Abstract
Background
Primary‐care veterinary clinical records can offer data to determine generalizable epidemiological data on seizures occurrence in the dog population.
Objectives
To identify and examine epidemiologic characteristics of seizure occurrence in dogs under primary veterinary care in the UK participating in the VetCompass™ Programme.
Animals
455,553 dogs in VetCompass™’.
Methods
A cross‐sectional analysis estimated the 1‐year period prevalence and risk factors for dogs with seizures during 2013.
Results
The overall 1‐year period prevalence for dogs having at least one seizure during 2013 was 0.82% (95% CI 0.79‐0.84). Multivariable modelling identified breeds with elevated odd ratios [OR] compared with the Labrador Retriever (e.g. Pug OR: 3.41 95% CI 2.71‐4.28, P < 0.001). Males had higher risk for seizures (Male/Entire OR: 1.47 95% CI 1.30–1.66; Male/Neutered OR: 1.34 95% CI 1.19–1.51) compared to entire females. Age (3.00 ‐ ≤ 6.00 OR: 2.13 95% CI 1.90‐2.39, P < 0.001, compared to animals aged 0.50–≤ 3.00 years), and bodyweight (≥ 40.00kg, OR: 1.24 95% CI 1.08–1.41, P = 0.002, compared to animals weighing < 10.0 kg) were identified as risk factors for seizures.
Conclusion and clinical Importance
Seizures are a relatively common clinical finding in dogs. The results for breed, age, sex and bodyweight as risk factors can assist veterinarians in refining differential diagnosis lists for dogs reported with behaviors that may have been seizures. In addition, the prevalence values reported here can support pharmacovigilance with baseline data from the overall population.
Keywords: Canine, Epilepsy, Convulsion, Frequency, First‐opinion, Breed
Abbreviations
- CI
confidence interval
- EPR
electronic patient record
- IQR
interquartile range
- OR
odds ratio
- PMS
practice management system
- ROC
receiver operating characteristic
- RVC
Royal Veterinary College
- UK
United Kingdom
- CNS
central nervous system
- DYAR
dog years at risk
- IVETF
International Veterinary Epilepsy Task Force
1. INTRODUCTION
Seizures in dogs are sudden, short lasting and transient events that are characterized by motor, autonomic or behavioral features.1 They are considered common events in dogs.2 Causes including genetic and insult‐triggered brain diseases, peripheral diseases, electrolyte imbalances, intoxications and idiopathic.3 Seizure activity can have substantial impact on quality of life3, 4, 5 as well as causing emotional distress for owners.6, 7 To date, most studies have focused on the subset of cases that are classified as epileptic.8, 9
Seizures are either epileptic or reactive according to the classification of the IVETF. Epileptic seizures are manifestations of excessive synchronous neuronal activity in the brain that is usually self‐limiting and that are promoted by a predisposition of the brain for ictogenesis.1, 10 Epilepsy is usually defined as requiring at least two unprovoked epileptic seizures more than 24 hours apart11 and reactive seizures (also called provoked seizures) are considered as natural responses from the normal brain to some transient disturbance in function (e.g. metabolic or toxic) which is reversible when the cause or disturbance is rectified.1, 3 Analysis of a large pet insurance database reported an epilepsy incidence of 18 per 10,000 dog years at risk (DYAR) in Sweden for either idiopathic epilepsy or epileptic convulsions.8 A study using primary‐care veterinary clinical data reported a prevalence of 0.62% for epilepsy in dogs in the UK.9 A cross sectional study compared period prevalence of epileptic seizures in a 2.5‐year observation period between first opinion practices (0.43%) and a referral clinic in southern Germany (1.78%).12 Among the referral subset of dogs seen at a veterinary teaching hospital in Germany, 2.6% had a history of seizures reported.13 Epidemiological studies using first opinion databases that explore seizures in general rather than relying on semi‐arbitrary classification into various subsets might offer novel and more clinically pertinent insights into this disease in dogs. There are breed predispositions to various forms of epilepsy, but predisposition studies often fail to describe the prevalence of seizures either in dogs overall and in individual breeds.
This study aimed to identify and explore seizure occurrence in the general dog population under primary veterinary care in the UK. The specific objectives were to estimate the 1‐year period prevalence for dogs that showed seizures and to also identify demographic risk factors associated with dogs that showed seizures, with breed as a risk factor of special interest. These results could be especially valuable for practitioners, insurance companies, pharmaceutical and nutrition manufacturers and pharmacovigilance agencies by providing a representative evidence‐base that can be generalized to the wider dog population and fill the evidential data gap on seizure occurrence.
2. MATERIALS AND METHODS
This study was based on data collected within the VetCompass™ Animal Surveillance System 14 at the Royal Veterinary College (RVC). VetCompass™ collates electronic patient record (EPR) data from hundreds of UK primary‐care practices for epidemiological research. Demographic information collected included species, breed, date of birth, sex and neuter status, while clinical information includes free text notes, treatment data as well as summarized diagnosis terms from the VeNom Codes standardized lists.15 Clinical data from participating practices are extracted from practice management systems (PMSs) using automated queries, uploaded securely to the RVC server and reformatted for entry into the VetCompass™ online database system.16
A cross‐sectional analysis using cohort clinical data of dogs attending VetCompass™ practices was used to estimate the 1‐year period prevalence and risk factors for dogs with at least one seizure during 2013.17 The denominator population for the current study comprised all dogs that had at least one EPR (clinical note, bodyweight or treatment) recorded in VetCompass™ during 2013 or dogs that had ‘at least one EPR before and one EPR after 2013’. These dogs were defined as being under veterinary care during 2013.
Sample size calculations estimated that a study with 149,698 dogs would be required to represent a disorder with 1% expected prevalence to a precision of 0.05% at a 95% confidence level from an estimated UK national population of 9.4 million dogs.18, 19 Ethical approval was granted by the RVC Ethics and Welfare Committee (reference number 2015 1369).
A two‐step letter‐string search process was used to identify seizure cases in the VetCompass™ dog population. In step one, all EPRs were initially screened for candidate seizure cases using a range of letter‐string search terms including seiz*, siez*, convul*, tonic*, clonic*, myoclo*, “had fit”, “had a fit”, epil*, diaz*, clonaz*, midazol*, phenobarb*, gabapen*, levetira*, bromide, pregaba*, zonis*, imepit*, pexi*, keppr*, hypnov*, epiph*, phenolep*, librom*, lyric*, alpraz* and zoneg*. In step two, the EPRs of all candidate cases which had been identified as possible seizure cases were manually read and evaluated for inclusion as a seizure case according to the study case definition. A seizure case was defined as any dog with at least one seizure event occurring during 2013 recorded in the EPR. All dogs with reported seizure activity in 2013 were grouped as seizure cases and all remaining dogs were grouped as non‐cases.
A “Purebred “variable classified all dogs of a recognizable breed as” purebred”, all dogs recorded with a designer breed name as “designer” 20 and all remaining dogs as “crossbred” . A “Breeds” variable included the breed name of any individual purebred or designer breed types with 15 or more seizure cases during 2013 while all remaining purebred and designer types were grouped as “Other purebreds and designers breed types” and a general category of crossbred dogs was also included. A “Kennel Club Breed Group” variable defined all KC recognized breeds according to their relevant UK KC breed groups (Gundog, Terrier, Utility, Hound, Working, Toy and Pastoral) and included all remaining dogs as “Breed not‐Kennel Club recognized”. The age for all dogs described the age at 31st December 2013. An age variable “Age” contained 7 age categories in years (0.00 ‐ ≤ 0.50, 0.50 ‐ ≤ 3.00, 3.00 ‐ ≤ 6.00, 6.00 ‐ ≤ 9.00, 9.00 ‐ ≤ 12.00, ≥ 12.00, unrecorded) according to the IVETF guidelines.21 The “Neuter status” variable recorded the neutering status recorded at the final EPR. Sex and neuter status were also combined into a single variable (Sex‐neuter) that reported the results across seven permutational categories. The variable “Adult (> 18 months) bodyweight” described the maximum bodyweight recorded during the study period for dogs older than 18 months and categorized adult bodyweight into 6 groups (< 10.00 kg, 10.00 ‐ ≤ 20.00 kg, 20.00 ‐ ≤ 30.00 kg, 30.00 ‐ ≤ 40.00 kg, ≥ 40.00 kg, unrecorded). A “Bodyweight relative to breed and sex mean” variable characterized the adult bodyweight of individual dogs as either below or equal/above the mean adult bodyweight for their breed and sex within the overall study population. This variable allowed the effect of adult bodyweight to be assessed within each breed/sex combination.
