Abstract
Objective
Cervical cancer is one of the most common malignant tumors. Our previous results showed that long non-coding RNA (lncRNA) XLOC_006390 plays an important role in cervical cancer. In this study, we have explored the mechanism of action of lncRNA XLOC_006390.
Methods
LncRNA XLOC_006390 was proposed to exercise its function as a competing endogenous RNA (ceRNA), and its potential targeted miRNAs was predicted through the database LncBase Predicted v.2. Two miRNAs, miR-331-3p, and miR-338-3p, were chosen for the study. Expression of miRNAs and lncRNA in cervical cancer cells and tissues was detected by reverse transcription polymerase chain reaction. To determine the correlation, silencing of XLOC_006390, over-expression of miR-331-3p, and miR-338-3p was performed in SiHa and Caski cell lines, respectively.
Results
Based on the interactive effect between miRNA and lncRNA, miR-331-3p and miR-338-3p were significantly downregulated in cervical cancer cells and tissues, and their expression levels were negatively related to that of lncRNA. Our results also showed that the expression of miR-331-3p target gene NRP2, miR-338-3p target genes PKM2, EYA2 was significantly downregulated when the XLOC_006390 was knocked down. Further, XLOC_006390 was found to facilitate cervical cancer tumorigenesis and metastasis by downregulating miR-331-3p and miR-338-3p expression.
Conclusion
Taken together, our study demonstrated that XLOC_006390 may serve as a ceRNA and reversely regulates the expression of miR-331-3p and miR-338-3p, thus facilitating cervical cancer tumorigenesis and metastasis.
Keywords: Cervical Cancer, Metastasis, Long Non-Coding RNA, microRNAs
INTRODUCTION
Cervical cancer is as one of the most common gynecological malignant tumors worldwide and has become a prominent public health issue [1]. It was reported that the incidence of cervical cancer ranked second in gynecological malignant tumors, with death rate ranking first among female malignant tumors of genital tract, hence becoming a disease threatening the health of females [2]. At present, operation, chemotherapy and radiotherapy are the most commonly used treatments for cervical cancer; however, due to drug resistance of cervical cancer cells against the therapeutic agents, chemotherapeutics see relatively poor effect in treating cervical cancer [1,3].
Competing endogenous RNAs (ceRNAs), a type of endogenous RNAs, compete for microRNA (miRNA) with other RNAs through miRNA response elements (MREs), which will influence the regulation of target genes by miRNA and ultimately the proliferation, apoptosis, migration, and invasion of many types of tumors [4]. Long non-coding RNAs (lncRNAs), a type of non-coding RNA with a sequence exceeding 200 nucleotides, exhibits multiple biological functions, such as chromatin modification, transcription, translation, splicing, and epigenetic regulation [5]. Various studies have shown that lncRNA is closely associated with the occurrence of cancers. It could regulate DNA methylation [6], histone modification [7], chromatin remodeling [8], and serve as the precursor of miRNA [9], ultimately inducing the occurrence of cancers. Recent research has showed that lncRNA could compete for MREs with the driver genes closely related to cancer occurrence and development as ceRNA, which would weaken the inhibition of miRNA upon target genes, indirectly regulate the expression level of target genes, and ultimately participate in cancer regulation process [10]. It has been demonstrated that lncRNA XLOC_006390 expression levels are associated with neural invasion, a histopathological characteristic, and an important prognostic factor of pancreatic cancer (PC) [11]. Microarray analysis results indicated that the level of lncRNA XLOC_006390 was significantly increased in the tissue of PC [12]. Our previous study showed that lncRNA XLOC_006390 saw high expression in cervical cancer tissues, and lncRNA XLOC_006390 influenced cervical cancer cell proliferation and migration by regulating downstream SET8 proteins [13]. However, how lncRNA XLOC_006390 specifically regulates SET8 protein remains unclear. Given the site of lncRNA XLOC_006390 and coding SET8 protein genes in chromosomes as well as lncRNA XLOC_006390 acting as intergenic lncRNA with a sequence reaching 4,899 nt, it is speculated that lncRNA XLOC_006390 may exercises its function as ceRNA. This study screens out absorbed miRNAs from miRNA pool with differential expression of cervical cancer based on the sequence characteristics of lncRNA XLOC_006390 and further analyzes the relationship among the miRNAs screened out for functional verification.
MATERIALS AND METHODS
1. miRNA-target prediction of XLOC_006390
miRNA-target prediction was performed by LncBase Predicted v.2 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex) [14]. The threshold was set as score >0.9.
