Abstract
Background
Bacterial urinary tract infections (UTIs) are common in companion animals. Increasing awareness of biofilm‐forming bacteria raises concern regarding the appropriate diagnosis, treatment, and prognosis of UTIs associated with these organisms.
Hypothesis/Objectives
To (1) describe the population of dogs with UTIs associated with biofilm‐forming Escherichia coli and (2) determine whether or not clinical differences exist between dogs with biofilm‐forming E. coli UTIs and dogs with nonbiofilm‐forming E. coli UTIs. We hypothesized that there would be no difference in the population characteristics, but that biofilm‐formation would be more prevalent in dogs with chronic, complicated, and asymptomatic UTIs.
Animals
Seventy‐six client‐owned dogs with E. coli UTIs, divided into 2 groups based on the biofilm‐forming capability of stored bacterial isolates as assessed by the crystal violet assay.
Methods
Retrospective cross‐sectional study. Medical records of the affected dogs were reviewed and their population and infection characteristics were compared.
Results
Most (52.6%) E. coli isolates were capable of forming biofilms. Biofilm‐forming E. coli had a lower likelihood (P < .001) of multidrug resistance than did nonbiofilm‐forming E. coli. No statistically significant differences were identified between the population or infection characteristics of the 2 groups of dogs.
Conclusions and Clinical Importance
Escherichia coli isolated from canine urinary tracts are frequently capable of forming biofilms. Because no reliable clinical features allowed exclusion of biofilm formation, the potential for biofilm formation should be considered whenever E. coli UTI is diagnosed. The association of antibiotic resistance and biofilm potential may affect treatment of UTIs, but additional investigation is warranted.
Keywords: chronic urinary tract infection, crystal violet, multidrug resistance, symptomatic urinary tract infection
Abbreviations
- BCS
body condition score
- BF+
biofilm‐forming
- BF−
nonbiofilm‐forming
- CFU
colony‐forming unit
- E. coli
Escherichia coli
- IQR
interquartile range
- LB
lysogeny broth
- LUTS
lower urinary tract signs
- MDR
multidrug resistance
- OD
optical density
- PBS
phosphate‐buffered saline
- UTI
urinary tract infection
1. INTRODUCTION
Bacterial urinary tract infection (UTI) is common in both humans and dogs and causes substantial morbidity,1 deleteriously affects quality of life, and has potential sequelae that can worsen prognosis, such as ascending infection and (in humans) neoplasia.2, 3 The prevalence of uncomplicated UTI in dogs is estimated to be 14%4 and fewer dogs (0.3%) develop chronic UTI,5 an umbrella term for relapsing infections, persistent infections, reinfections, and superinfections.6 Prolonged treatment often is recommended for chronic UTIs, which are subject to frequent treatment failures, increasing the cost for owners and morbidity for patients. Biofilm formation has been implicated in the development of complicated and recurrent UTIs in people,7 and is a risk factor for the development of multiple drug resistance (MDR)8 and pyelonephritis in children.9 The role of biofilm formation in UTIs of dogs is not well characterized.10
Escherichia coli is the most common organism causing UTI in both humans and dogs,11, 12, 13 accounting for 37%‐55% of UTIs in dogs.11, 13, 14, 15 Escherichia coli isolates are commonly capable of forming biofilms both in vivo and in vitro, but formation depends on a variety of factors, including growth medium.16 One study suggested that 31% of E. coli isolated from humans with UTIs may have biofilm‐forming capability,9 and another report suggested 47% of E. coli isolated from dogs with UTIs form biofilms within 24 hours.10
A biofilm is a complex organizational structure of sessile bacteria and their associated extracellular matrix. Reports have suggested biofilm formation in up to 80% of all microbial infections in humans,17 and a recent case series in India reported that 13.5% of uropathogenic E. coli isolated from humans have biofilm‐forming capability.18 The biofilm provides protective benefits to the bacteria including nutrient sharing19 and antibiotic resistance, antibiotic tolerance, or both by various mechanisms8 that result in increased pathogenicity and higher likelihood of treatment failure.20 In the veterinary literature, biofilm studies, in particular those involving E. coli, are uncommonly reported, and are limited to case reports of nonurinary diseases such as wounds (Staphylococcus [pseud]intermedius, Staphylococcus epidermidis, Streptococcus canis),21 otitis externa (Pseudomonas aeruginosa),22 implant infections (S. pseudintermedius),23 and experimental endometritis in horses (P. aeruginosa).24 Similarly, few studies in veterinary medicine describe the increased pathogenicity that biofilms confer, which includes increased mortality in kittens with gastrointestinal dysbiosis (Enterococcus faecalis, E. coli),25 and implied increased severity of bovine mastitis.26 We are unaware of any published study that compares the population or clinical characteristics of dogs with biofilm‐forming E. coli UTIs to nonbiofilm‐forming E. coli UTIs.
