Abstract
Background
Preanalytic protein adsorption to polymer and glass container surfaces may decrease urine protein concentration measurements and urine protein: creatinine ratios (UPC).
Hypothesis/Objectives
Urine stored in PC or glass containers will have lower UPC than urine stored in HP containers. The specific objective was to determine whether clinically relevant differences in UPC would be detected after storage in glass, PC, or HP containers using common storage times and temperatures.
Animals
Twelve client‐owned dogs with proteinuria.
Methods
Prospective, nonmasked study, divided into 2 phases. The first phase was a pilot study involving multiple (n = 5) measurements at each storage condition using 24‐hours urine samples from 2 dogs with persistent renal proteinuria of different magnitude. The second phase used urine samples from 10 dogs with proteinuria of variable magnitude. Sample aliquots were stored in HP, PC, and glass containers at 24°C for 4 hours, 4°C for 12 hours, and −20°C for 72 hours. The UPC of each was measured after storage and compared with baseline.
Results
Statistically significant but clinically irrelevant differences were found in phase 1. In phase 2, storage conditions did not affect urinary protein or creatinine concentrations or UPC.
Conclusions and Clinical Importance
Collection and storage of canine urine samples in clean HP, PC, or glass containers at 24°C for 4 hours, 4°C for 12 hours, or −20°C for 72 hours is unlikely to result in clinically relevant decreases in measured UPC values.
Keywords: adsorption, homopolymer polypropylene (HP), plastic, propylene copolymer (PC), proteinuria
Abbreviations
- ACVIM
American College of Veterinary Internal Medicine
- HP
homopolymer polypropylene
- IRIS
International Renal Interest Society
- PC
propylene copolymer
- UPC
urine protein:creatinine ratio
1. INTRODUCTION
Studies in humans and other animals suggest an association between persistent renal proteinuria and rate of progression of chronic kidney disease.1, 2, 3, 4, 5 In dogs, a positive correlation between magnitude of proteinuria and time to uremic crisis or death has been reported.5 Additionally, an association between use of medications that typically cause a reduction in magnitude of proteinuria in patients with chronic kidney disease and improved renal outcome measures has been reported in human and veterinary patients.6, 7, 8, 9, 10, 11, 12 The urine protein : creatinine ratio (UPC) is a common method for assessing the magnitude of proteinuria in dogs, and strongly correlates with 24‐hours urine protein excretion.13 Consensus statements published by the American College of Veterinary Internal Medicine (ACVIM) and the International Renal Interest Society (IRIS) have used UPC as the basis for recommendations related to clinical interpretation of the magnitude of renal proteinuria, including monitoring efficacy of treatment.6, 14, 15, 16
In healthy dogs, voided urine or urine collected by cystocentesis may be used for UPC measurement.17 In practice, owners frequently are asked to collect urine at home and bring the sample to a veterinary hospital for analysis. Because animals with severe renal proteinuria can have marked day‐to‐day variation in the magnitude of their proteinuria, the UPC of multiple collected urine samples can be averaged together, or equal volumes of multiple urine samples can be pooled together for a single UPC measurement.16, 18, 19 Common materials used to collect and store urine at home include polymer plastic food storage containers, glass containers, or medical‐grade homopolymer polypropylene (HP) containers.
At low concentrations, adsorption to the surface of polymer and glass containers can lead to significant decreases in measured albumin concentration in human urine samples.20, 21, 22, 23 Clear food plastic storage containers are commonly made of propylene copolymers (PC), which decrease refraction of light and increase clarity.24, 25 Homopolymer polypropylene containers are more appealing for biospecimen storage because of their resistance to protein adsorption.26 Specific hydrophilic coatings or nonionic detergents can be applied to polymers to help prevent adsorption of protein and other biologic material to the surface of the container, but these containers are costly and infrequently used for urine collection and storage.
