Abstract
Background
Atrioventricular accessory pathways (APs) in dogs have been reported rarely. Data regarding clinical presentation and long‐term outcome after radiofrequency catheter ablation (RFCA) are limited.
Hypothesis/Objectives
To study clinical features, electrophysiologic characteristics, and outcome of RFCA in dogs with APs.
Animals
Eighty‐nine dogs presented consecutively for RFCA of APs.
Methods
Case series.
Results
Labrador retrievers (47.2% of dogs) and male dogs (67.4% of dogs) were most commonly affected. Labrador retrievers were more likely to be male than non‐Labrador breeds (P = .043). Clinical signs were nonspecific and most commonly included lethargy and gastrointestinal signs. Concealed APs were more prevalent in Labrador retrievers than other breeds (P = .001). Right‐sided APs (91.7%) predominated over left‐sided (8.3%). Tachycardia‐induced cardiomyopathy (TICM) occurred in 46.1% of dogs, with complete resolution or substantial improvement noted on one‐month postablation echocardiograms. Radiofrequency catheter ablation successfully eliminated AP conduction long term in 98.8% of dogs in which it was performed. Complications occurred in 5/89 dogs. Recurrence in 3 dogs was eliminated long term with a second procedure.
Clinical Importance/Conclusions
Accessory pathways are challenging to recognize in dogs because of nonspecific clinical signs, frequency of concealed APs that show no evidence of their presence during sinus rhythm, and intermittent occurrence of tachyarrhythmias resulting from APs. Tachycardia‐induced cardiomyopathy commonly occurs with AP‐mediated tachycardias and should be considered in any dog presenting with a dilated cardiomyopathic phenotype because of its good long‐term prognosis with rhythm control. Radiofrequency catheter ablation is a highly effective method for eliminating AP conduction and providing long‐term resolution.
Keywords: accessory pathway, congestive heart failure, orthodromic atrioventricular reciprocating tachycardia, tachycardia, tachycardia‐induced cardiomyopathy, ventricular preexcitation
Abbreviations
- AF
atrial fibrillation
- AP
atrioventricular accessory pathway
- APC
atrial premature complex
- AV
atrioventricular
- CHF
congestive heart failure
- CS
coronary sinus
- EP
electrophysiology or electrophysiologic
- EPS
electrophysiologic study
- HB
His Bundle
- HRA
high right atrium
- LA:Ao
left atrium to aortic ratio
- LV
left ventricle or left ventricular
- LVESVI
left ventricular end‐systolic volume index
- LVFS
left ventricular fractional shortening
- LVIDdN
normalized left ventricular internal diameter at end‐diastole
- LVIDsN
normalized left ventricular internal diameter at end‐systole
- OAVRT
orthodromic atrioventricular reciprocating tachycardia
- RF
radiofrequency energy
- RFCA
radiofrequency catheter ablation
- RV
right ventricle or right ventricular
- RVA
right ventricular apex
- TICM
tachycardia‐induced cardiomyopathy
- VA
ventricular‐to‐atrial
- VF
ventricular fibrillation
- VPC
ventricular premature complex
- VPE
ventricular preexcitation
- VT
ventricular tachycardia
1. INTRODUCTION
Atrioventricular accessory pathways (APs) are anomalous bypass tracts typically composed of working myocardial cells.1 Such APs can form the retrograde limb of a macroreentrant circuit using the atrioventricular (AV) node as the antegrade limb, resulting in a regular, narrow complex tachyarrhythmia known as orthodromic atrioventricular reciprocating tachycardia (OAVRT). If the AP is capable of antegrade conduction, an ECG recorded during sinus rhythm may demonstrate ventricular preexcitation (VPE), characterized by a short PR interval and slurred upstroke to the QRS, known as a delta wave. The degree of VPE depends upon (1) AV nodal and His‐Purkinje conduction time, (2) conduction time of the sinus impulse to the AP, and (3) conduction time through the AP.2, 3 Ventricular preexcitation may be present consistently in sinus rhythm (manifest APs) or only intermittently. Wolff‐Parkinson‐White syndrome (WPW) is the combination of VPE during sinus rhythm and periods of symptomatic tachyarrhythmia. An AP only capable of retrograde conduction is known as a concealed AP, and the ECG during sinus rhythm will have a normal PR interval and QRS complex.
Rarely, an AP capable of antegrade conduction may participate as the antegrade limb of a macroreentrant tachycardia circuit, while the AV node forms the retrograde limb. This results in a wide complex tachyarrhythmia known as antidromic AV reciprocating tachycardia. A major risk for sudden cardiac death occurs when a patient having an AP with rapid antegrade conduction properties develops atrial fibrillation (AF). Atrial impulses can be rapidly conducted over the antegrade AP, resulting in ventricular fibrillation (VF).4
Tachycardia‐induced cardiomyopathy (TICM) can result from frequent or sustained tachyarrhythmias, including OAVRT. It is defined as myocardial dysfunction caused by a rapid ventricular rate that is completely or partially reversible after normalization of the heart rate.5, 6 Our knowledge of APs and TICM in dogs currently is limited. Single case reports and small case series have been reported.7, 8, 9, 10, 11, 12, 13
Radiofrequency catheter ablation (RFCA) is a minimally invasive technique targeting a tachyarrhythmic focus or reentrant circuit with radiofrequency energy to permanently disrupt that tachyarrhythmia.14 This procedure has been reported in a small number of dogs with APs.8, 9, 10, 11, 12, 13 Study goals were (1) describe the clinical, electrocardiographic, echocardiographic, and electrophysiologic findings in a large consecutive series of dogs with APs and (2) report RFCA procedural and long‐term outcomes, with assessment of potential curative properties for RFCA in a clinical population of dogs.
2. MATERIALS AND METHODS
2.1. Animals
Eighty‐nine dogs were referred to the principal author for electrophysiologic study (EPS) and RFCA of APs between September 1999 and February, 2017. Data from clinical history, CBC, serum biochemical profiles, echocardiograms, electrocardiograms, Holter monitors, and thoracic radiographs were collected by consultation with owners and referring cardiologists or internists.
Complete echocardiographic evaluation (2D, M‐mode, Doppler) was performed by a single cardiologist (KW) at the time of presentation for EPS. Measurements of LV systolic function included fractional shortening (LVFS), normalized LV internal diameter at end‐systole (LVIDsN), ejection fraction (LVEF), and LV end‐systolic volume index (LVESVI) using the modified Simpson's formula in the left parasternal 4‐chamber and 2‐chamber views. Left ventricular systolic dysfunction was defined as having at least 3 of these 4 measurements outside of the reference ranges (LVFS <25%, LVIDsN > 1.14, LVEF <50%, LVESVI > 35 ml/M2).15, 16 Normalized left ventricular internal diameter at end‐diastole (LVIDdN) and 2D right parasternal short‐axis LA‐to‐aortic (LA:Ao) ratio were evaluated, with dilatation defined as LVIDdN > 1.73 and 2D LA:Ao > 1.5.15, 17, 18 The presence and severity of valvular regurgitation and concurrent congenital or acquired cardiac defects were recorded.
Twelve‐lead electrocardiograms were obtained on the day of presentation, followed by telemetric monitoring until the time of discharge. Analyzed data included presence or absence of VPE (on any ECG), tachycardia cycle length, RP′ interval during OAVRT, presence or absence of P′ waves in the ST segment/T wave following at least some sinus complexes, other arrhythmias present before ablation, and antiarrhythmic drugs used.
2.2. Electrophysiologic study and radiofrequency catheter ablation
Orally administered antiarrhythmic drugs were discontinued for 5 half‐lives prior to EPS. Some dogs were hemodynamically unstable with their tachycardia, however, and required an IV injection of diltiazem or lidocaine to convert them to sinus rhythm within 24 hours before the procedure. Dogs were premedicated with butorphanol and midazolam, followed by anesthetic induction with etomidate, and maintenance with the lowest concentration of isoflurane needed to maintain a stable plane of anesthesia. Multielectrode catheters (Boston Scientific quadripolar fixed curve and decapolar fixed and steerable curve diagnostic electrophysiology catheters) were advanced through percutaneously placed catheter introducers in both femoral and external jugular veins to the right ventricular apex (RVA), high right atrium (HRA), His bundle region (HB), and coronary sinus (CS; Figure 1).14 Electrical stimuli were delivered at twice diastolic threshold from a programmable stimulator (Bloom DTU‐215 Programmable Cardiac Stimulator) to the distal electrodes of first the HRA and then RVA. Trains of 8 S1 extrastimuli followed by a progressively more premature single S2 extrastimulus were used to determine effective refractory periods, whereas incrementally faster trains of 8 extrastimuli were used to determine fastest 1‐to‐1 conduction properties of the AP and normal conduction system in antegrade and retrograde directions, respectively.19 High right atrial or RVA extrastimuli were used to induce the clinical tachyarrhythmia and any other inducible rhythms. An AP was confirmed using ≥1 of these criteria: (1) ventricular activation occurring before the HB potential (VPE) during sinus rhythm or atrial pacing; (2) eccentric atrial activation during ventricular pacing and narrow complex tachyarrhythmia; (3) rapid, nondecremental VA conduction during decremental RV pacing with identical retrograde atrial activation at all cycle lengths (particularly important if concentric retrograde atrial activation occurs).20, 21, 22 Rare APs exhibit slow, decremental retrograde conduction properties. To distinguish OAVRT from AV nodal reentry or atrial tachycardia with 1:1 AV nodal conduction, AP participation in the tachycardia circuit was confirmed by advancing (or delaying in the case of a decrementally conducting AP) the identical retrograde atrial activation sequence or terminating the tachycardia without subsequent atrial activation after introduction of a single RV extrastimulus into the tachycardia during His bundle refractoriness.20, 21, 22 Continuation of the tachycardia despite AV block or earliest atrial activation distant from the AV annuli excluded OAVRT and strongly supported atrial tachycardia. A fixed VA (and RP′) interval during tachycardia and tachycardia termination by a premature RV stimulus that does not conduct to the atrium were seen with OAVRT, not atrial tachycardia.20, 21, 22
Figure 1.
