Abstract
Background
Hypovitaminosis D is common in humans with tuberculosis, and adequate serum 25‐hydroxyvitamin D [25(OH)D] concentrations may improve response to therapy. The pathomechanism of Blastomyces dermatitidis is similar to that of Mycobacterium tuberculosis, but the 25(OH)D status of dogs with blastomycosis has not been investigated.
Objectives
To determine if dogs with blastomycosis have lower 25(OH)D concentrations compared with healthy controls and to explore the prognostic value of 25(OH)D concentrations in blastomycosis.
Animals
35 control dogs (16 client‐owned, healthy dogs and 19 healthy, random‐source hound mixes) and 22 dogs with blastomycosis.
Methods
Prospective study. Serum concentrations of 25(OH)D, parathyroid hormone (PTH), ionized calcium were measured, and biochemistry and hematology profiles were performed. The 25‐hydroxyvitamin D concentrations were compared between groups, and factors associated with 25(OH)D variation were investigated in dogs with blastomycosis. Dogs with blastomycosis were followed for up to 5 years after discharge and factors associated with survival were investigated.
Results
Dogs with blastomycosis had significantly lower concentrations of 25(OH)D and PTH and higher concentrations of ionized calcium than did control dogs. In dogs with blastomycosis, 25(OH)D concentrations were independently associated with neutrophil count, pCO2, and with bone and skin involvement. The 25‐hydroxyvitamin D concentration was not associated with survival in dogs with blastomycosis, whereas lactate concentrations; bone, skin, and lymph node involvement; number of affected sites; and, presence of respiratory signs were associated with survival.
Conclusions and Clinical Importance
Dogs with blastomycosis had lower 25(OH)D concentrations than did healthy controls. Despite no impact on survival, investigating the effect of 25(OH)D supplementation on recovery is warranted.
Keywords: Calcium, canine, fungal, parathyroid hormone, vitamin D
Abbreviations
- 25(OH)D
25‐hydroxyvitamin D
- 1,25(OH)2D
1,25‐dihydroxyvitamin D
- iCa
ionized calcium
- pCO2
partial pressure of carbon dioxide
- PTH
parathyroid hormone
- TB
tuberculosis
- WBC
white blood cell
1. INTRODUCTION
Vitamin D, long known for its role in calcium homeostasis,1 recently has been recognized for its immunoregulatory functions.2, 3 One of vitamin D's key effects is its role in mucosal immunity and early defense mechanisms against certain infections.4 Research into macrophage–pathogen interactions indicated that vitamin D plays a crucial role in endogenous antimicrobial defenses.5 Hypovitaminosis D has been implicated as a cause of impaired immune function in respiratory tract infections6, 7 and has been found to be a risk factor for pulmonary tuberculosis (TB) caused by Mycobacterium tuberculosis.8, 9, 10 Studies involving M. tuberculosis identified vitamin D's ability to stimulate differentiation of monocyte precursors into mature, macrophage‐like cells and an immunomodulatory role for vitamin D.11, 12 Vitamin D prevents infection by M. tuberculosis through the binding of its biologically active form, 1,25‐dihydroxyvitamin D [1,25(OH)2D], to the vitamin D receptor, a nuclear receptor that regulates gene expression in cells important to immune function.13, 14 Vitamin D‐dependent modulation of monocyte‐macrophage function is essential to innate and adaptive immunity.2
Blastomyces dermatitidis infections share clinical parallels with M. tuberculosis infections suggesting similar susceptibility factors, mechanisms or both. Both pathogen infections begin with inhalation, transmission to the alveoli, and then uptake of the pathogen by alveolar macrophages. Pulmonary alveolar macrophages typically prevent host infection of B. dermatitidis by killing conidia and inhibiting their conversion to the yeast phase.15 If pulmonary defenses fail to clear the conidia, normal body temperature along with other factors allow transformation to the yeast phase.16 B. dermatitidis can evade these host defenses by means of a surface adhesion virulence factor.17 The factor is present only in the yeast phase and is necessary for adherence to macrophages; it binds the fungus to macrophages via complement receptors18 in a manner very similar to M. tuberculosis.19 Internalization of the virulence factor via receptor‐specific endocytosis leads to suppression of normal macrophage and neutrophil functions.20 Suppression of macrophage function is also the means by which M. tuberculosis evades host defenses.21
In specific regions of the United States, B. dermatitidis causes substantial morbidity and mortality in both canine and human populations.22 Dogs appear to be at increased risk of infection23 and may act as a sentinel for human infections.24, 25 Preferred sites of dissemination include bone, eyes, lymph nodes, lung, skin, prostate, testes,26 and the brain.27, 28 Granuloma formation is characteristic of both B. dermatitidis and M. tuberculosis, again suggesting similar immunologic processes in the host response.16, 29
Using TB as a model for blastomycosis in dogs, our objectives were threefold: 1 To compare serum 25(OH)D, parathyroid and ionized calcium (iCa) concentrations in dogs with blastomycosis and healthy control dogs from the same geographical area; 2 To evaluate the association between serum 25(OH)D, parathyroid and iCa concentrations and results from CBC, serum biochemical profile, venous blood gas analysis, number of body sites affected, the clinical presence of respiratory or ocular signs, or evidence of pulmonary changes on radiographs; and 3 To evaluate associations between the variables from objective 2 and survival in dogs with blastomycosis. We hypothesized that vitamin D concentrations would be significantly lower in dogs with blastomycosis compared with healthy controls, and that survival would be impacted by 25(OH)D concentrations in dogs with blastomycosis.