There also are a variety of temperature conditions under which clients may store urine samples before analysis by a veterinarian. Samples may be stored at room temperature, refrigerated (4°C), or frozen (–20°C). In 1 prior study, canine urine was stored in an unspecified container type at room temperature for up to 4 hours and at −20°C for up to 3 months without significantly affecting UPC.27 Storage of human urine at −20°C for as little as 2 weeks has been reported to produce modifications to the albumin molecule that may cause mild preanalytic decreases in measured urine albumin concentration.28, 29 Several other studies confirm a time‐dependent decrease in measured urine albumin concentration at −20°C.30, 31, 32 Although some studies suggest that urine can be stored at −70°C or −80°C for much longer periods of time with clinically insignificant changes in urine albumin concentration, these studies do still consistently identify a small, time‐dependent decrease in measured concentrations.32, 33, 34 Measurement of urine albumin after storage also may be affected by urine pH and method of analysis.34, 35 Urine creatinine concentrations in urine samples of humans appear to be stable at temperatures ≤–20°C for extended storage times of up to several years.33, 36, 37
The primary objective of our study was to determine whether significant differences in measured UPC of proteinuric canine urine samples are observed before and after storage in glass, PC, or HP containers. A secondary objective was to determine whether observed differences would be likely to produce different clinical recommendations.
2. MATERIALS AND METHODS
Ours was a prospective, nonmasked study. Samples were collected from client‐owned dogs with a history of proteinuria. The research protocol was approved by the University of Florida Institutional Animal Care and Use Committee and University of Florida College of Veterinary Medicine's Veterinary Hospital Research Review Committee.
2.1. Study Populations
For the first phase of the study, urine was collected at home over a 24‐hours period from dogs that had previously been evaluated by the small animal internal medicine service as having probable persistent renal proteinuria. Inclusion criteria included documentation of at least 3 prior UPC >0.5, and urinalysis and culture performed on a urine sample collected by cystocentesis within 2 weeks before enrollment. Dogs were excluded if they had an active urine sediment (≥5 WBC/hpf, ≥20 RBC/hpf, or observed bacteriuria) at the time of urine collection, or a positive aerobic urine culture within the previous 2 weeks. During the collection period, urine was stored at 4°C in a homopolymer urine collection container of a type commonly sent home with human patients for 24‐hours urine collection (Medline 24‐hours urine collection bottle, Medline Industries, Northfield, Illinois). At the end of the collection period, the urine was immediately brought to the UF Small Animal Hospital where a urine sediment examination was performed.
For the second phase of the study, urine was collected from proteinuric dogs that presented either to the internal medicine service or the emergency service. Inclusion criteria for this group included documentation of a UPC >0.5 and a urinalysis and culture performed on a sample obtained by cystocentesis within 2 weeks of enrollment. Dogs were excluded if they had an active urine sediment (as defined above) within 24 hours of urine collection, or a positive aerobic urine culture within 2 weeks of urine collection. Urine was collected either at home into a HP urine specimen cup, or in the hospital by voiding or cystocentesis. Urine was stored in a HP urine specimen cup at 4°C until analysis. Urine was processed within 12 hours of collection.
2.2. Sample processing and urine protein measurements
For phase 1, 120 mL of urine from the 24‐hours urine collection container was divided evenly into four 60‐mL conical tubes and centrifuged at 1500 rpm for 10 minutes. The supernatant was separated into aliquots. Ten 1.5‐mL aliquots were placed in cryopreservation tubes and stored at −80°C until analysis. Forty‐five 3‐mL aliquots were put into 15 glass (Ball 4‐ounce glass jelly jars), 15 PC (Glad 4‐ounce mini round food storage container), and 15 HP (Fisherbrand 4‐ounce specimen containers) containers. Five of each container type were stored at 24°C for 4 hours, 4°C for 12 hours, and −20°C 72 hours. At the end of these storage periods, 1.5‐mL aliquots from each storage condition were transferred into 2 cryopreservation tubes and stored at −80°C until analysis.
For the second phase of the study, 30‐mL urine samples from each dog were centrifuged at 1500 rpm for 10 minutes. Two 1.5‐mL aliquots of supernatant from each sample were placed into cryopreservation tubes and stored at −80°C until analysis. Nine 3 mL aliquots of supernatant were put into 3 glass (Ball 4‐ounce glass jelly jars), 3 PC (Glad 4‐ounce mini round food storage container), and 3 HP (Fisherbrand 4‐ounce specimen containers) containers. One of each container type then was stored at 24°C for 4 hours, 4°C for 12 hours, and −20°C for 72 hours. At the end of these storage periods, 1.5‐mL aliquots were transferred into 2 cryopreservation tubes and stored at–80°C until analysis.