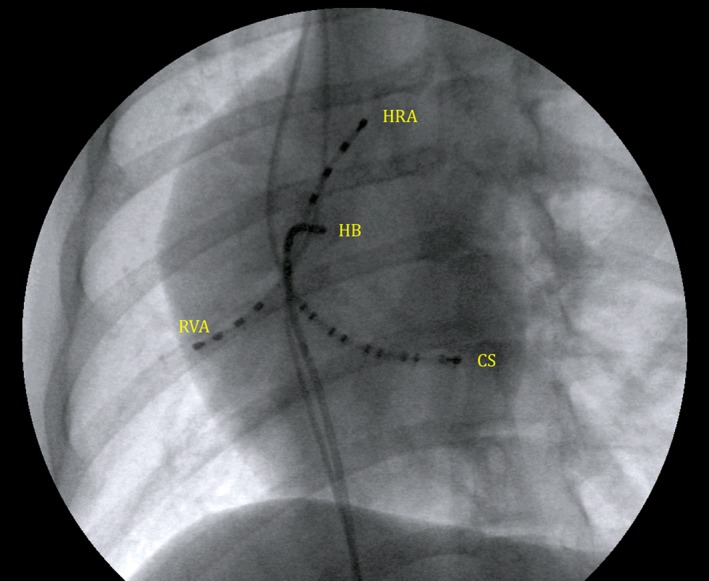
A 30° left anterior oblique fluoroscopic view of multielectrode catheter positioning for the initial EP study. HRA, high right atrium; HB, His bundle region; CS, coronary sinus; RVA, right ventricular apex. All multielectrode catheter poles are labeled by convention in numerical order starting at the distal (tip) electrode
Once the baseline EP study was completed, the HRA catheter was replaced by a 6‐7F deflectable quadripolar ablation catheter (Blazer II Temperature Ablation Catheter) positioned along the tricuspid annulus (Figure 2A). If a left‐sided accessory pathway was present, a separate 7F catheter introducer was inserted into the right femoral artery. The ablation catheter was advanced to the mitral annulus by prolapsing it across the aortic valve (Figure 2B). Detailed AP mapping and ablation were performed through the distal electrode bipole of the ablation catheter. The site of earliest retrograde atrial activation during RVA pacing and OAVRT was used to identify the atrial insertion site of the AP, whereas the earliest ventricular activation during sinus rhythm or HRA pacing identified the ventricular insertion site in manifest APs. An AP potential, which is a discrete, sharp, low‐amplitude signal between the ventricular and atrial electrograms for retrograde APs or atrial and ventricular electrograms for antegrade APs, also was sought in the the distal ablation dipole recording before RF delivery.20, 21, 23, 24, 25 Most commonly, the atrial insertion of AP was targeted for ablation during RV pacing. In some cases with manifest preexcitation, the ventricular AP insertion was targeted during preexcited sinus rhythm. The distal electrode pair on the mapping catheter was coupled to a temperature‐controlled cardiac radiofrequency (RF) ablation unit (EPT 1000TC Cardiac Ablation Unit). Temperature modulated mode was used for all ablations. The RF generator was set to 60°C‐65°C with a maximal delivered power of 50 W for APs not located in the midseptal or anteroparaseptal regions. For those pathways near the normal conduction system, the temperature was decreased to 40°C and maximal power decreased to 20 watts. If AP conduction was not abolished within 8 seconds, RF delivery was discontinued and fine mapping continued. When AP conduction terminated within 8 seconds of the start of RF delivery, energy delivery was continued for 60 seconds. Except for the first 3 dogs, EP testing continued for 1 hour to ensure AP conduction did not recur.
Figure 2.
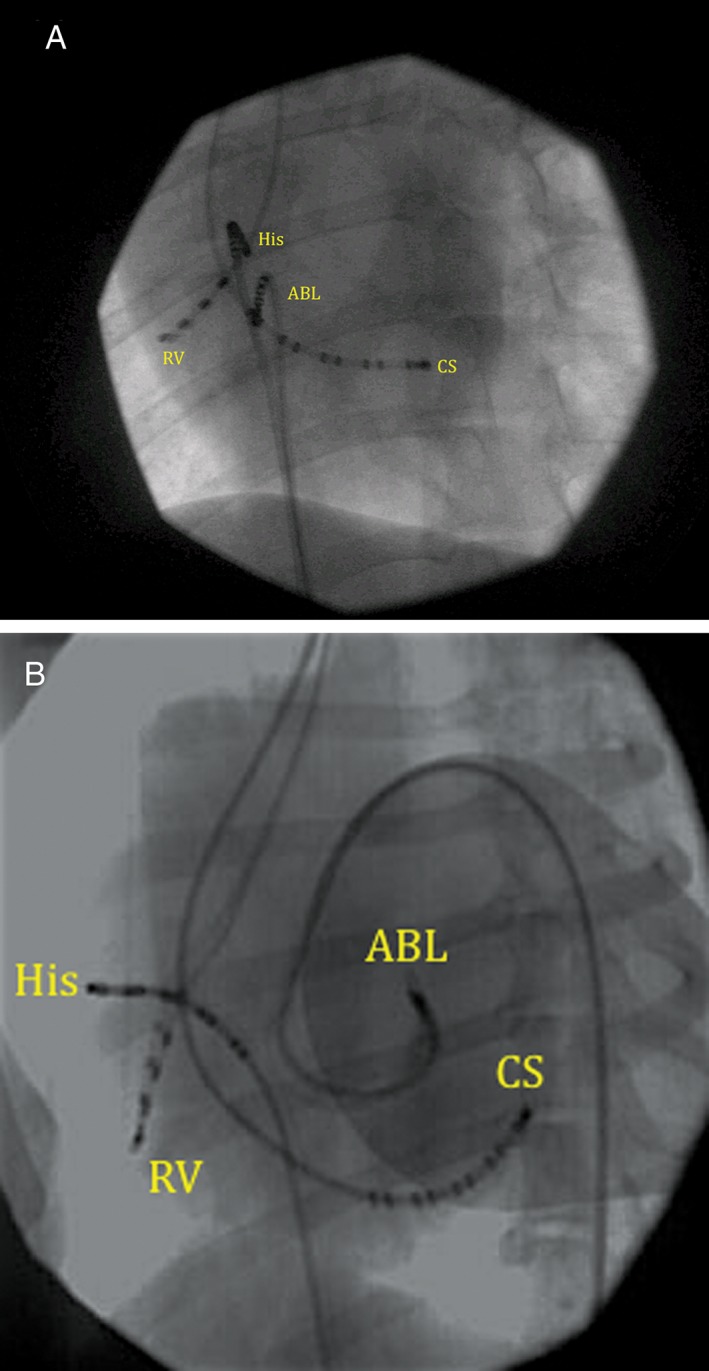
A, Left anterior oblique fluoroscopic view of a right posteroseptal accessory pathway ablation. ABL: ablation catheter at the ninth electrode of the CS catheter, which was located at the coronary sinus os. All multielectrode catheter poles are labeled by convention in numerical order starting at the distal (tip) electrode. CS, coronary sinus decapolar catheter; His, decapolar catheter with the tip electrodes located at the His bundle region. RV, quadripolar catheter located in the right ventricle. B, Left anterior oblique fluoroscopic view of a left anterior accessory pathway ablation. ABL, ablation catheter that has been prolapsed retrograde across the aortic valve and is deflected under the septal mitral valve leaflet. CS, coronary sinus decapolar catheter. His, decapolar catheter with the tip electrodes originally located at the His bundle region; however, it had flipped away from the septum at the time of this image. RV, quadripolar catheter located in the right ventricle
Recorded EP data included antegrade and retrograde AP properties (fastest 1:1 conduction cycle length, effective refractory period, stimulus‐atrial interval during RVA pacing, VA interval during OAVRT), AP location, time to AP block during RF delivery, and complications encountered. Accessory pathway location was defined based on the left anterior oblique view as left or right free wall or septal/paraseptal. The free wall regions were further divided into lateral, anterolateral, and posterolateral. The septal/paraseptal regions were further divided into anteroparaseptal, midseptal, and posteroparaseptal.11, 26
2.3. Follow‐up
Each dog was monitored in the intensive care unit with telemetry for at least 16 hours after the procedure before discharge. One to 2 months after ablation, 24‐hour Holter monitoring was performed in the dog's home environment. An echocardiogram also was to be performed by the referring cardiologist at this time.
Owners were contacted annually thereafter to assess patient status. Long‐term outcome and survival were assessed by identifying the date and cause of death by contacting owners, referring cardiologists, and family veterinarians. Cause of death was classified as cardiac (further divided into whether deaths were associated with APs) or noncardiac.
2.4. Statistical analysis
Statistical analyses were performed using commercially available statistics software programs (Stata IC/13.1, StataCorp LP, College Station, TX and StatXact, Cytel Software Corporation, Cambridge, MA). Continuous variables were expressed as median (interquartile range) for non‐normally distributed variables or mean (±standard deviation) for normally distributed variables. Descriptive statistics were used for demographic and categorical clinical data, expressed as number of dogs displaying the variable of interest out of total number of dogs that possibly could display that variable.