2. Clinical samples
Cervical cancer specimens and corresponding adjacent normal tissues were collected from 20 cancer patients undergoing surgery for cervical cancer in Affiliated Hospital of Qingdao University, China. The Medical Ethics Committee of Affiliated Hospital of Qingdao University approved this study (2015-08-04-01). Written informed consent was obtained from all the 20 participants.
3. Cell culture and transfection
Cervical cancer cell lines, SiHa, HeLa, CaSki, c-41, and c-33A (ATCC; USA), were cultured in RPMI-1640 (Gibco™; Thermo Fisher Scientific, Waltham, MA, USA) containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS), and incubated at 37°C, and 5% CO2. The XLOC_006390 siRNA (5′-UUA AGC UAA CGU UUA CCG CAG TT-3′) and miRNA mimics and inhibitors were purchased from GenePharma (Shanghai, China). Knockdown and overexpression of XLOC_006390 was carried out by transfection with the XLOC_006390 siRNA and recombinant XLOC_006390 pcDNA3.1(+) plasmid, respectively. Both SiHa and Caski cells were plated in 6-well plates for 24 hours and then transfected for 48 hours with the XLOC_006390 siRNA or negative control siRNA using Lipofectamine 2000 (Thermo Fisher Scientific). The transfected cells were used for the following studies.
4. Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using the CCK-8 kit (Dojindo Molecular Technologies, Inc., Shanghai, China). After the transfections, 100 µL cells (5×103 cells per well) were seeded in 96-well plates. The 10 µL of CCK-8 solution was added to each well at 0, 24, 48, and 72 hours. After 1 hour of incubation, the absorbance was measured at 450 nm on a microplate reader (Promega Corporation, Fitchburg, WI, USA).
5. Flow cytometry analysis of cell apoptosis
Apoptotic cells were identified using the Propidium Iodide (PI) and FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. After 24 hours of transfection, SiHa or Caski cells were cultured for 72 hours before determining apoptosis. The cells were harvested, washed twice with cold phosphate-buffered saline (PBS), and resuspended in 1× binding buffer. Then, the cells were stained with 5 µL Annexin V-FITC for 15 minutes and then 5 µL PI for 10 minutes in the dark at room temperature. The cells were examined using a FACSCanto II flow cytometer (BD Biosciences Europe, Heidelberg, Germany).
6. Wound healing assay
SiHa or Caski cells (5×104) were seeded in 24-well plates and incubated at 37°C. The confluent cells were scratched with a 200-µL pipette tip. The plates were washed with fresh medium after 24 hours incubation to remove non-adherent cells. The plate morphology was photographed, and the wound area was determined using an inverted microscope (Olympus, Tokyo, Japan).
7. Cell invasion assay
Cell invasion assay was performed using a Transwell system (24-wells; Corning Costar, Lowell, MA, USA). Briefly, SiHa or Caski cells (1×105 per well) were trypsinized and seeded into the upper chamber with serum free opti-MEM media. The low chamber was filled with 800 µL medium containing 10% FBS. After 48 hours of incubation, the cells on the lower side of the filter were fixed in 3.8% formaldehyde for 20 minutes and stained with 0.1% crystal violet solution. The number of cells in five randomly selected fields was counted under a light microscope and analyzed statistically.
8. Immunofluorescence
The cells were fixed with pre-chilled 4% paraformaldehyde. Then, the cells were washed thrice with PBS, permeabilized with PBS containing 0.1% Triton X-100, and washed thrice with PBS containing 0.05% Tween 20. PBS containing 3% bovine serum albumin (BSA) was then applied to block the samples for 1 hour at room temperature. The cells were incubated with antibodies against NRP2, PKM2, and EYA2 (1:2,000; Abcam, Cambridge, MA, USA) for 1 hour at room temperature. After washing, the cells were incubated with Alexa-Fluor-488-conjugated anti-rabbit-IgG for NRP2, PKM2 detection, and Alexa-Fluor-594-conjugated anti-mouse-IgG for EYA2 detection (1:1,000, Invitrogen™; Thermo Fisher Scientific) as well as DAPI. The samples were mounted and photographed with Zeiss Axio Imager Z1 fluorescence microscope (Zeiss, German).
