Abstract
Background
Tamoxifen, a selective estrogen receptor modulator, decreased airway neutrophilia and improved clinical signs in an experimental model of equine asthma, and induced neutrophilic apoptosis in vitro.
Hypothesis/Objectives
Tamoxifen reduces airway neutrophilia and improves lung function in severe asthmatic horses.
Animals
Twelve severe asthmatic horses from a research herd.
Methods
Randomized controlled blinded study design. The effects of a 12‐day oral treatment with tamoxifen (0.22 mg/kg, q24h) or dexamethasone (0.06 mg/kg, q24h) on lung function, endoscopic tracheal mucus score and bronchoalveolar lavage fluid cytology were compared.
Results
Tamoxifen significantly improved the pulmonary resistance (R L; mean reduction of 1.15 cm H2O/L/s [CI: 0.29‐2.01, P = .007] on day 13), but had no effect on the other variables evaluated. Dexamethasone normalized lung function (mean reduction of R L of 2.48 cm H2O/L/s [CI: 1.54‐3.43, P < .0001] on day 13), without affecting airway neutrophilia.
Conclusions and Clinical Importance
Results of this study do not support the use of tamoxifen at the dose studied as an antineutrophilic medication in the treatment of asthmatic horses in chronic exacerbation.
Keywords: airway neutrophilia, dexamethasone, estrogen, heaves
Abbreviations
- BALF
bronchoalveolar lavage fluid
- CI
95% confidence interval
- EL
pulmonary elastance
- NETs
neutrophil extracellular traps
- PL
transpulmonary pressure
- RL
pulmonary resistance
1. INTRODUCTION
Severe equine asthma, also known as heaves or recurrent airway obstruction, is a common and incurable respiratory disease of adult horses. Exacerbations are triggered by inhalation of environmental antigens, most commonly those found in hay. The disease is characterized by airway hyper‐responsiveness, mucus hypersecretion, intraluminal neutrophilia, and structural changes affecting the airways (remodeling).1 Through the release of pro‐inflammatory mediators, proteases, and extracellular traps, neutrophils are potentially major perpetuators of lung damage2, 3 and their presence has been associated with the dysfunction of peripheral airways in asthmatic patients.4 Usual therapies (corticosteroids and bronchodilators), while improving the lung function, do not normalize airway luminal neutrophilia and tissue remodeling of asthmatic horses.5, 6 Furthermore, clinical signs relapse quickly after cessation of medication.7 Although antigen avoidance controls airway inflammation, pulmonary remodeling is incompletely reversed even after a year at pasture.5 Therefore, therapies targeting airway neutrophilia are required to determine if control of pulmonary inflammation can improve lung function and remodeling in severe equine asthma.8
Tamoxifen is a synthetic selective estrogen receptor modulator. Because of its antagonism of estrogen‐dependent growth and its inhibitory effect on breast epithelial cells proliferation, its major use has been in the treatment of breast cancer.9, 10 Nonetheless, tamoxifen appears to have a broader spectrum of activity as it showed beneficial effects in estrogen‐receptor negative cancers, in selected immune disorders, and potentially in spinal cord injury.11, 12, 13, 14 As estrogen administration has been associated with both improvement and, contrariwise, to the development of asthma in women, studying the impact of an estrogen receptor modulator could help delineate the role of sex hormones in asthma.15, 16 Recently, it showed promising results for the treatment of severe equine asthma by reducing the neutrophilic chemotactic response and respiratory burst production and by inducing apoptosis of peripheral and pulmonary neutrophils in vitro.17, 18 Tamoxifen was also studied in healthy adult horses in which an asthma‐like inflammation was experimentally reproduced by exposure to Aspergillus fumigatus contaminated hay. In this experiment, tamoxifen increased the apoptosis of peripheral and pulmonary neutrophils and improved clinical condition, airway neutrophilia, and mucus accumulation.19 We therefore hypothesized that tamoxifen, by decreasing airway neutrophilia, would improve the lung function of severely asthmatic horses. These objectives were to study the effects of tamoxifen on airway luminal inflammation, on tracheal mucus accumulation and on pulmonary function testing of asthmatic horses during continuous antigen exposure.
2. MATERIALS AND METHODS
2.1. Ethics statement
All experimental procedures were performed in accordance with the Canadian Council for Animal Care guidelines and were approved by the Animal Care Committee of the Faculty of Veterinary Medicine of the Université de Montréal (Protocol # Rech‐1324).