Least‐squares linear regression was used for analyses between 2 continuous variables. Analyses between variables with 2 categories were performed using logistic regression with robust variance estimation to account for clustering of observations, whereas analyses of ≥3 categories used Kruskal–Wallis tests. A Mann–Whitney test was used to compare fastest HRA pacing cycle length exhibiting 1:1 antegrade conduction over the AP between intermittent and manifest preexcitation groups. Analyses between pre‐ and post‐RFCA echocardiographic variables were done with Wilcoxon signed‐rank tests. P values <.05 were considered statistically significant.
3. RESULTS
3.1. Signalment
Twenty‐three different breeds were affected (Table 1). The most common breed was the Labrador Retriever (42/89, 47.2%), followed by Golden Retrievers (7/89, 7.9%), Boxers (5/89, 5.6%), and English Bulldogs (4/89, 4.5%). Sixty of the 89 dogs (67.4%) were males. Affected Labradors were significantly more likely to be male (33/42) than non‐Labrador breeds (27/47; P = .043). Median age at the time of presentation was 2.3 years (range, 0.33‐9.7 years), with no difference between Labrador Retrievers and other breeds (P = .429). Median time from diagnosis of VPE or a narrow complex tachycardia to presentation for ablation was 4 months (range, 0.5 months to 7.8 years).
Table 1.
Clinical characteristics of 89 dogs with accessory atrioventricular pathways
| BREED | No. of cases |
|---|---|
| Labrador Retriever | 42 |
| Golden Retriever | 7 |
| Boxer | 5 |
| Mixed breed | 5 |
| English Bulldog | 4 |
| Bernese Mountain Dog | 3 |
| German Shepherd Dog | 2 |
| Australian Shepherd | 2 |
| American Staffordshire Terrier | 2 |
| Beagle | 2 |
| Doberman | 2 |
| German Shorthaired Pointer | 2 |
| Alaskan Malamute | 1 |
| French Bulldog | 1 |
| American Bulldog | 1 |
| English Springer Spaniel | 1 |
| Weimaraner | 1 |
| Bassett Hound | 1 |
| Boerbel Mastiff | 1 |
| Boston Terrier | 1 |
| Deutsch Langerhaar | 1 |
| Pembroke Welsh Corgi | 1 |
| Border Collie | 1 |
| Sex | No. of cases |
| Male | 19 |
| Male Castrated | 41 |
| Female | 10 |
| Female Spayed | 19 |
| Clinical signs noted by owner | No. of cases |
| Intermittent lethargy/weakness | 62 |
| Hyporexia | 47 |
| Vomiting | 33 |
| Head bobbing/body or thorax bobbing | 27 |
| Labored breathing | 19 |
| Syncope/collapse | 13 |
| Seeks owner | 8 |
| Abdominal distention | 8 |
| Diarrhea | 6 |
| Coughing | 5 |
| Marked weight loss/cachexia | 3 |
| Reclusive | 1 |
3.2. Clinical presentation
Presenting clinical signs are shown in Table 1. The most common sign was intermittent lethargy or weakness (62/89, 69.7%), followed by hyporexia (47/89, 52.8%) and vomiting (33/89, 37%). Three dogs (3.3%) had marked weight loss or cachexia. Head bobbing or chest pulsing was reported in 27/89 (30.3%). Syncope occurred in 13/89 (14.6%) and resuscitated cardiac arrest before referral in 2/89 (2.2%).
Documented CHF occurred in 34/89 dogs (38.2%). Of these, 14 (15.7%) exhibited left‐sided CHF only, 11 (12.4%) right‐sided CHF only, and 9 (10.1%) biventricular CHF. No relationship was found between presence of CHF and either fastest OAVRT cycle length before antiarrhythmic drugs (P = .374) or age at the time of ablation (P = .455). Labrador Retrievers were more likely to have biventricular CHF than non‐Labrador breeds (P = .010).
Cardiac medications administered before presentation are listed in Table 2. Thirty‐eight dogs (42.7%) were taking 1‐2 medications, whereas 51 dogs (57.3%) were receiving ≥3 medications. Only minor CBC and serum biochemical profile abnormalities were found, the most common being a mild increase in ALT activity (9/89 dogs).
Table 2.
Medications administered prior to RFCA
| Medication | No. of cases | Median dosage mg/kg/day (range) |
|---|---|---|
| Diltiazem | 67 | 4.19 (2.00‐12.12) |
| Sotalol | 55 | 3.76 (1.86‐9.53) |
| Enalapril | 27 | 0.80 (0.22‐1.49) |
| Furosemide | 26 | 3.06 (0.96‐5.32) |
| Pimobendan | 24 | 0.53 ((0.30‐0.78) |
| Procainamide | 24 | 52.44 (30.1‐96.62) |
| Digoxin | 19 | 0.009 (0.006‐0.020) |
| Mexiletine | 12 | 18.22 (15‐20.93) |
| Atenolol | 6 | 1.08 (0.72‐2.44) |
| Propafenone | 5 | 23.36 (9.91‐25.28) |
| Benazepril | 5 | 0.71 (0.28‐0.95) |
| Spironolactone | 3 | 2.39 (2.23‐2.54) |
| Amiodarone | 2 | 11.38 (7.54‐15.23) |
| Verapamil | 2 | 4.99 (3.05‐6.92) |
| Propranolol | 2 | 1.95 (1.95‐1.95) |
| Doxycycline | 2 | 9.25 (8.47‐10.07) |
| L‐carnitine | 1 | 81.6 (81.6‐81.6) |
| Taurine | 1 | 40.8 (40.8‐40.8) |
| Flecainide | 1 | 5.0 (5.0‐5.0) |
| Prednisone | 1 | 0.30 (0.30‐0.30) |
3.3. Electrographic findings
Narrow complex tachyarrhythmias were seen in all dogs (Table 3). Two dogs initially presented to veterinarians with wide complex tachyarrhythmias thought to be ventricular tachycardia (VT). Both wide complex tachyarrhythmias became narrow complex tachyarrhythmias as IV treatment resulted in mild rate slowing and resolution of prior intraventricular conduction delay. In 4 other dogs, the initial 3‐5 complexes of OAVRT were wide complex because of right bundle branch block, which resolved with continuation of the tachycardia. Median documented tachycardia cycle length before antiarrhythmic medications was 180 ms (range, 140‐240 ms), corresponding to heart rates between 250 and 428 bpm. No significant difference was found between OAVRT cycle length of Labrador Retrievers and other breeds (P = .645). P′ waves (confirmed retrograde AP conduction at EPS) could be identified within the ST segment or T wave during tachycardia in at least 1 surface ECG lead of 87/89 dogs with a median RP’ interval of 80 ± 11 ms (Figure 3A). P′ waves within the ST segment or T wave of sinus complexes occurred intermittently in 70/89 dogs, having a fixed RP′ interval equal to RP′ measured during OAVRT (Figure 3B). P′ waves generally were visualized best in the caudal (inferior) limb leads (II, aVF, or III), but in 18% of dogs, the cranial (superior) limb leads provided the best visualization of the P′ onset.
Table 3.
Electrocardiographic and electrophysiologic data from 89 dogs with accessory atrioventricular pathways
| OAVRT measurements | Mean ± SD or median (range) | P value |
|---|---|---|
| OAVRT cycle length (HR) prior to medication | 180 ms (140‐240 ms)* | *<.0001 |
| OAVRT cycle length during EPS | 219 ms (146‐290 ms)* | |
| Surface ECG RP′ interval during OAVRT prior to EPS | 77.6 ± 9.4 ms** | **.0033 |
| Surface ECG RP′ interval during OAVRT at EPS | 75.7 ± 8.2 ms** | |
| Surface ECG P′R interval during OAVRT prior to EPS | 107 ± 19 ms*** | ***<.0001 |
| Surface ECG P′R interval during OAVRT at EPS | 141 ± 22 ms*** | |
| VA interval to distal ablation dipole at site of success | 51 ms (32‐80 ms) | |
| Shortest VA interval to standard catheter dipole | 61.5 ± 15.5 ms | |
| Shortest cycle length at which retrograde AP conducts 1:1 during decremental RV pacing | 190 ms (150‐290 ms) | |
| AH in OAVRT | 112 ms (48‐206 ms)^ | ^<.0001 |
| AH in sinus rhythm | 70 ms (40‐125 ms)^ | |
| HV in OAVRT | 34.5 ms (24‐49 ms)# | #<0.0001 |
| HV in sinus rhythm (includes ventricular preexcitation) | 31 ms (−26 to 49 ms)# | |
| Ventricular preexcitation measurements | ||
| PR interval on preexcited complexes prior to EPS | 60 ms (40‐90 ms)## | ##0.7058 |
| PR interval on preexcited complexes at EPS | 60 ± 15 ms## | |
| PR interval on nonpreexcited complexes prior to EPS | 110 ms (80‐140 ms) | |
| Shortest cycle length at which antegrade AP conducts 1:1 during decremental HRA pacing | 303.6 ± 127.5 ms | |
| Antegrade AP effective refractory period | 243.1 ± 65.0 ms |
Figure 3.