After data checking and cleaning in Excel (Microsoft Office Excel 2013, Microsoft Corp.), statistical analyses were conducted with IBM SPSS Statistics 24®. The 1‐year period prevalence with 95% confidence intervals (CI) described the probability for each dog of having at least one seizure at any time during the 1‐year 2013 study period. The CI estimates were derived from standard errors, based on approximation to the normal distribution.22 Descriptive statistics characterized the count and proportions of cases and non‐case dogs for each of the study variables. Binary logistic regression modelling was used to evaluate univariable associations between risk factors and being a seizure case. Because breed was a factor of primary interest for the study, purebred status and Kennel Club Breed group (highly correlated with breed), and adult (>18months) bodyweight (a defining characteristic of individual breeds) were excluded from the initial breed multivariable modelling. Instead, each of these variables individually replaced the breed variable in the main final breed model in order to evaluate their effects after taking account of the other variables and these results were reported. Risk factors with liberal associations in univariable modelling (P < 0.2) were taken forward for multivariable logistic regression modelling evaluation. Model development used automated backwards stepwise elimination. The area under the ROC curve was used to assess the quality of the model fit. Statistical significance was set at P < 0.05.
3. RESULTS
The study population comprised 455,553 dogs attending primary care practices in the UK participating in VetCompass™. Initial screening identified 10,584 candidate cases. After manual review, 3,731 (35.25%) dogs met the seizure inclusion criteria giving an overall 1‐year period prevalence of 0.82% (95% CI 0.79 ‐ 0.84) for dogs having at least one seizure. Individual breeds with the highest seizure prevalence included Pug (1.88% of the breed affected, 95% CI 1.52 ‐ 2.24), Boxer (1.77%, 95% CI 1.44 ‐ 2.09), Basset Hound (1.74%, 95% CI 1.02 ‐ 2.46), Border Terrier (1.67%, 95% CI 1.33 ‐ 2.01) and Border Collie (1.45%, 95% CI 1.24 ‐ 1.66) (Table 1).
Table 1.
Prevalence of seizures in commonly affected dog breeds
| Breed | No. Cases | No. Dogs in study | Median age in years of cases(IQR) | Prevalence % | 95% CIa |
|---|---|---|---|---|---|
| Pug | 101 | 5376 | 4.95 (2.76‐6.99) | 1.88 | 1.52‐2.24 |
| Boxer | 111 | 6288 | 9.52 (6.95‐11.04) | 1.77 | 1.44‐2.09 |
| Basset Hound | 22 | 1263 | 6.22 (3.19‐9.36) | 1.74 | 1.02‐2.46 |
| Border Terrier | 91 | 5449 | 7.06 (4.85‐11.35) | 1.67 | 1.33‐2.01 |
| Border Collie | 178 | 12268 | 6.43 (4.18‐12.18) | 1.45 | 1.24‐1.66 |
| Beagle | 48 | 3508 | 5.71 (5.46‐7.35) | 1.37 | 0.98‐1.75 |
| King Charles Spaniel | 21 | 1666 | 5.18 (3.03‐11.33) | 1.26 | 0.72‐1.80 |
| Dogue de Bordeaux | 23 | 1858 | 3.76 (2.05‐5.91) | 1.24 | 0.73‐1.74 |
| British Bulldog | 39 | 3374 | 2.54 (1.58‐4.46) | 1.16 | 0.80‐1.52 |
| Weimaraner | 18 | 1568 | 8.30 (6.90‐10.73) | 1.15 | 0.62‐1.68 |
| Yorkshire Terrier | 178 | 15426 | 8.23 (5.33‐12.44) | 1.15 | 0.99‐1.32 |
| Cavalier King Charles Spaniel | 112 | 10143 | 6.95 (4.91‐9.20) | 1.10 | 0.90‐1.31 |
| Patterdale Terrier | 26 | 2433 | 5.58 (3.35‐8.31) | 1.07 | 0.66‐1.48 |
| Pomeranian | 22 | 2147 | 4.95 (1.81‐6.97) | 1.02 | 0.60‐1.45 |
| Labrador Retriever | 323 | 33321 | 7.21 (4.48‐9.91) | 0.97 | 0.87‐1.06 |
| Toy Poodle | 18 | 1881 | 7.60 (4.06‐12.83) | 0.69 | 0.52‐1.40 |
| Golden Retriever | 54 | 5670 | 8.33 (5.02‐12.40) | 0.95 | 0.70‐1.21 |
| German Shepherd Dog | 116 | 12520 | 8.59 (5.35‐10.98) | 0.93 | 0.76‐1.09 |
| Chihuahua | 101 | 11782 | 4.04 (1.85‐6.67) | 0.86 | 0.69‐1.02 |
| Akita | 17 | 2105 | 7.36 (3.45‐13.32) | 0.81 | 0.42‐1.19 |
| Miniature Schnauzer | 31 | 3857 | 6.30 (3.04‐10.66) | 0.80 | 0.52‐1.09 |
| French Bulldog | 19 | 2397 | 2.18 (1.56‐6.00) | 0.79 | 0.44‐1.15 |
| Jack Russell Terrier | 218 | 27691 | 8.59 (5.39‐12.83) | 0.79 | 0.68‐0.89 |
| Labradoodle | 23 | 3132 | 4.47 (3.73‐6.80) | 0.73 | 0.44‐1.03 |
| Miniature Dachshhund | 20 | 2792 | 5.64 (4.07‐8.14) | 0.72 | 0.40‐1.03 |
| Staffordshire Bull Terrier | 228 | 32635 | 7.97 (4.40‐10.97) | 0.70 | 0.61‐0.79 |
| Crossbred | 681 | 98931 | 8.23 (4.57‐12.71) | 0.96 | 0.64‐0.74 |
| Lurcher | 22 | 3222 | 5.78 (3.17‐11.32) | 0.68 | 0.40‐0.97 |
| Husky | 28 | 4162 | 4.55 (1.66‐5.91) | 0.67 | 0.42‐0.92 |
| West Highland White Terrier | 73 | 12017 | 10.94 (5.78‐13.83) | 0.61 | 0.47‐0.75 |
| Springer Spaniel | 35 | 5800 | 10.04 (5.71‐12.78) | 0.60 | 0.40‐0.80 |
| Lhasa Apso | 38 | 6840 | 8.80 (4.75‐12.91) | 0.56 | 0.38‐0.73 |
| Bichon | 36 | 6607 | 8.67 (3.84‐12.63) | 0.54 | 0.37‐0.72 |
| English Springer Spaniel | 29 | 5384 | 7.86 (3.92‐11.71) | 0.54 | 0.34‐0.73 |
| Unrecorded | 10 | 2009 | 9.18 (4.58‐14.76) | 0.50 | 0.19‐0.81 |
| Rottweiler | 24 | 5321 | 6.34 (4.91‐10.69) | 0.45 | 0.27‐0.63 |
| Designer | 33 | 7492 | 3.11 (1.89‐4.98) | 0.44 | 0.29‐0.59 |
| Cocker Spaniel | 62 | 15827 | 8.08 (4.72‐11.15) | 0.39 | 0.29‐0.49 |
| Shih‐tzu | 57 | 15038 | 8.24 (4.75‐13.56) | 0.38 | 0.28‐0.48 |
| Other purebreds and designers breed types | 445 | 54353 | 7.32 (4.19‐10.90) | 0.82 | 0.74‐0.89 |
| Overall total | 3731 | 455553 | 7.27 (4.23‐11.07) | 0.82 | 0.79‐0.84 |
One‐year period prevalence of seizures in commonly affected dog breeds under primary veterinary care in the UK; aCI confidence interval.
The most common breeds among the non‐case dogs were the Labrador Retriever 32,998 (7.3%), Staffordshire Bull Terrier 32,407 (7.2%), Jack Russell Terrier 27,473 (6.1%), Cocker Spaniel 15,765 (3.5%), Yorkshire Terrier 15,248 (3.4%) as well as 100,249 (22.2%) crossbreds and 13,768 (3.0%) designers (Table 2). Of the non‐case dogs, 337,805 (74.8%) were purebred and 232,080 (51.4%) were male. Of the males, 91,692 (20.3%) were entire and 102,968 (22.8%) were neutered (neuter status was not available for the remainer). The median adult bodyweight for non‐cases was 16.60 kg (interquartile range [IQR]: 9.00 ‐ 28.00, range: 0.30 ‐ 112.00) and the median age was 4.09 years (IQR: 1.70 ‐ 7.58, range: 0.00 ‐ 24.68) (Fig 1).
Table 2.