2.2. Animals
Twelve severe asthmatic horses (7 mares and 5 geldings) from this research herd were studied. Horses were mixed breeds, aged 14 ± 4 years and weighed 514 ± 51 kg. The horses were diagnosed with severe asthma based on history and previous results of pulmonary function and bronchoalveolar lavage fluid (BALF) cytology. These horses had historically >25% neutrophils on BALF cytology and a transpulmonary pressure (P L) change above 15 cm of H2O when stabled and fed hay and airway obstruction was reversible by antigen avoidance. The presence of a concomitant medical condition was excluded with a physical examination and complete blood count.
To induce chronic exacerbation of the disease as seen in clinical practice, the animals were stabled 3 weeks before the study and were fed dry timothy hay and sweet feed twice daily. The management remained the same throughout the study period. No treatment was administered at least 7 weeks before the trial. Horses were conditioned to wear a mask and to stand in a stock.
2.3. Pulmonary function tests
Lung function was measured in standing unsedated animals, except for one horse that required sedation before each pulmonary function test (xylazine [Rompum, Bayer, Mississauga, ON, Canada], 0.4 mg/kg, IV).6 Briefly, esophageal pressure was measured as an index of the transpulmonary pressure (P L) with a balloon sealed over the end of a polyethylene catheter placed in the distal third of the esophagus. Flow rates were obtained by the use of a heated pneumotachograph and a differential pressure transducer fitted to a mask placed over the horse's nose. The system (Flexiware 7.6, SCIREQ, Montréal, QC, Canada) allowed electronic integration of the flow signal to provide tidal volume. Before each experiment, the system was calibrated by forcing known flow of air through the pneumotachograph with a blower‐rotameter and by applying known pressure with a water manometer on the differential pressure transducer used to measure esophageal pressure. Values of pulmonary resistance (R L) and elastance (E L) were obtained by applying the data to the multiple regression equation for the single compartment model of the lung (P L = E L V + R L V + K) where V is the volume, V the airflow, and K the transpulmonary end‐expiratory pressure. All the valid breaths were used for analysis.
2.4. Endoscopic tracheal mucus scoring and bronchoalveolar lavage
Horses were sedated with xylazine (Rompum, Bayer, Mississauga, ON, Canada; 0.5 mg/kg, IV) and butorphanol (Torbugesic, Zoetis, Florham Park, New Jersey); 20‐30 μg/kg, IV) and tracheoscopy was performed with a 1.6 m videoendoscope (Evis Exera II CV‐180, Olympus Canada Inc., Richmond Hill, ON, Canada). Tracheal mucus score was evaluated during reviewing of video recordings by an investigator blinded to the treatment group.20 Bronchoalveolar lavage was performed as previously described.6 Briefly, after topical anesthesia with 0.5% lidocaine (Lurocaine; lidocaine hydrochloride 20 mg/mL, Vétoquinol N.‐A. Inc., , Lavaltrie, QC, Canada), two 250 mL‐boluses of warm sterile isotonic saline (0.9% Sodium Chloride Injection, USP, Baxter, Mississauga, ON, Canada) were sequentially instilled into a main bronchus through the videoendoscope and then aspirated with a suction pump. The samples were kept on ice until reaching the laboratory within 90 minutes. Cytocentrifuged preparations of BALF (400 μL, unfiltered) were made and cells were stained with a modified Wright–Giemsa solution (DiffQuick, Fisher Scientific, Waltham, Massachusetts). Differential leucocyte counts from 400 cells were performed by an investigator blinded to the treatment group.
2.5. Study protocol
After randomization based on pulmonary resistance ranking value, six horses received tamoxifen citrate (Apo‐tamox, Apotex Inc., Toronto, ON, Canada); 20 mg/tablet, 0.22 mg/kg, PO)19 once daily and six horses received dexamethasonei (Dexamethasone powder, Dominion Veterinary Laboratories Ltd. Winnipeg, MB, Canada); 10 mg/packet, 0.06 mg/kg, PO) once daily for 12 days. Pulmonary function tests were performed before treatment on day 1, and on days 6 and 13. Endoscopic tracheal mucus scores and bronchoalveolar lavages were performed on days 1 and 13. The attitude, appetite, and a clinical respiratory score21 were evaluated daily by a blinded investigator.