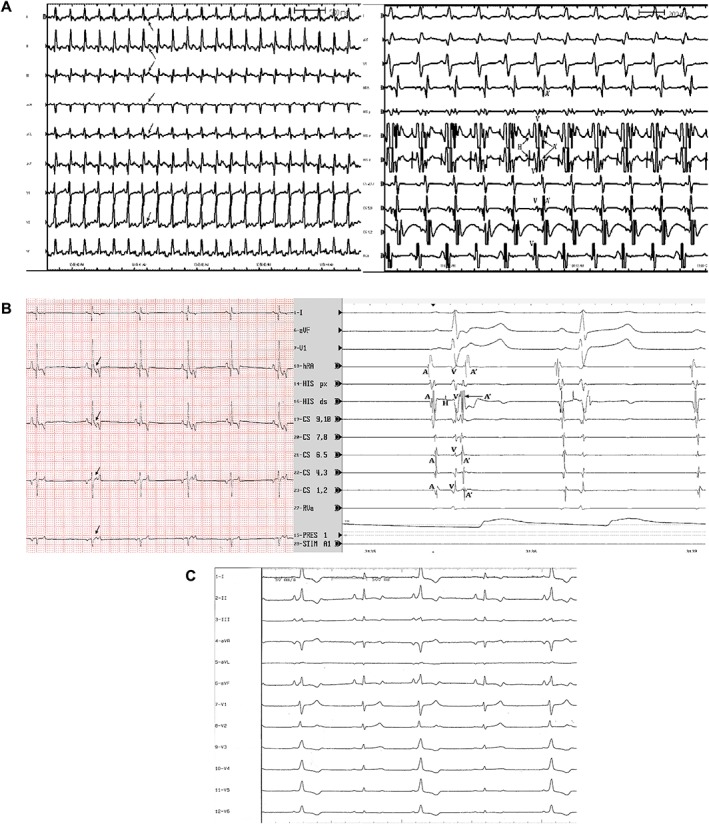
A, Surface ECG leads and intracardiac electrograms demonstrating retrograde accessory pathway activation during orthodromic atrioventricular reciprocating tachycardia. The figure on the left displays 9 of the 12 ECG leads recorded in the awake state just prior to electrophysiologic study. A narrow complex tachycardia at a cycle length of 221 ms is present. Arrows point to representative retrograde P′ waves visible within the early ST segment following each QRS complex. Paper speed 50 mm/s, calibration 10 mm/mV. The figure on the right is at the time of electrophysiologic study. Three surface ECG leads and eight intracardiac dipole recordings are shown. Orthodromic atrioventricular tachycardia is present. Electrograms are labeled as follows: A′, retrograde atrial depolarization over the accessory pathway; V, ventricular depolarization; H, His bundle activation. The earliest retrograde atrial activation is seen in the proximal coronary sinus for this right posterolateral free wall accessory pathway. Sweep speed is 100 mm/s. B, Surface ECG leads and intracardiac electrograms demonstrating intermittent retrograde accessory pathway conduction during sinus rhythm. The figure on the left is a baseline ECG during sinus rhythm in the awaken state. Note that the second and fifth sinus complexes have a deflection within the ST segment (arrows) that is not present on the first, third, and fourth complexes. This deflection represents retrograde accessory pathway conduction of the sinus impulse once it reaches the ventricles. Atrial depolarization results and is seen as a retrograde P′ wave on the surface ECG. The RP′ interval is always the same. Paper speed 25 mm/s, calibration 10 mm/mV. The figure on the right is at the time of electrophysiologic study. Three surface ECG leads and nine intracardiac leads are shown (100 mm/s). The first sinus complex conducts retrograde over a concealed right posteroparaseptal accessory pathway, while the second sinus complex does not. A, sinus nodal depolarization of the atria; H, His bundle electrograms; V, ventricular depolarization; A′, retrograde depolarization of the atria over the accessory pathway. Note that no A′ is visible on the second sinus complex. C, Twelve‐lead ECG from a 3‐year‐old Boxer with a midseptal accessory pathway at the time of electrophysiologic study (50 mm/s, 10 mm/mV). The first, third, and fifth complexes of this sinus rhythm have a shorter PR interval, slurred upstroke to the QRS complex, and prolonged QRS duration compared to the second and fourth complexes. This represents intermittent ventricular preexcitation. Antegrade accessory pathway conduction manifests on the surface ECG in this dog for every other sinus complex
Forty‐three of 89 dogs (48.3%) exhibited VPE sometime during sinus rhythm. Twenty‐three of these dogs had manifest VPE, whereas 20 had intermittent VPE (Figure 3C). Ventricular preexcitation was seen only on pre‐ablation Holter monitoring but not on baseline ECGs in 2 dogs. Median preexcited PR interval was 59 ms (range, 40‐90 ms), whereas the median PR interval on nonpreexcited complexes was 110 ms (range, 80‐140 ms). Median QRS duration during VPE was 80 ms (range, 55‐120 ms). Fourteen dogs had preexcited PR intervals ≥60 ms, and 10 dogs had preexcited QRS durations ≤60 ms. Labrador retrievers exhibited VPE significantly less frequently (12/42 or 28.5% of Labradors) than did non‐Labradors (31/47 or 66.0% of other breeds, P = .001).
Other arrhythmias were noted before ablation in 53/89 dogs. These included single ventricular premature complexes (VPCs, 36/89), ventricular triplets or nonsustained VT (16/89), single atrial premature complexes (APCs, 17/89), paroxysmal AF (6/89), and asystole requiring resuscitation (2/89).
3.4. Echocardiographic findings at presentation
Echocardiographic measurements are shown in Table 4. Left ventricular diastolic chamber dilatation (LVIDdN >1.73) at presentation was seen in 31/89 dogs, whereas 30/89 dogs had LA dilatation (2D RPS SAX LA:Ao > 1.5, all of which also had LV dilatation). Subjective right atrial dilatation (20/89 dogs) and right ventricular dilatiaton (16/89 dogs) were seen less frequently. Forty‐one of 89 dogs (46.1%) met criteria for LV systolic dysfunction. No relationship was found between presence of systolic dysfunction and fastest OAVRT cycle length before antiarrhythmic drugs (P = .42), time from initial diagnosis (P = .87), or age at time of ablation (P = .08). Labrador Retrievers were not more likely to have systolic dysfunction than non‐Labrador breeds (P = 0.368). Mitral regurgitation was trace to mild in 44/89 dogs and moderate to severe in 20/89 dogs. Tricuspid regurgitation was trace to mild in 53/89 dogs and moderate to severe 17/89 dogs. Congenital or acquired (other than TICM) structural heart disease occurred in 12/89 dogs and included tricuspid dysplasia (5/89), mitral dysplasia (2/89), mitral and tricuspid valve endocardiosis (3/89), patent ductus arteriosus (1/89), and subaortic stenosis (1/89).
Table 4.
Echocardiographic data of 89 dogs with accessory atrioventricular pathways prior to radiofrequency catheter ablation
| Echocardiographic measurement | Mean ± SD or median (range) |
|---|---|
| LVIDdN | 1.64 (1.26‐2.3) |
| LVIDsN | 1.18 ± 0.21 |
| LVEF | 53 ± 12% |
| LVESVI | 37.8 ± 21.9 ml/M2 |
| LVFS | 25% (5%‐42.8%) |
| LA:Ao ratio in 2D right parasternal short‐axis view | 1.35 (1.00‐2.12) |
| Mitral regurgitation | |
| None | 25 |
| Trace or mild | 44 |
| Moderate | 15 |
| Severe | 5 |
| Tricuspid regurgitation | |
| None | 19 |
| Trace or mild | 53 |
| Moderate | 12 |
| Severe | 5 |
3.5. Electrophysiologic study
Ninety‐six APs were identified in the 87 dogs completing the mapping procedure (Table 3). Nine dogs (10.3%) had 2 APs, whereas 78 dogs (89.7%) had a single AP. Four of 41 Labrador Retrievers (9.8%) had 2 APs, whereas 5 of 46 non‐Labradors (10.9%) had 2 APs (P = 1.00). Median OAVRT cycle length during EPS was 219 ms (range, 146‐290 ms), significantly longer than that recorded in the awake state (P < 0.0001). The majority of this prolongation came from an increased P′R interval (Table 3), rather than R′P interval, although both were prolonged in the anesthetized state. Incessant OAVRT in many dogs necessitated IV use of antiarrhythmic agents (diltiazem or lidocaine) the night before or morning of RFCA, with potential effects on tachycardia cycle length. Rapid retrograde AP conduction properties were uniformly noted with 1:1 retrograde VA conduction at pacing cycle lengths <200 ms in 73/89 dogs. Only 1 AP had decremental retrograde conduction properties.
Antegrade AP conduction occurred during sinus rhythm or atrial pacing in 42/96 APs (43.8%) and typically was less robust than retrograde conduction. Only 5 APs conducted antegradely 1:1 at pacing cycle lengths <200 ms (mean for all antegrade APs, 303 ± 127.5 msec). All but 1 AP that conducted antegradely also conducted retrogradely. Accessory pathways exhibiting manifest VPE conducted 1:1 in the antegrade direction at significantly faster atrial pacing cycle lengths (median, 240 ms) than APs showing intermittent VPE (median, 450 ms; P = .0003). Labrador Retrievers were significantly less likely than other breeds to have an AP capable of antegrade conduction (12/41 versus 30/46; P = .001).