2. MATERIALS AND METHODS
2.1. Control animals
The control population (n = 35) consisted of client‐owned, healthy dogs (n = 16) as well as healthy, random‐source hound mixes (n = 19). Routine laboratory screening (CBC, serum biochemical profile) in addition to a complete physical examination was performed in both groups to rule out subclinical illness. Neither group of healthy dogs was receiving any medications other than routine heartworm and flea prophylaxis.
2.2. Clinical animals
Client‐owned dogs (n = 22) presented to the Veterinary Teaching Hospital and diagnosed with blastomycosis were included in the study. Diagnosis was made based on appropriate clinical signs and positive cytological or histopathological identification of the organism or positive urine antigen test results (MVista® Blastomyces dermatitidis Quantitative Antigen EIA, MiraVista Diagnostics, Indianapolis, IN). Dogs were excluded if they had been treated with any glucocorticoids or antifungal drugs > 5 days before presentation. The study was approved by the University’s Institutional Animal Care and Use Committee.
Serum samples were collected and stored at −20°C or −80° C until submission to an outside laboratory (Diagnostic Center for Population and Animal Health, Michigan State University, Lansing, MI). Vitamin D [25(OH)D] was measured by radioimmunoassay, parathyroid hormone (PTH) was measured using an immunoradiometric assay and iCa was measured using an ion selective membrane (NOVA 8 analyzer, NOVA Biomedical, Waltham, MA). Medical records for each clinical patient were examined and any data regarding routine laboratory screening (CBC, serum biochemical profile), lactate concentrations, venous blood gas analytes, whether thoracic radiographs were taken (and their findings), and sites of infection were collected.
2.3. Statistical analysis
Statistical analyses were performed using a commercially available software package (STATA, version 14.0, StataCorp LP, College Station, TX). Continuous data were assessed for Gaussian distribution by histogram evaluation and the Shapiro‐Wilk test (Gaussian if P value >.05). As none of the continuous variables were normally distributed, a Mann‐Whitney test was used to compare the age and the analyte between dogs with and without blastomycosis. A 2‐tailed Fisher exact test was used to compare the 2 groups in term of sex. Variables that were significantly different between the 2 groups were assessed as potential confounders for the association between 25(OH)D concentration and blastomycosis.
Serum 25(OH)D, PTH, iCa and total calcium, and phosphorus concentration distributions were assessed for Gaussian distribution as previously described. An F‐test was performed for those variables identified as Gaussian in order to ascertain equality of variance between the 2 groups (ie, group of healthy dogs and group of dogs with blastomycosis). A Student's t‐test or Mann‐Whitney test was used as appropriate for univariate assessment of differences between dogs with and without blastomycosis. The association between 25(OH)D concentrations and the potential confounders was assessed by linear regression. The associations between 25(OH)D concentrations and the number of affected sites and radiographic findings in dogs with blastomycosis were assessed by ordered logistic regressions. The associations between 25(OH)D concentrations and the type of affected site and ocular and respiratory clinical signs in dogs with blastomycosis were assessed by analysis of variance (ANOVA) models.
The potential associations among 25(OH)D concentrations, PTH concentrations, iCa concentrations, CBC, serum biochemical profile, lactate concentrations, venous blood gas analytes, and survival in dogs with blastomycosis were studied using Cox proportional hazards regression models. The potential associations between affected site, number of affected sites, presence of radiographic pulmonary lesions, and presence of ocular and respiratory signs and survival in dogs with blastomycosis were assessed using log rank tests and Kaplan‐Meier curves.
When models were used, assumptions of Gaussian distribution and homoscedasticity of residuals were checked graphically and by the Shapiro‐Wilk, and the Breusch‐Pagan and Cook‐Weisberg tests, respectively. Distribution and residuals were considered as Gaussian and homoscedastic, respectively, when P value >.05 for the Shapiro‐Wilk and the Breusch‐Pagan/Cook‐Weisberg tests. When necessary, the dependent variable was log transformed to validate these assumptions. For all statistical analyses, significance was set at P value <.05.
3. RESULTS
3.1. Study population
Both healthy dog (n = 35) and clinical dog (n = 22) samples were analyzed for 25(OH)D concentrations. The PTH and iCa concentrations were not measured in all dogs because of insufficient sample amounts or issues that occurred during shipment of samples to the outside laboratory. For cases without sufficient sample volume, testing was prioritized in this order: 25(OH)D, PTH, and iCa. Nineteen healthy dogs and the 22 clinical case samples had PTH measured. For those samples sent to the outside laboratory with insufficient sample volume to run all 3 tests, iCa results were taken from results obtained in our hospital using an in‐house point‐of‐care analyzer (Critical Care X‐Press, NOVA Biomedical, Waltham, MA) similar to that used by the outside laboratory. Nineteen healthy dog and 21 clinical case samples had iCa performed; 3 of the 21 clinical case samples were measured in‐house and 18 were measured at the outside reference laboratory.
There was no difference in terms of age between groups (P = .13), with a median age for healthy dogs of 3 years (range, 2‐9) and 4.1 years (range, 0.8‐9) for the dogs with blastomycosis. There were 23 (15 intact) females and 12 (4 intact) males in the healthy group and 10 spayed females and 12 (4 intact) males in the affected dogs, which represented a significant difference in partition between groups (P = .001). The breeds within the healthy group included research bred hound‐crosses (n = 19), mixed breed dogs (n = 6), Labrador retriever (n = 2), and 1 each of other breeds (n = 8). The dogs with blastomycosis consisted of Labrador retriever (n = 6), Golden retriever (n = 4), Doberman (n = 2), mixed‐breed dog (n = 2), and 1 each of other breeds (n = 8).