2.6. Statistical analysis
Bronchoalveolar lavage, lung function, and the clinical respiratory score data were analyzed with repeated‐measures two‐way ANOVA with “group” as the between‐subject factor and “time” (days of treatment) as the within‐subject factor with Bonferroni corrections for multiple comparisons. Mucus scores were evaluated within each treatment group with Wilcoxon matched‐pairs signed rank tests and among treatment groups with Mann‐Whitney U tests. Data are described as mean difference with 95% confidence interval (CI). P values <.05 were considered statistically significant. GraphPad Prism 7 (GraphPad Prism 7, GraphPad Software, Inc, La Jolla, California) was used for statistical calculations.
3. RESULTS
No adverse effect was observed in the tamoxifen group. On the ninth day of treatment, a horse treated with dexamethasone developed hypocalcemic and hypomagnesemic tetany unresponsive to treatment. Euthanasia was humanely elected for this animal and at necropsy, loss of the principal cells of the parathyroid gland was observed. The exact cause of this finding was undetermined. All data from that horse were excluded from analysis.
3.1. Pulmonary function tests and clinical respiratory scores
Horses were in clinical exacerbation of the disease at baseline (P L > 15 cm H2O, R L > 1 cm H2O/L/s, and E L > 1 cm H2O/L). A group‐time interaction was noted for the R L, E L, and P L values as dexamethasone significantly improved lung function which normalized in all treated horses when evaluated on day 6 (mean reduction of R L of 2.29 cm H2O/L/s [CI: 1.35‐3.24, P < .0001], mean reduction of E L of 4.44 cm H2O/L [CI: 2.51‐6.37, P < .0001], and mean reduction of P L of 32.44 cm H2O [CI: 20.69‐44.20, P < 0.0001]) and 13 (mean reduction of R L of 2.48 cm H2O/L/s [CI: 1.54‐3.43, P < .0001], mean reduction of E L of 4.45 cm H2O/L [CI: 2.52‐6.38, P < .0001], and mean reduction of P L of 34.65 cm H2O [CI: 22.89‐46.41, P < .0001]; Figures 1, 2, 3). Treatment with tamoxifen had no significant effect on P L (mean increase of 2.19 cm H2O on day 13 [CI: −12.93 to 8.54, P > .99]) and E L (mean increase of 1.74 cm H2O/L on day 13 [CI: −3.51 to 0.02, P = .054]). However, a significant reduction of R L was noted at the end of the treatment period with tamoxifen (mean reduction of 1.15 cm H2O/L/s [CI: 0.29‐2.01, P = .007]). The clinical respiratory scores were improved in the dexamethasone group only, on day 6 (mean reduction of 1.6/8 [CI: 0.2‐3, P = .01]), 8 (mean reduction of 1.8/8 [CI: 0.4‐3.2, P = .004]), 10 (mean reduction of 1.8/8 [CI: 0.4‐3.2, P = .004]), and 11 (mean reduction of 2.2/8 [CI: 0.8‐3.6, P = .0002]; Figure 4).
Figure 1.
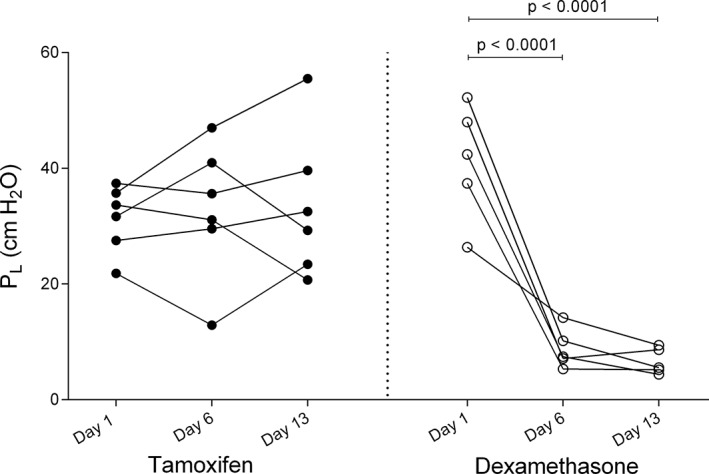
Values of transpulmonary pressure (P L) on day 1 (before administration of medication), day 6 and day 13 of treatment with tamoxifen (black circles) and dexamethasone (white circles)
Figure 2.
