Abstract
Objective
The present study is to evaluate the biological functions of long non-coding RNA (lncRNA), X-inactive specific transcript, X-inactive specific transcript (XIST) in human epithelial ovarian cancer (EOC).
Methods
XIST was upregulated in EOC cell lines, CAOV3 and OVCAR3 cells by lentiviral transduction. The effects of XIST overexpression on cancer cell proliferation, invasion, chemosensitivity and in vivo tumor growth were investigated, respectively. Possible sponging interaction between XIST and human microRNA hsa-miR-214-3p was further evaluated. Furthermore, hsa-miR-214-3p was overexpressed in XIST-upregulated CAOV3 and OVCAR3 cells to evaluate its effect on XIST-mediated EOC regulation.
Results
Lentivirus-mediated XIST upregulation had significant anticancer effects in CAOV3 and OVCAR3 cells by suppressing cancer cell proliferation, invasion, increasing cisplatin chemosensitivity and inhibiting in vivo tumor growth. Hsa-miR-214-3p was confirmed to directly bind XIST, and inversely downregulated in XIST-upregulated EOC cells. In EOC cells with XIST upregulation, secondary lentiviral transduction successfully upregulated hsa-miR-214-3p expression. Subsequently, hsa-miR-214-3p upregulation functionally reversed the anticancer effects of XIST-upregulation in EOC.
Conclusion
Upregulation of lncRNA XIST may suppress EOC development, possibly through sponging effect to induce hsa-miR-214-3p downregulation.
Keywords: Epithelial Ovarian Cancer, lncRNA, X (inactive)-Specific Transcript (XIST), miRNA, hsa-miR-214-3p, Proliferation Therapy, Neoplasm Invasion
INTRODUCTION
Epithelial ovarian cancer (EOC) is one of the most common and malignant gynecologic cancers in the world [1,2,3,4]. In the United States, approximate 22,440 new cases of EOC would be discovered, and approximate 14,080 cancer patients would die of EOC every year [1]. In China, nearly half million cases of EOC are diagnosed and about 225,000 EOC patients die of EOC every year [2]. Although tremendous efforts had been devoted, from both academic research and clinical practice, to understanding the underlying molecular mechanisms of EOC oncogenesis and pathology, as well as developing efficient diagnostic methods and treatment options for EOC patients, the improvement on patients' prognosis and long-term survival after initial diagnosis has been marginal [1,2,3]. Thus, much more research is needed to identify novel biomarkers and therapeutic targets to benefit patients with EOC.
During the past decade, groups of non-coding transcripts, or long non-coding RNAs (lncRNAs), have been identified to be dysregulated in human cancers and play critical roles in cancer regulations by interacting with messenger RNAs or epigenetic factors of microRNA (miRNAs) [5,6,7,8]. Among them, lncRNA X-inactive specific transcript (XIST) was originally identified to be located in the X inactivation center of human chromosome, and play important roles in inducing X inactivation in female cells and dosage equilibration in male cells [9]. While the exact biological functions of XIST in human cell development are still under investigation, emerging evidence has demonstrated that XIST may be dysregulated in various types of human cancers, and interact with other transcriptional factors to regulate cancer cell pathological development [10,11,12,13]. In human EOC, XIST was identified to be lowly expressed in EOC cell lines, and might be associated with EOC chemotherapy resistance [14,15]. However, no functional mechanisms of XIST had ever been revealed in regulating EOC development, such as proliferation, invasion or chemosensitivity.
Thus in the present study, we used lentiviral transduction technology to force XIST overexpression (or upregulation) in 2 of known EOC cell lines with very low XIST expressions, CAOV3 and OVCAR3 cells [14]. Then, we investigated the possible biological functions of XIST upregulation on regulating EOC proliferation, invasion, in vivo tumor growth and cisplatin chemosensitivity. Moreover, we explored the possibility of XIST sponging other epigenetic regulator, human miRNA 214-3p (hsa-miR-214-3p), a known oncogenic factor in EOC [16], to further elucidate the associated signaling pathway of XIST in modulating EOC.