9. RNA extraction and quantitative reverse transcription polymerase chain reaction (RT-PCR)
RNA was isolated from cervical cancer cells and tumor samples with TRIzol reagent (Invitrogen™; Thermo Fisher Scientific) and RNeasy Plus Micro Kit (QIAGEN, Germantown, MD, USA) according to the manufacturer's instructions. Then, reverse transcription was conducted to synthesize the Bestar qPCR RT Kit (DBI Bioscience, Shanghai, China). The quantitative RT-PCR was performed in Applied Biosystems 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA), using 20 ng template in 25-µL reaction volume with 2×Power SYBR® Green PCR Master Mix (Invitrogen™; Thermo Fisher Scientific) and gene specific primer pairs. The gene expression levels for all miRNAs and protein genes were normalized to U6 RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression, respectively, using the comparative Ct method. The data is expressed as the mean±standard deviation (SD) of 3 independent experiments.
10. Western blot analysis
Cervical cancer cells were harvested and lysed in the RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations were determined using the BCA protein assay kit (Thermo Fisher Scientific). The proteins (30 μg) were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking with BSA, the membranes were incubated with primary antibodies against NRP2, EYA2, PKM2, and GAPDH (Abcam). GAPDH was loaded as an internal reference. The bands were treated with goat anti-rabbit IgG-HRP secondary antibody (1:2,000; Abcam) and were developed using chemiluminescence substance (Thermo Fisher Scientific).
11. Statistical analysis
The data was analyzed with Prism 5.0 (GraphPad Software, San Diego, CA, USA). All the experiments were performed in triplicates and the data was expressed as the means±SD). One-way analysis of variance with multiple comparisons using Dunnett's test was used for multiple comparison. A value of p<0.05 was considered as statistically significantly.
RESULTS
1. XLOC_006390 reversely regulated the expression of miR-331-3p and miR-338-3p
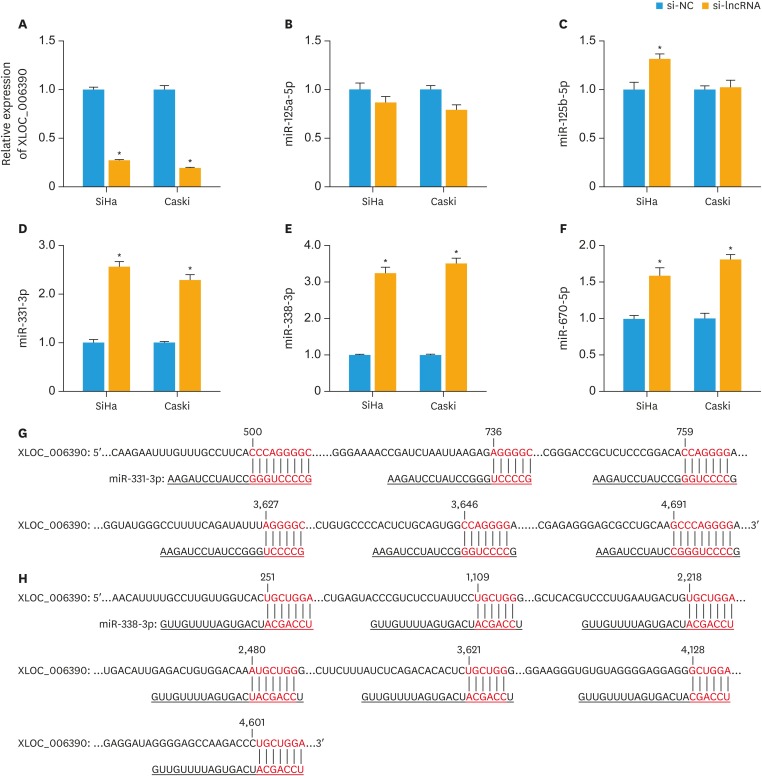
To examine whether XLOC_006390 can serve as a ceRNA in the regulation of predicted miRNAs, we knocked down XLOC_006390 expressions in both SiHa and Caski cells by siRNA transfection. The efficacy of knockdown was confirmed by RT-PCR analysis (p<0.05 vs. the negative comtrol [NC] group; Fig. 1A). The expression of 6 miRNAs, miR-125b-5p, miR-125a-5p, miR-7-2-3p, miR-670-5p, miR-331-3p, and miR-338-3p, was detected after transfection based on the prediction of XLOC_006390 sponge miRNAs in LncBase Predicted v.2 database and previous studies [15,16,17,18,19,20]. Among these miRNAs, the expression of miR-331-3p, and miR-338-3p was significantly increased when the XLOC_006390 was knocked down (p<0.05 vs. the NC group; Fig. 1B-F), while no increase in expression of miR-7-2-3p was observed. MiR-331-3p and miR-338-3p were reported to regulate multiple cancer tumorigenesis and metastasis by targeting many genes in human cells (listed in Supplementary Table 1, Supplementary Figs. 1, and 2) [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Therefore, miR-331-3p and miR-338-3p were chosen for further studies. The potential binding sites for XLOC_006390 on miR-331-3p and miR-338-3p are indicated in Fig. 1G and H.