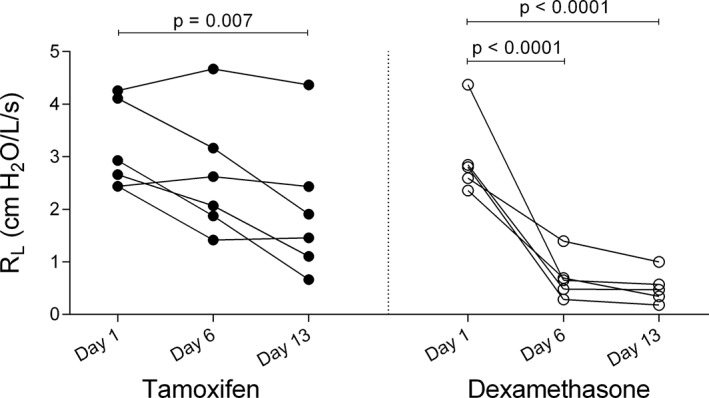
Values of pulmonary resistance (R L) on day 1 (before administration of medication), day 6 and day 13 of treatment with tamoxifen (black circles) and dexamethasone (white circles)
Figure 3.
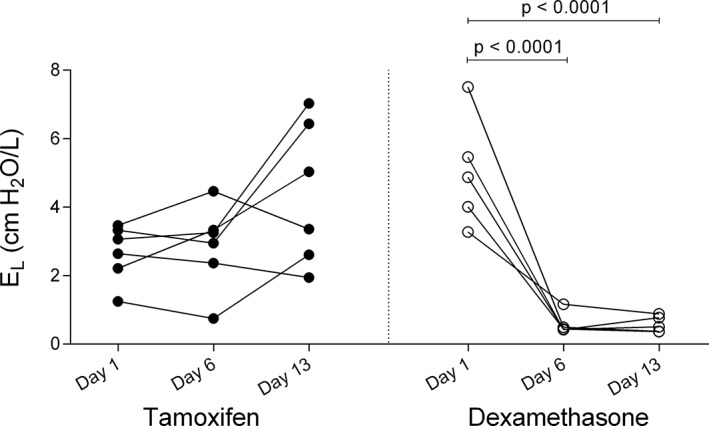
Values of pulmonary elastance (E L) on day 1 (before administration of medication), day 6 and day 13 of treatment with tamoxifen (black circles) and dexamethasone (white circles)
Figure 4.
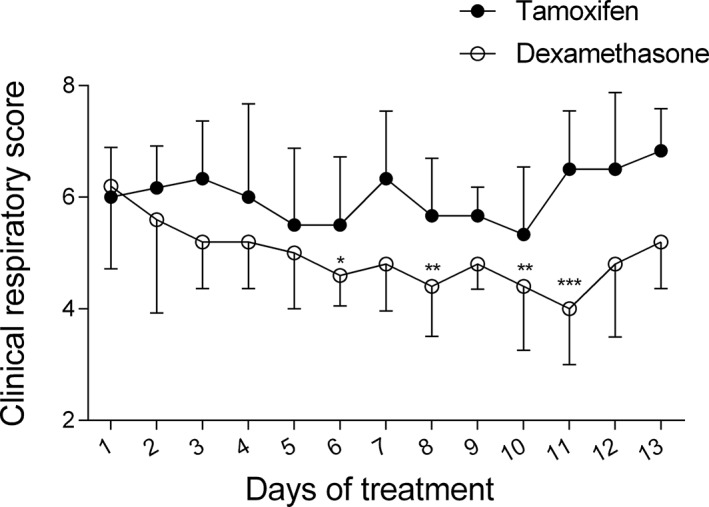
Daily clinical respiratory scores (means and standard deviations) from day 1 (before administration of medication) to day 13 of treatment with tamoxifen (black circles) and dexamethasone (white circles). *P < .05; **P < .01; ***P < .001 (significant differences from baseline)
3.2. Tracheal mucus scores
No difference was observed in the tracheal mucus scores among treatment groups at baseline or after treatment (mean reduction of 0.8/5 [CI: −2.8 to 1.1, P = .56] in the tamoxifen group and mean reduction of 0.5/5 [CI: −2.0 to 1.0, P = .75) in the dexamethasone group on day 13; Figure 5).
Figure 5.