For both phases, after storage at −80°C, the urine was thawed before analysis. Urine protein concentration was measured using a modified pyrogallol red‐molybdate method on an automated chemical analyzer (Siemens Dimension Xpand Plus integrated chemistry system, Siemens Medical Solutions and Diamond Diagnostics, Holliston, Massachusetts). If necessary because of markedly increased urine protein concentration, the sample was analyzed after 1 : 2 dilution. Urine creatinine concentrations were measured using a modified Jaffe protocol using the same chemistry analyzer.
2.3. Classifications based on ACVIM consensus guidelines
To determine if storage of samples would lead to different clinical recommendations, the samples from phase 2 were assigned a specific clinical response category based on the 2004 ACVIM consensus statement on the assessment and management of proteinuric dogs.6 For samples from dogs with azotemia, categories included “intervention” (UPC ≥0.5) and “nonintervention” (UPC <0.5). For samples from nonazotemic dogs, categories included “no action” (UPC <0.5), “monitor” (UPC ≥0.5 and <1), “investigate” (UPC ≥1 and <2), and “intervene” (UPC ≥2). In addition, to determine if differences in UPC based on storage condition could be confused with the significant day‐to‐day variation that can occur in animals with proteinuria, an alternative “significant change” in UPC was considered to be a change in UPC from the control sample of 35% when baseline UPC was > 8, 50% when baseline UPC was 1–8 and 80% when baseline UPC was < 1.6, 18
2.4. Statistical analysis
Values are reported as means and pooled standard deviations or medians and ranges. Comparisons among methods were performed with statistical software (SAS System for Windows 9.4, SAS Institute Inc, Cary, North Carolina). A probability of error <5% was considered significant. A prospective power analysis was performed, and the study was designed to detect a 10% decrease in UPC in a severely proteinuric sample (from 10 to 9) and a 33% decrease in UPC in a mildly proteinuric sample (from 0.6 to 0.4). Distributions were assessed both visually and using the Shapiro‐Wilk test. In phase 1, values with non‐normal distributions were compared among treatment combinations and control values for high and low protein urine samples separately using a Kruskal‐Wallis test. A Wilcoxon rank‐sum test was used for post‐hoc comparisons. Values after storage at −80°C were compared post‐hoc with all values obtained after storage at temperatures ≥–20°C, and then values were compared between individual treatments and controls with a Bonferroni correction. Values with normal distributions were compared using a general linear model procedure (SAS procedure PROC GLM) with temperature and container type as factors in the model. Dunnett's test was used to compare treatment values to control values. In phase 2, concentrations and ratios were compared using a Friedman's analysis of variance (ANOVA) with treatment combination or control as a repeated factor for each dog urine sample.
3. RESULTS
3.1. Populations
Urine samples from 2 dogs with suspected persistent renal proteinuria were included in phase 1. The first dog was a 14‐year old spayed female Staffordshire terrier with prior UPC ranging from 0.5 to 0.9. The second dog was a 12‐year old spayed female Cavalier King Charles spaniel with prior UPC ranging from 6.1 to 12.3. This dog initially was excluded because of a positive urine culture, but later was recruited when documented to have a negative urine culture 1 month after discontinuation of antibiotic treatment. Prior diagnostic testing for these patients was variable, but included serial serum biochemistry, CBC, urinalysis, urine culture, UPC, and blood pressure measurements, as well as at least 1 set of thoracic radiographs, and abdominal ultrasound evaluation. Neither patient had a kidney biopsy performed or specific diagnosis.
Urine samples from 10 dogs were used in phase 2. Samples from 4 additional dogs were excluded. Two had active urine sediment, and 2 others had positive cultures. Four of these dogs had no previous documentation of proteinuria, whereas the other 6 had prior UPC values ranging from 0.9 to 13.5. Of the 10 dogs included, 6 were spayed females and 4 were neutered males. The median age was 10.1 years (range, 1.5–12 years). There were 2 Yorkshire terriers and 2 Labrador retrievers, but no other dog breed was represented more than once. Documented underlying diseases included protein‐losing nephropathy (6), nephrolithiasis (1), endocarditis (1), acute kidney injury of unknown cause (1), and multiple myeloma (1).