Table 1 shows a comparison of signalment, CBC, serum biochemistry, and venous blood gas variables between dogs with and without blastomycosis. In addition to 25(OH)D, PTH, and iCa, significant differences were found between the dogs with and without blastomycosis for sex, pH, partial pressure of carbon dioxide (pCO2), bicarbonate, lactate, white blood cell (WBC) count, neutrophils, monocytes, platelets, as well as serum creatinine, total protein, and potassium concentrations and alkaline phosphatase activity.
Table 1.
Comparison of signalment, complete blood count, biochemistry and venous blood gas variables between control and clinical dogs
| Variable | Reference range | Control (n = 35) | Clinical (n = 22) | P value |
|---|---|---|---|---|
| Gender | MI: 4 [11%] MC: 8 [23%] FI: 15 [43%] FS: 8 [23%] |
MI: 4 [18%] MC: 8 [36%] FI: 0 [0%] FS: 10 [46%] |
.001 | |
| 25(OH)D (nmol/L) | 60‐215 | 132 [81‐209] (n = 35) | 79 [33‐125] (n = 22) | <.001 |
| PTH (pmol/L) | 0.5‐5.8 | 1.5 [0.8‐6.7] (n = 19) | 0.5 [0.3‐1.5](n = 21) | <.001 |
| iCa (mmol/L) | 1.25‐1.45 | 1.16 [1.05‐1.29] (n = 19) | 1.30 [1.08‐1.45] (n = 21) | <.001 |
| pH | 7.39‐7.49 | 7.35 [7.32‐7.44] (n = 9) | 7.43 [7.25‐7.56] (n = 16) | .006 |
| pCO2 | 23.1‐37.1 | 39.1 [30.7‐41.7] (n = 8) | 27.2 [21.2‐34.8] (n = 18) | <.001 |
| HCO3 | 16‐24 | 21.7 [20.3‐23.2] (n = 8) | 19.0 [13.6‐21.8] (n = 18) | <.001 |
| Lactate | 0.44‐2.93 | 0.9 [0.8‐2.3] (n = 8) | 1.9 [0.6‐9.3] (n = 17) | .006 |
| WBC (×103/μL) | 6.0‐17.0 | 6.2 [3.7‐16.2] (n = 25) | 17.0 [8.4‐41.8] (n = 13) | <.001 |
| Neutrophils (×103/μL) | 3.0‐11.5 | 3.9 [1.8‐12.8] (n = 25) | 13.9 [5.4‐35.1] (n = 13) | <.001 |
| Monocytes (×103/μL) | 0.2‐1.4 | 0.2 [0.04‐1.0] (n = 25) | 0.9 [0.2‐1.9] (n = 13) | <.001 |
| PLT (×103/μL) | 200‐900 | 231 [174‐400] (n = 25) | 333 [160‐425] (n = 13) | .008 |
| Creat (mg/dL) | 0.5‐1.5 | 0.9 [0.6‐1.5] (n = 16) | 0.7 [0.5‐1.1] (n = 21) | .002 |
| TP (g/dL) | 5.1‐7.0 | 6.0 [5.5‐6.4] (n = 9) | 6.9 [5.4‐7.5] (n = 12) | .004 |
| Potassium (mmol/L) | 3.9‐5.5 | 3.9 [3.5‐4.4] (n = 16) | 4.3 [3.6‐4.9] (n = 21) | .02 |
| AlkP (U/L) | 7‐92 | 33 [22‐143] (n = 9) | 80 [41‐850] (n = 14) | .04 |
Results are presented as median (range) for all continuous variables, and as number of dogs (percentage) for categorical variables. Only values which had a significant P value were included (significance level set at P < .05). MI, male intact; MC, male castrated; FI, female intact; FS, female spayed; 25(OH)D, 25‐hydroxyvitamin D; PTH, parathyroid hormone; Ica, ionized calcium; pCO2, partial pressure carbon dioxide; HCO3, bicarbonate; WBC, white blood cell; PLT, platelet; creat, creatinine; TP, total protein; AlkP, alkaline phosphatase.
3.2. Vitamin D, PTH, and ICA concentrations in dogs with blastomycosis
The median 25(OH)D concentration in the clinical group of dogs (79 nmol/L; range, 33‐125) was significantly lower (P < .001) than in the control group (132 nmol/L; range, 81‐209). Six dogs (27.3%) with blastomycosis had 25(OH)D concentrations below the lower end of the normal reference range. The median PTH concentration in the clinical dogs (0.5 pmol/L; range, 0.3‐1.5) was significantly lower (P < .001) than in control dogs (1.5 pmol/L; range, 0.8‐6.7). Median iCa was significantly higher (P < .001) in clinical dogs (1.3 mmol/L; range, 1.08‐1.45) as compared with control dogs (1.16 mmol/L; range, 1.05‐1.29; Figure 1).
Figure 1.

Boxplots displaying the 25(OH)D (a), parathyroid hormone (b), ionized calcium (c), and phosphorus (d) concentrations of dogs in the control (n = 35) and clinical (n = 22) groups. PTH and iCa were available in 19 of 35 controls and 21 of 22 affected dogs. *Significant difference between the control and the clinical groups, as defined by P < .05. The box represents the interquartile range (ie, from 1st to 3rd quartile), the line represents the median, the whiskers represent the highest and lowest value within 1.5× the interquartile range, and the circles indicate outliers
Vitamin D concentrations in dogs with blastomycosis were significantly associated with WBC count, neutrophil count, and monocyte count (P < .001 for all associations). However, only the association with neutrophil count remained significant (P = .001) when both neutrophil and monocyte counts were entered in the model. For every 1 × 103/μL increase in neutrophil count, there was a 3.2 nmol/L decrease in 25(OH)D. Vitamin D concentrations in dogs with blastomycosis also were significantly associated with pCO2 (P < .001). This association was independent of the neutrophil count; for every 1 mm Hg decrease in pCO2, there was a 3.9 nmol/L decrease in 25(OH)D.