The aims of our retrospective cross‐sectional study were to: (1) describe the populations of dogs with E. coli UTI that do and do not exhibit biofilm‐forming capability and (2) compare the clinical characteristics of dogs with biofilm‐forming E. coli bacteriuria to dogs with nonbiofilm‐forming E. coli bacteriuria. Based on studies in humans, we hypothesized that, although the patient population characteristics would be indistinguishable, isolates from dogs with biofilm‐forming E. coli UTIs would be associated with a higher prevalence of chronic, asymptomatic, complicated UTIs, or some combination of these.
2. MATERIALS AND METHODS
2.1. Study design
Retrospective cross‐sectional study performed at North Carolina State University.
2.2. Case selection and data collection
Escherichia coli isolates from canine urine were randomly banked by the (NCSU Microbiology and Molecular Diagnostics Laboratory) from May 2011 through April 2017 for research purposes. All of the banked isolates (n = 78) were included in our study. Medical records for all dogs with included isolates were reviewed for the following data: sex and neuter status; breed, weight (kg); age (years); 9‐point body condition score (BCS); presence of lower urinary tract signs (LUTS); characterization of UTI as uncomplicated, complicated or pyelonephritis; presence of pyuria; presence of an MDR strain of E. coli; recent exposure to antibiotics; quantification of bacterial growth (colony‐forming units [CFU]/mL); and, chronicity of UTI as defined below. All available information from incomplete records was included and missing data were excluded from statistical analysis.
2.3. Definitions
Chronicity of infection included cases with documentation of persistent infections, reinfections, infection relapses, or superinfections, as defined elsewhere.6 Because some patients with these types of infections were asymptomatic, such patients are hereafter collectively referred to as patients with chronic bacteriuria. Uncomplicated lower UTIs were defined as those with no known predisposing cause (eg, anatomic abnormalities, micturition disorders, metabolic or endocrine disease, immunosuppression), whereas the presence of a predisposing cause for infection or being a male dog defined a complicated UTI. Pyelonephritis, for the purpose of our study, required the presence of fever, abdominal pain, ultrasonographic changes consistent with pyelonephritis (eg, perinephric free fluid, pyelectasia, hyperechoic renal cortices, or some combination of these), or was based on the diagnosis of the attending clinician. Lower urinary tract signs included pollakiuria, dysuria, discolored urine, malodorous urine, stranguria, or any combination of these. Antibiotic MDR was defined as resistance to ≥ 3 classes of antibiotics using Clinical Laboratory Standards Institute interpretations based on organism, sample location, and animal species, as used in previous studies.11, 27, 28 Recent exposure to antibiotics was characterized as documented antibiotic use within 2 months before urine culture.
2.4. Urine collection and culture
Medical records indicated that all urine samples were collected either by cystocentesis or sterile catheterization according to the attending clinician's preference. According to standard hospital protocol, urine then was placed into portable culture transport medium (A.C.T. I, Remel Inc, Lenexa, Kansas) and processed for culture within 24 hours. Ten microliters of urine were aseptically plated according to laboratory standard operating procedure onto 5% sheep blood agar (B.A.P., Remel Inc, Lenexa, Kansas) and MacConkey agar (MacConkey Agar, Remel Inc, Lenexa, Kansas) plates and incubated for 24 hours at 37°C. Presumptive E. coli isolates were evaluated for purity and subjected to identification and antimicrobial susceptibility testing using an automated system (Sensititre, Thermo Fisher Scientific, Waltham, Massachusetts). Confirmed isolates were stored at −80°C with 25% glycerol for future characterization.
2.5. Crystal violet assay to assess biofilm‐forming capability
Isolates were plated for overnight growth onto sheep blood agar (B.A.P., Remel Inc, Lenexa, Kansas) and evaluated for purity before the crystal violet assay, which previously has been used to determine biofilm potential.29 Briefly, each E. coli isolate was enriched individually overnight in 3 mL of lysogeny broth at 37°C. After enrichment, isolates were adjusted to 0.5 McFarland (approximately 1.5 × 108 CFU/mL) and 10 µL were transferred into 90 µL of M9 broth (Ingredients from Sigma Aldrich, St. Louis, Missouri) in a 96‐well flat‐bottom polystyrene tissue culture treated plate (MBEC 96‐well biofilm inoculator, Innovotech, Edmonton, AB, Canada). After overnight incubation, the bacterial suspension was removed and the wells were washed with 200 µL of phosphate buffered saline (PBS) 3 times to remove any remaining, unattached bacteria. The remaining biofilm was stained with 0.1% crystal violet solution and allowed to sit at room temperature for 15 minutes, after which excess stain was removed by washing the plate 3 times with PBS. After the final wash, the plate was treated with ethanol to solubilize the crystal violet. Plates then were evaluated for absorbance by determining the optical density (OD) of each well at a wavelength of 570 nm (OD570). The OD was recorded as a proportion as compared to a robust biofilm‐forming E. coli control strain (E. coli ATCC 25922, Manassas, Virginia). Each isolate was evaluated in triplicate, and the mean was determined by averaging the proportion of each isolate individually. Isolates were allocated into 4 groups based on the distribution of all evaluated isolates’ relative absorption compared to the positive control: minimal (OD570 ratio ≤ 0.25), mild (OD570 ratio 0.25 to 0.74), moderate (OD570 ratio 0.75 to 1.24), or heavy (OD570 ratio ≥ 1.25). Because of the low number of isolates with minimal or heavy biofilm formation and thus low statistical power, those isolates with minimal or mild absorbance (ie, OD570 ratio ≤ 0.74) were considered nonbiofilm‐forming (BF−) and those with moderate or heavy absorbance (ie, OD570 ratio ≥ 0.75) were considered biofilm‐forming (BF+).