3.2. Urine protein : creatinine ratio results
In phase 1, for the urine sample from patient #1 with a pre‐storage UPC ratio of 0.7, mean urine protein concentrations, urine creatinine concentrations, and UPCs differed slightly among combinations of storage conditions (P < .05) but differences between mean ratios were small (≤.01) and a statistical difference between UPCs under individual conditions or all conditions combined and control values was not identified (Table 1). For the urine sample from patient #2 with a prestorage UPC of 8.6, there was an effect of storage temperature on mean UPC ratio (P ≤ .0005), but there was no evidence of an effect of container type (Table 2). Mean UPCs were slightly higher (9%) in samples stored at temperatures ≥–20°C (all experimental conditions) than in control samples stored at −80°C (P = .002) and were slightly higher in samples stored in the refrigerator than in samples stored in a freezer (P < .05) because creatinine concentrations decreased more than protein concentrations with storage.
Table 1.
Repeated measures of urine protein, urine creatinine, and UPC values from a urine sample with a prestorage UPC of 0.7 stored under different conditions
| Control (–80°C) | 24°C for 4 hours | 4°C for 12 hours | –20°C for 72 hours | All treatments | Pooled standard deviation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Container | HP | PC | HP | Glass | PC | HP | Glass | PC | HP | Glass | – | |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 45 | |
| Protein (mg/dL)a | 72.2 | 74.6 | 77.4 | 81.3b | 71.6 | 75.0 | 72.0 | 72.6 | 71.6 | 71.2 | 74 | 5.0 |
| Creatinine (mg/dL)a | 108 | 110 | 116 | 122b | 107 | 114 | 106 | 110 | 107 | 107 | 110 | 7.8 |
| UPC ratioa | 0.67 | 0.68 | 0.66 | 0.67 | 0.67 | 0.66 | 0.68 | 0.66 | 0.67 | 0.67 | 0.67 | 0.01 |
Abbreviations: HP, homopolymer polypropylene; PC, propylene copolymer; UPC, Urine protein:creatinine ratio.
While an overall difference between storage conditions was detected (P < .05), mean values for most storage conditions and mean values when all treatments (experimental storage conditions) were combined were not different from controls (P > .05).
Statistically different from control (P < .05).
Table 2.
Repeated measures of urine protein, urine creatinine, and UPC values from a urine sample with a prestorage UPC of 8.6 stored under different conditions
| Control (–80°C) | 24°C for 4 hours | 4°C for 12 hours | –20°C for 72 hours | All treatments | Pooled standard deviation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Container | HP | PC | HP | Glass | PC | HP | Glass | PC | HP | Glass | – | |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 45 | |
| Protein (mg/dL) | 369 | 410 | 362 | 403 | 330 | 346 | 301a | 386 | 440a | 410 | 376 | 33 |
| Creatinine (mg/dL) | 46.0 | 48.8 | 40.0 | 46.8 | 36.4a | 39.2a | 33.1a | 46.0 | 51.9 | 48.4 | 43.3 | 3.7 |
| UPC ratio | 8.0 | 8.4 | 9.0a | 8.6a | 9.1a | 8.8a | 9.1a | 8.4 | 8.5 | 8.5 | 8.7a | 0.4 |
Abbreviations: HP, homopolymer polypropylene; PC, propylene copolymer; UPC, Urine protein:creatinine ratio.
Statistically different from control (P < .05).
In phase 2, the baseline UPC from the 10 dogs ranged from 0.8 to 26.6. One of these dogs had mild proteinuria (0.8), 5 had moderate proteinuria (2.5–6.6), and 4 had severe proteinuria (11.4–26.6). No evidence of a statistically significant difference was identified among different storage conditions with respect to either the final protein or creatinine concentrations, or UPCs, or the change in concentration of protein or creatinine, or change in UPC from baseline after storage (Figure 1). The average absolute and percentage change in UPC from baseline for all treatments were −0.6 and −4%, respectively, with pooled standard deviations of 1.1 and 7%, respectively.
Figure 1.