Vitamin D concentrations in dogs with blastomycosis were significantly associated with serum creatinine and lactate concentrations although these associations did not remain significant when neutrophil count was added into the regression models. Vitamin D concentrations in dogs with blastomycosis also were significantly associated with blood pH although this association did not remain significant when pCO2 was added into the model.
Body sites where blastomycosis was found included bone (n = 4), eyes (n = 14), lung (n = 18), lymph node (n = 12), and skin (n = 5). Four dogs had only 1 site affected, 10 had 2 sites affected, 5 had 3 sites affected, 4 had 4 sites affected, and 2 dogs had all 5 sites affected. No association was found between 25(OH)D concentrations and the number of body sites affected (P = .41), but vitamin D concentrations were lower when both bone and skin were affected together (P = .04). Thoracic radiographs were taken on 21 of the 22 clinical dogs. Radiographic findings included diffuse miliary pattern (n = 7), mass lesion (n = 4), consolidated lung lobe (n = 2), mass lesion and miliary pattern (n = 2), cranial mediastinal lymphadenopathy (n = 1), tracheobronchial lymphadenopathy (n = 1), and diffuse nodules (n = 1). Three dogs had normal thoracic radiographs. No association was found between the 25(OH)D concentrations and the presence of respiratory and ocular signs or pulmonary radiographic findings.
3.3. Survival study in dogs with blastomycosis
No significant association was found between 25(OH)D, PTH, or iCa concentrations and survival. Among all of the variables tested, the only ones that were significantly associated with survival were lactate concentrations (P = .02), bone as an affected site (P = .01), lymph node as an affected site (P = .008), skin as an affected site (P < .001), number of affected sites (P = .005), and presence of respiratory signs (P < .001), whereas pulmonary involvement and the presence of pulmonary radiographic lesions were not (Figure 2).
Figure 2.
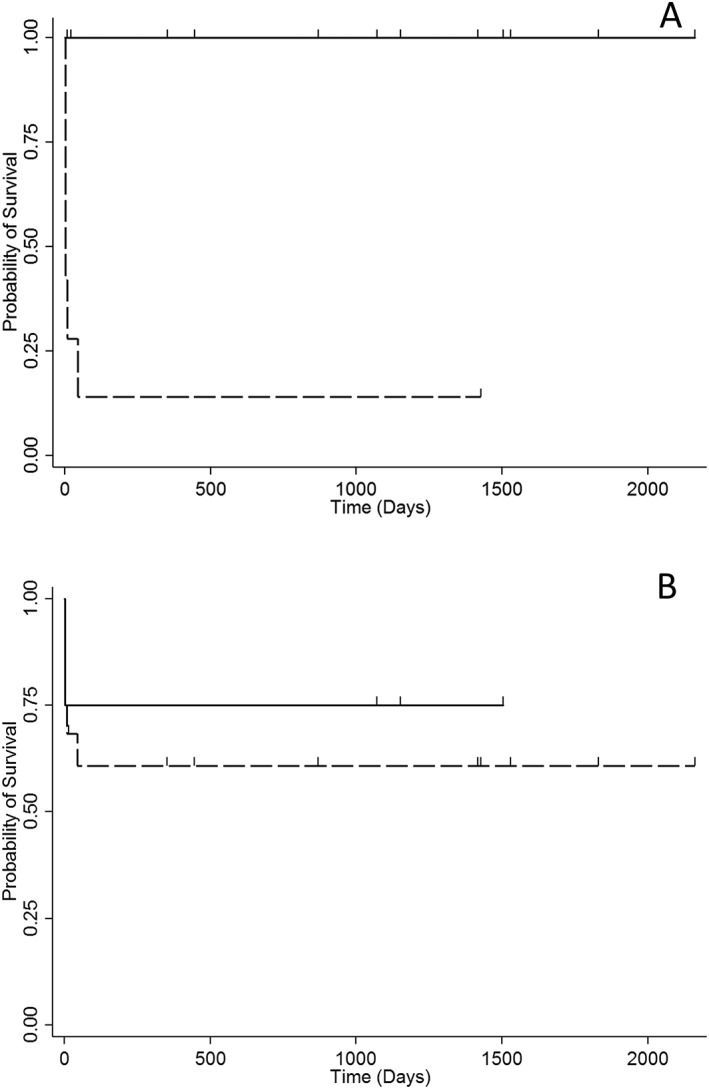
Kaplan‐Meier survival curve for 22 dogs with blastomycosis with (dashed line) and without (solid line) respiratory signs (A), and with (dashed line) and without (solid line) involvement of the lungs (B). Dogs were censored from analysis if they were still alive at follow‐up or at the time they were lost to follow‐up, and are represented by small spikes on the graph
4. DISCUSSION
Dogs with blastomycosis had lower 25(OH)D and PTH concentrations and higher iCa concentrations than did the controls. The median concentrations for each of these analytes still were within the normal reference range, but 27.3% of the dogs with blastomycosis had 25(OH)D concentrations that fell below the normal reference range. Vitamin D concentrations also were associated with WBC count and more specifically, with neutrophil count, in that as the neutrophil count rose, the 25(OH)D concentration decreased. Decreased survival was noted with increased lactate concentrations, if bone, lung, or skin was involved, as the number of affected sites increased and if there were respiratory signs present. Survival was not impacted by lower 25(OH)D concentrations.