Descriptive and univariable regression results
| Variable | Category | Case No. (%) | Non‐case No. (%) | Odds ratio | 95% CIa | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|---|---|
| Purebred | Crossbred | 681 (18.3) | 98250 (21.7) | Base | < 0.001 | ||
| Designer | 78 (2.1) | 13768 (3.0) | 0.82 | 0.65‐1.03 | 0.092 | ||
| Purebred | 2962 (79.4) | 337805 (74.8) | 1.27 | 1.16‐1.38 | < 0.001 | ||
| Unrecorded | 10 (0.3) | 1999 (0.4) | 0.72 | 0.39‐1.35 | 0.31 | ||
| Breeds | Labrador Retriever | 323 (8.7) | 32998 (7.3) | Base | < 0.001 | < 0.001 | |
| Pug | 101 (2.7) | 5275 (1.2) | 1.96 | 1.56‐2.45 | < 0.001 | ||
| Boxer | 111 (3.0) | 6177 (1.4) | 1.84 | 1.48‐2.28 | < 0.001 | ||
| Basset Hound | 22 (0.6) | 1241 (0.3) | 1.81 | 1.17‐2.80 | 0.008 | ||
| Border Terrier | 91 (2.4) | 5358 (1.2) | 1.74 | 1.37‐2.19 | < 0.001 | ||
| Border Collie | 178 (4.8) | 12090 (2.7) | 1.50 | 1.25‐1.81 | < 0.001 | ||
| Beagle | 48 (1.3) | 3460 (0.8) | 1.42 | 1.04‐1.92 | 0.025 | ||
| King Charles Spaniel | 21 (0.6) | 1645 (0.4) | 1.30 | 0.84‐2.03 | 0.24 | ||
| Dogue de Bordeaux | 23 (0.6) | 1835 (0.4) | 1.28 | 0.84‐1.96 | 0.26 | ||
| British Bulldog | 39 (1.0) | 3335 (0.7) | 1.19 | 0.86‐1.67 | 0.30 | ||
| Yorkshire Terrier | 178 (4.8) | 15248 (3.4) | 1.19 | 0.99‐1.43 | 0.061 | ||
| Weimaraner | 18 (0.5) | 1550 (0.3) | 1.19 | 0.74‐1.91 | 0.48 | ||
| Cavalier King Charles Spaniel | 112 (3.0) | 10031 (2.2) | 1.14 | 0.92‐1.42 | 0.23 | ||
| Patterdale Terrier | 26 (0.7) | 2407 (0.5) | 1.10 | 0.74‐1.65 | 0.63 | ||
| Pomeranian | 22 (0.6) | 2125 (0.5) | 1.06 | 0.69‐1.63 | 0.80 | ||
| Toy Poodle | 18 (0.5) | 1863 (0.4) | 0.99 | 0.61‐1.59 | 0.96 | ||
| Golden Retriever | 54 (1.4) | 5616 (1.2) | 0.98 | 0.74‐1.31 | 0.90 | ||
| German Shepherd Dog | 116 (3.1) | 12404 (2.7) | 0.96 | 0.77‐1.18 | 0.68 | ||
| Chihuahua | 101 (2.7) | 11681 (2.6) | 0.88 | 0.71‐1.11 | 0.28 | ||
| Akita | 17 (0.5) | 2088 (0.5) | 0.83 | 0.51‐1.36 | 0.46 | ||
| Miniature Schnauzer | 31 (0.8) | 3826 (0.8) | 0.83 | 0.57‐1.20 | 0.32 | ||
| French Bulldog | 19 (0.5) | 2378 (0.5) | 0.82 | 0.51‐1.30 | 0.39 | ||
| Jack Russell Terrier | 218 (5.8) | 27473 (6.1) | 0.81 | 0.68‐0.96 | 0.017 | ||
| Labradoodle (Designer) | 23 (0.6) | 3109 (0.7) | 0.76 | 0.49‐1.16 | 0.20 | ||
| Miniature Dachshund | 20 (0.5) | 2772 (0.6) | 0.74 | 0.47‐1.16 | 0.19 | ||
| Staffordshire Bull Terrier | 228 (6.1) | 32407 (7.2) | 0.72 | 0.61‐0.85 | < 0.001 | ||
| Crossbred | 681 (18.3) | 98250 (21.7) | 0.71 | 0.62‐0.81 | < 0.001 | ||
| Lurcher (Designer) | 22 (0.6) | 3200 (0.7) | 0.70 | 0.46‐1.08 | 0.11 | ||
| Husky | 28 (0.8) | 4134 (0.9) | 0.69 | 0.47‐1.02 | 0.063 | ||
| West Highland White Terrier | 73 (2.0) | 11944 (2.6) | 0.62 | 0.48‐0.81 | < 0.001 | ||
| Springer Spaniel | 35 (0.9) | 5765 (1.3) | 0.62 | 0.44‐0.88 | 0.007 | ||
| Lhasa Apso | 38 (1.0) | 6802 (1.5) | 0.57 | 0.41‐0.80 | 0.001 | ||
| Bichon | 36 (1.0) | 6571 (1.5) | 0.56 | 0.40‐0.79 | 0.001 | ||
| English Springer Spaniel | 29 (0.8) | 5355 (1.2) | 0.55 | 0.38‐0.81 | 0.002 | ||
| Rottweiler | 24 (0.6) | 5297 (1.2) | 0.46 | 0.31‐0.70 | < 0.001 | ||
| Designer | 33 (0.9) | 7459 (1.6) | 0.45 | 0.32‐0.65 | < 0.001 | ||
| Cocker Spaniel | 62 (1.7) | 15765 (3.5) | 0.40 | 0.31‐0.53 | < 0.001 | ||
| Shih‐tzu | 57 (1.5) | 14981 (3.3) | 0.39 | 0.29‐0.52 | < 0.001 | ||
| Unrecorded | 10 (0.3) | 1999 (0.4) | 0.51 | 0.27‐0.96 | 0.037 | ||
| Other purebreds and designers breed types | 445 (11.9) | 53908 (11.9) | 0.84 | 0.73‐0.97 | 0.02 | ||
| Kennel Club Breed Groups | Breed not Kennel Club recognized | 1074 (28.8) | 151937 (33.6) | Base | < 0.001 | ||
| Gundog | 589 (15.8) | 72359 (16.0) | 1.15 | 1.04‐1.27 | 0.006 | ||
| Hound | 139 (3.7) | 15832 (3.5) | 1.23 | 1.03‐1.47 | 0.021 | ||
| Pastoral | 331 (8.9) | 29374 (6.5) | 1.59 | 1.41‐1.80 | < 0.001 | ||
| Terrier | 462 (12.4) | 58643 (13.0) | 1.12 | 0.99‐1.24 | 0.052 | ||
| Toy | 617 (16.5) | 56704 (12.6) | 1.54 | 1.39‐1.70 | < 0.001 | ||
| Utility | 307 (8.2) | 44855 (9.9) | 0.97 | 0.85‐1.10 | 0.62 | ||
| Working | 212 (5.7) | 22118 (4.9) | 1.36 | 1.17‐1.57 | < 0.001 | ||
| Adult (>18months) bodyweight (kg) | < 10.00 | 878 (23.5) | 98359 (21.8) | Base | < 0.001 | < 0.001 | |
| 10.00 ‐ ≤ 20.00 | 830 (22.3) | 91219 (20.2) | 1.02 | 0.93‐1.12 | 0.69 | ||
| 20.00 ‐ ≤ 30.00 | 641 (17.2) | 67845 (15.0) | 1.06 | 0.95‐1.17 | 0.29 | ||
| 30.00 ‐ ≤ 40.00 | 526 (14.1) | 46004 (10.2) | 1.28 | 1.15‐1.43 | < 0.001 | ||
| ≥ 40.00 | 313 (8.4) | 24860 (5.5) | 1.41 | 1.24‐1.61 | < 0.001 | ||
| Unrecorded | 543 (14.6) | 123535 (27.2) | 0.49 | 0.44‐0.55 | < 0.001 | ||
| Bodyweight relative to breed and sex mean | Lower | 1636 (43.9) | 182105 (40.3) | Base | < 0.001 | ||
| Equal/Higher | 1550 (41.5) | 145848 (32.4) | 1.18 | 1.10‐1.27 | < 0.001 | ||
| Unrecorded | 545 (14.6) | 123869 (27.3) | 0.49 | 0.44‐0.54 | < 0.001 | ||
| Age (years) | 0.00 ‐ ≤ 0.50 | 25 (0.7) | 25508 (5.6) | 0.28 | 0.19‐0.42 | < 0.001 | < 0.001 |
| 0.50 ‐ ≤ 3.00 | 522 (14.0) | 148813 (32.9) | Base | ||||
| 3.00 ‐ ≤ 6.00 | 923 (24.7) | 114584 (25.4) | 2.30 | 2.06‐2.56 | < 0.001 | ||
| 6.00 ‐ ≤ 9.00 | 832 (22.3) | 76360 (16.9) | 3.10 | 2.78‐3.46 | < 0.001 | ||
| 9.00 ‐ ≤ 12.00 | 636 (17.1) | 47228 (10.5) | 3.84 | 3.42‐4.31 | < 0.001 | ||
| ≥ 12.00 | 767 (20.6) | 33180 (7.3) | 6.59 | 5.89‐7.37 | < 0.001 | ||
| Unrecorded | 26 (0.7) | 6149 (1.4) | 1.21 | 0.81‐1.79 | 0.35 | ||
| Sex | Female | 1592 (42.7) | 217440 (48.1) | Base | < 0.001 | ||
| Male | 2131 (57.1) | 232080 (51.4) | 1.25 | 1.17‐1.34 | < 0.001 | ||
| Not recorded | 8 (0.2) | 2302 (0.5) | 0.48 | 0.24‐0.95 | 0.036 | ||
| Neuter status | Entire | 1150 (30.8) | 177066 (39.1) | Base | < 0.001 | ||
| Neutered | 1767 (47.3) | 203252 (45.0) | 1.34 | 1.24‐1.44 | < 0.001 | ||
| Unrecorded | 814 (21.8) | 71504 (15.9) | 1.75 | 1.60‐1.92 | < 0.001 | ||
| Sex‐neuter | Female/Entire | 412 (11.0) | 83498 (18.5) | Base | < 0.001 | ||
| Female/Neutered | 832 (22.3) | 100164 (22.2) | 1.68 | 1.50‐1.90 | < 0.001 | ||
| Female/Unrecorded | 348 (9.3) | 33778 (7.5) | 2.09 | 1.81‐2.41 | < 0.001 | ||
| Male/Entire | 731 (19.6) | 91692 (20.3) | 1.62 | 1.43‐1.82 | < 0.001 | ||
| Male/Neutered | 934 (25.0) | 102968 (22.8) | 1.84 | 1.64‐2.06 | < 0.001 | ||
| Male/Unrecorded | 466 (12.5) | 37420 (8.3) | 2.52 | 2.21‐2.88 | < 0.001 | ||
| Unrecorded/Unrecorded | 8 (0.2) | 2302(0.5) | 0.70 | 0.35‐1.42 | 0.33 |
Descriptive and univariable regression results for risk factors associated with seizure events in dogs under veterinary primary‐care in the UK. Percentages shown in brackets aCI confidence interval.