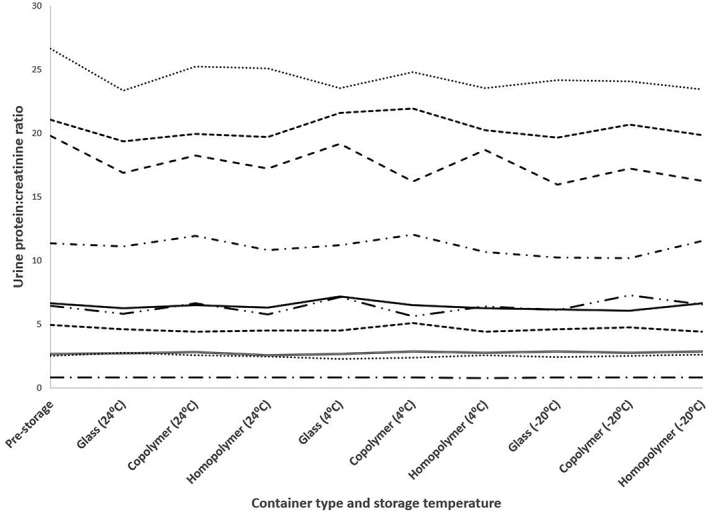
Urine protein: creatinine ratio values of urine from 10 dogs in phase 2 at the time of collection (prestorage) and after storage at various times and temperatures
3.3. American College of Veterinary Internal Medicine consensus classification
No change in UPC values after storage in either phase was sufficient for the UPC to move to the other side of a clinical response category from the ACVIM consensus guidelines, and only 4 samples were reasonably close (within 0.5 or 25% of baseline value) to a cut‐off point.
4. DISCUSSION
Some small differences were identified in the UPC values at different storage conditions in phase 1, but not phase 2 of the study. Although statistically significant, the changes were very small and would be very unlikely to result in different clinical interpretations. The results suggest that canine urine samples can be collected in clean HP, PC, or glass containers and stored at 24°C for 4 hours, 4°C for 12 hours, and −20°C 72 hours with only clinically insignificant changes to the UPC values. Nonetheless, there were some unexpected differences in urine protein concentration, urine creatinine concentration, and UPC among the storage conditions in the 2 phases and there were some limitations of our study.
In phase 1, although significant differences in some measured variables were noted between different combinations of storage variables and controls for the mildly proteinuric patient, the absolute differences were minimal, and likely can be attributed solely to analyzer variability. Regardless of the cause, these differences are likely clinically insignificant, and would not lead to different IRIS classification or different clinical recommendations based on ACVIM consensus guidelines. For the severely proteinuric sample in phase 1, samples stored at 4°C had significantly higher UPC ratios when compared to samples stored at 24°C, −20°C, or −80°C. These differences are primarily a result of decreases in creatinine concentrations rather than altered protein concentrations. It is unlikely that this result is secondary to creatinine adsorption or evaporation, because a lower creatinine concentration was not observed at this temperature in the mildly proteinuric group or in phase 2 of the study. Therefore, it seems more likely to be a consequence of analytical error or variation. In phase 2 of the study, there was no evidence of a difference in mean protein or creatinine concentrations, mean UPC ratio or change in concentration or ratio among storage variables. The slight differences in actual UPC values suggest that storage of urine samples under the conditions used in our study would have minimal impact on clinical interpretations of magnitude of proteinuria.
One limitation of our study is the method of protein measurement. Although albumin is the most abundant protein in the urine of animals with protein‐losing nephropathy, the specific types of protein in the urine samples was not confirmed in our study. It is possible that some of the measured protein was not albumin or that the measurement did not account for all the proteins present. The modified pyrogallol red‐molybdate method has excellent recovery of albumin and globulin proteins, but can have poor recovery of Tamm‐Horsfall mucoprotein as well as several other proteins and peptides that can be found in the urine of humans.38 In humans, the urinary proteome can contain over 100 000 different peptides, with at least 5000 occurring in >20% of patients.39 Similar to dogs, albumin is the most common protein in the urine of people with renal disease, but humans also can have a number of other peptides and protein fragments in the urine, including peptides from globulins, collagens, fibrinogen, hemoglobin, and a number of other proteins.39 Each protein and peptide in urine has a different isoelectric point and, depending on the pH of the urine, can be more or less likely to adsorb to the surface of polymers or glass. If an individual dog were to have a greater or lesser proportion of albumin and globulin proteins in its urine proteome relative to the dogs in our study and if the other proteins in that dog's urine reacted differently to the different container types, the UPC results might be different. However, we did have 1 dog with multiple myeloma in our study that might have been expected to have a different urine proteome than a typical dog with renal proteinuria and that dog did not appear to have any more or less variation in its UPC than did the other dogs.