Accessory pathway location was distributed as shown in Figure 4. The majority of APs (88/96 or 91.7%) were located along the tricuspid annulus, with only 8/96 (8.3%) located along the mitral annulus. Only 3 of these 8 left‐sided APs required a retrograde aortic approach, but 1 owner declined this approach, precluding detailed AP mapping and ablation. The other 5 left‐sided APs could be ablated from a right‐sided approach within the proximal CS or middle cardiac vein. The most common AP location was right posteroparaseptal (45/96, 46.9%), followed by right free wall (36/96, 37.5%). Rapid termination of AP conduction, as shown in Figure 5, was noted at the successful ablation site. Ventricular‐to‐atrial timing to the distal ablation catheter bipole at the site of success was 51 ms (range, 32‐80 ms). Time to AP block from RF onset was 3.8 seconds (range, 1.2‐8.5 seconds).
Figure 4.
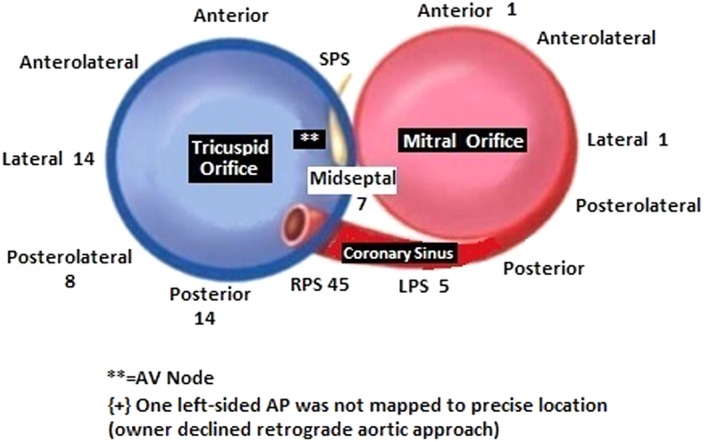
Diagram corresponding to a left anterior oblique fluoroscopic view showing the mitral and tricuspid valve annuli (11, 24). The number of accessory pathways found in this group of 89 dogs at each location is marked. RPS, right posteroparaseptal; LPS, left posteroparaseptal; SPS, superoparaseptal or anteroparaseptal; AV, atrioventricular. The location of the AV node is marked by the symbol **
Figure 5.
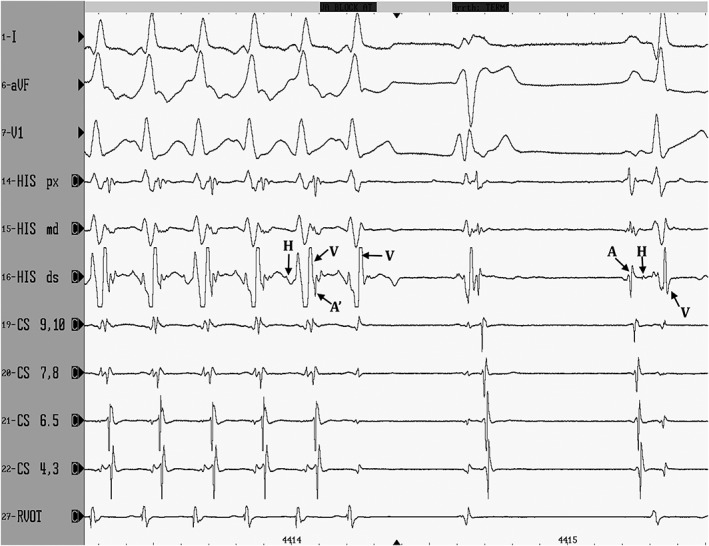
Three surface ECG leads and eight intracardiac dipoles are displayed during radiofrequency catheter ablation (sweep speed 100 mm/s). The first 5 complexes represent orthodromic atrioventricular reciprocating tachycardia with retrograde atrial activation (labeled as A′ in the fifth complex) over a right posteroparaseptal accessory pathway. Earliest retrograde atrial activation in these standard leads occurs in the CS 9, 10 dipole. With the sixth complex, the accessory pathway is successfully ablated, as ventricular activation (V) is not followed by retrograde atrial activation. In this dog, OAVRT terminates 3.4 s after the onset of radiofrequency energy (RF onset is not shown in this figure), and sinus rhythm resumes after a ventricular complex. Small His bundle electrograms are marked with H. Sinus nodal activation of the atria is labeled A
Twenty‐four dogs had retrograde AV nodal conduction after successful AP ablation, whereas complete VA block during RV pacing was noted in the remaining 61 dogs having successful ablation of all APs. Ventricular‐to‐atrial intervals over the retrograde AV node had a median of 134 ms (range, 72‐205 ms), 83 ms (range, 40‐125 ms) longer than those seen with retrograde APs.
Other arrhythmias encountered during EPS included nonsustained AF (14/89), sustained AF requiring synchronized cardioversion (2/89), automatic right atrial tachycardia (3/89), automatic left atrial tachycardia (1/89), complete AV block requiring pacemaker implantation (1/89), VF (3/89; which quickly followed preexcited AF in 2 of these 3 dogs), and electromechanical dissociation (1/89). Dual antegrade AV nodal pathways were identified post‐ablation in 3/89 dogs, whereas dual retrograde AV nodal pathways were present in 2/89 dogs. Four dogs had single atypical AV nodal echoes during ventricular extrastimulus pacing (VA conduction over the retrograde slow pathway followed by antegrade conduction over the fast pathway). No dog had inducible AV nodal reentrant tachycardia.
3.6. Procedural and long‐term outcomes
Eighty‐six dogs survived EPS/RFCA. Three dogs died during induction of general anesthesia or EPS. Two of these dogs developed VF, whereas the third (with the most severe myocardial dysfunction) developed electromechanical dissociation. A fourth dog developed VF during the procedure, but was resuscitated. Persistent, profound neurologic deficits 5 days postprocedure led to this patient's euthanasia. All these cases occurred earlier in the study period. The remaining 85 dogs were followed until time of death, loss to follow‐up, or writing of this article.
Ninety‐four APs were ablated. One dog died before ablation of its right posteroparaseptal AP (due to electromechanical dissociation), and the owner of the smallest study patient declined detailed mapping of a left‐sided AP via a retrograde aortic approach. Recurrence of AP conduction occurred in 3 dogs; 2 were early in this study (1999) when an intraoperative hour‐long waiting period after acute disruption of AP conduction was not followed. These 2 dogs had recurrence within the first month, whereas the third dog had recurrence 3 months after the first ablation. Repeat RFCA was successful long‐term in all 3 dogs. Holter monitoring confirmed absence of any evidence of antegrade (VPE) or retrograde AP conduction (AP echoes or OAVRT) in 76 of 77 dogs, in which it was performed after their last EPS. Retrograde AP conduction remained in the French bulldog with a left‐sided AP that was not ablated. Other arrhythmias found on the 1‐month follow‐up Holter monitoring included: single APCs (>30/24 hours) in 5 dogs, atrial tachycardia in 4 dogs (all also had single APCs), VPCS (>30/24 hours) in 9 dogs, ventricular couplets in 1 dog (also having single VPCs), and nonsustained VT in 2 dogs. The dog that developed complete AV block during the study with subsequent pacemaker placement had a ventricular paced rhythm throughout the Holter monitoring period. All dogs with atrial or ventricular tachyarrhythmias documented on the 1‐month post‐RFCA Holter tracing also had evidence of these arrhythmias before ablation. Three additional dogs developed VT 6 months to 2 years after ablation. One of these was an English Bulldog that developed arrhythmogenic RV cardiomyopathy, and the other 2 were active hunting dogs, in which myocarditis was suspected. Follow‐up Holter data were not available for 8 of the 85 dogs. These 8 dogs were treated earlier in the study period and were followed until death. None of these 8 dogs died because of cardiac causes. Long‐term success rate of a single RFCA procedure in dogs surviving to ablation was 95%. Overall long‐term success rate when including dogs undergoing a successful second RFCA procedure was 98.8%.
Of the 41 dogs that met systolic dysfunction criteria before RFCA, follow‐up echocardiograms were available in 29. Significant improvement in systolic function 1‐month post‐RFCA was documented. The most commonly measured LV function values on post‐RFCA echocardiograms performed by referring cardiologists were LVFS and LVIDsN. The mean increase in LVFS 1 month after RFCA was 12.9% (pre‐ versus post‐LVFS, P < .0001), whereas the mean decrease in LVIDsN was 0.31 (pre‐ versus post‐LVIDsN, P = .0001).
Of the 85 dogs surviving >5 days post‐RFCA, 45 were confirmed alive at the time of manuscript preparation, a mean of 4.0 ± 3.2 years after RFCA. These owners have reported resolution of their dogs’ presenting clinical signs after RFCA. Three dogs are receiving antiarrhythmic agents for ventricular tachyarrhythmias noted on Holter monitoring. One of these dogs had ventricular tachyarrhythmias documented before RFCA; the other 2 developed them 6 months to 2 years after ablation. Two dogs are receiving antiarrhythmic drugs for atrial tachyarrhythmias documented before ablation. Both of these were older dogs (8 and 9.7 years) with moderate mitral valve endocardiosis at presentation for RFCA. Thirty‐four dogs have died (11) or were euthanized (23). The median age at time of death in these dogs was 11.3 years (range, 2.83‐15 years), with median time from RFCA of 8.0 years (range, 1.2‐13.3 years). Two deaths were cardiac‐related; 32 were not (Table 5). The 2 cardiac‐related deaths occurred in 1 Boxer and 1 English Bulldog, both with documented ARVC, that died suddenly 5 years and 4 years after RFCA, respectively. Six dogs were lost to follow‐up (1 between 1 and 2 years post‐RFCA, 3 between 2 and 4 years post, and 2 between 7 and 10 years post).
Table 5.