Fig. 1. XLOC_006390 reversely regulates the expression of miR-331-3p and miR-338-3p. (A) The knockdown efficacy of XLOC_006390 in SiHa and Caski cells confirmed by RT-PCR analysis. (B-F) The expression of miR-125a-5p (B), miR-125b-5p (C), miR-331-3p (D), miR-338-3p (E), and miR-670-5p (F) detected by RT-PCR. (G, H) Potential binding sites for XLOC_006390 on miR-331-3p and miR-338-3p, respectively.
RT-PCR, reverse transcription polymerase chain reaction; si-NC, small RNA without targeting genes.
*p<0.05 vs. the si-NC group.
2. miR-331-3p and miR-338-3p are significantly downregulated in cervical cancer
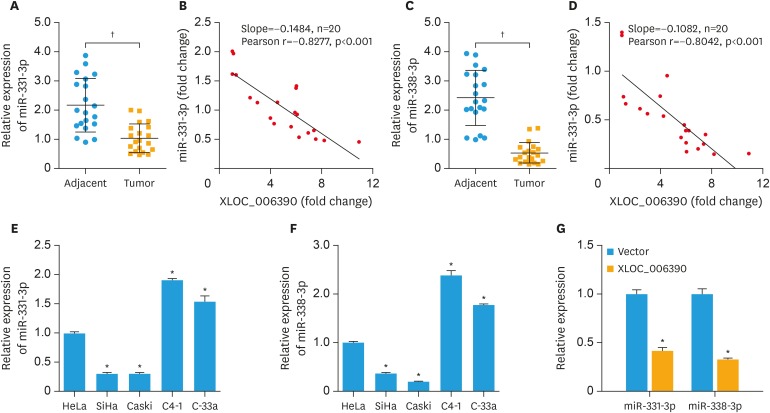
In order to evaluate the changes in expression levels of miR-331-3p and miR-338-3p in cervical cancer, 20 cancer patients undergoing surgery for cervical cancer were recruited in this study. The expression levels of miR-331-3p and miR-338-3p were significantly downregulated in the cervical cancer tissues compared to the corresponding adjacent normal tissues (p<0.001; Fig. 2A and C). In addition, the expression of XLOC_006390 was reversely correlated to the expression of miR-331-3p and miR-338-3p (Fig. 2B and D). The results indicated a negative correlation between XLOC_006390 and miR-331-3p or miR-338-3p expressions in cervical cancer. The expression levels of miR-331-3p and miR-338-3p in cervical cancer cell lines were further determined. As shown in Fig. 2E and F, the expression levels of miR-331-3p and miR-338-3p were significantly downregulated in both SiHa and Caski cells, whereas those were significantly upregulated in C4-1 and C-33a cells compared to Hela cells (p<0.05). The expression of miR-331-3p and miR-338-3p in all these cell lines showed a reversed pattern compared to XLOC_006390 as reported by our previous study [13]. Moreover, XLOC_006390 was overexpressed in C4-1 cells, and the result (Fig. 2G) showed that the overexpression of XLOC_006390 significantly downregulated the expression of both miR-331-3p and miR-338-3p (p<0.05 vs. the vector group).
Fig. 2. Expression of miR-331-3p and miR-338-3p was significantly downregulated in cervical cancer. (A, B) The expression level of miR-331-3p was significantly downregulated in the cervical cancer tissues compared to the corresponding adjacent normal tissues, and the expression of XLOC_006390 was reversely correlated to the expression of miR-331-3p. (C, D) The expression level of miR-338-3p was significantly downregulated in the cervical cancer tissues compared to the corresponding adjacent normal tissues, and the expression of XLOC_006390 was reversely correlated to the expression of miR-338-3p. (E, F) The expression level of miR-331-3p and miR-338-3p in HeLa, SiHa, Caski, C4-1, and C-33a cells detected by RT-PCR. (G) Over-expression of XLOC_006390 significantly downregulated the expression of both miR-331-3p and miR-338-3p in C4-1 cells.
RT-PCR, reverse transcription polymerase chain reaction.
*p<0.05, †p<0.001 vs. the adjacent normal tissues, the HeLa group or Vector group.