2.6. Statistics
Continuous population characteristics (age, BCS, weight) were tested for normality with the Shapiro‐Wilk test. Continuous parametric variables are presented as a mean ± standard deviation, and continuous nonparametric variables are presented as median and interquartile range (IQR). Discrete variables (eg, sex, breed, biofilm group, and pyuria) are presented as counts and proportions.
The population characteristics of the BF+ and BF− groups (age, BCS, weight, sex, and breed) were compared for similar composition with the Wilcoxon rank‐sum test for nonparametric continuous variables, the Student's t test for parametric continuous variables, and chi‐square test or Fisher's exact test as appropriate based on cell counts for discrete variables, including the frequency of missing data. A logistic regression model was fit using biofilm formation as the outcome, and putative factors including sex, pyuria, LUTS, MDR, antibiotic exposure, infection class, and chronicity as independent variables. Regressions with putative interactions (eg, pyuria and LUTS, MDR and antibiotic exposure) were performed, but showed poorer fit than the logistic model, and were not included in the results. Additionally, pairwise comparisons for discrete dichotomous variables were performed in contingency tables, using a chi‐square test or Fisher's exact test if any cell in a contingency table had a count < 5.
Statistical analysis was performed using open‐source statistical software30 with significance set at P = .05.
3. RESULTS
Seventy‐eight individual E. coli isolates were acquired from 76 dogs. For the 2 dogs with repeat sampling, the isolates were acquired 104 and 29 days apart, respectively, and both had reported resolution of infection between samples. For these 2 dogs’ population characteristics, only data from their first presentations were used. Mean age was 9.05 ± 3.50 years, median weight was 17.9 kg (IQR, 9.95‐30.15 kg), and median BCS was 5.5 (IQR, 4–7). There were 3 (3.9%) intact males, 21 (27.6%) castrated males, 3 (3.9%) intact females, and 49 (64.5%) spayed females. Population characteristic findings are summarized in Table 1. Breeds represented included Labrador Retriever (n = 12, 15.8%); mixed breed dog (n = 8, 10.5%); German Shepherd Dog (n = 4, 5.3%); Golden Retriever (n = 4, 5.3%); 3 each (3.9%) of Australian Shepherd, Great Dane, Shih Tzu, and Pug; 2 each (2.6%) of Basset Hound, Cardigan Welsh Corgi, Beagle, Dachshund, French Bulldog, Miniature Schnauzer, Standard Poodle, and Yorkshire Terrier; and, 1 each (1.3%) of 20 additional breeds.
Table 1.
Population characteristics (n = 76)
| Mean | SD | Median | Q1 | Q3 | P | ||
|---|---|---|---|---|---|---|---|
| Age (years) | BF+ | 8.75 | 3.58 | ||||
| BF− | 9.39 | 3.42 | .43 | ||||
| BCS | BF+ | 5.00 | 4.00 | 6.00 | |||
| BF− | 6.00 | 4.75 | 7.00 | .49 | |||
| Weight (kg) | BF+ | 19.00 | 10.30 | 30.30 | |||
| BF− | 15.75 | 9.53 | 29.58 | .46 | |||
| Sex | BF+ | BF− | P | ||||
|---|---|---|---|---|---|---|---|
| MI | 1 | 2 | |||||
| MC | 12 | 9 | |||||
| Fl | 3 | 0 | |||||
| FS | 25 | 24 | .44 |
Aggregate population data for all dogs included in the study, n = 76. Abbreviations: BCS, body condition score (9‐point scale); BF+, dogs with biofilm‐forming E. coli isolates; BF−, dogs with nonbiofilm‐forming E. coli isolates; FI, intact females; FS, spayed females; MC, castrated males; MI, intact males; Q1, first quartile; Q3, third quartile; SD, standard deviation.
