity, and prolongation was expected and corresponded with the findings from previous studies.4, 5, 6 After flecainide infusion, the QRS duration was longer in the AF horses compared to the controls and one could speculate that this could have contributed to the occurrence of abnormal QRS complexes. As seen in Table 1, these primarily occurred when the horses were in AF. Prolongation of the QTc interval is not consistently reported in horses. One study found a prolongation only at higher doses of flecainide,5 whereas a second study found prolongations similar to what we observed.4 We could show that the ventricular repolarization was prolonged independently from QRS prolongation by calculating the JTc interval. This prolongation is linked to the risk of torsades de pointes ventricular tachycardia in humans.40 However, flecainide does not induce JTc prolongation in humans, and flecainide‐induced torsades de pointes ventricular tachycardia is rare.41, 42 A JTc prolongation after flecainide infusion also was found in another study,4 but the association between this prolongation and torsades de pointes ventricular tachycardia in horses remains to be proven.
Spontaneous cardioversion rates after experimentally induced AF of hours or days in duration is difficult to predict because limited information is available in the literature. One study found that 4/6 horses subjected to pacing‐induced AF of 7 day's duration cardioverted spontaneously within 3 days of no pacing.15 Our study did not include any control horses receiving saline, which would have allowed us to study spontaneous cardioversion. This design feature is a limitation of this study as we are unable to tell whether spontaneous cardioversion may have occurred on the specified procedure days. However, the fact that most AF episodes were self‐sustained for hours to weeks before treatment, and cardioversion was achieved within a short time of flecainide infusion, strongly indicates that cardioversion was induced by the antiarrhythmic effects of flecainide.
The ECGs were analyzed no more than 30 minutes before and 60 minutes after flecainide infusion. Furthermore, ECG recordings were obtained mainly on procedure days and during AF induction. Additional uninterrupted ECG recordings or a larger study population could have provided a larger set of data and made comparisons more valid. Statistical significance should be evaluated with the number of animals studied in mind.
In conclusion, the efficacy of flecainide for AF cardioversion in horses with experimentally induced AF decreases with the duration of AF. Increased HR was correlated with the number of abnormal ventricular complexes after flecainide infusion, and further investigation into rate‐controlling drugs administered in conjunction with flecainide is warranted. Until further studies are available, flecainide should be used with great caution in horses because the drug can lead to severe proarrhythmic events and sudden death.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge St. Jude Medical A/S Denmark for their kind assistance with pacemakers and pacemaker leads. Furthermore, we acknowledge all staff members at The Large Animal Teaching Hospital, Department of Veterinary Clinical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen involved in the project. All experiments were performed at the Large Animal Teaching Hospital, Department of Veterinary Clinical Sciences, University of Copenhagen, Agrovej 8, 2630 Taastrup, Denmark. The study was generously funded by The Kustos Foundation of 1881 and The Augustinus Foundation. Merle Fenner is funded by the European Union Horizon 2020 research and innovation program under the Marie Sklodowska‐Curie grant agreement No 675351. Some data from this manuscript were presented at the 2017 ACVIM Forum, National Harbor, MD.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the local ethical committee at the Department of Veterinary Clinical Sciences, University of Copenhagen and The Danish Animal Experiments Inspectorate (license number 2015–15–0201‐00693), and was performed in accordance with the European Commission Directive 86/609/EEC.