In our study, urine samples came into contact with multiple polymers before and after the specific experimental storage conditions, including collection containers, conical centrifugation tubes, syringes, pipettes, and cryopreservation tubes. Although all of these are homopolymer containers, it is possible that their use exacerbated protein loss because of adsorption, or that the proteins and peptides that are more likely to adsorb to polymer surface had already done so before reaching the study containers. However, the lack of a significant decrease in UPC or protein concentration relative to creatinine concentration compared with prestorage UPC in phase 1 suggests that these changes, if present, would likely be clinically insignificant. Previously published articles evaluating storage of human urine samples suggest that there would be less urine albumin binding to the homopolymer surfaces than to a glass or propylene surface, but this may not apply to canine urine.20, 21, 22, 23 Therefore, we cannot rule out the possibility that collection and storage in glass or PC containers only (no contact with a homopolymer of any kind) before analysis would result in even less variation in measured protein concentrations.
Although our study was designed for a power to detect a 10% decrease in UPC in a severely proteinuric sample (from 10 to 9) and a 33% decrease in UPC in a mildly proteinuric sample (from 0.6 to 0.4) in phase 1, smaller changes in urine protein concentration might go undetected unless a larger population was evaluated. Furthermore, although the mean absolute and percentage changes in UPC from baseline for all storage conditions in phase 2 were relatively small (–0.6 and −4%, respectively), a few samples from the most severely proteinuric dogs had a UPC difference as high as 18%.
A secondary objective of our study was to determine whether protein adsorption could lead to different clinical recommendations. Because of the small amount of protein adsorption, changes in clinical recommendations are most likely to occur when prestorage urine samples are near cut‐offs for different clinical recommendations (ie, UPC ratios near 2.0 in non‐azotemic animals, or near 0.5 in azotemic animals based on the ACVIM consensus statement or near 0.5 for IRIS recommendations). Unfortunately, in our study, only 1 dog in phase 1 (mean prestorage UPC 0.67) and 1 dog in phase 2 (prestorage UPC 0.8) had UPC ratios near the 0.5 cut‐off, and only 2 dogs in phase 2 (prestorage UPCs of 2.5 and 2.7) had UPC ratios near the 2.0 cut‐off. Although none of these patients had a difference in UPC large enough to fall on different sides of a cut‐off point, additional studies focusing on patients near these cut‐off points (especially the 0.5 value) would be required to better determine whether container type or storage temperature might actually lead to different recommendations in some of these patients. Prior studies have suggested that to suspect an actual change over time as a result of disease progression or response to treatment, rather than merely day‐to‐day variation, relatively substantial changes in UPC must occur.18 Extrapolating from those studies, our study evaluated criteria for significant change from baseline UPC values of <1, 1–8, or >8 of 80, 50, or 35%, respectively. However, these are not validated criteria and some clinicians may choose to accept smaller changes as clinically important.
In conclusion, canine urine samples likely can be collected and stored in clean HP, PC, or glass containers at 24°C for 4 hours and −20°C for 72 hours with minimal effect on UPC values. Caution should be used when storing urine at 4°C for ≥12 hours, because results of our study indicate that doing so may lead to an increase in UPC ratios, although it is also unlikely to cause clinically relevant changes.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The research protocol was approved by the University of Florida IACUC and University of Florida College of Veterinary Medicine's Veterinary Hospital Research Review Committee.
ACKNOWLEDGMENTS
The authors thank A. Lamar for help with recruitment, obtaining consent, and ensuring proper sample handling. This study was completed at University of Florida Small Animal Hospital at 2015 SW 16th Ave, Gainesville, FL 32608. This article was supported by the 2014 University of Florida CVM Resident's Intramural Competitive Grants Program. Data from this manuscript were presented at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, MD.