Among the 78 isolates of E. coli, 7 (9.0%) had minimal biofilm, 30 (38.5%) had mild biofilm, 33 (42.3%) had moderate biofilm, and 8 (10.3%) had heavy biofilm formation. Collectively, these findings resulted in 37 (47.4%) BF− isolates and 41 (52.6%) BF+ isolates.
Of the 78 isolates, 42 (53.8%) were associated with pyuria, 26 (33.3%) with LUTS, 45 (57.7%) with MDR, 36 (46.2%) with antibiotic exposure, and 34 (43.6%) with chronic infection. Uncomplicated infections accounted for 9 (11.5%) isolates, complicated infections for 63 (80.8%) isolates, and pyelonephritis for 5 (6.4%) isolates. Table 2 presents the counts and proportions of the infection characteristics associated with all 78 isolates.
Table 2.
Clinical infection characteristics
| Group | Total | MDR | Pyuria | Antibiotic use | LUTS | Chronic infection | Uncomplicated infection | Complicated infection | Pyelonephritis |
|---|---|---|---|---|---|---|---|---|---|
| All samples | 78 | 45 (58%) | 42 (54%) | 36 (46%) | 26 (33%) | 34 (44%) | 9 (12%) | 63 (81%) | 5 (6%) |
| BF+ | 41 | 14 (34%)* | 23 (56%) | 16 (39%) | 14 (34%) | 15 (37%) | 6 (15%) | 34 (83%) | 1 (2%) |
| BF− | 37 | 31 (84%)* | 19 (51%) | 20 (54%) | 12 (32%) | 19 (51%) | 3 (8%) | 29 (78%) | 4 (11%) |
Aggregate clinical sign data associated with all isolates, n = 78. Percentages represent proportion within groups. Abbreviations: BF+, dogs with biofilm‐forming E. coli isolates; BF−, dogs with nonbiofilm‐forming E. coli isolates; LUTS, lower urinary tract signs; MDR, multidrug resistance. * = significant difference within group with P < .01.
Figure 1 shows distributions of age, BCS, and weight. No statistically significant differences were found between the population characteristics of the biofilm groups (BF+ versus BF−), including frequency of missing data, which accounted for 8.2% of all observations (45/546). No difference was found among the breeds represented in the BF+ and BF− groups (P = .56, data not shown).
Figure 1.
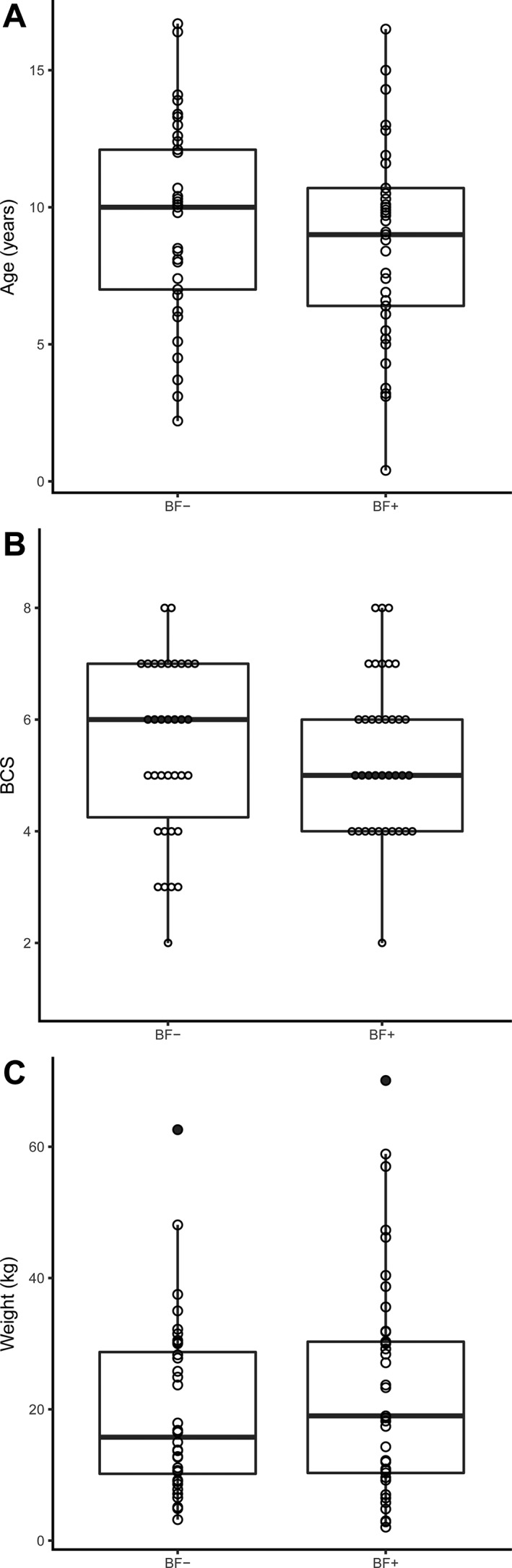
Box‐and‐whiskers plots of population characteristics. Legend: Box‐and‐whiskers plot of (A) age (years), (B) BCS (9‐point scale), and (C) weight (kg) of all dogs (n = 76) in the study. The box represents the middle 50%, or IQR, while the bold horizontal line represents the median. Whiskers, the vertical lines above and below the box, show 1.5 times the IQR. Filled circles are outliers. Open circles represent each individual case. Abbreviations: BCS, body condition score; BF−, dogs with nonbiofilm‐forming E. coli isolates; BF+, dogs with biofilm‐forming E. coli isolates
The distribution of infection characteristics between BF− and BF+ groups is depicted in Figure 2. The only statistically significant predictor of biofilm‐forming capability when using the logistic regression model was MDR. A higher frequency of MDR was found in dogs with UTIs associated with nonbiofilm forming E. coli (31/37, 84%) than in those with biofilm‐forming E. coli (14/41, 34%; P < .001). Residual plotting showed a subjectively good fit of the model. In addition to logistic regression, confirmatory testing using a chi‐square (or Fisher's exact test where appropriate) also showed an association between MDR and BF− E. coli (P < .001). Other pair‐wise contingency tables failed to detect a difference between the BF− and BF+ groups for any other infection characteristics.