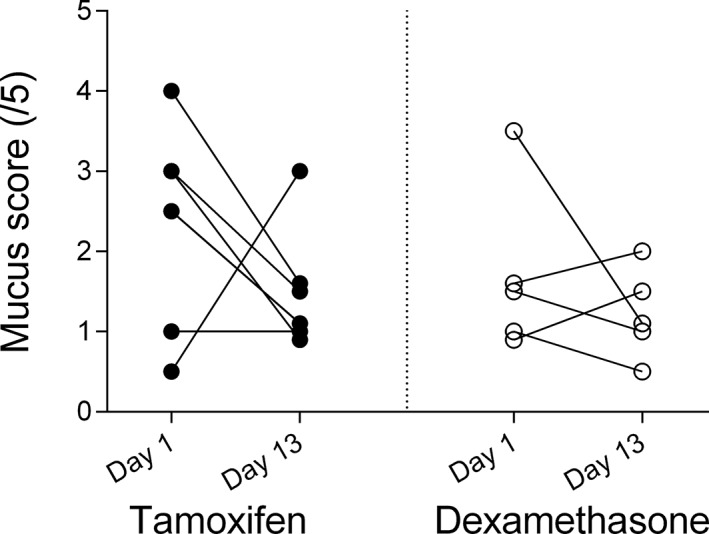
Tracheal mucus scores on day 1 (before administration of medication) and day 13 of treatment with tamoxifen (black circles) and dexamethasone (white circles)
3.3. BALF cytology
At the beginning of the study, the percentage of neutrophils in the BALF was above normal (>5%) in 10 of the 11 remaining horses. The horse lacking airway neutrophilia was excluded from analysis concerning BALF cytology. The two‐way ANOVA showed a group effect (P = .02) which was possibly related to a higher neutrophilia at baseline in the tamoxifen group. Neither tamoxifen (mean reduction of 17.3% [CI: −15.1 to 49.8, P = .35]) nor dexamethasone (mean reduction of 3.3% [CI: −29.2 to 35.7, P > .99]) improved the BALF neutrophilia (Figure 6).
Figure 6.
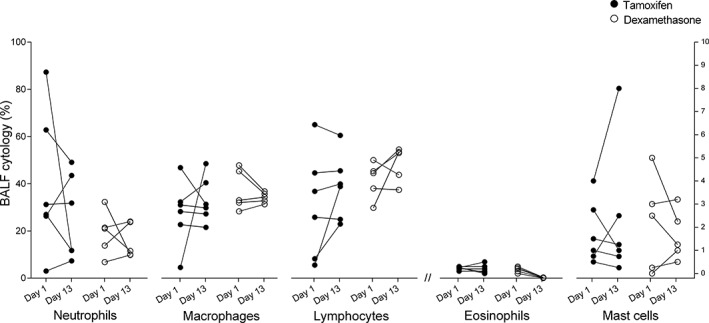
Percentage of each leucocyte population in the bronchoalveolar lavage fluid (BALF) before (day 1) and after treatment (day 13) with tamoxifen (black circles) and dexamethasone (white circles). The left Y axis applies to the percentage of neutrophils, macrophages and lymphocytes. The right Y axis applies to the percentage of eosinophils and mast cells
4. DISCUSSION
The results of this randomized controlled study failed to detect an effect of a short‐term treatment with tamoxifen on airway neutrophilia in severe asthmatic horses, refuting this hypothesis. A statistically significant diminution, but not a normalization, of airway resistance was observed, without improvement of the pulmonary elastance. As expected, dexamethasone normalized lung function while neither reducing airway inflammation6 nor macroscopic mucus accumulation 22.
Tamoxifen's effectiveness in the treatment of breast cancer is partly attributed to the apoptosis of tumor cells mediated by oxidative stress and increased ceramide intracellular level among other mechanisms.23 Tamoxifen also causes ceramide intracellular accumulation in neutrophils24, therefore, we suspected that it could lead to neutrophilic apoptosis and concurrent improvement of airway inflammation. However, we did not observe a decrease of airway neutrophilia with tamoxifen which contrasts with previous reports. Indeed, in one study, tamoxifen administered every other day for three doses reduced experimentally induced intraluminal inflammation in healthy horses.19 However, the pathways responsible for neutrophil accumulation in healthy horses after hay exposure likely differ from that of asthmatic horses which might explain these different results. In addition, because the airway neutrophilia is transient when hay is introduced to healthy horses and considering that antigenic exposure was ceased when the treatment was initiated, the improvement reported might have been the normal kinetic of airway inflammation regulation, rather than an effect of tamoxifen.25 Moreover, the duration of the antigenic challenge was of a short duration (1 week) when compared with this study (3 weeks), which could have contributed the discordance of the results. The chronicity of the exacerbation in this study could have impeded the anti‐inflammatory efficacy of this medication. However, to be useful clinically, a treatment would have to be effective under these conditions.