Causes of death or euthanasia in dogs with atrioventricular pathways undergoing radiofrequency catheter ablation
| Cause of death/euthanasia | Number of dogs |
|---|---|
| Neoplasia | 18 |
| Progressive neurologic dysfunction | 5 |
| Ventricular fibrillation at time of study | 3 |
| Sudden cardiac death related to arrhythmogenic right ventricular dysplasia | 2 |
| Electromechanical dissociation at time of study | 1 |
| Acute necrotizing pancreatitis | 1 |
| Hepatic failure | 1 |
| Pneumonia | 1 |
| Laryngeal collapse | 1 |
| Gunshot wound | 1 |
| Aggression | 1 |
| Progressive lower airway disease | 1 |
| Progressive gastrointestinal signs thought to be neoplastic in origin (owner declined advanced diagnostics) | 1 |
| Marked polyuria/polydipsia and weight loss (owner declined advanced diagnostics) | 1 |
4. DISCUSSION
Ours is the largest study of APs in dogs to date. Several novel findings could aid in the identification and treatment of this often elusive condition. The first is confirmation of predisposition of Labrador Retrievers to APs. Representing nearly half (47%) of dogs presented for RFCA of APs, Labrador Retrievers appear overrepresented in this population. Although the most popular breed in the United States, Labrador Retrievers represent only 15% of all registered dogs according to American Kennel Club statistics, falling well short of the percentage found in our study.
A male predominance also was found in our study, with Labrador Retrievers more likely than non‐Labrador breeds to be male. In a prior European case series of 10 dogs, 6 were Labrador Retrievers and all were male.11 Other case reports include Labrador Retrievers (2), Boxers (2), Bulldogs (2), Alaskan Malamute (1), Beagle (1), and Golden Retriever (1).7, 8, 9, 10, 12, 13 Seven of the 9 dogs in these small studies were male. The possibility of a sex‐linked or sex‐modified mode of inheritance for APs in Labrador Retrievers can be postulated. Conflicting reports of male predominance have been reported in studies of humans with APs.27, 28, 29, 30, 31, 32, 33
The prevalence of gastrointestinal signs as the manifestation of tachycardia in these often young, large breed dogs is important to note, as many initially were misdiagnosed with dietary indiscretion or potential gastrointestinal foreign bodies. The common occurrence of inappetence and vomiting in these dogs should prompt veterinarians to add OAVRT (or another tachyarrhythmia) to their differential diagnostic list for this presentation. If tachycardia is auscultated at presentation, then an ECG should be performed, rather than assuming sinus tachycardia in response to dehydration or discomfort. If tachycardia is not detected at presentation, an intermittent tachyarrhythmia still should be considered, particularly if no other cause is found on routine diagnostic testing. In this setting, Holter or event monitoring would be useful.
Surface ECGs can provide useful clues to the presence of an AP. The rate of OAVRT in dogs not on antiarrhythmic drugs is rapid, (median, 333 bpm; range, 250‐428 bpm) in our study. The OAVRT rate under general anesthesia was significantly slower, (median, 274 bpm; range, 207‐411 bpm). Whether evaluating the awake or anesthetized OAVRT rates in our study, they are faster than in a previously reported group of 14 dogs, in which the anesthetized OAVRT rate was 229 ± 42 bpm.34 Whereas focal atrial tachycardias (FAT) had faster ventricular rates than OAVRT in that study, median OAVRT rate in our awake dogs was substantially higher and median OAVRT rate in our anesthetized dogs was the same as the anesthetized mean FAT ventricular rate (278 bpm) in the prior study. Isoflurane can affect AV conduction as well as atrial, ventricular, and AP refractory periods.35, 36, 37 Significant prolongation of the P′R interval and lesser prolongation of the R′P interval in the anesthetized state versus the unmedicated, awake state (Table 3) was seen in our group of dogs. This finding would suggest a greater effect of general anesthesia on the AV nodal conduction system as compared to the AP. Hemodynamically compromising, incessant OAVRT in many dogs not receiving PO antiarrhythmic drugs necessitated conversion with IV diltiazem (less commonly lidocaine) the night before anesthesia. Lingering drug effects also may have played a role in slower tachycardia cycle lengths and longer P′R intervals.
Retrograde P′ waves could be seen within the ST segment of 98.8% of dogs on a 6‐ or 12‐lead ECG, representing retrograde conduction over the AP and resulting in a short RP’ interval of 80 ± 11 ms. This is similar to the RP’ interval of 85 ± 16.8 ms and the frequency of identifiable P′ waves in OAVRT reported in another study.34 Intermittent P′ waves in the ST segments of sinus complexes were seen in the majority of dogs (78.7%) as well. Retrograde P′ waves, however, often were identifiable only in certain leads, and thus the more leads recorded, the better the chance of identification. Also, identification of retrograde P′ waves following a sinus QRS complex was enhanced with Holter monitoring or extended multiple lead ECG recordings. Occurrence of wide complex tachycardia does not eliminate the possibility of OAVRT, as 2 dogs had sustained OAVRT with right bundle branch block. The mimicking of VT by OAVRT conducted with aberrancy has been reported previously in 1 dog.12
Several dogs in our study experienced paroxysmal AF before or at the time of EPS or both. A higher occurrence of AF in people with APs has been reported, particularly in males and in those with manifest APs.38, 39 Neither of these predilections was found in our dogs with AF. Right‐sided APs and shorter OAVRT cycle lengths in children are associated with degeneration of OAVRT into AF and a higher risk of sudden death.40 Our dogs had predominantly right‐sided APs and short OAVRT cycle lengths, potentially predisposing them to AF.
The distinct predominance of right‐sided APs in these dogs coincides with prior smaller studies.7, 8, 9, 10, 11, 12, 13 Right posteroparaseptal was the most common location, followed by right free wall. This distribution is distinctly different than reported in humans, where ~60% of APs are left free wall, 25% are septal pathways, and 15% are right free wall pathways.1 Multiple APs were identified in 10% of dogs in our study, similar to the 3%‐13% of human AP patients with multiple APs.41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Five of 19 (26.3%) previously reported dogs with APs had multiple pathways.7, 8, 9, 10, 11, 12, 13 This percentage is higher than observed in our case series or in larger studies of humans. A large retrospective study of 1088 pediatric AP patients found that congenital heart disease increased the risk of multiple APs threefold from 8% to 26% in that population.51 Only 1 of the 9 dogs (11.1%) with congenital heart disease in our study had multiple APs.
Antegrade conduction over the AP during sinus rhythm or atrial pacing (VPE) in dogs of our study occurred less frequently than reported in humans. Antegrade AP conduction was documented in 43.8% of our dogs. This percentage is compared to 64% in a large study of children and 63%‐83% in human adults.51, 52 Ventricular preexcitation often is more subtle on a surface ECG in dogs than in humans. Thirty‐three percent of VPE dogs in our series had preexcited PR intervals of 60‐90 ms, which is within commonly cited normal reference ranges.53 Considering Labrador Retrievers alone, occurrence of antegrade AP conduction decreases to 29.2%. Concealed APs are more challenging to diagnose. Manifest or intermittent antegrade AP conduction will point to an AP′s presence during sinus rhythm (VPE), whereas concealed APs are silent on the surface ECG unless the dog is in OAVRT or retrograde AP echoes occur during sinus rhythm. The predominance of Labrador Retrievers and their tendency to have concealed APs poses a diagnostic challenge. Clinicians should maintain heightened awareness for APs and pursue extended ECG monitoring, such as Holter monitors, to detect salvoes of tachycardia or intermittent preexcitation in this breed.
Although supraventricular tachyarrhythmias are thought to be more benign than ventricular tachyarrhythmias, our study highlights their potentially lethal nature. Atrial fibrillation can degenerate rapidly into VF in a dog with rapid antegrade AP conduction. This scenario occurred in 2 dogs in our study during EPS. Two other dogs had resuscitated sudden cardiac death before presentation and 13 experienced syncope.
Myocardial dysfunction secondary to frequent or sustained OAVRT (TICM) can lead to CHF and resultant euthanasia or death. Its unique feature is reversibility of this dysfunction upon control of the arrhythmia.5, 8, 54 Most of our knowledge regarding pathophysiologic and morphologic features of TICM is based on a canine model of rapid pacing.55 Occurrence of TICM relates both to the rapidity and frequency of a given tachyarrhythmia. The sustained heart rate that triggers TICM is not well established in any species. It may manifest months to years after onset of the tachyarrhythmia, particularly in individuals with slower or more intermittent tachyarrhythmias. Experimental dogs and human patients with higher tachyarrhythmia rates develop TICM earlier.55, 56 Time to onset of ventricular dysfunction also is dependent on other factors, including underlying structural heart disease and patient age.6 We found no relationship between LV systolic dysfunction and fastest OAVRT cycle length before antiarrhythmic drugs or age at time of ablation. Possible explanations include the following: (1) We did not analyze the frequency of OAVRT, an important factor in TICM development. Doing so would require an implantable loop recorder for accurate assessment in dogs. (2) Systolic function measurements were made at presentation for RFCA, when most dogs were already on antiarrhythmic and other cardiac medications. (3) A larger patient population may be needed to achieve statistical significance.