Carstensen H, Hesselkilde EZ, Fenner M, et al. Time‐dependent antiarrhythmic effects of flecainide on induced atrial fibrillation in horses. J Vet Intern Med. 2018;32:1708–1717. 10.1111/jvim.15287
REFERENCES
- 1. Reef VB, Bonagura J, Buhl R, et al. Recommendations for management of equine athletes with cardiovascular abnormalities. J Vet Intern Med. 2014;28:749‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230‐246. [DOI] [PubMed] [Google Scholar]
- 3. Reef VB, Levitan CW, Spencer PA. Factors affecting prognosis and conversion in equine atrial fibrillation. J Vet Intern Med. 1988;2:1‐6. [DOI] [PubMed] [Google Scholar]
- 4. Haugaard MM, Pehrson S, Carstensen H, et al. Antiarrhythmic and electrophysiologic effects of flecainide on acutely induced atrial fibrillation in healthy horses. J Vet Intern Med. 2015;29:339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohmura H, Nukada T, Mizuno Y, Yamaya Y, Nakayama T, Amada A. Safe and efficacious dosage of flecainide acetate for treating equine atrial fibrillation. J Vet Med Sci. 2000;62:711‐715. [DOI] [PubMed] [Google Scholar]
- 6. van Loon G, Blissitt KJ, Keen JA, Young LE. Use of intravenous flecainide in horses with naturally‐occurring atrial fibrillation. Equine Vet J. 2004;36:609‐614. [DOI] [PubMed] [Google Scholar]
- 7. De Clercq D, van Loon G, Baert K, et al. Intravenous amiodarone treatment in horses with chronic atrial fibrillation. Vet J. 2006;172:129‐134. [DOI] [PubMed] [Google Scholar]
- 8. De Clercq D, van Loon G, Baert K, et al. Effects of an adapted intravenous amiodarone treatment protocol in horses with atrial fibrillation. Equine Vet J. 2007;39:344‐349. [DOI] [PubMed] [Google Scholar]
- 9. De Clercq D, van Loon G, Tavernier R, Verbesselt R, Deprez P. Use of propafenone for conversion of chronic atrial fibrillation in horses. Am J Vet Res. 2009;70:223‐227. [DOI] [PubMed] [Google Scholar]
- 10. Broux B, De Clercq D, Decloedt A, et al. Pharmacokinetics and electrophysiological effects of sotalol hydrochloride in horses. Equine Vet J. 2017;50:377‐383. [DOI] [PubMed] [Google Scholar]
- 11. Haugaard MM, Hesselkilde EZ, Pehrson S, et al. Pharmacologic inhibition of small‐conductance calcium‐activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart Rhythm. 2015;12:825‐835. [DOI] [PubMed] [Google Scholar]
- 12. Carstensen H, Kjaer L, Haugaard MM, et al. Antiarrhythmic effects of combining dofetilide and ranolazine in a model of acutely induced atrial fibrillation in horses. J Cardiovasc Pharmacol. 2018;71:26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi Y, Ishikawa Y, Ohmura H. Treatment of recent‐onset atrial fibrillation with quinidine and flecainide in thoroughbred racehorses: 107 cases (1987‐2014). J Am Vet Med Assoc. 2018;252:1409‐1414. [DOI] [PubMed] [Google Scholar]
- 14. Birettoni F, Porciello F, Rishniw M, della Rocca G, Di Salvo A, Sgorbini M. Treatment of chronic atrial fibrillation in the horse with flecainide: personal observation. Vet Res Commun. 2007;31(Suppl 1):273‐275. [DOI] [PubMed] [Google Scholar]
- 15. De Clercq D, van Loon G, Tavernier R, Duchateau L, Deprez P. Atrial and ventricular electrical and contractile remodeling and reverse remodeling owing to short‐term pacing‐induced atrial fibrillation in horses. J Vet Intern Med. 2008;22:1353‐1359. [DOI] [PubMed] [Google Scholar]
- 16. Dembek KA, Hurcombe SD, Schober KE, Toribio RE. Sudden death of a horse with supraventricular tachycardia following oral administration of flecainide acetate. J Vet Emerg Crit Care (San Antonio). 2014;24:759‐763. [DOI] [PubMed] [Google Scholar]
- 17. Robinson S, Feary D. Sudden death following oral administration of flecainide to horses with naturally occuring atrial fibrillation. Aust Equine Vet. 2008;27:49‐51. [Google Scholar]
- 18. Aliot E, Capucci A, Crijns HJ, Goette A, Tamargo J. Twenty‐five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;2014(130):2071‐2104. [DOI] [PubMed] [Google Scholar]
- 20. Buhl R, Ersboll AK, Eriksen L, Koch J. Changes over time in echocardiographic measurements in young Standardbred racehorses undergoing training and racing and association with racing performance. J Am Vet Med Assoc. 2005;226:1881‐1887. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen PJ, Kanters JK, Buhl R, Klaerke DA. Normal electrocardiographic QT interval in race‐fit Standardbred horses at rest and its rate dependence during exercise. J Vet Cardiol. 2013;15:23‐31. [DOI] [PubMed] [Google Scholar]