MATERIALS AND METHODS
1. Ethic approval
The approval to conduct the present study was granted by the Clinical Research & Ethic Committees at the China-Japan Union Hospital of Jilin University, Jilin University, Sinopharm Changchun A-Think Pharmaceutical Co., Ltd. (Changchun, China) in Changchun of Jilin Province and Medicine Hospital of Kaifeng in Kaifeng of Henan Province in China.
2. EOC cell lines
Human EOC cell lines, CAOV3 and OVCAR3 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). EOC cells were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin and 100 µg/mL streptomycin (Sinopharm) in a in humidified atmosphere at 37°C with 5% CO2.
3. XIST upregulation assay
Lentiviral vectors expressing the full-length human XIST (L/XIST), and a non-specific empty lentiviral vector (L/NS) were purchased from Genechem Company (Shanghai, China). CAOV3 and OVCAR3 were transduced with L/XIST or L/NS using the TransDux MAX Lentivirus Transduction Reagent (System Biosciences, Palo Alto, CA, USA) and polybrene (8-μg/mL; Sinopharm) at multiplicity of infection between 20 and 30. Forty-eight hours later, cells were collected and underwent a selection procedure in culture medium containing blasticidin (10 μg/mL; Sinopharm) for 72 hours. Healthy CAOV3 and OVCAR3 cells were then collected and passaged for 3–5 times. quantitative reverse transcription polymerase chain reaction (qRT-PCR) was conducted to verify XIST upregulation.
4. RNA extraction and qRT-PCR
Total RNA was extracted and purified from CAOV3 and OVCAR3 cells using a Trizol™ Plus RNA Purification Kit (Invitrogen) according to the manufacturer' protocol. Reverse transcription was conducted using a High-Capacity RNA-to-cDNA Kit (Invitrogen) according to the manufacturer's protocol. qRT-PCR was conducted on an ABI PRISM 7300 PCR sequence detection system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. The expression of lncRNA XIST was probed using a TaqMan non-coding RNA assay (Applied Biosystems). The expression of hsa-miR-214-3p was probed using a TaqMan advanced miRNA Assay (Applied Biosystems). Relative gene expression levels were calculated as fold changes using the (2−ΔΔCt) method.
5. Proliferation assay
CAOV3 and OVCAR3 cells were seeded in a 96-well plate at a density of 3×103 cells/well for 96 hours. Every 24 hour, a CyQUANT™ Cell Proliferation Assay Kit (Invitrogen) was conducted according to the manufacturer's protocol. EOC proliferation was characterized by using a SpectraMax iD5 Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA, USA) to measure absorbance of 570 nm.
6. Invasion assay
In a 24-well transwell assay, the inserts (8-μm; Costar, Washington, D.C., USA) were pre-coated with 1% gelatin (Sinopharm) overnight. CAOV3 and OVCAR3 cells were seeded onto the upper chamber in serum-free culture medium at a density of 1×105 cells/well. The lower chamber was filled with regular culture medium (w/10% FBS). Twenty-four hours later, inserts and medium were removed. Invading EOC cells, which were attached to the bottoms of wells, were quickly fixed by 70% ethanol (Sinopharm) and stained with 0.1% crystal violet (Sinopharm) for 20 minutes at room temperature. The transwell plate was then moved onto an IX91 inverted microscope (Olympus, Tokyo, Japan) and examined under a 40X objective. In each well, cells were counted in 5 randomly selected fields. The resulted invading cell numbers were all normalized to the cell numbers under control condition.
7. Chemosensitivity assay
CAOV3 and OVCAR3 cells were seeded in a 96-well plate at a density of 5×104 cells/well, and then treated with various concentrations of cisplatin (Sinopharm) at 0, 0.25, 0.5, 1, 4, 8, and 16 µM. Twenty-four hours later, viable cells were evaluated using the CyQUANT™ Cell Proliferation Assay Kit (Invitrogen). All measured absorbances were normalized to the absorbance under control conditions.
8. In vivo tumor xenograft assay
Lentiviral transduced CAOV3 cells were inoculated into subcutaneous spaces under dorsal skin in 5-week-old athymic nude mice. CAOV3 xenografts were evaluated weekly by measuring their subcutaneous lengths (Ls) and widths (Ws) and then calculating their in vivo tumor volumes (Vs) using the equation, V=l*W*W/2. After 5 weeks, mice were killed and CAVO3 xenografts were exposed.