Figure 2.
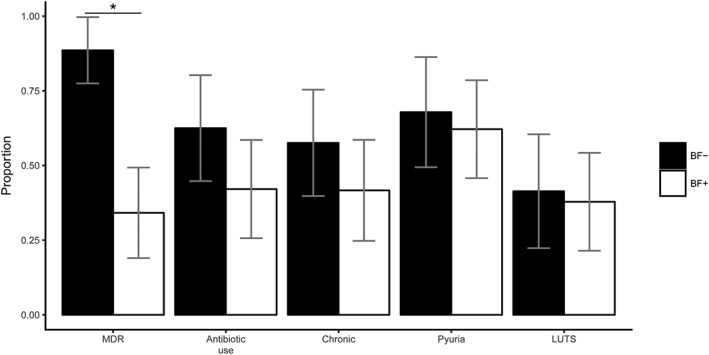
Infection characteristics. Legend: Bars represent the proportion of isolates (n = 78) associated with the infection characteristics described on the x‐axis. Black bars represent nonbiofilm‐forming E. coli isolates, and white bars represent biofilm‐forming E. coli isolates. Error bars show the 95% confidence interval. Abbreviations: BF−, dogs with nonbiofilm‐forming E. coli isolates; BF+, dogs with biofilm‐forming E. coli isolates; MDR, multidrug resistance; chronic, dogs with chronic bacteriuria; LUTS, dogs with lower urinary tract signs. *Denotes statistical significance (P < .001)
As a post hoc exploratory data analysis, in addition to the statistical comparisons described above for the entire patient population, we performed subpopulation analyses and stratified for dogs with symptomatic UTIs (n = 26), dogs with asymptomatic bacteriuria (n = 40), and dogs with chronic bacteriuria (n = 34). These data showed the same patterns of statistical significance as did the overall population except for a significantly different sex distribution between BF+ and BF− dogs with chronic bacteriuria (P = .03), and that the association between MDR and dogs with BF− isolates was not sustained in the population of dogs with symptomatic UTIs (P = .21). Because of the small sample sizes of these subpopulations and likelihood of multiple comparisons affecting interpretation, these results are not described further, but are available as Supporting Information material online (Tables S1‐S4). Similar post hoc analysis was performed with all 4 levels of biofilm formation (ie, none, mild, moderate, and heavy rather than BF− and BF+) to ensure no level of biofilm formation had skewed the results disproportionately. Results from these contingency tables had an identical pattern of significance as that seen in the general population.
4. DISCUSSION
The goal of our retrospective cross‐sectional study was to describe and compare the population and infection characteristics of dogs with UTIs caused by E. coli with and without biofilm‐forming capability. We found that approximately half of E. coli isolates had biofilm‐forming capability. Furthermore, dogs harboring these infections were clinically indistinguishable from dogs with nonbiofilm‐forming E. coli UTIs, and thus any dog with an E. coli UTI, regardless of age, sex, breed, or body condition could carry BF+ E. coli.
The prevalence of biofilm‐forming E. coli in our study (52.6%) is similar to that shown in previous studies in dogs (47%)10 and people (31%).9 Given the overlap of bacterial species causing UTI in both dogs and humans,14, 31 this similarity was expected and suggests that biofilm formation is a common capability of E. coli isolates that infect urinary tracts. The finding that dogs that have biofilm‐forming E. coli UTIs are clinically indistinguishable from other dogs with E. coli UTI, also is suggested in people, where the prevalence of asymptomatic bacteriuria with biofilm‐forming UTIs and nonbiofilm‐forming UTIs is the same.32 Other studies, however, have suggested that biofilms may be associated with asymptomatic UTI.33 Because biofilms confer protective benefit to the bacteria, our inability to clinically suspect biofilm formation suggests that further research is required before determining the value of biofilm‐specific treatments in the management of dogs with UTI. Before considering the effect of treatments, comparing the outcomes (eg, resolution of infection) of biofilm‐forming E. coli compared with nonbiofilm‐forming E. coli would be indicated. Because of the frequency of biofilm‐forming ability that we found among E. coli isolates, screening any dog with an E. coli UTI would be a feasible approach to generate populations of dogs for prospective evaluation of outcomes.