The finding of significantly lower concentrations of serum 25(OH)D in dogs with blastomycosis compared with healthy dogs supports the hypothesis that dogs with lower vitamin D concentrations may be more at risk of infection by this geographically ubiquitous pathogen. Although no studies exist correlating vitamin D concentrations to risk of contracting blastomycosis, this presumption is corroborated by several studies in humans which lower serum 25(OH)D concentrations were found to be associated with higher risk of active tuberculosis,8, 9, 10 in some cases regardless of nutrition.30 In a case series of 3 dogs with blastomycosis that developed calcinosis cutis, 1 dog had a 25(OH)D concentration at the low end of normal whereas another had a low vitamin D concentration.31 Future studies could address the question of whether low 25(OH)D concentrations are a result of infection or whether they predispose the dog to infection by testing 25(OH)D concentrations before and after anti‐fungal treatment. Improvement in 25(OH)D concentrations with therapy would support low 25(OH)D concentrations to be a result of infection.
The importance of sufficient vitamin D concentrations for resistance to infection has long been appreciated but poorly understood. Hypovitaminosis D has been linked to increased risk for respiratory tract infection,6 TB,9, 32, 33 non‐tubercular mycobacterial lung disease,34 and human immunodeficiency virus.35 Vitamin D plays a key role in the host response to M. tuberculosis.36 Host defenses against mycobacteria involve the process of autophagy and the activation of endogenous antimicrobial peptides. Autophagy describes the ability of the host to eliminate intracellular pathogens by the maturation of phagosomes into phagolysosomes.37 Vitamin D improves macrophage killing of M. tuberculosis by these mechanisms.5, 38 The ability to escape this macrophage activity characterizes the virulence of M. tuberculosis 39, 40 and is speculated to occur when vitamin D concentrations are low or insufficient.30, 33
Cats with mycobacterial infections were found to have significantly lower vitamin D concentrations than healthy controls, but similar vitamin D concentrations to cats with systemic illness. One argument for this finding is that cats that are ill are not eating and therefore do not ingest enough vitamin D to maintain normal concentrations.32 Cats and dogs obtain their vitamin D through diet and not sunlight exposure.41 In this latter study, many of the cats with mycobacterial infections were eating and the conclusion was that the low vitamin D concentrations were likely a result of the disease process.32 Unfortunately in our study, many of the medical records did not contain information on whether the dog was eating, therefore a similar conclusion cannot be drawn for the dogs in our study.
Alternatively, it is possible that the lower 25(OH)D concentrations were due to increased conversion to calcitriol within the macrophages and consumption of 25(OH)D as a substrate. Renal PTH‐vitamin D‐calcium homeostasis is under tight feedback mechanisms but such mechanisms do not occur with extrarenal vitamin D production. Indeed, 1‐α‐hydroxylase becomes overexpressed in macrophages in some granulomatous diseases as a result of inflammation.42 The rationale is that higher 1,25(OH)2D activity is the body's response to combat the pathogen.43 A previous study showed that macrophages increase production of endogenous antimicrobial peptides in the presence of 1,25(OH)2D.36 We did not measure 1,25(OH)2D concentrations in our study and cannot determine if the decreased concentrations of 25(OH)D are a consequence of increased 1,25(OH)2D production, although the combination of lower PTH concentrations and higher iCa concentrations in dogs with blastomycosis compared with healthy control may support this hypothesis. Some have argued that because production of 1,25(OH)2D occurs within macrophages or within tissues, testing serum concentrations would not reflect this extrarenal production.44
Our finding of comparatively lower PTH concentrations in dogs with blastomycosis also has been reported in patients with pulmonary TB. The lower PTH concentrations could simply be due to negative feedback stimulation at the parathyroid gland from increased calcium concentrations.1 Additional theories for this finding include abnormal calcium metabolism or suppression of the parathyroid glands by cytokines or other inflammatory mediators.45 Previous reports in people have noted an inverse relationship between PTH and 25(OH)D concentrations up to a certain concentration of 25(OH)D at which PTH concentrations plateau. It is this inflection point that has been used to determine a normal or sufficient 25(OH)D concentration.46, 47
One possible explanation for the low PTH concentrations is that 1,25(OH)2D concentrations are increased, leading to a negative feedback effect on PTH production. In a study of patients with untreated TB, significantly lower 25(OH)D concentrations were found in the patients compared with healthy matched controls, but no differences in 1,25(OH)2D concentrations or PTH were found, disputing the presumption that 1,25(OH)2D concentrations are higher and therefore, through negative feedback, cause the PTH concentrations to decrease.48 Studies have speculated that much of the extrarenal production of 1,25(OH)2D remains confined to the cell and may not be reflected by serum concentrations. Parathyroid cells co‐express the CYP27B1‐hydroxylase and the vitamin D receptor, so it is possible that internally 1,25(OH)2D concentrations do increase and by negative feedback lead to decreased production of PTH.44
The normal serum calcium concentration in most dogs is consistent with a previous study evaluating calcium concentrations in dogs with blastomycosis.49 We did find higher iCa concentrations in the dogs with blastomycosis, but it should be noted that the higher iCa concentrations still were within the normal range. In a study of 125 dogs with blastomycosis, only 2 of 47 dogs had increased serum total calcium concentrations and only 1 of 26 dogs had serum iCa concentrations above the reference range.50 Another study found only 2 dogs of 38 with increased serum iCa concentrations.51 Vitamin D concentrations were not measured in either study. Granulomatous disease is known to cause hypercalcemia by unchecked 1,25(OH)2D activity within macrophages,52 but this mechanism has not been proven in dogs with blastomycosis. Hypercalcemia has been documented in a dog with granulomatous lymphadenitis53 and in a dog with gastric pythiosis.54 A cat with blastomycosis had hypercalcemia, increased 1,25(OH)2D concentrations and a 25(OH)D concentration below normal. Treatment with antifungal therapy resolved these abnormalities.55
Significant differences were found between the 2 groups of dogs for certain variables on the CBC, serum biochemical profile and venous blood gas analytes. Dogs with blastomycosis had higher numbers of total WBC, neutrophils, monocytes, and platelets. Leukocytosis, typically mild, has been found in other studies of blastomycosis27, 56, 57 often with neutrophilia. Monocytosis and increased platelet count also have been previously documented.27, 57 These findings support the presence of an ongoing systemic inflammatory response. Increased neutrophil and monocyte counts in dogs with lower vitamin D concentrations are not surprising given that a similar association has been noted in human patients with TB. Adequate response to therapy in human TB patients is associated with a decrease in neutrophil and monocyte counts.58, 59 Humans with TB also are noted to have higher platelet numbers. Platelets are believed to play a part in the inflammatory process because their numbers are associated with acute phase reactants.60
Dogs with lower vitamin D concentrations also had higher serum creatinine, total protein, and potassium concentrations and alkaline phosphatase activity. For venous blood gas analytes, dogs with blastomycosis had higher pH, and lactate concentration and lower PCO2 and bicarbonate concentrations. The increased total protein concentration has been a consistent finding in dogs with blastomycosis.27, 50, 51 The differences in serum creatinine and potassium concentrations, although statistically significant, were minor and concentrations were still within the normal reference range.