9. Dual-luciferase reporter assay
Based on several online lncRNA-miRNA biding databases (Starbase v2.0, MiRWalk 2.0 and MiRNet), we identified a putative hsa-miR-214-3p binding site on human XIST gene. Then, the DNA sequence on human XIST gene which included the putative hsa-miR-214-3p binding site was sub-cloned into a firefly luciferase vector (GenePharma, Shanghai, China) to create a luciferase plasmid Luc-XIST. Alternatively, the hsa-miR-214-3p binding site was mutated, and the mutant XIST sequence was also sub-cloned into the luciferase vector to create another luciferase plasmid Luc-XIST(m). In human HEK293T cells, they were co-transfected with a synthetic hsa-miR-214-3p mimics (miR-214-3p, GenePharma) or a non-specific miRNA mimics (miR-NC, GenePharma), along with Luc-XIST or Luc-XIST(m). Forty-eight hours later, relative firefly luciferase activities were measured using a Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA), and then normalized to renilla luciferase activities, according to the manufacturer's protocol.
10. MiR-214-3p upregulation assay
Lentiviral vectors containing hsa-miR-214-3p mimics (L/miR214), and a non-specific miRNA mimics (L/miR) were purchased from Genechem Company. In CAOV3 and OVCAR3 cells already transduced with L/XIST, they were further transduced with L/miR214 or L/miR using the TransDux MAX Lentivirus Transduction Reagent (System Biosciences) and polybrene (8-μg/mL; Sinopharm) at multiplicity of infection between 15 and 20. Forty-eight hours later, healthy cells were collected and passaged for 3–5 times. qRT-PCR was conducted to verify hsa-miR-214-3p upregulation.
11. Statistical analysis
All experiments were biologically repeated for at least 3 times. Data were presented as mean±standard error of the mean. A version 13.0 SPSS software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Student's t-test, or 1-way ANOVA followed by Turkey's post hoc test was applied for comparisons. The p<0.05 was considered to represent significant difference.
RESULTS
1. XIST upregulation suppressed EOC proliferation and invasion
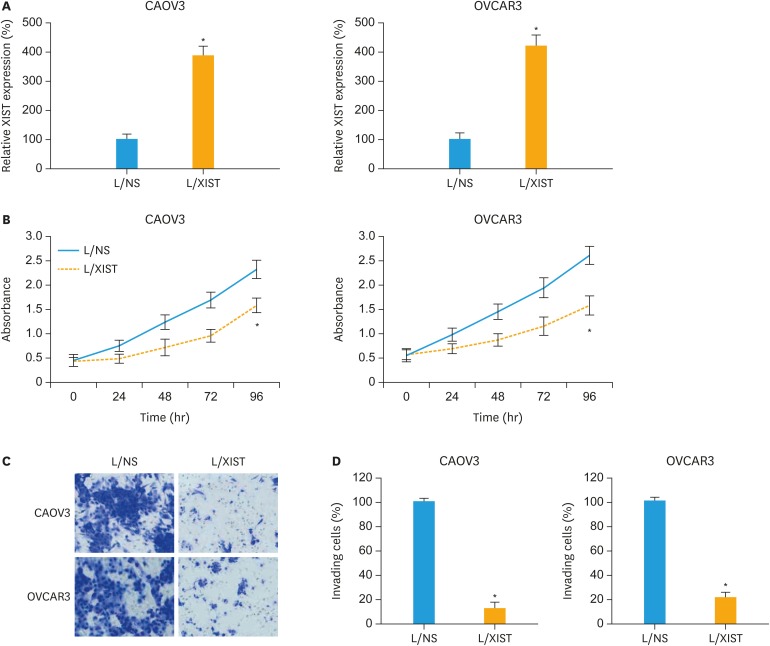
CAOV3 and OVCAR3 cells were transduced lentivirus to upregulate expression of lncRNA XIST. qRT-PCR demonstrated that, in EOC cells stably transduced with L/XIST, XIST expressions were significantly higher than in EOC cells transduced with L/NS (Fig. 1A, p<0.05).