Our data also showed a robust association between nonbiofilm‐forming E. coli and phenotypic expression of MDR when isolates were evaluated after bacterial culture of the urine. This finding contrasts with available literature, which generally has shown associations between biofilm‐forming bacteria and MDR.10, 18, 34 In the aforementioned literature, the definitions of resistance are variable and bacteria other than E. coli are considered, thus making extrapolation to our current data problematic. There are several possible explanations for why biofilm‐forming E. coli may appear less resistant to antimicrobials. First, the antimicrobial tolerance of biofilm‐forming E. coli may not be related to the presence of a resistance gene, but rather is a property of the metabolic activity of the biofilm community. Consequently, such antimicrobial tolerance is not assessed using standard laboratory protocols for antimicrobial susceptibility testing. In determining susceptibility, isolates are evaluated in a planktonic growth state, and thus antimicrobial tolerance imparted by biofilm formation would not be incorporated into a susceptibility report. Still, the E. coli may be unresponsive to antimicrobial treatment. Other proposed mechanisms of antibiotic tolerance in biofilm communities include an oxygen gradient that limits antibiotic activity, the presence of a persister cell population, and limited antibiotic diffusion through the extracellular matrix.20 These factors are unlikely to affect a traditional antimicrobial susceptibility report, but should be considered in vivo. Second, if demonstrating biofilm‐forming characteristics when their antimicrobial susceptibility is assessed, because they are in a different growth state, these isolates may have not been identified as MDR even if resistance genes were present. Given the prevalence and potential contribution of biofilms to virulence, further understanding of this phenomenon may be important for clinical management of UTI in dogs.
Host‐microbe interaction is a complicated field of study and is important for managing disease. Research in other species suggests that the pathogenicity of E. coli may be due, in part, to its ability to form intracellular biofilm communities within urothelial cells. This was demonstrated in mice both in vitro35 and in vivo,36 and humans in vitro,37 and is postulated to be a source of re‐infection,38 even after negative urine culture. Thus, E. coli that are very highly associated with biofilm potentially may have been missed during bacterial culture, because they would not be excreted in urine. Additionally, the antimicrobial susceptibility of this subgroup would not have been assessed. The role of biofilms as solely pathogenic is not entirely established. One recent study suggested biofilms may even help establish a steady state of nonpathogenic asymptomatic bacteriuria that could limit the growth of pathogenic urinary bacteria by competing for resources.39 Increased understanding of biofilm formation, including steps of formation and mechanisms of conferring antibiotic resistance, may serve as potential therapeutic targets, which is an area of active investigation.40 It remains to be seen, however, if improved understanding of the role of biofilm in UTIs in dogs, can be used to develop novel therapies that may be useful in managing chronic UTI.
This primary limitation of our study is sample size. A post hoc power analysis suggested that, in order to detect a 20% effect size (the average seen in our study), 272 E. coli isolates would be required. This does not indicate the results of our study are invalid, but means the difference between groups may be small. This raises the question of whether an even smaller difference, albeit statistically significant, would be clinically relevant. Several other possibilities may explain the absence of detectable differences between populations and other (non‐MDR) infection characteristics. For example, it is possible that a subtle difference exists between the populations of dogs with E. coli UTIs that can and cannot form biofilms, but that the dogs’ caregivers are incapable of detecting it. In other words, anamnesis plays a crucial role in developing a clinical suspicion of UTI. Although people can report feelings of discomfort to a physician even without overt clinical signs, dogs are not able to do so. Furthermore, clinical signs in dogs may be inapparent because they may urinate unobserved or owners may be unaware of the associated clinical signs. Although prospective enrollment and larger sample size may ameliorate the issue of power, limitations that result from inherent difficulty in observation cannot be addressed.
Although our study was retrospective, which resulted in missing data, the impact of this missing data was limited because it had similar prevalence between the groups. Data retrieval from medical records at a referral institution is difficult by nature. For example, definition of chronic bacteriuria required documentation of previous or subsequent UTI, but some UTIs may have been diagnosed by a primary care veterinarian without information being included in the medical record. Future studies in which dogs are prospectively enrolled and followed may help improve our observations and ability to study specific risk factors.