Tachycardia‐induced cardiomyopathy should be considered in all patients with a DCM phenotype of unknown origin that have a tachyarrhythmia. The dramatic difference in prognosis of TICM treated by eliminating the tachyarrhythmic substrate versus idiopathic DCM warrants diligence to rule out this important underlying cause. Data from several studies and individual case reports have shown that rhythm control results in significant improvement of ventricular systolic function and resolution of clinical signs of CHF.8, 54, 56, 57, 58 Follow‐up data in our study confirm the excellent long‐term prognosis for recovery from CHF and ventricular dysfunction in dogs when the tachyarrhythmia is eliminated by RFCA. Significant improvement in LV systolic function was seen on 1‐month follow‐up echocardiograms and all heart failure medications were discontinued at that time, apart from 2 dogs that remained on medications such as pimobendan because of underlying structural heart disease unrelated to their APs. Interestingly, similar to some older dogs with PDAs that are successfully corrected, some TICM dogs had a persistent mild decrease in LVFS postablation. These same dogs, however, still had resolved CHF and improved LA and LV dimensions. Systolic function measurements did not deteriorate when pimobendan was discontinued.
Finally, our study confirms the efficacy of RFCA for eliminating APs, thus curing dogs of associated tachyarrhythmias and often totally reversing myocardial dysfunction. The long‐term success rate of a single RFCA in the dogs undergoing ablation was 95% (91% success rate when including dogs dying before AP ablation). Overall long‐term success rate when including dogs undergoing successful second RFCA procedures was 98.8% (95% when including dogs dying before AP ablation). American Heart Association and American College of Cardiology clinical practice guidelines currently recommend RFCA as first‐line therapy in AP patients with AVRT, preexcited AF, or both, citing several large case series that support this approach.59 These series report success rates of 93% to 95% and 3% risk of major complications in patients followed for 6 months to 8 years.45, 46, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 Successful RFCA eliminates the need for continued antiarrhythmic drug therapy (unless other arrhythmia substrates are present), repeated hospitalizations for tachyarrhythmia breakthrough or CHF, and lifelong follow‐ups with a veterinary specialist. Given the expense associated with these factors, an RFCA procedure is economically beneficial as well.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The work was completed at MedVet Medical & Cancer Centers for Pets (Cincinnati and Columbus, Ohio locations), The CARE Center (Cincinnati, OH), and Cincinnati Children's Hospital Medical Center Comparative Cardiac Catheterization Laboratory. This study was supported in part by a grant from the Morris Animal Foundation (D02CA‐052). A portion of this study was presented at the 2013 American College of Veterinary Internal Medicine Forum, Seattle, WA.
Wright KN, Connor CE, Irvin HM, Knilans TK, Webber D, Kass PH. Atrioventricular accessory pathways in 89 dogs: Clinical features and outcome after radiofrequency catheter ablation. J Vet Intern Med. 2018;32:1517–1529. 10.1111/jvim.15248
REFERENCES
- 1. Chugh A, Morady F. Preexcitation, atrioventricular reentry, and variants In: Zipes DP, Jalife J, eds. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia: Elsevier Saunders; 2014:755‐765. [Google Scholar]
- 2. De la Fuente D, Sasyniuk B, Moe GK. Conduction through a narrow isthmus in canine atrial tissue. A model of the WPW syndrome. Circulation. 1971;44:803‐809. [DOI] [PubMed] [Google Scholar]
- 3. Slama R, Coumel P, Bouvrain T. Type A Wolff‐Parkinson‐White syndromes, inapparent or latent in sinus rhythm. Arch Mol Coeur Vaiss. 1973;66:639‐653. [PubMed] [Google Scholar]
- 4. Josephson ME. Preexcitation syndromes In: Josephson's Clinical Cardiac Electrophysiology: Techniques and Interpretation. Baltimore: Wolters Kluwer; 2016:336‐440. [Google Scholar]
- 5. Lishmanov A, Chockalingam P, Senthilkumar A, Chockalingam A. Tachycardia‐induced cardiomyopathy: evaluation and therapeutic options. Congest Heart Fail. 2010;16:122‐126. [DOI] [PubMed] [Google Scholar]
- 6. Fenelon G, Wijns W, Andries E, Brugada P. Tachycardiomyopathy: mechanism and clinical implications. Pacing Clin Electrophysiol. 1996;19:95‐106. [DOI] [PubMed] [Google Scholar]
- 7. Atkins CE, Kanter R, Wright K, et al. Orthodromic reciprocating tachycardia and heart failure in a dog with a concealed accessory pathway. J Vet Intern Med. 1995;9:43‐49. [DOI] [PubMed] [Google Scholar]
- 8. Wright KN, Mehdirad AA, Giacobbe P, et al. Radiofrequency catheter ablation of atrioventricular accessory pathways in 3 dogs with subsequent resolution of tachycardia‐induced cardiomyopathy. J Vet Intern Med. 1999;13:361‐371. [DOI] [PubMed] [Google Scholar]
- 9. Santilli RA, Spadacini G, Moretti P, et al. Radiofrequency catheter ablation of concealed accessory pathways in two dogs with symptomatic atrioventricular reciprocating tachycardia. J Vet Cardiol. 2006;8:157‐165. [DOI] [PubMed] [Google Scholar]
- 10. Foster SF, Hunt GB, Thomas SP. Tachycardia‐induced cardiomyopathy in a young Boxer dog with supraventricular tachycardia due to an accessory pathway. Aust Vet J. 2006;84:326‐331. [DOI] [PubMed] [Google Scholar]
- 11. Santilli RA, Spadacini G, Moretti P. Anatomic distribution and electrophysiologic properties of accessory pathways in dogs. J Am Vet Med Assoc. 2007;231:393‐398. [DOI] [PubMed] [Google Scholar]
- 12. Santilli RA, Diana A, Baron Toaldo M. Orthodromic atrioventricular tachycardia conducted with intraventricular conduction disturbance mimicking ventricular tachycardia in an English Bulldog. J Vet Cardiol. 2012;14:363‐370. [DOI] [PubMed] [Google Scholar]
- 13. Santilli RA, Santos LFN, Perego M. Permanent junctional reciprocating tachycardia in a dog. J Vet Cardiol. 2013;15:225‐230. [DOI] [PubMed] [Google Scholar]
- 14. Wright KN. Interventional catheterization for tachyarrhythmias. Vet Clin North Am Small Anim Pract. 2004;34:1171‐1185. [DOI] [PubMed] [Google Scholar]
- 15. Cornell CC, Kittleson MD, Della Torre P. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18:311‐321. [DOI] [PubMed] [Google Scholar]
- 16. Fuentes VL. Echocardiography and Doppler ultrasound In: Smith FWK, Tilley LP, Oyama MA, Sleeper MM, eds. Manual of Canine and Feline Cardiology. Philadelphia: Elsevier Health Sciences; 2015:77‐92. [Google Scholar]
- 17. Hansson K, Haggstrom J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 2002;43:568‐575. [DOI] [PubMed] [Google Scholar]
- 18. Wesselowski S, Borgarelli M, Bello NM, Abbott J. Discrepancies in the identification of left atrial enlargement using left atrial volume versus left atrial‐to‐aortic ratio in dogs. J Vet Intern Med. 2014;28:1527‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Josephson ME. Electrophysiologic investigation: general concepts In: Josephson's Clinical Cardiac Electrophysiology: Techniques and Interpretation. Baltimore: Wolters Kluwer; 2016:22‐69. [Google Scholar]
- 20. Kalahasty G, Wood MA. Ablation of posteroseptal accessory pathways In: Huang SK, Miller JM, eds. Catheter Ablation of Cardiac Arrhythmias. Philadelphia: Elsevier; Saunders; 2015:447‐460. [Google Scholar]
- 21. Shephard RK, Wood MA. Ablation of free wall accessory pathways In: Huang SK, Miller JM, eds. Catheter Ablation of Cardiac Arrhythmias. Philadelphia: Elsevier Saunders; 2015:421‐446. [Google Scholar]
- 22. Josephson ME. Supraventricular tachycardias In: Josephson's Clinical Cardiac Electrophysiology: Techniques and Interpretation. Baltimore: Wolters Kluwer; 2016:171‐280. [Google Scholar]
- 23. Radbill AE, Fish FA. Mapping and ablation of supraventricular tachycardia in pediatric and congenital heart disease patients. Prog Pediatric Cardiol. 2013;35:65‐77. [Google Scholar]
- 24. Macedo PG, Patel SM, Bisco SE, Asirvatham SJ. Septal accessory pathway: anatomy, causes for difficulty, and approach to ablation. Indian Pacing Electrophysiol J. 2010;10:292‐309. [PMC free article] [PubMed] [Google Scholar]
- 25. Wissner E, Ooyang F, Metzner A. Ablation of supraventricular tachyarrhythmias In: Zipes DP, Jalife J, eds. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia: Elsevier Saunders; 2014:1239‐1248. [Google Scholar]
- 26. Liang JJ, DeSimone CV, Lachman N. Cardiac anatomy for catheter mapping and ablation of arrhythmias In: Huang SK, Miller JM, eds. Catheter Ablation of Cardiac Arrhythmias. Philadelphia: Elsevier Saunders; 2015:85‐103. [Google Scholar]