Figure 1.
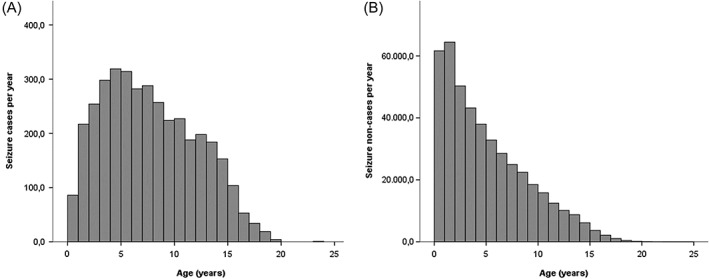
A: Seizure cases per year (n = 3,704) in the UK dog population under primary veterinary care B: Seizure non‐cases per year (n = 445,674) in the UK dog population under primary veterinary care
Of the seizure cases, 2,962 (79.4%) were purebred, 2,131 (57.1%) were male. Of the males 731 (19.6%) were entire and 934 (25.0%) were neutered (neuter status was not available for the remainer). Across both sexes combined, 1,767 (47.3%) dogs were neutered. Dogs with seizures had a median adult bodyweight of 18.20 kg (IQR: 9.30 ‐ 30.45, range: 0.80 ‐ 86.90) and median age of 7.27 years (IQR: 4.23 ‐ 11.07, range: 0.11 ‐ 23.23) (Fig 1). The most common breeds among the seizure cases were Labrador Retriever (323/3,731 cases, 8.7% of all cases), Staffordshire Bull Terrier (228/3,731, 6.1%), Jack Russell Terrier (218/3,731, 5.8%), Border Collie (178/3,731, 4.8%) and Yorkshire Terrier (178/3,731, 4.8%) as well as crossbreds (681/3,731, 18.3%) and designer dogs (78/3,731, 2.1%), (Table 2).
Data completeness varied between the variables assessed: breed 100.0%, age 98.6%, sex 99.5%, bodyweight (> 18 months) 72.8% and neuter 84.1%. Univariable logistic regression modelling identified five variables that were liberally associated with seizures and were further evaluated in the main multivariable logistic regression modelling: breed, age, bodyweight relative to breed and sex mean, neuter status, sex and sex‐neuter (Table 2). The final breed multivariable logistic regression model showed good discrimination (area under the ROC curve: 0.714). After accounting for the effects of the other variables evaluated, 11 breed types had significantly increased odds of seizures compared with Labrador Retriever dogs. The breeds with the highest odd ratios (OR) included the Pug (OR: 3.41 95% CI 2.71 ‐ 4.28, P < 0.001), Basset Hound (OR: 2.13 95% CI 1.38 ‐ 3.30, P < 0.001), Dogue de Bordeaux (OR: 2.10 95% CI 1.37 ‐ 3.22, P < 0.001), Boxer (OR: 1.95 95% CI 1.57 ‐ 2.42, P < 0.001) and Beagle (OR: 1.91 95% CI 1.40 ‐ 2.59, P < 0.001). Breeds with a significant lower OR of seizures in comparison to Labrador Retrievers were the Shih‐tzu (OR: 0.53, 95% CI 0.40 ‐ 0.70, P < 0.001), West Highland White Terrier (OR: 0.52, 95% CI 0.40 ‐ 0.67, P < 0.001), English Springer Spaniel (OR: 0.50, 95% CI 0.34 ‐ 0.74, P < 0.001) and Cocker Spaniel (OR: 0.44, 95% CI 0.34 ‐ 0.58, P < 0.001). Crossbred dogs presented as well significantly reduced odds for seizures in comparison to Labrador Retriever (OR: 0.75 95% CI 0.66 ‐ 0.86 P < 0.001). Compared with dogs aged between 0.50 ‐ ≤ 3.00 years, dogs aged 3.00 ‐ ≤ 6.00 years had 2.13 times the odds (95% CI 1.90 ‐ 2.39, P < 0.001) for seizures. Dogs younger than 0.50 years had reduced odds (OR: 0.30 95% CI 0.20 ‐ 0.45, P < 0.001) for seizures. The results showed a general trend towards increasing odds with increasing age. Males, regardless of neuter status had higher odds of seizures compared with entire females (Table 3).
Table 3.