Moyle PS, Specht A, Hill R. Effect of common storage temperatures and container types on urine protein : creatinine ratios in urine samples of proteinuric dogs. J Vet Intern Med. 2018;32:1652–1658. 10.1111/jvim.15232
Funding information 2014 University of Florida CVM Resident's Intramural Competitive Grants Program
REFERENCES
- 1. Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis. 1996;27:765–775. [DOI] [PubMed] [Google Scholar]
- 2. Eddy AA. Proteinuria and interstitial injury. Nephrol Dial Transplant. 2004;19:277–281. [DOI] [PubMed] [Google Scholar]
- 3. Locatelli F, Marcelli D, Comelli M, et al. Northern Italian Cooperative Study Group: Proteinuria and blood pressure as causal components of progression to end‐stage renal failure. Nephrol Dial Transplant. 1996;11:461–467. [DOI] [PubMed] [Google Scholar]
- 4. Jepson RE, Brodbelt D, Vallance C, Syme HM, Elliott J. Evaluation of predictors of the development of azotemia in cats. JVet Intern Med. 2009;23:806–813. [DOI] [PubMed] [Google Scholar]
- 5. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. JAm Vet Med Assoc. 2005;226:393–400. [DOI] [PubMed] [Google Scholar]
- 6. Lees GE, Brown SA, Elliott J, Grauer GE, Vaden SL; American College of Veterinary Internal Medicine . Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement. JVet Intern Med. 2005;19:377–385. [DOI] [PubMed] [Google Scholar]
- 7. GISEN Group . Randomized placebo‐controlled trial effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non‐diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 8. Wapstra FH, Navis G, de Jong PE, de Zeeuw D. Prognostic value of the short‐term antiproteinuric response to ACE inhibition for prediction of GFR decline in patients with nondiabetic renal disease. Exp Nephrol. 1996;4:47–52. [PubMed] [Google Scholar]
- 9. Brown SA, Finco DR, Brown CA, et al. Evaluation of the effects of inhibition of angiotensin converting enzyme with enalapril in dogs with induced chronic renal insufficiency. Am J Vet Res. 2003;64:321–327. [DOI] [PubMed] [Google Scholar]
- 10. Grodecki KM, Gains MJ, Baumal R, et al. Treatment of X‐linked hereditary nephritis in Samoyed dogs with angiotensin converting enzyme (ACE) inhibitor. JComp Pathol. 1997;117:209–225. [DOI] [PubMed] [Google Scholar]
- 11. Grauer GF, Greco DS, Getzy DM, et al. Effects of enalapril versus placebo as a treatment for canine idiopathic glomerulonephritis. JVet Intern Med. 2000;14:526–533. [DOI] [PubMed] [Google Scholar]
- 12. Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. JVet Intern Med. 2007;21:402–409. [DOI] [PubMed] [Google Scholar]
- 13. White JV, Olivier NB, Reimann K, Johnson C. Use of protein‐to‐creatinine ratio in a single urine specimen for quantitative estimation of canine proteinuria. JAm Vet Med Assoc. 1984;185:882–885. [PubMed] [Google Scholar]
- 14. IRIS Staging of CKD . International Renal Interest Society. http://www.iris-kidney.com/guidelines/staging.html. Last accessed December 2017.
- 15. IRIS Canine GN Study Group Diagnosis Subgroup, Littman MP, Daminet S, et al. Consensus recommendations for the diagnostic investigation of dogs with suspected glomerular disease. JVet Intern Med. 2013;27Supp1:19–26. [DOI] [PubMed] [Google Scholar]
- 16. IRIS Canine GN Study Group Standard Therapy Subgroup, Brown S, Elliott J, et al. Consensus recommendations for standard therapy of glomerular disease in dogs. JVet Intern Med. 2013;27Supp1:27–43. [DOI] [PubMed] [Google Scholar]
- 17. Beatrice L, Nizi F, Callegari D, et al. Comparison of urine protein‐to‐creatinine ratio in urine samples collected by cystocentesis versus free catch in dogs. JAm Vet Med Assoc. 2010;236:1221–1224. [DOI] [PubMed] [Google Scholar]
- 18. Nabitty MB, Boggess MM, Kashtan CE, Lees GE. Day‐to‐day variation of the urine protein:creatinine ratio in female dogs with stable glomerular proteinuria caused by X‐linked hereditary nephropathy. JVet Intern Med. 2007;21:425–430. [DOI] [PubMed] [Google Scholar]