The effects of tamoxifen on neutrophils are controversial with in vitro studies suggesting an anti‐inflammatory effect by the induction of apoptosis, a reduction of the chemotactic response and respiratory burst production, a decreased production of 5‐lipoxygenase and a diminution of the neutrophilic infiltration to the site of injury.17, 18, 19, 26, 27 In contrast, other experiments have shown activation of neutrophils by an enhancement of their chemotaxis, phagocytic and bactericidal activity, and neutrophil extracellular traps (NETs) formation.24 Because aberrant NETs production is a feature of human and equine asthma, medications increasing their formation might be detrimental for the treatment of this condition.3, 28 Furthermore, it has been suggested that the immunomodulation associated with tamoxifen is mediated by a shift from a Th1 to a Th2 response, possibly related to an inhibition of the maturation of dendritic cells.11, 29 Because a predominant Th2‐type response has been associated with exacerbation in severe equine asthma, at least in some horses 30, 31, a shift in cellular signaling might explain the lack of efficacy in this study. Of note, a case report describes human asthma exacerbations induced by tamoxifen, but the mechanisms of those deteriorations were not determined.32 Taken together, the usefulness of this medication raises interrogations in a disease where immunological pathways involved are complex and incompletely understood.
The main limitation of this trial is the small number of horses which might have precluded the detection of small differences in the airway neutrophilia, the primary outcome of this study. In addition, the mild neutrophilia (<25%) observed in five horses in the present trial could have reduced our capacity to identify improvement of luminal inflammation. Even though the BALF cytology is a mainstay in asthma diagnosis, the degree and onset of neutrophilic influx might not be constant.33 From these five horses, only one had airway neutrophilia defined as normal (<5%) despite its disease being well characterized by being part of the research herd for several years, and the presence of severe airway obstruction (R L of 4.3 cm H2O/L/s and E L of 2.2 cm H2O/L) at the onset of the study. Nevertheless, the changes in airway neutrophilia with tamoxifen were inconsistent, and did not lead to normalization in any horses. The sample size was based on power analysis calculated from the Perez's study19 results where six animals in each group was sufficient to observe significant improvement in airway inflammation, clinical score, and mucus accumulation with tamoxifen. However, considering the mild reduction of neutrophilia and the variability of the data in the current study, about 30 severe asthmatic animals would have been required to obtain significant result with a power of 80% and alpha set at 0.05. Such a small effect would question the usefulness of this drug for the treatment of severe equine asthma. Finally, we cannot exclude that the lack of improvement because of inappropriate dosage. The dose was chosen because it was reportedly effective at decreasing the airway neutrophilia in experimental airway inflammation in horses and was similar to the dosage used for breast‐cancer treatment.10, 19 The pharmacokinetics of the molecule is currently studied (G. Morán, personal communication). The results of this study should be interpreted in light of the limitations raised above.
The clinical significance of the improvement in R L with tamoxifen in the present study is difficult to conceptualize because the other parameters of lung function and the clinical respiratory scores were unchanged. Resistance is associated with airflow limitations in central airways. Therefore, it is possible that tamoxifen‐mediated specific bronchodilator effect on central bronchi independently from an effect on airway luminal neutrophils and without improving airflow in the periphery of the lung. Selective estrogen receptor modulators can act as a receptor antagonist or agonist depending on the target cells. Interestingly, estrogen possesses bronchodilator property which might be related to its receptor colocalization with beta‐2 adrenergic receptors in airway smooth muscle cells.34 Some studies associate estrogen with improvement of asthma clinical signs or lung function15, whereas others suggest a detrimental effect of this sex hormone16. Therefore, an estrogen‐mediated bronchodilation would be a possible explanation for the reduced R L observed in this study, however more research on the role of sex hormones in asthma is required. Alternatively, the bronchodilation could have been mediated by an interaction with calcium‐channels. A reduction of vascular smooth muscle cell contractility has been reported with tamoxifen and the authors suggested that the effect might be related to an inhibition of voltage‐dependant calcium channels.35
Consistent with the favorable safety profile of tamoxifen in humans, we did not observe adverse events with a short‐term administration. Reported adverse effects after prolonged use include endometrial cancers and thromboembolic accidents.9 The refractory hypocalcemic and hypomagnesemic tetany observed in a dexamethasone treated horse was associated with the loss of principal cells in the parathyroid gland which possibly decreased the production of parathyroid hormone. Because glucocorticoids alter calcium metabolism by increasing urinary excretion and decreasing intestinal absorption, the primary underlying condition might have been worsened by the dexamethasone treatment.36, 37
In conclusion, a short‐term tamoxifen treatment failed to improve airway inflammation of severely asthmatic horses in chronic exacerbation, precluding the use of this medication at the current posology to assess the role of pulmonary neutrophils in the disease. The treatment resulted in a reduction of R L, but in contrast to dexamethasone, the lung function did not normalize. Nevertheless, deciphering the mechanisms responsible for the improvement in lung function of severe asthmatic horses with tamoxifen is of interest and justifies determining its pharmacokinetics for future studies.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank Serena Ceriotti and Amandine Vargas for technical support through this study. This study was performed at the Faculty of Veterinary Medicine of the Université de Montréal. It was supported by the Equine Research Fund of the Université de Montréal and an unrestricted research grant from Zoetis and the Canadian Institutes of Health Research. The results of this study were presented at the 35th VCRS annual symposium, Champaign, IL, USA, October 1‐4th, 2017.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All experimental procedures were performed in accordance with the Canadian Council for Animal Care guidelines and were approved by the Animal Care Committee of the Faculty of Veterinary Medicine of the Université de Montréal (Protocol # Rech‐1324).