Final breed multivariable logistic regression results
| Variable | Odds ratio | 95%CIa | p‐value | Variable P‐value | |
|---|---|---|---|---|---|
| Breeds | Labrador Retriever | Base | < 0.001 | ||
| Pug | 3.41 | 2.71‐4.28 | < 0.001 | ||
| Basset Hound | 2.13 | 1.38‐3.30 | 0.001 | ||
| Dogue de Bordeaux | 2.10 | 1.37‐3.22 | 0.001 | ||
| Boxer | 1.95 | 1.57‐2.42 | < 0.001 | ||
| Beagle | 1.91 | 1.40‐2.59 | < 0.001 | ||
| French Bulldog | 1.87 | 1.17‐2.98 | 0.009 | ||
| British Bulldog | 1.84 | 1.31‐2.58 | < 0.001 | ||
| Border Terrier | 1.79 | 1.42‐2.27 | < 0.001 | ||
| Pomeranian | 1.57 | 1.01‐2.43 | 0.043 | ||
| Chihuahua | 1.50 | 1.20‐1.89 | < 0.001 | ||
| Border Collie | 1.41 | 1.17‐1.69 | < 0.001 | ||
| King Charles Spaniel | 1.39 | 0.89‐2.17 | 0.15 | ||
| Patterdale Terrier | 1.26 | 0.84‐1.88 | 0.27 | ||
| Cavalier King Charles Spaniel | 1.22 | 0.98‐1.52 | 0.070 | ||
| Yorkshire Terrier | 1.14 | 0.95‐1.37 | 0.16 | ||
| Labradoodle | 1.13 | 0.74‐1.74 | 0.56 | ||
| Weimaraner | 1.13 | 0.70‐1.82 | 0.62 | ||
| Akita | 1.11 | 0.68‐1.81 | 0.68 | ||
| Toy Poodle | 1.06 | 0.66‐1.72 | 0.80 | ||
| Husky | 1.05 | 0.71‐1.55 | 0.81 | ||
| German Shepherd Dog | 1.02 | 0.82‐1.26 | 0.88 | ||
| Designer | 1.00 | 0.70‐1.44 | 0.99 | ||
| Miniature Schnauzer | 0.97 | 0.67‐1.40 | 0.854 | ||
| Miniature Dachshund | 0.83 | 0.53‐1.31 | 0.435 | ||
| Golden Retriever | 0.83 | 0.62‐1.11 | 0.215 | ||
| Staffordshire Bull Terrier | 0.81 | 0.68‐0.96 | 0.016 | ||
| Jack Russell Terrier | 0.79 | 0.66‐0.94 | 0.007 | ||
| Crossbred | 0.75 | 0.66‐0.86 | < 0.001 | ||
| Bichon | 0.69 | 0.49‐0.97 | 0.034 | ||
| Lurcher | 0.68 | 0.44‐1.05 | 0.083 | ||
| Springer Spaniel | 0.66 | 0.47‐0.94 | 0.022 | ||
| Lhasa Apso | 0.65 | 0.46‐0.91 | 0.011 | ||
| Rottweiler | 0.54 | 0.36‐0.82 | 0.004 | ||
| Shih‐tzu | 0.53 | 0.40‐0.70 | < 0.001 | ||
| West Highland White Terrier | 0.52 | 0.40‐0.67 | < 0.001 | ||
| English Springer Spaniel | 0.50 | 0.34‐0.74 | < 0.001 | ||
| Cocker Spaniel | 0.44 | 0.34‐0.58 | < 0.001 | ||
| Unrecorded | 0.72 | 0.38‐1.37 | 0.31 | ||
| Other purebreds and designers breed types | 0.89 | 0.77‐1.03 | 0.11 | ||
| Age (years) | 0.00 ‐ ≤ 0.50 | 0.30 | 0.20‐0.45 | < 0.001 | < 0.001 |
| 0.50 ‐ ≤ 3.00 | Base | < 0.001 | |||
| 3.00 ‐ ≤ 6.00 | 2.13 | 1.90‐2.39 | < 0.001 | ||
| 6.00 ‐ ≤ 9.00 | 2.89 | 2.57‐3.25 | < 0.001 | ||
| 9.00 ‐ ≤ 12.00 | 3.63 | 3.21‐4.11 | < 0.001 | ||
| ≥ 12.00 | 6.63 | 5.88‐7.47 | < 0.001 | ||
| Unrecorded | 1.46 | 0.98‐2.18 | 0.065 | ||
| Bodyweight relative to breed and sex mean | Lower | Base | < 0.001 | < 0.001 | |
| Equal/Higher | 1.06 | 0.99‐1.14 | 0.082 | ||
| Unrecorded | 0.76 | 0.69‐0.84 | < 0.001 | ||
| Sex‐neuter | Female/Entire | Base | < 0.001 | < 0.001 | |
| Female/Neutered | 1.14 | 1.01‐1.28 | 0.039 | ||
| Female/ Unrecorded | 1.43 | 1.24‐1.65 | < 0.001 | ||
| Male/Entire | 1.47 | 1.30‐1.66 | < 0.001 | ||
| Male/Neutered | 1.34 | 1.19‐1.51 | < 0.001 | ||
| Male/ Unrecorded | 1.73 | 1.51‐1.99 | < 0.001 | ||
| Not recorded/ Unrecorded | 1.00 | 0.49‐2.05 | 0.99 |
Multivariable logistic regression model for risk factors associated with seizures in dogs under primary‐care in the UK; aCI confidence interval.
As described in the methods, three variables (Purebred status, Kennel Club Breed groups, Adult (> 18months) bodyweight individually replaced the breed variable in the final multivariable breed model. Purebred dogs had 1.28 times the odds (95% CI 1.18 ‐ 1.39, P < 0.001) compared with crossbred dogs. Compared with “Breeds not Kennel Club recognized”, the “Toy” Kennel Club Breed group showed 1.68 times the odds (95% CI 1.52 ‐ 1.86 P < 0.001), the “Working” group had 1.49 the odds (95% CI 1.29 ‐ 1.73, P < 0.001) and the “Pastoral” group had 1.42 the odds (95% CI 1.26 ‐ 1.61, P < 0.001). Dogs with an adult (> 18months) bodyweight ≥ 40.00 kg had 1.24 times the odds (95% CI 1.08 ‐ 1.41, P = 0.002) compared with dogs < 10.00 kg (Table 4).
Table 4.
Multivariable logistic regression results for variables that replaced breed
| Variable | Category | Odds ratio | 95%CIa | p‐value | Variable P‐value |
|---|---|---|---|---|---|
| Purebred | Crossbred | Base | < 0.001 | ||
| Purebred | 1.28 | 1.18‐1.39 | < 0.001 | ||
| Designer | 1.18 | 0.93‐1.50 | 0.16 | ||
| Unrecorded | 0.94 | 0.50‐1.78 | 0.86 | ||
| Kennel Club Breed Groups | Breed not Kennel Club recognized | Base | < 0.001 | ||
| Gundog | 1.05 | 0.95‐1.16 | 0.34 | ||
| Hound | 1.20 | 1.01‐1.44 | 0.042 | ||
| Pastoral | 1.42 | 1.25‐1.60 | < 0.001 | ||
| Terrier | 1.03 | 0.92‐1.15 | 0.59 | ||
| Toy | 1.68 | 1.52‐1.86 | < 0.001 | ||
| Utility | 1.09 | 0.96‐1.24 | 0.16 | ||
| Working | 1.49 | 1.28‐1.72 | < 0.001 | ||
| Adult (>18months) bodyweight (kg) | < 10.00 | Base | < 0.001 | ||
| 10.00 ‐ ≤ 20.00 | 0.92 | 0.84‐1.01 | 0.092 | ||
| 20.00 ‐ ≤ 30.00 | 0.92 | 0.83‐1.02 | 0.110 | ||
| 30.00 ‐ ≤ 40.00 | 1.11 | 1.00‐1.24 | 0.061 | ||
| ≥ 40.00 | 1.24 | 1.08‐1.41 | 0.002 | ||
| Unrecorded | 0.74 | 0.66‐0.83 | < 0.001 |
Results for variables that replaced the breed variable in the final breed multivariable logistic regression model (with age category, bodyweight relative to breed mean sex and neuter status) to evaluate risk factors associated with seizures in dogs under primary veterinary care in the UK. Bodyweight relative to breed mean was also removed for adult bodyweight, aCI confidence interval.
4. DISCUSSION
This study explored the occurrence of seizures in a UK dog population under primary veterinary care. It benefitted from a large sample size of over 450,000 dogs. The study reports an overall 1‐year period prevalence of 0.82% and identified the Pug, Boxer, Basset Hound, Border Terrier and Border Collie as the breeds with the highest seizure prevalence. Increasing age, being male, being purebred and weighing over 40kg were highlighted as significant risk factors. These results can assist veterinary clinicians, researchers, breeders and pharmacovigilance agencies by providing a reliable evidence resource. This study also highlights the value of developing similar large‐scale systems for collection and analysis of veterinary clinical data outside of the UK in order to generate evidence that will be more generalizable to their national animal populations.
The VetCompass™ database was selected as a useful data source for the current study that aimed to provide a prevalence estimate for dogs with seizures with high levels of validity, generalizability and precision for the general population of dogs in the UK. Primary‐care veterinary clinical data might lack diagnostic precision for complicated disorders due to financial constraints on clinical work‐up, but are much less affected by the selection biases and therefore the results are thought to have greater validity than referral studies that preferentially select for dogs that are by definition sick and generally affected with more complicated disease and more severe presentations and owned by more motivated owners.23 Primary‐care data collected and merged from many hundreds of clinics now offers study sample sizes of sufficiently large size to allow much greater numerical precision in results than has previously been possible.23 It is estimated that 70% of UK dogs are registered with a veterinary practice and therefore results of studies using primary‐care data offer good prospects of generalization to the wider dog population and clinical relevance of the emergent results to veterinarians.19, 24
The current study reported a 1‐year prevalence of 0.82% for dogs affected by at least one seizure. To our knowledge, no previous studies have reported on the prevalence of seizures in the general dog population that can be used for direct comparison to this result. An earlier VetCompass™ study using primary‐care data reported a prevalence of 0.62% for epilepsy of unknown origin in the UK.9 This earlier study covered a longer period from January 2010 to April 2011 and focused specifically on the subset of dogs with “epilepsy of unknown origin”, excluding any dogs which had a clinically identified underlying disease causing epilepsy, whereas the current study included all dogs with seizures from any etiology during a 1‐year period. Thus, the previous reported prevalence is lower than the value reported in the current study as would be expected. Analysis of claim data from a large insurance database in Sweden reported a 0.75% prevalence of insured dogs with a recorded claim for epilepsy.8 However, this prevalence among insured dogs might be higher than in the wider dog population under veterinary care because certain breed types perceived to have higher health risks may be preferentially insured.25 Furthermore, the study dogs contributed an average of 4.2 years to the insurance study compared with the 1‐year period included in the current study. Further studies have also reported on referral populations.13, 26, 27 One of these studies reported a total seizure prevalence of 2.6% in a hospital population in Germany comprising 394 seizure case dogs admitted between January 2002 and March 2008.13 This prevalence may exceed the 0.82% reported in the current UK primary‐care study because primary‐care caseloads include 24.2% of dogs that have no diseases recorded and many of the remaining have minor ailments.23, 28 However, further factors that could partially account for the differences need to be considered and may include geographic and temporal differences between the studies as well as differing sample size and breed compositions.