- 19. LeVine DN, Zhang D, Harris T, Vaden SL. The use of pooled vs serial urine samples to measure urine protein:creatinine ratios. Vet Clin Pathol. 2010;39:53–56. [DOI] [PubMed] [Google Scholar]
- 20. Hara F, Shiba K. Nonspecific binding of urinary albumin on preservation tube. Jpn J Clin Chem. 2003;32:28–29. [Google Scholar]
- 21. Robinson MK, Caudill SP, Koch DD, et al. Albumin adsorption onto surfaces of urine collection and analysis containers. Clin Chim Acta. 2014;431:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiyokawa I, Sogawa K. Adsorption of urinary proteins on the conventionally used urine collection tubes: possible effects on urinary proteome analysis and prevention of the adsorption by polymer coating. Int J Proteomics. 2011;2011:502845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suelter C, Deluca M. How to prevent losses of protein by adsorption to glass and plastic. Anal Biochem. 1983;135:112–119. [DOI] [PubMed] [Google Scholar]
- 24. Mayer C, Calafut T. Polypropylene: The definitive user's guide and databook. Norwich, NY: Plastic Design Library; 1998. [Google Scholar]
- 25. Bailey M, Brauer D. Polypropylene: new array of polymer variations expand end‐use applications. McGraw‐Hill: Modern Plastics Encyclopedia; 1995. [Google Scholar]
- 26. Kofanova OA, Mommaerts K, Betsou F. Tube polypropylene: A neglected critical parameter for protein adsorption during biospecimen storage. Biopreserv Biobank. 2015;13:296–298. [DOI] [PubMed] [Google Scholar]
- 27. Rossi G, Giori L, Campagnola S, Zatelli A, Zini E, Paltrinieri S. Evaluation of factors that affect analytic variability of urine protein‐to‐creatinine ratio determination in dogs. Am J Vet Res. 2012;73:779–787. [DOI] [PubMed] [Google Scholar]
- 28. Osberg I, Chase HP, Garg SK, et al. Effects of storage time and temperature on measurement of small concentrations of albumin in urine. Clin Chem. 1990;36:1428–1430. [PubMed] [Google Scholar]
- 29. Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. [DOI] [PubMed] [Google Scholar]
- 30. Schultz CJ, Dalton RN, Turner C, Neil HA, Dunger DB. Freezing method affects the concentration and variability of urine proteins and the interpretation of data on microalbuminuria. Diabet Med. 2000;17:7–14. [DOI] [PubMed] [Google Scholar]
- 31. Giampietro O, Penno G, Clerico A, Cruschelli L, Cecere M. How and how long to store urine samples before albumin radioimmunoassay: A practical response. Clin Chem. 1993;3:533–536. [PubMed] [Google Scholar]
- 32. MacNeil ML, Mueller PW, Caudill SP, Steinberg KK. Considerations when measuring urinary albumin: precision, substances that may interfere, and conditions for sample storage. Clin Chem. 1991;37:2120–2123. [PubMed] [Google Scholar]
- 33. Parekh RS, Kao WHL, Meoni LA, et al. Reliability of urinary albumin, total protein, and creatinine assays after prolonged storage: The family investigation of nephropathy and diabetes. Clin J Am Soc Nephrol. 2007;2:1156–1162. [DOI] [PubMed] [Google Scholar]
- 34. Brinkman JW, de Zeeuw D, Lambers Heerspink HJ, et al. Apparent loss of urinary albumin during long‐term frozen storage: HPLC vs immunonephelometry. Clin Chem. 2007;53:1520–1526. [DOI] [PubMed] [Google Scholar]
- 35. Kania K, Byrnes EA, Beilby JP, Webb SA, Strong KJ. Urinary proteases degrade albumin: implications for measurement of albuminuria in stored samples. Ann Clin Biochem. 2010;47:151–157. [DOI] [PubMed] [Google Scholar]
- 36. Spierto FW, Hannon WH, Gunter EW, Smith SJ. Stability of urine creatinine. Clin Chim Acta. 1997;264:227–232. [DOI] [PubMed] [Google Scholar]
- 37. Miki K, Sudo A. Effect of urine pH, storage time, and temperature on stability of catecholamines, cortisol, and creatinine. Clin Chem. 1998;44:1759–1762. [PubMed] [Google Scholar]
- 38. Orsonneau JL, Douet P, Massoubre C, Lustenberger P, Bernard S. An improved pyrogallol red‐molybdate method for determining total urinary protein. Clin Chem. 1989;35:2233–2236. [PubMed] [Google Scholar]
- 39. Coon JJ, Zürbig P, Dakna M, et al. CE‐MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl. 2008;2:964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]