3. Knockdown of XLOC_006390 downregulated the expression of miR-331-3p and miR-338-3p target genes
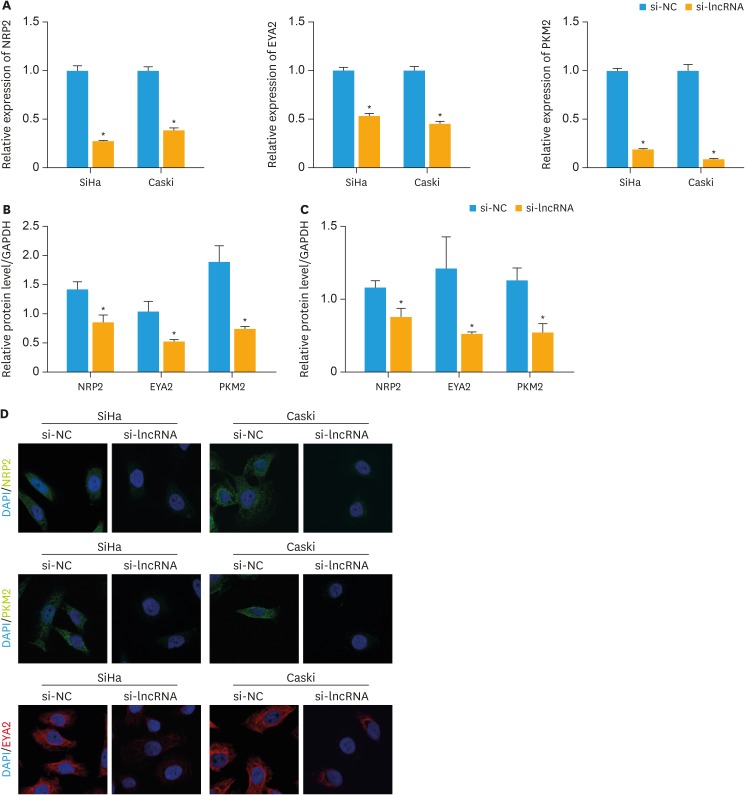
To investigate whether XLOC_006390 could affect the related target genes of miR-331-3p and miR-338-3p, the expression of miR-331-3p target gene NRP2 and miR-338-3p target genes PKM2 and EYA2 was determined. As shown in Fig. 3A, mRNA expression of NRP2, PKM2, and EYA2 was significantly downregulated when XLOC_006390 was knocked down (p<0.05 vs. the NC group). In addition, similar patterns were observed by the western blot analysis (Fig. 3B and C, Supplementary Fig. 2A and B) and the immunofluorescence (Fig. 3D). These findings indicated that XLOC_006390 could negatively regulate the expression of miR-331-3p and miR-338-3p thereby thus affecting the expression of related miRNA target genes.
Fig. 3. Knockdown of XLOC_006390 downregulated the expression of miR-331-3p and miR-338-3p target genes. (A) The expression of miR-331-3p target gene NRP2 and miR-338-3p target genes PKM2 and EYA2 in SiHa and Caski cells detected by RT-PCR. (B, C) Western blot analysis of miR-331-3p target gene NRP2 and miR-338-3p target genes PKM2 and EYA2 in SiHa (B) and Caski (C) cells. (D) Immunofluorescence study of the expressions of NRP2, PKM2, and EYA2 in the XLOC_006390 knockdown SiHa and Caski cells.
si-NC, small RNA without targeting genes.
*p<0.05 vs. the si-NC group.