Fig. 1. Effects of XIST upregulation on EOC proliferation and invasion. (A) CAOV3 and OVCAR3 cells were stably transduced with lentivirus. qRT-PCR was then applied to compare XIST expressions between EOC cells transduced with L/NS and EOC cells transduced with L/XIST. (B) A proliferation assay was performed for 96 hours to compare cancer proliferation between EOC cells transduced with L/NS and EOC cells transduced with L/XIST. Every 24-hour, absorbance was measured at 570 nm. (C) A transwell assay was performed. 24 hours later, lentiviral transduced EOC cells that invaded onto the bottoms of wells were stained by 0.1% crystal violet. (D) The numbers of invading cells were compared between EOC cells transduced with L/NS and EOC cells transduced with L/XIST.
EOC, epithelial ovarian cancer; L/NS, a non-specific empty lentiviral vector; L/XIST, lentiviral vectors expressing the full-length human XIST; qRT-PCR, quantitative reverse transcription polymerase chain reaction; XIST, X-inactive specific transcript.
*p<0.05.
Biochemical functions of cancer proliferation and invasion were then compared in lentiviral transduced EOC cells. In as 96-hours cancer proliferation assay, it was demonstrated that cancer proliferating rates were significantly suppressed in CAOV3 and OVCAR3 cells transduced with L/XIST, than in EOC cells transduced with L/NS (Fig. 1B, p<0.05). In addition, in a 24-hours transwell assay, it was shown that much less EOC cells invaded into the bottom chambers of transwell (Fig. 1C). Quantitative comparison on the number of invading EOC cells confirmed that cancer invasion was significantly suppressed in CAOV3 and OVCAR3 cells transduced with L/XIST, than in EOC cells transduced with L/NS (Fig. 1D, p<0.05).
Thus, these experiments suggested that XIST upregulation had significant anticancer effects on proliferation and invasion in EOC cell lines.
2. XIST upregulation increased EOC chemosensitivity and suppressed EOC xenograft
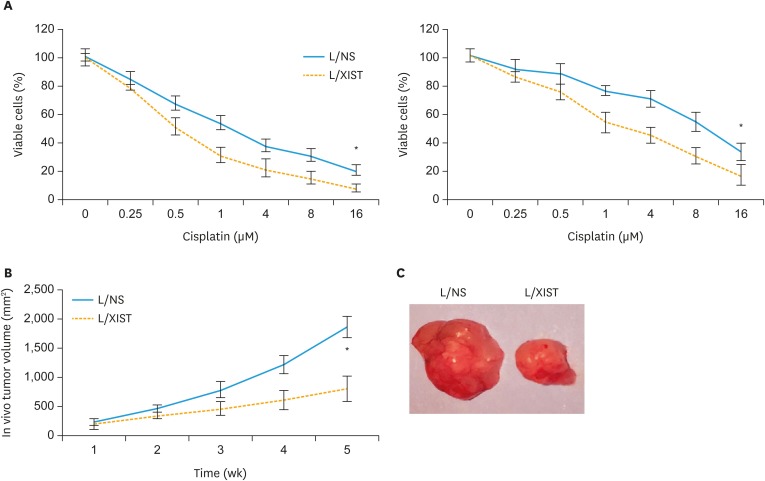
Lentiviral transduced CAOV3 and OVCAR3 cells were incubated with different concentrations of cisplatin to compare their chemosensitivity. Twenty-four hours later, quantitative measurement on viable cancer cells demonstrated that, cisplatin chemosensitivity was significantly increased in CAOV3 and OVCAR3 cells transduced with L/XIST, than in EOC cells transduced with L/NS (Fig. 2A, p<0.05).
Fig. 2. Effects of XIST upregulation on EOC chemosensitivity and in vivo tumor growth. (A) Lentiviral transduced CAOV3 and OVCAR3 cells were tested with cisplatin at concentrations (μM) of 0, 0.25, 0.5, 1, 4, 8 and 16 for 24 hours. A viability assay was then performed to compare cisplatin sensitivity between EOC cells transduced with L/NS and EOC cells transduced with L/XIST. (B) Lentiviral transduced CAOV3 cells were subcutaneously injected into carrier mice. The in vivo growth of CAOV3 xenograft was monitored by weekly measurement on in vivo tumor volumes. (C) After 5 weeks, L/NS-transduced and L/XIST-transduced CAOV3 xenografts were extracted and compared.