The higher pH and lower PCO2 concentrations in blood may reflect respiratory alkalosis in those dogs with pulmonary involvement. The lower bicarbonate concentrations may represent metabolic acidosis secondary to increased lactate concentrations or could be compensatory to respiratory alkalosis. Again, the differences are minor and may simply reflect normal variation in the population.
Interestingly, pulmonary involvement and the presence of pulmonary radiographic lesions were not associated with survival as had been shown in a previous study,61 indicating that the prognosis for pulmonary blastomycosis was poor only if the dog presented with respiratory clinical signs. Therefore, finding a pulmonary lesion of blastomycosis in a dog is not necessarily associated with a worse outcome, even when the radiographic pattern is miliary or diffuse, as long as the dog does not show respiratory clinical signs.
Our study had several limitations. We studied a small number of dogs and may not have had enough power to identify all the associations that existed. Because of the clinical nature of our study, not all animals received the same diagnostic testing and therefore we may have missed lesions that could have affected the comparisons. Although statistical significance was reached for many variables between the 2 groups, some differences were slight and did not fall outside of the normal reference ranges.
Similar to humans with TB, our study found comparatively lower vitamin D concentrations in dogs with blastomycosis. The significance of these findings is unknown at this time. It remains to be determined whether supplementation with vitamin D will improve response to therapy in dogs with blastomycoses or is simply a single risk factor in a multifactorial process.62 In humans with TB, supplementation with vitamin D has led to conflicting results,63, 64, 65 and these results may be the result of genetic polymorphisms for multiple metabolites in the vitamin D pathway. Genetic polymorphisms may influence susceptibility to infection as well as response to supplementation. One study demonstrated polymorphism of the vitamin D binding protein to be protective in people to blastomycosis.66 A recent study found that oral vitamin D supplementation in M. tuberculosis‐infected mice did not decrease the bacterial load but rather was beneficial in decreasing TB‐associated immunopathology.67
Ours is the first report to identify an association between serum vitamin D concentrations and blastomycosis in dogs. Additional studies are needed to determine the importance of these findings, evaluate if genetic polymorphisms exist in dogs, and determine if supplementation with vitamin D will benefit affected dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
[Correction added after first online publication 31 August 2018: Previous sentence wording has been changed]
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the University of Illinois IACUC.
O'Brien MA, McMichael MA, Le Boedec K. 25‐Hydroxyvitamin D concentrations in dogs with naturally acquired blastomycosis. J Vet Intern Med. 2018;32:1684–1691. 10.1111/jvim.15255
[Correction added after first online publication 31 August 2018: Article history has been corrected]
REFERENCES
- 1. Dittmer KE, Thompson KG. Vitamin D metabolism and rickets in domestic animals: a review. Vet Pathol. 2011;48:389‐407. [DOI] [PubMed] [Google Scholar]
- 2. Di Rosa M, Malaguarnera G, De Gregorio C. Immuno‐modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36‐43. [DOI] [PubMed] [Google Scholar]
- 3. O'Brien MA, Jackson MW. Vitamin D and the immune system: beyond rickets. Vet J. 2012;194:27‐33. [DOI] [PubMed] [Google Scholar]
- 4. Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090‐7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu P, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis . Curr Opin Immunol. 2008;20:371‐376. [DOI] [PubMed] [Google Scholar]
- 6. Bartley J. Vitamin D, innate immunity and upper respiratory tract infection. J Laryngol Otol. 2010;124:465. [DOI] [PubMed] [Google Scholar]
- 7. Sabetta JR, DePetrillo P, Cipriani RJ, et al. Serum 25‐hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5:e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta‐analysis. Int J Epidemiol. 2008;37:113‐119. [DOI] [PubMed] [Google Scholar]
- 9. Iftikhar R, Kamran SM, Qadir A, et al. Vitamin D deficiency in patients with tuberculosis. J Coll Physicians Surg Pak. 2013;23:780‐783. [PubMed] [Google Scholar]