- 22. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954‐1968. [DOI] [PubMed] [Google Scholar]
- 23. Ausma J, Litjens N, Lenders MH, et al. Time course of atrial fibrillation‐induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol. 2001;33:2083‐2094. [DOI] [PubMed] [Google Scholar]
- 24. McNamara RL, Bass EB, Miller MR, et al. Management of new onset atrial fibrillation. Evid Rep Technol Assess (Summ). 2000;12:1‐7. [PMC free article] [PubMed] [Google Scholar]
- 25. Cosio FG, Aliot E, Botto GL, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21‐27. [DOI] [PubMed] [Google Scholar]
- 26. Hesselkilde EZ, Carstensen H, Haugaard MM, et al. Effect of flecainide on atrial fibrillatory rate in a large animal model with induced atrial fibrillation. BMC Cardiovasc Disord. 2017;17:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nabar A, Rodriguez LM, Timmermans C, van Mechelen R, Wellens HJ. Class IC antiarrhythmic drug induced atrial flutter: electrocardiographic and electrophysiological findings and their importance for long term outcome after right atrial isthmus ablation. Heart. 2001;85:424‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crijns HJ, van Gelder IC, Lie KI. Supraventricular tachycardia mimicking ventricular tachycardia during flecainide treatment. Am J Cardiol. 1988;62:1303‐1306. [DOI] [PubMed] [Google Scholar]
- 29. Murdock CJ, Kyles AE, Yeung‐Lai‐Wah JA, Qi A, Vorderbrugge S, Kerr CR. Atrial flutter in patients treated for atrial fibrillation with propafenone. Am J Cardiol. 1990;66:755‐757. [DOI] [PubMed] [Google Scholar]
- 30. Kingma JH, Suttorp MJ. Acute pharmacologic conversion of atrial fibrillation and flutter: the role of flecainide, propafenone, and verapamil. Am J Cardiol. 1992;70:56A‐60A. discussion 60A‐61A. [DOI] [PubMed] [Google Scholar]
- 31. Van Loon G, Jordaens L, Muylle E, Nollet H, Sustronck B. Intracardiac overdrive pacing as a treatment of atrial flutter in a horse. Vet Rec. 1998;142:301‐303. [DOI] [PubMed] [Google Scholar]
- 32. Hudson KB, Brady WJ, Chan TC, Pollack M, Harrigan RA. Electrocardiographic manifestations: ventricular tachycardia. J Emerg Med. 2003;25:303‐314. [DOI] [PubMed] [Google Scholar]
- 33. Goldberger ZD, Rho RW, Page RL. Approach to the diagnosis and initial management of the stable adult patient with a wide complex tachycardia. Am J Cardiol. 2008;101:1456‐1466. [DOI] [PubMed] [Google Scholar]
- 34. Biffi M, Boriani G, Bronzetti G, Capucci A, Branzi A, Magnani B. Electrophysiological effects of flecainide and propafenone on atrial fibrillation cycle and relation with arrhythmia termination. Heart. 1999;82:176‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hondeghem LM. Antiarrhythmic agents: modulated receptor applications. Circulation. 1987;75:514‐520. [DOI] [PubMed] [Google Scholar]
- 36. Langenfeld H, Kohler C, Weirich J, Kirstein M, Kochsiek K. Reverse use dependence of antiarrhythmic class Ia, Ib, and Ic: effects of drugs on the action potential duration? Pacing Clin Electrophysiol. 1992;15:2097‐2102. [DOI] [PubMed] [Google Scholar]
- 37. Brugada J, Boersma L, Kirchhof C, Allessie M. Proarrhythmic effects of flecainide. Experimental evidence for increased susceptibility to reentrant arrhythmias. Circulation. 1991;84:1808‐1818. [DOI] [PubMed] [Google Scholar]
- 38. Yamashita K, Tsubakishita S, Futaok S, et al. Cardiovascular effects of medetomidine, detomidine and xylazine in horses. J Vet Med Sci. 2000;62:1025‐1032. [DOI] [PubMed] [Google Scholar]
- 39. Valverde A. Alpha‐2 agonists as pain therapy in horses. Vet Clin North Am Equine Pract. 2010;26:515‐532. [DOI] [PubMed] [Google Scholar]
- 40. Gowda RM, Khan IA, Wilbur SL, Vasavada BC, Sacchi TJ. Torsade de pointes: the clinical considerations. Int J Cardiol. 2004;96:1‐6. [DOI] [PubMed] [Google Scholar]
- 41. Nogales Asensio JM, Moreno Sanchez N, Doncel Vecino LJ, Villar Mariscal C, Lopez‐Minguez JR, Merchan Herrera A. Torsade‐de‐pointes in a patient under flecainide treatment, an unusual case of proarrhythmicity. Int J Cardiol. 2007;114:E65‐E67. [DOI] [PubMed] [Google Scholar]
- 42. Hellestrand KJ, Bexton RS, Nathan AW, Spurrell RA, Camm AJ. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982;48:140‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Loon G, Fonteyne W, Rottiers H, et al. Dual‐chamber pacemaker implantation via the cephalic vein in healthy equids. J Vet Intern Med. 2001;15:564‐571. [DOI] [PubMed] [Google Scholar]