The 1‐year period prevalence of 0.82% identifies seizures as a relatively frequent clinical presentation in the UK dog population under primary veterinary care. Seizures are a clinical manifestation that can result from a wide variety of causes, but are not limited to, genetic predisposition, primary brain disease, intoxication, cardiovascular disease, electrolyte disturbance and endocrine and metabolic disorders.23 The proportional contribution of reactive seizures to the total seizure case burden has been variously estimated at between 13.6% and 32% respectively.13, 26, 27 The differences between the study outcome might result from variation between study design (e.g. prospective versus retrospective analyses), countries, diagnostic procedures and animal population selection (e.g. primary‐care versus referral caseloads). The results of the current study underline the relevance of undergraduate training and continuing education about the multiple causes of seizure events, the optimal diagnostic procedures, and the management options for seizure events in dogs. These period prevalence data should also be considered as a useful background presence of seizures when considering suspected adverse events reported to pharmacovigilance monitoring systems.29, 30
Risk factors results can provide valuable information to improve veterinary diagnosis of disease and hence to improve animal welfare monitoring.31 The current study placed special focus on exploring breed as a risk factor for seizure occurrence because of prior evidence of genetic associations with epilepsy in dogs.26 As might have been expected, seizures proved to be more frequent in purebreds compared to crossbred and designer dogs although the purebred predisposition could be heavily influenced by the subgroup of dogs with idiopathic epilepsy. In a recent longitudinal study at Copenhagen University Hospital, dogs with idiopathic epilepsy exhibited a breed distribution of 83.6% purebred and 16.4% crossbred.26 However, hereditary factors might also contribute to structural epilepsy as well as non‐epileptic seizures cases as the genetic background might, for instance, predispose to epilepsy development after a brain injury or might predispose to a peripheral disease associated with non‐epileptic seizures.
Hybrid vigor (also known as heterosis or crossbred vigor) describes a phenomenon whereby the average performance of first‐generation crosses for specified traits outperforms the average performance of their parental breeds 32 and has been proposed to enhance the health of crossbred compared with purebred animals for over 150 years.33 Substantial evidence exists for these effects in production species of plants 34 and animals.35, 36, 37 However, pet dogs are generally not kept for production traits and the strength of evidence to support the true impact of hybrid vigor in dogs has recently been questioned.38 The findings in the current study that purebred dogs had 1.28 times higher odds of being seizure cases compared with crossbreds might add further weight to the existence of hybrid vigor in domestic dogs. Although the difference in effect shown might not appear to be substantial, it is likely that many of the crossbreds in our study were not first‐generation crosses and therefore would have lost much of the additional health gains shown by first‐generation crosses.32 It is possible that any true reduction in seizure prevalence in crossbreds may have been much higher specifically in the first‐generation subset of crosses but it was not possible to identify these dogs in the current study. The heterogeneity in genetics and health among the many individual breeds that exist among the purebred grouping should act as a further caveat to facile acceptance of the hybrid vigor concept.28 Relative changes in the proportions of some common breeds that are either predisposed or protected to certain diseases could dramatically affect the overall probability of disease in purebred dogs overall. From this it is clear that resolving the hybrid vigor question in dogs has quite some way to go.
Exploration of associations, in both predisposition and protective directions, between individual breeds and the occurrence of seizures was of particular interest in this study. After accounting for other relevant factors, the final multivariable identified predisposition to seizures in 11 breeds with odds ratios ranging up to 3.41 times higher than that of Labrador Retrievers. Although the Labrador Retriever itself has previously been reported as a predisposed breed for epilepsy with a reported lifetime prevalence of 3.1%,39 the current study elected to use the Labrador Retriever instead of crossbreds as the comparator breed in contrast to other previous studies9. Crossbred dogs, by definition, provide highly uncertain genetic structure, body conformation and parental‐breed contribution compared with their purebred counterparts that are more standardized with higher genetic homozygosity, breed standards for conformation and defined parentage 40 The high count of Labrador Retrievers in the study also enabled high statistical power to explore breed risks.41 However, interested readers who still wish to evaluate breed predisposition using crossbreds as the comparator category can still check for overlap of the 95% CI for each category; categories that do not overlap can be interpreted as showing statistically significant differences in their odds of diagnosis as seizure cases. The 11 breeds with significantly higher odds ratios compared to the Labrador Retriever were the Pug, Basset Hound, Dogue de Bordeaux, Boxer, Beagle, French Bulldog, British Bulldog, Border Terrier, Pomeranian, Chihuahua and Border Collie.
The majority of previous studies in this area have focused on epilepsy in dog breeds and often went even further by specifically targeting the epilepsy subset with a suspected inheritance of idiopathic epilepsy.10 Many of these earlier studies relied on relatively small groups of dogs or families that made it problematic to generalize the findings to the overall breed although recently some larger studies have reported breed‐specific epilepsy prevalence estimates.10
There are differing etiologies for epileptic seizures for Pugs (necrotizing encephalopathy) 42 and the Basset Hounds (Lafora disease).43 Seizure activity in the Dogue de Bordeaux is associated with a genetic predisposition but based on a small sample size of 5 dogs.44 Seizures and their etiology have also been investigated for 3 of the other predisposed breeds found in the current study: the Border Collie,45 Border Terrier,9, 46 Labrador Retriever,39 and the Chihuahua.47 However, there are no reliable reports on seizure activity in the French Bulldog, British Bulldog and Pomeranian. The findings of the current study go further than many of these previous studies and additionally include dogs with seizures that are both reactive as well as epileptic in order to give a more holistic view of the seizure predispositions that includes both genetic and environmental proclivities.
A protective effect for seizures was identified in Shih‐tzu, West Highland White Terrier, English Springer Spaniel and Cocker Spaniel. A previous primary‐care veterinary study similarly showed reduced risk to epilepsy of unknown origin in English Springer Spaniels and West Highland White Terrier in the UK.9 Breed health studies to date have heavily focused on predisposition to disease.28, 48 However, evidence to support protective disease effects, both between and within breeds, is increasing needed to support planned breed health reforms such as outcrossing to other breeds or to breed strains from other countries.49
Increased risk for epileptic seizures in male dogs is recognized across all dogs types 8, 9, 50, 51, 52, 53 as well as within specific breeds.10 Our analysis of UK primary‐care veterinary data that included both epileptic and reactive seizures also revealed a higher risk for male dogs. An interaction effect was noted between neutering and sex whereby the effect the neutering was not equal between the sexes. For this reason, the final multivariable model reported the values for each combination of sex and neuter status separately. Although neutering is a time dependent event whereby animals have an increased opportunity to become neutered as they age, the final multivariable model did take age into account and still showed an increased odds of seizures in neutered compared with entire bitches. This effect appears to be quite sex‐specific however and no significant effect of neutering on seizure risk in males was noted. However, a word of caution in relation to the potential benefits of neutering in bitches: studies such as the current cross‐sectional analysis can only show statistical association and must not be taken on their own as evidence of causality.31, 54 It is also possible that these associations stem from reverse causality effects whereby bitches that show seizures might be more likely to be removed from the breeding pool and then neutered rather than the other way around.52, 55 Future cohort analyses that follow animals over time to collect date information on the seizure onset and neutering as well as the rationale for the neutering decision are required to clarify the true role of neutering and seizure occurrence.