- 10. Sutaria N, Liu CT, Chen TC. Vitamin D status, receptor gene polymorphisms, and supplementation on tuberculosis: a systematic review of case‐control studies and randomized controlled trials. J Clin Transl Endocrinol. 2014;1:151‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abe E, Miyaura C, Sakagami H, et al. Differentiation of mouse myeloid leukemia cells induced by 1‐α, 25‐dihydroxyvitamin D3 . Proc Natl Acad Sci U S A. 1981;78:4990‐4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangelsdorf DJ, Koeffler HP, Donaldson CA, et al. 1,25‐Dihydroxyvitamin D3‐induced differentiation in a human promyelocytic leukemia cell line (HL‐60): receptor‐mediated maturation to macrophage‐like cells. J Cell Biol. 1984;98:391‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN‐gamma‐mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hmama Z, Nandan D, Sly L, et al. 1alpha,25‐dihydroxyvitamin D(3)‐induced myeloid cell differentiation is regulated by a vitamin D receptor‐phosphatidylinositol 3‐kinase signaling complex. J Exp Med. 1999;190:1583‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugar AM, Picard M, Wagner R, et al. Interactions between human bronchoalveolar macrophages and Blastomyces dermatitidis conidia: demonstration of fungicidal and fungistatic effects. J Infect Dis. 1995;171:1559‐1562. [DOI] [PubMed] [Google Scholar]
- 16. Bradsher RW. Macrophages and Blastomyces dermatitidis. Immunol Ser. 1994;60:553‐565. [PubMed] [Google Scholar]
- 17. Giles S, Klein B, Czuprynski C. The effect of canine macrophages on the adherence and growth of Blastomyces dermatitidis yeast: evidence of a soluble factor that enhances the growth of B. dermatitidis yeast. Microb Pathog. 1999;27:395‐405. [DOI] [PubMed] [Google Scholar]
- 18. Newman SL, Chaturvedi S, Klein BS. The WI‐1 antigen of Blastomyces dermatitidis yeasts mediates binding to human macrophage CD11b/CD18 (CR3) and CD14. J Immunol. 1995;154:753‐761. [PubMed] [Google Scholar]
- 19. Ferguson JS, Weis JJ, Martin JL, et al. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun. 2004;72:2564‐2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang MX, Brandhorst TT, Kozel TR, et al. Role of glucan and surface protein BAD1 in complement activation by Blastomyces dermatitidis yeast. Infect Immun. 2001;69:7559‐7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhatt K, Salgame P. Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol. 2007;27:347‐362. [DOI] [PubMed] [Google Scholar]
- 22. Werner A, Norton F. Blastomycosis. Compend Contin Educ Vet. 2011;33:E1‐E5. [PubMed] [Google Scholar]
- 23. Herrmann JA, Kostiuk SL, Dworkin MS, et al. Temporal and spatial distribution of blastomycosis cases among humans and dogs in Illinois (2001‐2007). J Am Vet Med Assoc. 2011;239:335‐343. [DOI] [PubMed] [Google Scholar]
- 24. Sarosi GA, Eckman MR, Davies SF, et al. Canine blastomycosis as a harbinger of human disease. Ann Intern Med. 1979;91:733‐735. [DOI] [PubMed] [Google Scholar]
- 25. Furcolow ML, Busey JF, Menges RW, et al. Prevalence and incidence studies of human and canine blastomycosis. II. Yearly incidence studies in three selected states, 1960‐1967. Am J Epidemiol. 1970;92:121‐131. [DOI] [PubMed] [Google Scholar]
- 26. Totten AK, Ridgway MD, Sauberli DS. Blastomyces dermatitidis prostatic and testicular infection in eight dogs (1992‐2005). J Am Anim Hosp Assoc. 2011;47:413‐418. [DOI] [PubMed] [Google Scholar]
- 27. Hecht S, Adams WH, Smith JR, et al. Clinical and imaging findings in five dogs with intracranial blastomycosis (Blastomyces dermatiditis). J Am Anim Hosp Assoc. 2011;47:241‐249. [DOI] [PubMed] [Google Scholar]
- 28. Legendre AM. Blastomycosis In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3 rd ed. St. Louis: Saunders Elsevier; 2006:569‐576. [Google Scholar]
- 29. Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol. 2013;35:563‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JH, Park J‐S, Cho Y‐J, et al. Low serum 25‐hydroxyvitamin D level: an independent risk factor for tuberculosis? Clin Nutr. 2014;33:1081‐1086. [DOI] [PubMed] [Google Scholar]
- 31. Gortel K, McKiernan BC, Johnson JK, et al. Calcinosis cutis associated with systemic blastomycosis in three dogs. J Am Anim Hosp Assoc. 1999;35:368‐374. [DOI] [PubMed] [Google Scholar]
- 32. Lalor SM, Mellanby RJ, Friend EJ, et al. Domesticated cats with active mycobacteria infections have low serum vitamin D (25(OH)D) concentrations. Transbound Emerg Dis. 2012;59:279‐281. [DOI] [PubMed] [Google Scholar]
- 33. Hong JY, Kim SY, Chung KS, et al. Association between vitamin D deficiency and tuberculosis in a Korean population. Int J Tuberc Lung Dis. 2014;18:73‐78. [DOI] [PubMed] [Google Scholar]
- 34. Jeon K, Kim SY, Jeong BH, et al. Severe vitamin D deficiency is associated with non‐tuberculous mycobacterial lung disease: a case‐control study. Respirology. 2013;18:983‐988. [DOI] [PubMed] [Google Scholar]
- 35. Havers F, Smeaton L, Gupte N, et al. 25‐Hydroxyvitamin D insufficiency and deficiency is associated with HIV disease progression and virological failure post‐antiretroviral therapy initiation in diverse multinational settings. J Infect Dis. 2014;210:244‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu PT, Stenger S, Li H, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science. 2006;311:1770‐1773. [DOI] [PubMed] [Google Scholar]
- 37. Jo E‐K. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12:1026‐1035. [DOI] [PubMed] [Google Scholar]
- 38. Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231‐243. [DOI] [PubMed] [Google Scholar]