EOC, epithelial ovarian cancer; L/NS, a non-specific empty lentiviral vector; L/XIST, lentiviral vectors expressing the full-length human XIST; XIST, X-inactive specific transcript.
*p<0.05.
In addition, an in vivo xenograft assay was carried out by subcutaneously inoculating lentiviral transduced CAOV3 cells into adult athymic nude mice. Weekly measurement on in vivo tumor volumes showed that the growth of CAOV3 xenograft was significantly suppressed in those transduced with L/XIST, than in tumor-grafts transduced with L/NS (Fig. 2B, p<0.05). After 5-week, CAOV3 xenografts were extracted. Imaging probe confirmed that L/XIST-transduced xenograft was significantly smaller than L/NS-transduced xenograft (Fig. 2C).
Thus, these experiments suggested that XIST upregulation had anticancer effects on chemosensitivity and in vivo tumor growth of EOC cell lines.
3. MiR-214-3p downregulation was associated with XIST upregulation in EOC
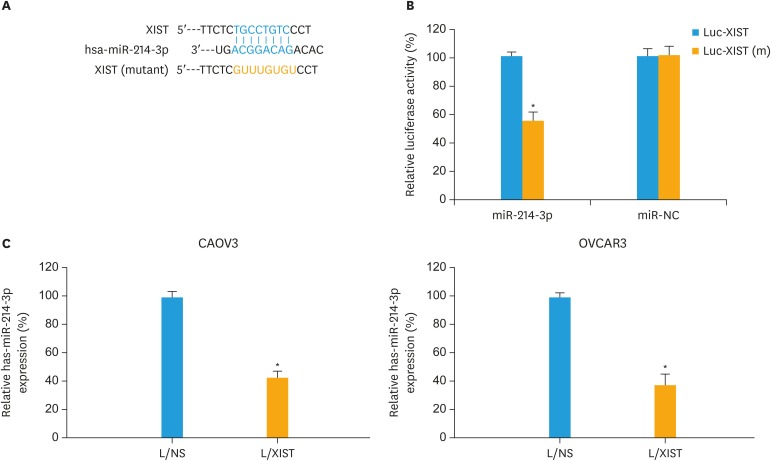
By researching several online lncRNA-miRNA binding databases (Starbase v2.0, MiRWalk 2.0 and MiRNet), a possible sponge interaction between hsa-miR-214-3p and XIST was identified (Fig. 3A). In order to verify the physical association between hsa-miR-214-3p and XIST, we constructed a luciferase plasmid containing the DNA sequence of hsa-miR-214-3p binding site on XIST (Luc-XIST). Alternatively, another luciferase plasmid containing the mutant hsa-miR-214-3p binding site was also created (Luc-XIST[m], Fig. 3A). Then, a dual-luciferase reporter assay demonstrated that hsa-miR-214-3p did physically interact with XIST (Fig. 3B, p<0.05).
Fig. 3. XIST sponged hsa-miR-214-3p in EOC. (A) Schematic drawing was demonstrated for the predictive binding site for hsa-miR-214-3p on XIST. Also, the DNA sequence was mutated at the hsa-miR-214-3p binding site on XIST. (B) Human HEK293T cells were co-transfected with luciferase plasmids of Luc-XIST or Luc-XIST(m), and synthetic miRNA mimics of miR-214-3p or miR-NC. A dual-luciferase reporter assay was then performed to measure relative luciferase activities in co-transfected HEK293T cells. (C) qRT-PCR was applied to compare hsa-miR-214-3p expressions between CAVO3 and OVCAR3 cells transduced with L/NS and those transduced with L/XIST.
EOC, epithelial ovarian cancer; L/NS, a non-specific empty lentiviral vector; L/XIST, lentiviral vectors expressing the full-length human XIST; qRT-PCR, quantitative reverse transcription polymerase chain reaction; XIST, X-inactive specific transcript.
*p<0.05.
In addition, in lentiviral transduced CAOV3 and OVCAR3 cells, qRT-PCR showed that, due to XIST upregulation, hsa-miR-214-3p expressions were significantly downregulated (Fig. 3C, p<0.05).