In agreement with previous studies, the current study identified increasing seizure risk with aging.39, 52 It is also worth noting that our study design reported the age of all cases during 2013 rather than reporting the age that these animal first became a case. This means that the inference from the results describes the probability for seizure cases existing within these age groups (prevalence) rather than the probability of new cases developing during these ages (incidence). However, a detailed investigation of seizure etiologies within the age groups was beyond the scope the study and would be required to further explore differing seizure etiologies across the age groups. A study of dogs referred for examination in Austria showed that idiopathic epilepsy was 3.25 times more likely to be the cause for seizure onset in dogs aged one to five years compared to structural epilepsy 56 whereas a US study of referred dogs identified that 65% of seizure cases older than five years were associated with structural epilepsy.57
Dogs weighing over 40.00 kg in absolute bodyweight had significantly higher odds and dogs weighing between 30.00 ‐ ≤ 40.00 kg had a tendency to higher odds for seizures compared with dogs weighing under 10.00 kg. Although these findings might suggest increased seizure risk in larger dogs, it is also possible that these associations with bodyweight might result from seizure disease management using anti‐convulsive treatment. Bodyweight gain due to polyphagia has been described as a common adverse effect of many antiepileptic drugs.58 It is also worth noting that no effect was identified within breeds between dogs that weighed over or under their breed and sex average bodyweight.
In common with other epidemiological studies using primary‐care veterinary clinical data, this study had some limitations.16, 59 Retrospective identification of cases with seizures relied on the diagnostic acumen and reporting of the veterinarians contributing data to the VetCompass™ Program. Information about seizure occurrence is often based on description by owners and the veterinarian might not often witness the actual seizure event directly, and therefore some seizures that occur when dogs are not under observation by the owner might be missed or conversely falsely classified as seizure. In addition, less severe, non‐generalized seizures might not even be recognized as seizures by the owner and therefore might not be reported to the veterinarian. Even in human patients with epilepsy, up to 38% of focal seizures go unrecognized 60, 61, 62 and similar under‐reporting of seizures has been considered in dogs.63 Thus, the results of the present study might underestimate the true absolute period prevalence of seizures in the UK canine population but these effects are unlikely to affect the results of the risk factor analyses where similar under‐reporting is expected across all categories within each variable.64
In conclusion, this analysis of over 450,000 dogs under primary veterinary care shows that the combined effects of epileptic and reactive seizures are a relatively common clinical finding with 0.82% of UK dogs affected in a 1‐year period. As seizure diagnosis and management therefore constitute an important role in clinical practice, these results highlighting particular breeds, sex and aging as significant risk factors should assist veterinarian practitioners to identify and alert owners to dogs at higher risk. Enhanced owner awareness could promote earlier, faster and more reliable diagnosis and treatment to support improved animal welfare. These results can also assist pharmacovigilance efforts by providing baseline data from the overall population to optimize interpretability of seizures when reported as suspected adverse reaction to medication use.
CONFLICT OF INTEREST DECLARATION
Heidrun Potschka received consulting fees from Bayer Animal Health and MSD Animal Health, speaker fees from Desitin and Zogenix, and funding for collaborative projects from Bayer Animal Health and Roche.
Holger Volk served as paid consultant for Boehringer Ingelheim and CEVA animal health. Served as contract researcher for: Nestle 2012–2014 and 2017‐ongoing, dietary modification of epilepsy in dogs; Desitin Pharma, 2012, the role of levetiracetam in a referral hospital; industrial Funding, 2014–2015, investigating the effects of imepitoin behavioural, physiologic and owner‐reported indicators of anxiety in dogs treated for idiopathic epilepsy. Received competitive research grants for: RCVS pump primer grant, 2010– 2013, pharmacometabonomic profiling of epileptic dogs; Waltham Foundation, 2011–2014, determination of plasma omega‐3 fatty acid status in dogs with primary epilepsy and relationship to antiepileptic drug metabolism; CASE BBSRC PhD studentship, 2012–2016 metabolic profiling of epilepsy in dogs; American Kennel Club, American Health Foundation, 2016‐2018, Investigating the Effect of a Ketogenic Medium Chain Triglycerides Supplement on the treatment of Canine Idiopathic Epilepsy and its behavioural comorbidities; BBSRC, 2017‐2020, Investigating the relationship between epilepsy, drug‐resistance and affective disorders in the domestic dog.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
ACKNOWLEDGMENTS
We acknowledge funding support from Bayer Animal Health GmbH for this study and the VetCompass™ team in the UK for data collection, collation and access. We acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Blythwood Vets, Vale Vets, Vets Now and other UK practices which collaborate in VetCompass™. Work was done at he Royal Veterinary College, Hawkshead Lane, Hatfield AL9 7TA, UK, Institute of Pharmacology, Toxicology and Pharmacy, Ludwig‐Maximilians‐University, Königinstr. 16, 80539 Munich, Germany, Bayer Animal Health GmbH, 51373 Leverkusen.
The study was supported by Bayer Animal Health GmbH, Kaiser‐Wilhelm ‐ Allee 10 51373 Leverkusen, Germany.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Erlen A, Potschka H, Volk HA, Sauter‐Louis C, O'Neill DG. Seizure occurrence in dogs under primary veterinary care in the UK: prevalence and risk factors. J Vet Intern Med. 2018;32:1665–1676. 10.1111/jvim.15290
REFERENCES
- 1. Berendt M, Farquhar RG, Mandigers PJ, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Risio L, Newton R, Freeman J, et al. Idiopathic epilepsy in the Italian Spinone in the United Kingdom: Prevalence, clinical characteristics, and predictors of survival and seizure remission. J Vet Intern Med. 2015;29:917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brauer C, Jambroszyk M, Tipold A. Metabolic and toxic causes of canine seizure disorders: A retrospective study of 96 cases. The Veterinary Journal. 2011;187:272‐275. [DOI] [PubMed] [Google Scholar]
- 4. Platt SR, McDonnell JJ. Status epilepticus: Clinical features and pathophysiology. Compendium on Continuing Education for the Practicing Veterinarian. 2000;22:660‐669. [Google Scholar]
- 5. Packer RMA, Volk HA. Epilepsy beyond seizures: a review of the impact of epilepsy and its comorbidities on health‐related quality of life in dogs. Vet Rec. 2015;177:306‐315. [DOI] [PubMed] [Google Scholar]
- 6. Packer RMA, Lucas R, Volk HA. Owner perception of focal seizures in canine epilepsy. Veterinary Record. 2017;180:150. [DOI] [PubMed] [Google Scholar]
- 7. Wessmann A, Volk H, Packer R, et al. Quality‐of‐life aspects in idiopathic epilepsy in dogs. Veterinary Record. 2016;179:229. [DOI] [PubMed] [Google Scholar]
- 8. Heske L, Nødtvedt A, Jäderlund KH, et al. A cohort study of epilepsy among 665,000 insured dogs: Incidence, mortality and survival after diagnosis. The Veterinary Journal. 2014;202:471‐476. [DOI] [PubMed] [Google Scholar]
- 9. Kearsley‐Fleet L, O'Neill DG, Volk HA, et al. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec. 2013;172:338. [DOI] [PubMed] [Google Scholar]
- 10. Hülsmeyer V‐I, Fischer A, Mandigers PJJ, et al. International Veterinary Epilepsy Task Force's current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet Res. 2015;11:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475‐482. [DOI] [PubMed] [Google Scholar]
- 12. Tauber C‐A. Epidemiologische Untersuchungen zum Vorkommen von Epilepsie bei Hund und Katze in der Kleintierpraxis. lmu. 2017. [Google Scholar]
- 13. Zimmermann R, Hülsmeyer V‐I, Sauter‐Louis C, Fischer A. Status Epilepticus and Epileptic Seizures in Dogs. J Vet Intern Med. 2009;23:970‐976. [DOI] [PubMed] [Google Scholar]
- 14. VetCompass . VetCompass: Health surveillance for UK companion animals. In. London: RVC Electronic Media Unit; 2017.
- 15.The VeNom Coding Group. VeNom Veterinary Nomenclature [http://www.venomcoding.org]. Accessed 15 March 2017.
- 16. O'Neill DG, Lee MM, Brodbelt DC, et al. Corneal ulcerative disease in dogs under primary veterinary care in England: epidemiology and clinical management. Canine Genet Epidemiol. 2017;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pearce N. Classification of epidemiological study designs. International Journal of Epidemiology. 2012;41:393‐397. [DOI] [PubMed] [Google Scholar]
- 18. Epi Info 7 CDC . Centers for Disease Control and Prevention (US): Introducing Epi Info 7 [http://wwwn.cdc.gov/epiinfo/7]. Accessed 20 January 2017.