Mainguy‐Seers S, Picotte K, Lavoie J‐P. Efficacy of tamoxifen for the treatment of severe equine asthma. J Vet Intern Med. 2018;32:1748–1753. 10.1111/jvim.15289
Funding information
Canadian Institutes of Health Research; Equine Research Fund of the Université de Montréal (unrestricted grant from Zoetis)
References
- 1. Pirie RS. Recurrent airway obstruction: a review. Equine Vet J. 2014;46:276‐288. [DOI] [PubMed] [Google Scholar]
- 2. Ciepiela O, Ostafin M, Demkow U. Neutrophils in asthma—a review. Respir Physiol Neurobiol. 2015;209:13‐16. [DOI] [PubMed] [Google Scholar]
- 3. Cote O, Clark ME, Viel L, et al. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS One. 2014;9:e96217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farah CS, Keulers LA, Hardaker KM, et al. Association between peripheral airway function and neutrophilic inflammation in asthma. Respirology. 2015;20:975‐981. [DOI] [PubMed] [Google Scholar]
- 5. Leclere M, Lavoie‐Lamoureux A, Joubert P, et al. Corticosteroids and antigen avoidance decrease airway smooth muscle mass in an equine asthma model. Am J Respir Cell Mol Biol. 2012;47:589‐596. [DOI] [PubMed] [Google Scholar]
- 6. Lavoie JP, Pasloske K, Joubert P, et al. Lack of clinical efficacy of a phosphodiesterase‐4 inhibitor for treatment of heaves in horses. J Vet Intern Med. 2006;20:175‐181. [DOI] [PubMed] [Google Scholar]
- 7. Rush BR, Raub ES, Rhoads WS, et al. Pulmonary function in horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am J Vet Res. 1998;59:1039‐1043. [PubMed] [Google Scholar]
- 8. Leclere M, Lavoie‐Lamoureux A, Lavoie JP. Heaves, an asthma‐like disease of horses. Respirology. 2011;16:1027‐1046. [DOI] [PubMed] [Google Scholar]
- 9. Traboulsi T, El Ezzy M, Gleason JL, et al. Antiestrogens: structure‐activity relationships and use in breast cancer treatment. J Mol Endocrinol. 2017;58:R15‐R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howell SJ, Johnston SR, Howell A. The use of selective estrogen receptor modulators and selective estrogen receptor down‐regulators in breast cancer. Best Pract Res Clin Endocrinol Metab. 2004;18:47‐66. [DOI] [PubMed] [Google Scholar]
- 11. Behjati S, Frank MH. The effects of tamoxifen on immunity. Curr Med Chem. 2009;16:3076‐3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colon JM, Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res. 2016;11:1208‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sereda D, Werth VP. Improvement in dermatomyositis rash associated with the use of antiestrogen medication. Arch Dermatol. 2006;142:70‐72. [DOI] [PubMed] [Google Scholar]
- 14. Few J, Thompson NW, Angelos P, Simeone D, Giordano T, Reeve T. Riedel's thyroiditis: treatment with tamoxifen. Surgery. 1996;120:993‐998. discussion 998‐999. [DOI] [PubMed] [Google Scholar]
- 15. Chandler MH, Schuldheisz S, Phillips BA, Muse KN. Premenstrual asthma: the effect of estrogen on symptoms, pulmonary function, and beta 2‐receptors. Pharmacotherapy. 1997;17:224‐234. [PubMed] [Google Scholar]
- 16. Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult‐onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995;152:1183‐1188. [DOI] [PubMed] [Google Scholar]
- 17. Borlone C, Morales N, Henriquez C, et al. In vitro effects of tamoxifen on equine neutrophils. Res Vet Sci. 2017;110:60‐64. [DOI] [PubMed] [Google Scholar]
- 18. Sarmiento J, Perez B, Morales N, et al. Apoptotic effects of tamoxifen on leukocytes from horse peripheral blood and bronchoalveolar lavage fluid. Vet Res Commun. 2013;37:333‐338. [DOI] [PubMed] [Google Scholar]