4. MiR-214-3p upregulation reversed the anticancer effects of XIST upregulation in EOC
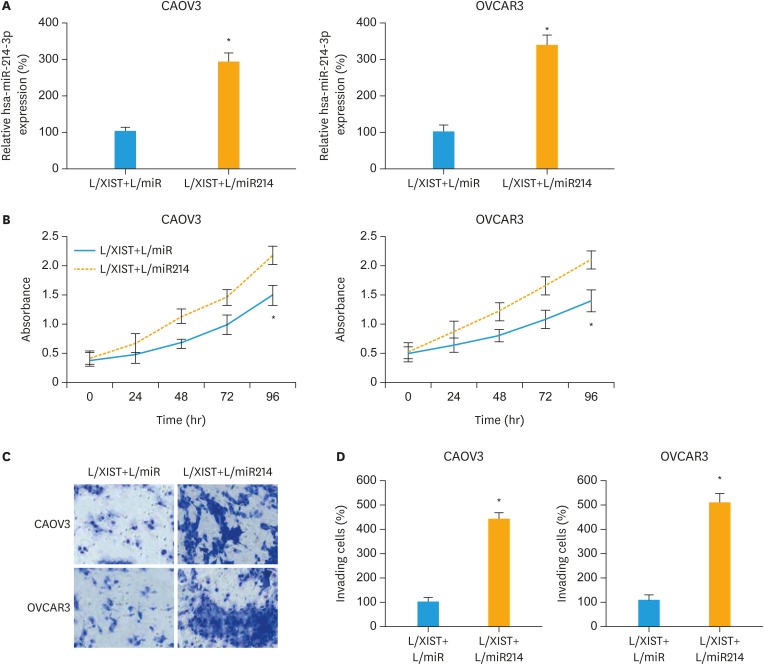
In CAOV3 and OVCAR3 cells transduced L/XIST, the downregulation of hsa-miR-214-3p was reversed by secondary lentiviral transduction. After that, qRT-PCR confirmed that hsa-miR-214-3p expressions were significantly upregulated in EOC cells stably transduced with a hsa-miR-214-3p mimics lentivirus (L/miR214), than in EOC cells transduced with a non-specific miRNA mimics lentivirus (L/miR) (Fig. 4A, p<0.05).
Fig. 4. Effects of hsa-miR-214-3p upregulation on XIST-mediated EOC proliferation and invasion. (A) CAOV3 and OVCAR3 cells, which were previously transduced with L/XIST, were then double transduced with L/miR or L/MIR214. qRT-PCR was applied to compare hsa-miR-214-3p expressions between EOC cells transduced with L/XIST+L/miR and EOC cells transduced with L/XIST+L/miR214. (B) A proliferation assay was performed for 96 hours to compare cancer proliferation between EOC cells transduced with L/XIST+L/miR and EOC cells transduced with L/XIST+L/miR214. Every 24-hour, absorbance was measured at 570 nm. (C) A transwell assay was also performed. 24 hours later, double-transduced EOC cells that invaded onto the bottoms of wells were stained by 0.1% crystal violet. (D) The numbers of invading cells were compared between EOC cells transduced with L/XIST+L/miR and EOC cells transduced with L/XIST+L/miR214.
EOC, epithelial ovarian cancer; L/XIST, lentiviral vectors expressing the full-length human XIST; qRT-PCR, quantitative reverse transcription polymerase chain reaction; XIST, X-inactive specific transcript.
*p<0.05.