Lastly, although the crystal violet assay is validated for assessing biofilm formation, it remains an in vitro approximation of what happens in vivo. That is, the formation of an in vitro biofilm may not correlate to biofilm formation in the lower urinary tract. The potential for disparity has been highlighted using E. coli isolated from people in which the growth of biofilm varied according to growth medium, bacterial source, and nutrient availability.16 Despite this, the assay remains a valid approximation29 used frequently in studies7, 9, 25, 32, 33, 41, 42, 43, 44 because of its affordability, feasibility, and lack of invasiveness. Assessing in vivo biofilm formation may require bladder biopsy, which would likely decrease potential subject enrollment.
In conclusion, our data show that the populations of dogs with BF+ and BF− E. coli UTIs are indistinguishable from each other. Despite a lack of difference, biofilm formation is a common capability of E. coli isolated from the canine urinary tract. Furthermore, a lower prevalence of phenotypic MDR susceptibility reports was found in dogs with biofilm‐forming E. coli. Future research is indicated to further understand the extent to which these bacteria cause morbidity, the long‐term outcome of patients with BF+ E. coli UTI, and whether or not use of treatments to target biofilms has a place in the treatment of dogs with UTIs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
Supporting information
Table S1.
Table S2.
Table S3.
Table S4.
ACKNOWLEDGMENTS
The authors thank Anna Rogers and Megan Fauls for their work in preparation of bacterial cultures, bacterial isolation, and performing the crystal violet assay. The work was performed at the NC State Veterinary Hospital.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This retrospective study was exempt from North Carolina State University IACUC review.
Kern ZT, Jacob ME, Gilbertie JM, Vaden SL, Lyle SK. Characteristics of dogs with biofilm‐forming Escherichia coli urinary tract infections. J Vet Intern Med. 2018;32:1645–1651. 10.1111/jvim.15231
REFERENCES
- 1. Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am J Med. 2002;113:5–13. [DOI] [PubMed] [Google Scholar]
- 2. Marques C, Gama LT, Belas A, et al. European multicenter study on antimicrobial resistance in bacteria isolated from companion animal urinary tract infections. BMC Vet Res. 2016;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vermeulen SH, Hanum N, Grotenhuis AJ, et al. Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br J Cancer. 2015;112:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ling GV. Therapeutic strategies involving antimicrobial treatment of the canine urinary tract. JAm Vet Med Assoc. 1984;185:1162–1164. [PubMed] [Google Scholar]
- 5. Norris CR, Williams BJ, Ling GV, Franti CE, Johnson, Ruby AL. Recurrent and persistent urinary tract infections in dogs: 383 cases (1969–1995). JAm Anim Hosp Assoc. 2003;36:484–492. [DOI] [PubMed] [Google Scholar]
- 6. Seguin MA, Vaden SL, Altier C, Stone E, Levine JF. Persistent urinary tract infections and reinfections in 100 dogs (1989–1999). JVet Intern Med. 2003;17:622–631. [DOI] [PubMed] [Google Scholar]
- 7. Soto SM, Smithson A, Horcajada JP, Martinez JA, Mensa JP, Vila J. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin Microbiol Infect. 2006;12:1034–1036. [DOI] [PubMed] [Google Scholar]
- 8. Ito A, Taniuchi A, May T, Kawata K, Okabe S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl Environ Microbiol. 2009;75:4093–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salo J, Sevander J‐J, Tapiainen T, et al. Biofilm formation by Escherichia coli isolated from patients with urinary tract infections. Clin Nephrol. 2009;71:501–507. [DOI] [PubMed] [Google Scholar]
- 10. Oliveira M, Dias F, Pomba C. Biofilm and fluoroquinolone resistance of canine Escherichia coli uropathogenic isolates. BMC Res Notes. 2014;7:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong C, Epstein SE, Westropp JL. Antimicrobial susceptibility patterns in urinary tract infections in dogs (2010–2013). JVet Intern Med. 2015;29:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ronald A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am J Med. 2002;113:14–19. [DOI] [PubMed] [Google Scholar]
- 13. Thompson MF, Litster AL, Platell JL, Trott DJ. Canine bacterial urinary tract infections: New developments in old pathogens. Vet J. 2011;190:22–27. [DOI] [PubMed] [Google Scholar]
- 14. Hall JL, Holmes MA, Baines SJ. Prevalence and antimicrobial resistance of canine urinary tract pathogens. Vet Rec. 2013;173:549. [DOI] [PubMed] [Google Scholar]
- 15. Olin SJ, Bartges JW. Urinary tract infections. Treatment/comparative therapeutics. Vet Clin North Am Small Anim Pract. 2015;45:721–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reisner A, Krogfelt KA, Klein BM, Zechner EL, Molin S. In vitro biofilm formation of commensal and pathogenic Escherichia coli strains: Impact of environmental and genetic factors. JBacteriol. 2006;188:3572–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mangan DF, Moy P, Agodoa L, et al. Research on Microbial Biofilms. 2002.