- 27. Wolbrette D, Naccarelli G, Curtis A, et al. Gender differences in arrhythmias. Clin Cardiol. 2002;25:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tadros R, Ton A, Fiset C, Nattel S. Sex differences in cardiac electrophysiology and clinical arrhythmias: epidemiology, therapeutics, and mechanisms. Can J Cardiol. 2014;30:783‐792. [DOI] [PubMed] [Google Scholar]
- 29. Deneke T, Mugge A, Muller P, de Groot JR. Therapeutic implications of gender differences in supraventricular cardiac arrhythmias: lessons of life cannot be learned in a day. Expert Rev Cardiovasc Ther. 2009;7:879‐882. [DOI] [PubMed] [Google Scholar]
- 30. Birati E, Eldar M, Belhassen B. Gender differences in accessory connections location: an Israeli study. J Interv Card Electrophysiol. 2012;34:227‐229. [DOI] [PubMed] [Google Scholar]
- 31. Huang SK, Hu YF, Chang SL, et al. Gender differences of electrophysiologic characteristics in patients with accessory pathways. Heart Rhythm. 2011;8:571‐574. [DOI] [PubMed] [Google Scholar]
- 32. Hsu JC, Tanel RE, Lee BK, et al. Differences in accessory pathway location by sex and race. Heart Rhythm. 2010;7:52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferro CR, de Assis CF, Mendonca MA, et al. Correlation of accessory pathway location with gender and their manifest or concealed presentation. Int J Cardiol. 2016;216:43‐45. [DOI] [PubMed] [Google Scholar]
- 34. Santilli RA, Perego M, Crosara S, et al. Utility of 12‐lead electrocardiogram for differentiating paroxysmal supraventricular tachycardias in dogs. J Vet Inter Med. 2008;22:915‐923. [DOI] [PubMed] [Google Scholar]
- 35. Atlee JL III, Yeager TS. Electrophysiologic assessment of the effects of enflurane, halothane, and isoflurane on properties affecting supraventricular reentry in chronically instrumented dogs. Anesthesiology. 1989;71:941‐952. [DOI] [PubMed] [Google Scholar]
- 36. Atlee JL, III , Brownlee SW, Burstrom RE. Conscious state comparisons of the effects of inhalation anesthetics on specialized atrioventricular conduction times in dogs. Anesthesiology. 1986;64:703‐710. [DOI] [PubMed] [Google Scholar]
- 37. Chang RKR, Stevenson WG, Wetzel GT, et al. Effects of isoflurane on electrophysiological measurements in children with the Wolff‐Parkinson‐White syndrome. Pacing Clin Electro. 1996;19:1082‐1088. [DOI] [PubMed] [Google Scholar]
- 38. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an Accessory Pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17:1352‐1356. [DOI] [PubMed] [Google Scholar]
- 39. Ma L, Li Y, Wang Y, et al. Relationship between accessory pathway location and occurrence of atrial fibrillation in patients with atrioventricular reentrant tachycardia. Exp Clin Cardiol. 2004;9:196‐199. [PMC free article] [PubMed] [Google Scholar]
- 40. A Harahsheh, W Du, H Singh, P Karpawich. Risk factors for atrioventricular tachycardia degenerating to atrial flutter/fibrillation in the young with Wolff‐Parkinson‐White syndrome. Pacing Clin Electrophsyiol. 2008;31:1307‐1312. [DOI] [PubMed] [Google Scholar]
- 41. Bromberg BI, Lindsay BD, Cain ME, Cox JL. Impact of clinical history and electrophysiologic characterization of accessory pathways on management strategies to reduce sudden death among children with Wolff‐Parkinson‐White syndrome. J Am Coll Cardiol. 1996;27:690‐695. [DOI] [PubMed] [Google Scholar]
- 42. Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm. 2004;1:168‐173. [DOI] [PubMed] [Google Scholar]
- 43. Chen SA, Hsia CP, Chiang CE, et al. Reappraisal of radiofrequency catheter ablation of multiple accessory pathways. Am Heart J. 1993;125:760‐771. [DOI] [PubMed] [Google Scholar]
- 44. Iturralde P, Guevara‐Valdivia M, Rodriguez‐Chavez L, et al. Radiofrequency ablation of multiple accessory pathways. Europace. 2002;4:273‐280. [DOI] [PubMed] [Google Scholar]
- 45. Kugler JD, Danford DA, Deal BJ, et al. Radiofrequency catheter ablation for tachyarrhythmias in children and adolescents. N Engl J Med. 1994;330:1481‐1487. [DOI] [PubMed] [Google Scholar]
- 46. Kugler JD, Danford DA, Houston KA, Felix G. Pediatric radiofrequency catheter ablation registry success, fluoroscopy time, and complication rate for supraventricular tachycardia: comparison of early and recent eras. J Cardiovasc Electrophysiol. 2002;13:336‐341. [DOI] [PubMed] [Google Scholar]
- 47. Pappone C, Manguso F, Santinelli R, et al. Radiofrequency ablation in children with asymptomatic Wolff‐Parkinson‐White syndrome. N Engl J Med. 2004;351:1197‐1205. [DOI] [PubMed] [Google Scholar]
- 48. Weng KP, Wolff GS, Young ML. Multiple accessory pathways in pediatric patients with Wolff‐ Parkinson‐White syndrome. Am J Cardiol. 2003;91:1178‐1183. [DOI] [PubMed] [Google Scholar]
- 49. Yeh SJ, Wang CC, Wen MS, et al. Radiofrequency ablation in multiple accessory pathways and the physiologic implications. Am J Cardiol. 1993;71:1174‐1180. [DOI] [PubMed] [Google Scholar]
- 50. Arya A, Haghjoo M, Jafari A, et al. Effect of conduction mode and location on electrophysiologic characteristics of accessory pathways. Am J Cardiol. 2005;95:1250‐1252. [DOI] [PubMed] [Google Scholar]
- 51. Zachariah JP, Walsh EP, Triedman J, et al. Multiple accessory pathways in the young: the impact of structural heart disease. Am Heart J. 2013;165:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller JM. Therapy of Wolff‐Parkinson‐White syndrome and concealed bypass tracts: part I. J Cardiovasc Electrophysiol. 1996;7:85‐93. [DOI] [PubMed] [Google Scholar]
- 53. Tilley LP, FWK Smith. Electrocardiography In: Tilley LP, FWK Smith, Jr, Oyama MA, Sleeper MM, eds. Manual of Canine and Feline Cardiology. Philadelphia: Elsevier; 2008:49‐77. [Google Scholar]
- 54. Calo L, De Ruvo E, Sette A, et al. Tachycardia‐induced cardiomyopathy: mechanisms of heart failure and clinical implications. J Cardiovasc Med. 2007;8:138‐143. [DOI] [PubMed] [Google Scholar]
- 55. Whipple GH, Sheffield LT, Woodman EG, et al. Reversible congestive heart failure due to chronic rapid stimulation of the normal heart. Proc N Engl Cardiovasc Soc. 1962;20:39‐40. [Google Scholar]
- 56. Umana E, Solares CA, Alpert MA. Tachycardia‐induced cardiomyopathy. Am J Med. 2003;114:51‐55. [DOI] [PubMed] [Google Scholar]
- 57. Deshmukh PM, Krishnamani R, Romanyshyn M. Association of angiotensin converting enzyme gene polymorphism with tachycardia cardiomyopathy. Int J Mol Med. 2004;13:455‐458. [PubMed] [Google Scholar]
- 58. Shinbane JS, Wood MA, Jensen DN, et al. Tachycardia‐induced cardiomyopathy: a review of animal models and clinical study. J Am Coll Cardiol. 1997;29:709‐715. [DOI] [PubMed] [Google Scholar]
- 59. Page RL, Joglar JA, Caldwell MA, et al. 2015. ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia. J Am Coll Cardiol. 2016;67:e27‐e115. [DOI] [PubMed] [Google Scholar]
- 60. Spector P, Reynolds MR, Calkins H, et al. Meta‐analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol. 2009;104:671‐677. [DOI] [PubMed] [Google Scholar]
- 61. Calkins H, Yong P, Miller JM, et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation. 1999;99:262‐270. [DOI] [PubMed] [Google Scholar]
- 62. Pappone C, Vicedomini G, Manguso F, et al. Wolff‐Parkinson‐White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130:811‐819. [DOI] [PubMed] [Google Scholar]
- 63. Jackman WM, Wang XZ, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff‐ Parkinson‐White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605‐1611. [DOI] [PubMed] [Google Scholar]
- 64. Calkins H, Langberg J, Sousa J, et al. Radiofrequency catheter ablation of accessory atrioventricular connections in 250 patients. Abbreviated therapeutic approach to Wolff‐Parkinson‐White syndrome. Circulation. 1992;85:1337‐1346. [DOI] [PubMed] [Google Scholar]
- 65. Dagres N, Clague JR, Kottkamp H, et al. Radiofrequency catheter ablation of accessory pathways. Outcome and use of antiarrhythmic drugs during follow‐up. Eur Heart J. 1999;20:1826‐1832. [DOI] [PubMed] [Google Scholar]
- 66. Schläpfer J, Fromer M. Late clinical outcome after successful radiofrequency catheter ablation of accessory pathways. Eur Heart J. 2001;22:605‐609. [DOI] [PubMed] [Google Scholar]
- 67. Belhassen B, Rogowski O, Glick A, et al. Radiofrequency ablation of accessory pathways: a 14 year experience at the Tel Aviv Medical Center in 508 patients. Isr Med Assoc J. 2007;9:265‐270. [PubMed] [Google Scholar]
- 68. Kugler JD, Danford DA, Houston K, et al. Radiofrequency catheter ablation for paroxysmal supraventricular tachycardia in children and adolescents without structural heart disease. Pediatric EP Society, Radiofrequency Catheter Ablation Registry. Am J Cardiol. 1997;80:1438‐1443. [DOI] [PubMed] [Google Scholar]
- 69. Telishevska M, Faelchle J, Buiatti A, et al. Irrigated tip catheters for radiofrequency ablation of right‐sided accessory pathways in adolescents. Pacing Clin Electrophysiol. 2017;40:1167‐1172. [DOI] [PubMed] [Google Scholar]