- 19. Asher L, Buckland E, Phylactopoulos CL, et al. Estimation of the number and demographics of companion dogs in the UK. BMC Vet Res. 2011;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliver JAC, Gould DJ. Survey of ophthalmic abnormalities in the labradoodle in the UK. Vet Rec. 2012;170:390. [DOI] [PubMed] [Google Scholar]
- 21. De Risio L, Bhatti S, Muñana K, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed. Oxford: Blackwell Science; 2003. [Google Scholar]
- 23. Bartlett PC, Van Buren JW, Neterer M, et al. Disease surveillance and referral bias in the veterinary medical database. Prev Vet Med. 2010;94:264‐271. [DOI] [PubMed] [Google Scholar]
- 24. Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the surveillance, epidemiology, and end results registry population: Factors relevant to epidemiologic and health care research. Journal of Clinical Epidemiology. 1997;50:939‐945. [DOI] [PubMed] [Google Scholar]
- 25. Egenvall A, Bonnett BN, Olson P, et al. Validation of computerized Swedish dog and cat insurance data against veterinary practice records. Preventive Veterinary Medicine. 1998;36:51‐65. [DOI] [PubMed] [Google Scholar]
- 26. Fredsø N, Toft N, Sabers A, et al. A prospective observational longitudinal study of new‐onset seizures and newly diagnosed epilepsy in dogs. BMC Vet Res. 2016;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steinmetz S, Tipold A, Löscher W. Epilepsy after head injury in dogs: A natural model of posttraumatic epilepsy. Epilepsia. 2013;54:580‐588. [DOI] [PubMed] [Google Scholar]
- 28. O′Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in dogs attending primary‐care veterinary practices in England. PLoS ONE. 2014;9:e90501‐e90516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodward KN. Veterinary pharmacovigilance. Part 3. Adverse effects of veterinary medicinal products in animals and on the environment. J Vet Pharmacol Ther. 2005;28:171‐184. [DOI] [PubMed] [Google Scholar]
- 30. Keck G, Ibrahim C. Veterinary pharmacovigilance: between regulation and science. Journal of Veterinary Pharmacology and Therapeutics. 2002;24:369‐373. [DOI] [PubMed] [Google Scholar]
- 31. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2nd ed. Charlottetown, Canada: VER Inc; 2009. [Google Scholar]
- 32. Nicholas FW. Introduction to Veterinary Genetics. 3rd ed. Oxford: Wiley‐Blackwell; 2010. [Google Scholar]
- 33. Darwin C. On the Origin of Species by means of Natural Selection, or the preservation of favoured races in the struggle for life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 34. Troyer AF. Adaptedness and heterosis in corn and mule hybrids. Crop Science. 2006;46:528‐543. [Google Scholar]
- 35. Lalev M, Mincheva N, Oblakova M, et al. Estimation of heterosis, direct and maternal additive effects from crossbreeding experiment involving two White Plymouth Rock lines of chickens. Bio Anim Husb. 2014;30:103‐114. [Google Scholar]
- 36. Ferreira VC, Rosa GJM, Berger YM, et al. Survival in crossbred lambs: Breed and heterosis effects. J Anim Sci. 2015;93:912‐919. [DOI] [PubMed] [Google Scholar]
- 37. Buckley F, Lopez‐Villalobos N, Heins BJ. Crossbreeding: implications for dairy cow fertility and survival. Animal. 2014;8:122‐133. [DOI] [PubMed] [Google Scholar]
- 38. Nicholas FW, Arnott ER, McGreevy PD. Hybrid vigour in dogs? The Veterinary Journal. 2016;214:77‐83. [DOI] [PubMed] [Google Scholar]
- 39. Berendt M, Gredal H, Pedersen LG, et al. A Cross‐Sectional Study of Epilepsy in Danish Labrador Retrievers: Prevalence and Selected Risk Factors. Journal of Veterinary Internal Medicine. 2002;16:262‐268. [DOI] [PubMed] [Google Scholar]
- 40. Mellanby RJ, Ogden R, Clements DN, et al. Population structure and genetic heterogeneity in popular dog breeds in the UK. Vet J. 2013;196:92‐97. [DOI] [PubMed] [Google Scholar]
- 41. Dohoo IR, Martin SW, Stryhn H. Methods in Epidemiologic Research. 2012. VER Inc.: Charlottetown, Prince Edward Island. [Google Scholar]
- 42. Levine JM, Fosgate GT, Porter B, et al. Epidemiology of Necrotizing Meningoencephalitis in Pug Dogs. Journal of Veterinary Internal Medicine. 2008;22:961‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaiser E, Krauser K, Schwartz‐Porsche D. Lafora disease (progressive myoclonic epilepsy) in the Bassett hound–possibility of early diagnosis using muscle biopsy? Tierarztl Prax. 1991;19:290‐295. [PubMed] [Google Scholar]
- 44. Escriou C, Quignon P, Menzer E, et al. Genetic Epilepsy In Cane Corso And Dogue De Bordeaux. Journal of Veterinary Internal Medicine. 2016;30:464. [Google Scholar]
- 45. Hülsmeyer V, Zimmermann R, Brauer C et al. Epilepsy in Border Collies: clinical manifestation, outcome, and mode of inheritance. Journal of Veterinary Internal Medicine. 2010;24:171‐178. [DOI] [PubMed] [Google Scholar]
- 46. Kloene J, Sewell AC, Hamann H, et al. Clinical investiagtion of seizures in Border Terriers. Kleintierpraxis. 2008;8:5‐12. [Google Scholar]
- 47. Higgins RJ, Dickinson PJ, Kube SA, et al. Necrotizing Meningoencephalitis in Five Chihuahua Dogs. Vet Pathol. 2008;45:336‐346. [DOI] [PubMed] [Google Scholar]
- 48. Bateson P. Independent inquiry into dog breeding. In. Cambridge: University of Cambridge; 2010. [Google Scholar]
- 49. Farrell L, Schoenebeck J, Wiener P, et al. The challenges of pedigree dog health: approaches to combating inherited disease. Canine Genet Epidemiol. 2015;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Casal ML, Munuve RM, Janis MA, et al. Epilepsy in Irish Wolfhounds. J Vet Intern Med. 2006;20:131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kathmann I, Jaggy A, Busato A, et al. Clinical and genetic investigations of idiopathic epilepsy in the Bernese mountain dog. J Small Anim Pract. 1999;40:319‐325. [DOI] [PubMed] [Google Scholar]
- 52. Short A, Dunne A, Lohi H, et al. Characteristics of epileptic episodes in UK dog breeds: an epidemiological approach. Veterinary Record‐English Edition. 2011;169:48. [DOI] [PubMed] [Google Scholar]
- 53. Van Meervenne SAE, Volk HA, Matiasek K, et al. The influence of sex hormones on seizures in dogs and humans. Vet J. 2014;201:15‐20. [DOI] [PubMed] [Google Scholar]
- 54. Woodward KN. Veterinary pharmacovigilance. Part 5. Causality and expectedness. J Vet Pharmacol Ther. 2005;28:203‐211. [DOI] [PubMed] [Google Scholar]
- 55. Flanders W, Augestad L. Adjusting for reverse causality in the relationship between obesity and mortality. Int J Obes. 2008;32:S42‐S46. [DOI] [PubMed] [Google Scholar]
- 56. Pákozdy A, Leschnik M, Tichy AG, et al. Retrospective clinical comparison of idiopathic versus symptomatic epilepsy in 240 dogs with seizures. Acta Vet Hung. 2008;56:471. [DOI] [PubMed] [Google Scholar]
- 57. Ghormley TM, Feldman DG, Cook JR Jr. Epilepsy in dogs five years of age and older: 99 cases (2006–2011). J Am Vet Med Assoc. 2015;246:447‐450. [DOI] [PubMed] [Google Scholar]
- 58. Bhatti SFM, De Risio L, Muñana K, et al. International Veterinary Epilepsy Task Force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet Res. 2015;11:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Neill DG, Riddell A, Church DB, et al. Urinary incontinence in bitches under primary veterinary care in England: prevalence and risk factors. J Small Anim Pract. 2017;58:685‐693. [DOI] [PubMed] [Google Scholar]
- 60. Kerling F, Mueller S, Pauli E, et al. When do patients forget their seizures? An electroclinical study. Epilepsy & Behavior. 2006;9:281‐285. [DOI] [PubMed] [Google Scholar]
- 61. Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64:1595‐1599. [DOI] [PubMed] [Google Scholar]
- 62. Tatum WO, Winters L, Gieron M, et al. Outpatient Seizure Identification: Results of 502 Patients Using Computer‐Assisted Ambulatory EEG. journal of Clinical Neurophysiology. 2001;18:14‐19. [DOI] [PubMed] [Google Scholar]
- 63. Packer RM, Berendt M, Bhatti S, et al. Inter‐observer agreement of canine and feline paroxysmal event semiology and classification by veterinary neurology specialists and non‐specialists. BMC Vet Res. 2015;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Elwood M. Critical appraisal of epidemiological studies and clinical trials. 3rd ed. Oxford, UK: Oxford University Press; 2007. [Google Scholar]