- 18. Mittal S, Sharma M, Chaudhary U. Biofilm and multidrug resistance in uropathogenic Escherichia coli. Pathog Glob Health. 2015;109:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398–a000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. JIntern Med. 2012;272:541–561. [DOI] [PubMed] [Google Scholar]
- 21. Swanson EA, Freeman LJ, Seleem MN, Snyder PW. Biofilm‐infected wounds in a dog. JAm Vet Med Assoc. 2014;244:699–707. [DOI] [PubMed] [Google Scholar]
- 22. Pye CC, Yu AA, Weese JS. Evaluation of biofilm production by Pseudomonas aeruginosa from canine ears and the impact of biofilm on antimicrobial susceptibility in vitro. Vet Dermatol. 2013;24:446–449, e98–99. [DOI] [PubMed] [Google Scholar]
- 23. Crawford EC, Singh A, Gibson TW, Weese JS. Biofilm‐associated gene expression in Staphylococcus pseudintermedius on a variety of implant materials. Vet Surg. 2016;45:499–506. May; [DOI] [PubMed] [Google Scholar]
- 24. Ferris RA, McCue PM, Borlee GI, et al. Model of chronic equine endometritis involving a Pseudomonas aeruginosa biofilm. Infect Immun. 2017;85:e00332‐e00317. Dec; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghosh A, Borst L, Stauffer SH, et al. Mortality in kittens is associated with a shift in ileum mucosa‐associated enterococci from enterococcus hirae to biofilm‐forming enterococcus faecalis and adherent escherichia coli. JClin Microbiol. 2013;51:3567–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melchior MB, Vaarkamp H, Fink‐Gremmels J. Biofilms: A role in recurrent mastitis infections? Vet J. 2006;171:398–407. [DOI] [PubMed] [Google Scholar]
- 27. Tuerena I, Williams NJ, Nuttall T, Pinchbeck G. Antimicrobial‐resistant Escherichia coli in hospitalised companion animals and their hospital environment. JSmall Anim Pract. 2016;57:339–347. [DOI] [PubMed] [Google Scholar]
- 28. KuKanich KS, Lubbers BV. Review of Enterococci Isolated from Canine and Feline Urine Specimens from 2006 to 2011. JAm Anim Hosp Assoc. 2015;51:148–154. [DOI] [PubMed] [Google Scholar]
- 29. Naves P, Del Prado G, Huelves L, et al. Measurement of biofilm formation by clinical isolates of Escherichia coli is method‐dependent. JAppl Microbiol. 2008;105:585–590. [DOI] [PubMed] [Google Scholar]
- 30.R version 3.3.2. R Core Team. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. 2016.
- 31. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. [DOI] [PubMed] [Google Scholar]
- 32. Watts RE, Hancock V, Ong C‐LY, et al. Escherichia coli isolates causing asymptomatic bacteriuria in catheterized and noncatheterized individuals possess similar virulence properties. JClin Microbiol. 2010;48:2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hancock V, Ferrières L, Klemm P. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol Lett. 2007;267:30–37. [DOI] [PubMed] [Google Scholar]
- 34. Sanchez CJ, Mende K, Beckius ML, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 2004;12:424–430. [DOI] [PubMed] [Google Scholar]
- 36. Justice SS, Hung C, Theriot JA, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci USA. 2004;101:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a Persistent Escherichia coli Reservoir during the Acute Phase of a Bladder Infection. Infect Immunol. 2001;69:4572–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosen DA, Hooton TM, Stamm WE, et al. Detection of Intracellular Bacterial Communities in Human Urinary Tract Infection. PLoS Med. 2007;4:e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrières L, Hancock V, Klemm P. Biofilm exclusion of uropathogenic bacteria by selected asymptomatic bacteriuria Escherichia coli strains. Microbiology. 2007;153:1711–1719. [DOI] [PubMed] [Google Scholar]
- 40. Soto SM. Importance of biofilms in urinary tract infections: New therapeutic approaches. Adv Biol. 2014;2014:1–13. [Google Scholar]
- 41. Tapiainen T, Hanni AM, Salo J, et al. Escherichia coli biofilm formation and recurrences of urinary tract infections in children. Eur J Clin Microbiol Infect Dis. 2014;33:111–115. [DOI] [PubMed] [Google Scholar]
- 42. Naves P, del Prado G, Huelves L, et al. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb Pathog. 2008;45:86–91. Aug; [DOI] [PubMed] [Google Scholar]
- 43. Soto SM, Smithson A, Martinez JA, et al. Biofilm formation in uropathogenic Escherichia coli strains: Relationship with prostatitis, urovirulence factors and antimicrobial resistance. JUrol. 2007;177:365–368. [DOI] [PubMed] [Google Scholar]
- 44. Merritt JH, Kadouri DE, O'Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2011;(Suppl. 22):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Table S4.