- 19. Perez B, Henriquez C, Sarmiento J, et al. Tamoxifen as a new therapeutic tool for neutrophilic lung inflammation. Respirology. 2016;21:112‐118. [DOI] [PubMed] [Google Scholar]
- 20. Gerber V, Straub R, Marti E, et al. Endoscopic scoring of mucus quantity and quality: observer and horse variance and relationship to inflammation, mucus viscoelasticity and volume. Equine Vet J. 2004;36:576‐582. [DOI] [PubMed] [Google Scholar]
- 21. Robinson NE, Olszewski MA, Boehler D, et al. Relationship between clinical signs and lung function in horses with recurrent airway obstruction (heaves) during a bronchodilator trial. Equine Vet J. 2000;32:393‐400. [DOI] [PubMed] [Google Scholar]
- 22. Courouce‐Malblanc A, Fortier G, Pronost S, et al. Comparison of prednisolone and dexamethasone effects in the presence of environmental control in heaves‐affected horses. Vet J. 2008;175:227‐233. [DOI] [PubMed] [Google Scholar]
- 23. Bekele RT, Venkatraman G, Liu RZ, et al. Oxidative stress contributes to the tamoxifen‐induced killing of breast cancer cells: implications for tamoxifen therapy and resistance. Sci Rep. 2016;6:21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corriden R, Hollands A, Olson J, et al. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun. 2015;6:8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leclere M, Lavoie‐Lamoureux A, Gelinas‐Lymburner E, et al. Effect of antigenic exposure on airway smooth muscle remodeling in an equine model of chronic asthma. Am J Respir Cell Mol Biol. 2011;45:181‐187. [DOI] [PubMed] [Google Scholar]
- 26. Tavares IA, Stamford IF, Bennett A. Tamoxifen inhibits 5‐lipoxygenase in human polymorphonuclear leucocytes. J Pharm Pharmacol. 1987;39:323‐324. [DOI] [PubMed] [Google Scholar]
- 27. Wei HY, Ma X. Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF‐kB pathway after spinal cord injury in rats. Neurol Sci. 2014;35:1763‐1768. [DOI] [PubMed] [Google Scholar]
- 28. Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Komi J, Lassila O. Nonsteroidal anti‐estrogens inhibit the functional differentiation of human monocyte‐derived dendritic cells. Blood. 2000;95:2875‐2882. [PubMed] [Google Scholar]
- 30. Lavoie JP, Maghni K, Desnoyers M, et al. Neutrophilic airway inflammation in horses with heaves is characterized by a Th2‐type cytokine profile. Am J Respir Crit Care Med. 2001;164:1410‐1413. [DOI] [PubMed] [Google Scholar]
- 31. Cordeau ME, Joubert P, Dewachi O, Hamid Q, Lavoie JP. IL‐4, IL‐5 and IFN‐gamma mRNA expression in pulmonary lymphocytes in equine heaves. Vet Immunol Immunopathol. 2004;97:87‐96. [DOI] [PubMed] [Google Scholar]
- 32. Smith RP, Dewar JA, Winter JH. Tamoxifen‐induced asthma. Lancet. 1993;341:772. [DOI] [PubMed] [Google Scholar]
- 33. Fairbairn SM, Page CP, Lees P, et al. Early neutrophil but not eosinophil or platelet recruitment to the lungs of allergic horses following antigen exposure. Clin Exp Allergy. 1993;23:821‐828. [DOI] [PubMed] [Google Scholar]
- 34. Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2012;303:L923‐L928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song J, Standley PR, Zhang F, et al. Tamoxifen (estrogen antagonist) inhibits voltage‐gated calcium current and contractility in vascular smooth muscle from rats. J Pharmacol Exp Ther. 1996;277:1444‐1453. [PubMed] [Google Scholar]
- 36. Glade MJ, Krook L, Schryver HF, Hintz HF. Calcium metabolism in glucocorticoid‐treated pony foals. J Nutr. 1982;112:77‐86. [DOI] [PubMed] [Google Scholar]
- 37. Liamis G, Milionis HJ, Elisaf M. A review of drug‐induced hypocalcemia. J Bone Miner Metab. 2009;27:635‐642. [DOI] [PubMed] [Google Scholar]