4. Upregulation of miR-331-3p and miR-338-3p inhibited cervical cancer cell migration and invasion and enhanced the cell apoptosis
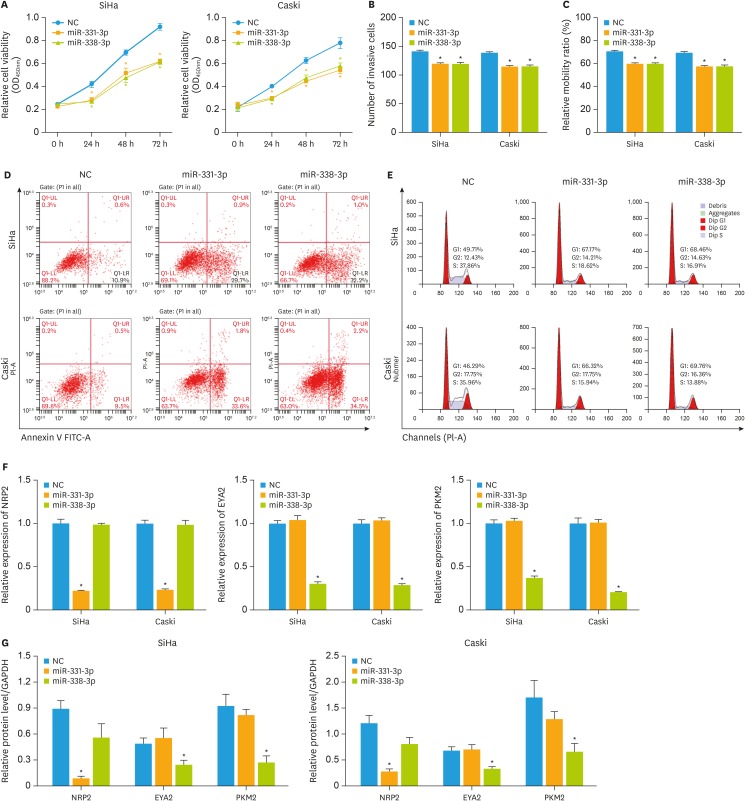
To further confirm the roles of miR-331-3p and miR-338-3p in the cervical cancer tumorigenesis and metastasis, we determined the overexpression of miR-331-3p and miR-338-3p in cervical cancer cells. After transfection for 24 hours, the viability of the cells was significantly reduced when compared to NC group (p<0.05) as detected by CCK-8 (Fig. 4A). In addition, the Transwell assay results showed that miR-331-3p and miR-338-3p over-expression inhibited the migration and invasion ability of cervical cancer cells (Fig. 4B and C), whereas increased the rate of apoptosis of SiHa and Caski cells (Fig. 4D). The cell cycle was also blocked by the miR-331-3p and miR-338-3p overexpression (Fig. 4E). Next, the mRNA and protein expression levels of the miR-331-3p and miR-338-3p target genes were detected. The RT-PCR results (Fig. 4F) showed that miR-331-3p and miR-338-3p overexpression could significantly decrease the expression of the target genes (p<0.05 vs. the NC group), which was also confirmed by the western blot analysis (Fig. 4G, Supplementary Fig. 2C). All these results indicated that miR-331-3p and miR-338-3p play critical roles in the cervical cancer tumorigenesis and metastasis.
Fig. 4. Up-regulations of miR-331-3p and miR-338-3p inhibit cervical cancer cell migration and invasion and enhance the cell apoptosis. (A) The viability of the cells transfected by miR-331-3p and miR-338-3p mimics was detected by CCK-8. Cell invasion (B) and migration (C) were detected by Transwell assay and wound healing assay, respectively. The rate of apoptosis (D) and cell cycle (E) of both SiHa and Caski cells was determined by flow cytometry analysis. The expression level of mRNA (F) and protein (G) of the miR-331-3p and miR-338-3p target genes was detected by RT-PCR and western blot analysis.
CCK-8, Cell Counting Kit-8; NC, negative control; RT-PCR, reverse transcription polymerase chain reaction.
*p<0.05 vs. the NC group.
5. XLOC_006390 facilitated cervical cancer tumorigenesis and metastasis by regulating miR-331-3p and miR-338-3p expression
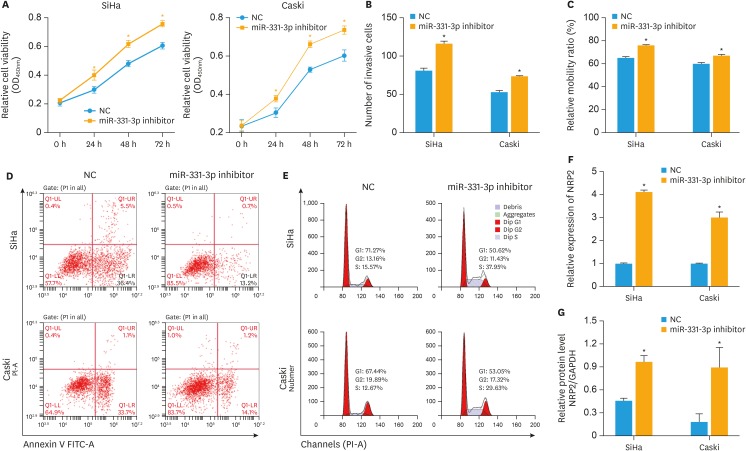
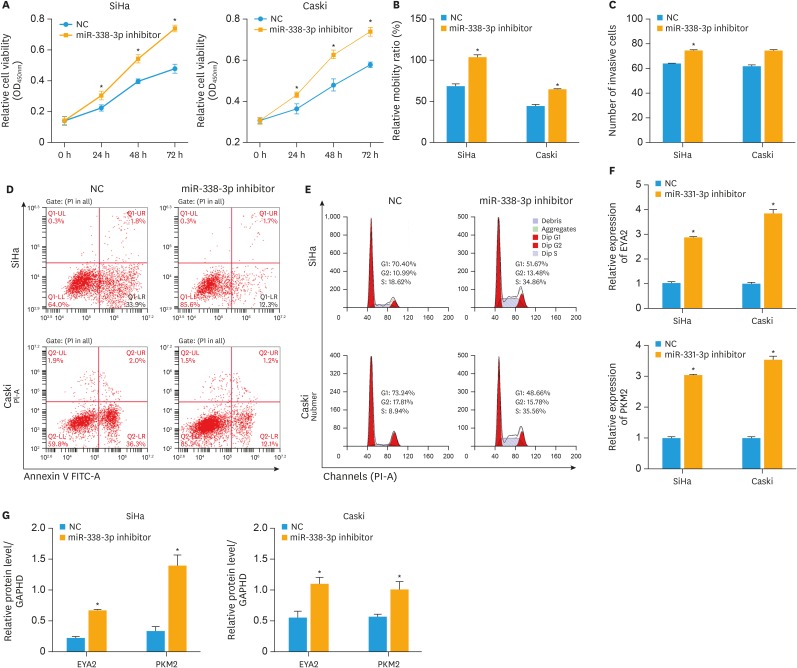
XLOC_006390 siRNA was co-transfected with miR-331-3p or miR-338-3p inhibitors. After transfection for 24 h, the viability of the cells was significantly increased compared to NC group (p<0.05) as detected by CCK-8 (Figs. 5A and 6A). The Transwell assay results showed that co-transfection of XLOC_006390 siRNA and miR-331-3p or miR-338-3p inhibitors increased invasion (Figs. 5B and 6B), migration (Figs. 5C and 6C), and ability of cervical