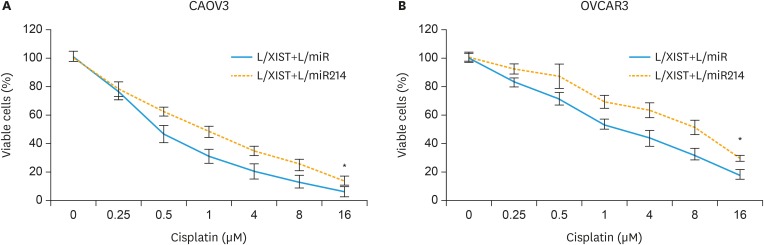
Then, we evaluated the biological functions in double-transduced EOC cells. Firstly, in the 96-hours cancer proliferation assay, it was demonstrated that cancer proliferation was significantly facilitated in CAOV3 and OVCAR3 cells transduced with L/XIST+L/miR214, than in EOC cells transduced with L/XIST+L/miR (Fig. 4B, p<0.05). Secondly, in the 24-hour transwell assay, it was shown that cancer invasion was also significantly enhanced in CAOV3 and OVCAR3 cells transduced with L/XIST+L/miR214, than in EOC cells transduced with L/XIST+L/miR (Fig. 4C and D, p<0.05). Thirdly, in the chemosensitivity assay, it demonstrated that cisplatin chemosensitivity was significantly decreased in CAOV3 and OVCAR3 cells transduced with L/XIST+L/miR214, than in EOC cells transduced with L/XIST+L/miR (Fig. 5A and B, p<0.05).
Fig. 5. Effects of hsa-miR-214-3p upregulation on XIST-mediated EOC chemosensitivity. (A, B) Double-transduced CAOV3 (A) and OVCAR3 (B) cells were tested with cisplatin at concentrations (μM) of 0, 0.25, 0.5, 1, 4, 8 and 16 for 24 hours. A viability assay was then performed to compare cisplatin sensitivity between EOC cells transduced with L/XIST+L/miR and EOC cells transduced with L/XIST+L/miR214.
EOC, epithelial ovarian cancer; L/XIST, lentiviral vectors expressing the full-length human XIST; XIST, X-inactive specific transcript.
*p<0.05.
Therefore, our experiments on double-transduced EOC cells clearly suggest that upregulation of hsa-miR-214-3p could inversely mediate the anticancer effects of XIST upregulation in EOC cell lines.
DISCUSSION
Mounting evidence has demonstrated that lncRNA may be dysregulated in various kinds of human cancers, and acting as prognostic biomarkers for cancer diagnosis and therapeutic targets for treatment options [5,7,8]. Among them, XIST was found to be aberrantly expressed in female cancer cell lines, including breast, ovarian or cervical cancers [14,17]. In addition, it was speculated that XIST might closely correlate with tumor suppressor BRCA1 to functionally affect cancer cell development [18,19,20]. In human EOC, lncRNA XIST was demonstrated to be downregulated in cancer cell lines and closely associated with chemotherapy resistance [10,15]. Yet, the regulatory mechanisms of XIST had never been identified in human EOC.
Thus, firstly in the present study, we took advantage of lentivirus-induced gene overexpression technology to stably overexpress (or upregulate) XIST in 2 EOC cells, CAOV3 and OVCAR3, which are known for their low or undetectable endogenous XIST expression levels [15]. We discovered that, XIST upregulation had significantly anticancer effects by suppressing EOC cell proliferation, invasion, in vivo tumor growth and increasing EOC cisplatin chemosensitivity. Thus, it seems like our data strongly supports a tumor suppressor role of XIST in human EOC. Interestingly, in other human cancers, such as non-small cell lung cancer and glioblastoma, XIST seemed to have opposite role as XIST acted as oncogenes in regulating cancer functions [12,13]. Most importantly, XIST was also reported to be relatively upregulated in other EOC cell lines, such as SKOV3 cells [14]. Therefore, caution and more research are needed before we may conclude XIST is primarily acting as a tumor suppressor in human EOC.
Secondly in the present study, we investigated the possible transcriptional factors to be associated with the anticancer modulation induced by XIST upregulation. Through bioinformatics study, we speculated epigenetic regulator, hsa-miR-214-3p, which is a known oncogene in EOC [16], might be the candidate. Our biochemical probes then confirmed this hypothesis. Dual-luciferase reporter assay and qRT-PCR analysis demonstrated that hsa-miR-214-3p can physically sponge on XIST, and hsa-miR-214-3p was inversely downregulated by XIST upregulation in EOC cells.
Thirdly and finally in the present study, functional involvement of hsa-miR-214-3p in modulating XIST-upregulation-induced anticancer effects was proven by our experiments of employing double transductions in CAOV3 and OVCAR3 cells. It showed, upregulating hsa-miR-214-3p significantly reversed the XIST-upregulation-induced suppression on EOC proliferation, invasion and cisplatin chemoresistance. To the best of knowledge, the present study is the first-ever research report discovering the sponging interaction between XIST and miR-214 in any known human or animal cell types.