- 39. Sun J, Wang X, Lau A, et al. Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS ONE. 2010;5:e8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hestvik AL, Hmama Z, Av‐Gay Y. Mycobacterial manipulation of the host cell. FEMS Microbiol Rev. 2005;29:1041‐1050. [DOI] [PubMed] [Google Scholar]
- 41. How KL, Hazewinkel AW, Mol JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol. 1994;96:12‐18. [DOI] [PubMed] [Google Scholar]
- 42. Monkawa T, Yoshida T, Hayashi M, et al. Identification of 25‐hydroxyvitamin D3 1alpha‐hydroxylase gene expression in macrophages. Kidney Int. 2000;58:559‐568. [DOI] [PubMed] [Google Scholar]
- 43. Richmond BW, Drake WP. Vitamin D, innate immunity, and sarcoidosis granulomatous inflammation: insights from mycobacterial research. Curr Opin Pulm Med. 2010;16:461‐464. [DOI] [PubMed] [Google Scholar]
- 44. Adams JS, Rafison B, Witzel S, et al. Regulation of the extrarenal CYP27B1‐hydroxylase. J Steroid Biochem Mol Biol. 2014;144(Pt A):22‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deniz O, Tozkoparan E, Yonem A, et al. Low parathormone levels and hypercalcaemia in patients with pulmonary tuberculosis: relation to radiological extent of disease and tuberculin skin test. Int J Tuberc Lung Dis. 2005;9:317‐321. [PubMed] [Google Scholar]
- 46. Dawson‐Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65:67‐71. [DOI] [PubMed] [Google Scholar]
- 47. Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439‐443. [DOI] [PubMed] [Google Scholar]
- 48. Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40:187‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dow SW, Legendre AM, Stiff M, Greene C. Hypercalcemia associated with blastomycosis in dogs. J Am Vet Med Assoc. 1986;188:706‐709. [PubMed] [Google Scholar]
- 50. Crews LJ, Feeney DA, Jessen CR, et al. Utility of diagnostic tests for and medical treatment of pulmonary blastomycosis in dogs: 125 cases (1989‐2006). J Am Vet Med Assoc. 2008;232:222‐227. [DOI] [PubMed] [Google Scholar]
- 51. Crews LJ, Sharkey LC, Feeney DA, et al. Evaluation of total and ionized calcium status in dogs with blastomycosis: 38 cases (1997‐2006). J Am Vet Med Assoc. 2007;231:1545‐1549. [DOI] [PubMed] [Google Scholar]
- 52. Tebben PJ, Singh RJ, Kumar R. Vitamin D‐mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37:521‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mellanby RJ, Mellor P, Villiers EJ, et al. Hypercalcaemia associated with granulomatous lymphadenitis and elevated 1,25 dihydroxyvitamin D concentration in a dog. J Small Anim Pract. 2006;47:207‐212. [DOI] [PubMed] [Google Scholar]
- 54. LeBlanc CJ, Echandi RL, Moore RR, et al. Hypercalcemia associated with gastric pythiosis in a dog. Vet Clin Pathol. 2008;37:115‐120. [DOI] [PubMed] [Google Scholar]
- 55. Stern JA, Chew DJ, Schissler JR, et al. Cutaneous and systemic blastomycosis, hypercalcemia, and excess synthesis of calcitriol in a domestic shorthair cat. J Am Anim Hosp Assoc. 2011;47:e116‐e120. [DOI] [PubMed] [Google Scholar]
- 56. Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980‐1995). J Am Vet Med Assoc. 1998;213:658‐664. [PubMed] [Google Scholar]
- 57. McMichael MA, O'Brien M, Smith SA. Hypercoagulability in dogs with blastomycosis. J Vet Intern Med. 2015;29:499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brahmbhatt S, Black GF, Carroll NM, et al. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin Exp Immunol. 2006;146:243‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coussens AK, Wilkinson RJ, Hanifa Y, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A. 2012;109:15449‐15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Şahin F, Yazar E, Yıldız P. Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip Respir Med. 2012;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med. 1996;10:365‐371. [DOI] [PubMed] [Google Scholar]
- 62. de Borst MH, de Boer RA, Stolk RP, et al. Vitamin D deficiency: universal risk factor for multifactorial diseases? Curr Drug Targets. 2011;12:97‐106. [DOI] [PubMed] [Google Scholar]
- 63. Salahuddin N, Ali F, Hasan Z, et al. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo‐controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis. 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tukvadze N, Sanikidze E, Kipiani M, et al. High‐dose vitamin D3 in adults with pulmonary tuberculosis: a double‐blind randomized controlled trial. Am J Clin Nutr. 2015;102:1059‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double‐blind, placebo‐controlled trial. Lancet Infect Dis. 2015;15:528‐534. [DOI] [PubMed] [Google Scholar]
- 66. Sainsbury JP, Trajtman A, Stalker AT, et al. Vitamin D binding protein polymorphism protects against development of blastomycosis. J Mycol Med. 2014;24:328‐331. [DOI] [PubMed] [Google Scholar]
- 67. Reeme AE, Robinson RT. Dietary vitamin d3 suppresses pulmonary immunopathology associated with late‐stage tuberculosis in c3heb/fej mice. J Immunol. 2016;196:1293‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]