cancer cells. Moreover, the flow cytometry results showed that rate of apoptosis of SiHa and Caski cells was decreased compared to NC group (Figs. 5D and 6D) and the cell cycle was also stimulated by the co-transfection of XLOC_006390 siRNA and miR-331-3p or miR-338-3p inhibitors (Figs. 5E and 6E). Next, the RT-PCR results (Figs. 5F and 6F) showed that co-transfection of XLOC_006390 siRNA and miR-331-3p or miR-338-3p inhibitors significantly increased the expression of their target genes (p<0.05 vs. the NC group). The same pattern was also confirmed by the western blot analysis (Figs. 5G and 6G, Supplementary Fig. 2D and E). All these results indicated that XLOC_006390 could facilitate cervical cancer tumorigenesis and metastasis via regulation of miR-331-3p and miR-338-3p.
Fig. 5. XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis by regulating of miR-331-3p. XLOC_006390 siRNA was co-transfected with miR-331-3p inhibitor. (A) The viability of the SiHa and Caski cells transfected by miR-331-3p inhibitor was detected by CCK-8. Cell invasion (B) and migration (C) capacities were detected by Transwell assay and wound healing assay, respectively. The rate of apoptosis (D) and cell cycle (E) of both SiHa and Caski cells was determined by flow cytometry analysis. The expression level of mRNA (F) and protein (G) of the miR-331-3p target gene was determined by RT-PCR and Western blot analysis.
CCK-8, Cell Counting Kit-8; NC, negative control; RT-PCR, reverse transcription polymerase chain reaction.
*p<0.05 vs. the NC group.
Fig. 6. XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis by regulating of miR-338-3p. XLOC_006390 siRNA was co-transfected with miR-338-3p inhibitor. (A) The viability of the SiHa and Caski cells transfected by miR-338-3p inhibitor was detected by CCK-8. Cell invasion (B) and migration (C) capacities were detected by Transwell assay and wound healing assay, respectively. The rate of apoptosis (D) and cell cycle (E) of both SiHa and Caski cells was determined by flow cytometry analysis. The expression level mRNA (F) and protein (G) of the miR-338-3p target genes was determined by RT-PCR and western blot analysis.
CCK-8, Cell Counting Kit-8; NC, negative control; RT-PCR, reverse transcription polymerase chain reaction.
*p<0.05 vs. the NC group.
DISCUSSION
Cervical cancer is one of the most common malignant tumors, and its incidence is only preceded by breast cancer [1,2]. In recent years, more attention is paid to study the role of lncRNA in cervical cancer [39,40]. In our previous study, lncRNA XLOC_006390 was found to exhibit abnormal expression in cervical cancer cells and influence cervical cancer cell proliferation and migration by regulating downstream SET8 proteins [13].