It is worth noting that, downstream signaling pathway of hsa-miR-214-3p, presumably an apoptotic gene phosphatase and tensin homolog (PTEN) was involved in the oncogenic regulation of hsa-miR-214-3p in EOC [16]. At the present time, it is unknown whether XIST upregulation may also act on PTEN, either through indirectly sponging on miR-214 or direct interaction with PTEN, to exert its anticancer effects in EOC cells. Therefore, future work is much needed to elucidate the associated transcriptional network of XIST, in order to develop lncRNA-oriented EOC diagnosis and treatment strategies.
Overall, our study revealed a novel epigenetic regulation of an lncRNA/miRNA axis, XIST/miR-214-3p, in EOC. We demonstrated that XIST was significantly downregulated in EOC cells and its upregulation suppressed EOC cancer cell proliferation, invasion and chemoresistance, very likely through a sponging effect on inhibiting hsa-miR-214-3p.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Data curation: W.C., W.P.
- Formal analysis: W.C., L.C., W.P.
- Investigation: L.C.
- Methodology: W.C., L.C.
- Resources: L.D.
- Software: X.C., L.D.
- Validation: X.C., L.D.
- Writing - original draft: X.C.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Cannioto RA, Trabert B, Poole EM, Schildkraut JM. Ovarian cancer epidemiology in the era of collaborative team science. Cancer Causes Control. 2017;28:487–495. doi: 10.1007/s10552-017-0862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Vecchia C. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev. 2017;26:55–62. doi: 10.1097/CEJ.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 5.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieczorek E, Reszka E. mRNA, microRNA and lncRNA as novel bladder tumor markers. Clin Chim Acta. 2018;477:141–153. doi: 10.1016/j.cca.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 9.Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 10.Weakley SM, Wang H, Yao Q, Chen C. Expression and function of a large non-coding RNA gene XIST in human cancer. World J Surg. 2011;35:1751–1756. doi: 10.1007/s00268-010-0951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvador MA, Wicinski J, Cabaud O, Toiron Y, Finetti P, Josselin E, et al. The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin Cancer Res. 2013;19:6520–6531. doi: 10.1158/1078-0432.CCR-13-0877. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Sun CC, Gong C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun. 2016;478:811–817. doi: 10.1016/j.bbrc.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J, et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015;359:75–86. doi: 10.1016/j.canlet.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 14.Benoît MH, Hudson TJ, Maire G, Squire JA, Arcand SL, Provencher D, et al. Global analysis of chromosome X gene expression in primary cultures of normal ovarian surface epithelial cells and epithelial ovarian cancer cell lines. Int J Oncol. 2007;30:5–17. [PubMed] [Google Scholar]
- 15.Huang KC, Rao PH, Lau CC, Heard E, Ng SK, Brown C, et al. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol Cancer Ther. 2002;1:769–776. [PubMed] [Google Scholar]
- 16.Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami T, Zhang C, Taniguchi T, Kim CJ, Okada Y, Sugihara H, et al. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23:6163–6169. doi: 10.1038/sj.onc.1207808. [DOI] [PubMed] [Google Scholar]
- 18.Silver DP, Dimitrov SD, Feunteun J, Gelman R, Drapkin R, Lu SD, et al. Further evidence for BRCA1 communication with the inactive X chromosome. Cell. 2007;128:991–1002. doi: 10.1016/j.cell.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Pageau GJ, Lawrence JB. BRCA1 foci in normal S-phase nuclei are linked to interphase centromeres and replication of pericentric heterochromatin. J Cell Biol. 2006;175:693–701. doi: 10.1083/jcb.200602055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirchia SM, Ramoscelli L, Grati FR, Barbera F, Coradini D, Rossella F, et al. Loss of the inactive X chromosome and replication of the active X in BRCA1-defective and wild-type breast cancer cells. Cancer Res. 2005;65:2139–2146. doi: 10.1158/0008-5472.CAN-04-3465. [DOI] [PubMed] [Google Scholar]