MiRNA, a proto-oncogene and anti-oncogene, is closely associated with the occurrence of cancer [41]. Various studies have studied the correlation between miRNA and cervical cancer incidence is increasing and showed a close relationship between them [42,43,44]. Based on the interactive effect between miRNA and lncRNA, this study screened out the miRNAs that interact with lncRNA XLOC_006390 and further analyzes its molecular mechanism. The target miRNAs of XLOC_006390 were predicted by LncBase Predicted v.2. Among the predicted miRNAs, miR-125b-5p, miR-125a-5p, miR-940, miR-7-2-3p, miR-670-5p, miR-331-3p, miR-338-3p, and miR-944 were all reported to play an important role in different cancers. Moreover, miR-944, miR-940, miR-7, and miR-331-3p were also involved in cervical cancer, among which miR-944 and miR-940 were demonstrated to exercise the function of promoting cervical cancer cell proliferation, migration, and invasion [45,46]. Considering that XLOC_006390 also promotes the survival of cervical cancer cells, our experiment excluded the study of miR-944 and miR-940 from the angle of ceRNA role of lncRNA. Both miR-7 and miR-331-3p were reported to inhibit the metastasis and invasion of cervical cancer [23,47]. However, the mechanism by which miR-7 and miR-331 inhibit the survival of cervical cancer cells remains unclear. In our study, based on the prediction of XLOC_006390 sponge miRNAs in LncBase Predicted v.2 database and previous studies [15,16,17,18,19,20], 6 miRNAs, miR-125b-5p, miR-125a-5p, miR-7-2-3p, miR-670-5p, miR-331-3p, and miR-338-3p, were chosen for further analysis. Previous evidences have demonstrated overexpression of miR-670-5p in HCC and in hepatoma-derived cells Hep3B. The expression of miR-670-5p promoted cellular proliferation and decreased expression of PROX1. On the other hand, overexpression of PROX1 significantly inhibited cell proliferation, thereby implying that miR-670-5p plays an important role in enhancing proliferation activity by modulating PROX1 expression at posttranscriptional level [18]. The effect of overexpression of miR-670-5p was similar to that of lncRNA XLOC_006390 on cancer cells. lncRNA can act as ceRNA to facilitate cervical cancer progression through negative regulation of miRNA [48]. Of note, we found that the levels of miR-331-3p and miR-338-3p were significantly increased when the XLOC_006390 was knocked down, indicating a sponge role of lncRNA XLOC_006390 to miR-331-3p and miR-338-3p. Moreover, the expression levels of miR-331-3p and miR-338-3p were significantly downregulated in cervical cancer tissues compared to the corresponding adjacent normal tissues, which was in line with our aim on the regulation of tumor genesis via lncRNA sponging miRNAs. Further, the expression of miR-331-3p target gene NRP2 and miR-338-3p target genes PKM2 and EYA2 was significantly downregulated when the XLOC_006390 was knocked down. These results indicated that XLOC_006390 may serve as a ceRNA and reversely regulates the expression of miR-331-3p and miR-338-3p in cervical cancer cells.
The above finding showed that XLOC_006390, as carcinogenic gene regulating cervical cancer progression based on ceRNA mechanism, exhibited an obviously high expression in cervical cancer tissues. It competitively binds miR-331-3p and miR-338-3p with mRNAs related to cervical cancer development, antagonizes the inhibiting effect of miRNA against the target genes, indirectly upregulating the expression level of mRNA, and promoting the occurrence and development of cervical cancer. In this study, upregulation of miR-331-3p and miR-338-3p was observed to inhibit the cervical cancer cell migration and invasion and enhance the cell apoptosis, which indicates that miR-331-3p and miR-338-3p play critical roles in the cervical cancer tumorigenesis and metastasis. Due to different effects of target genes regulated by lncRNAs, lncRNAs are involved in multiple facets of cervical cancer process, including cervical cancer cell proliferation, migration and invasion, prognosis, and survival of patients [10]. XLOC_006390 was found to facilitate cervical cancer tumorigenesis and metastasis by regulating miR-331-3p and miR-338-3p. Interestingly, in our previous study, we shed light on the role of lncRNA XLOC_006390 in the progression and metastasis of cervical cancer [13]. Based on the prediction of XLOC_006390 sponge miRNAs in LncBase Predicted v.2 database, miR-7-2-3p was included. However, no expression of miR-7-2-3p was observed in control or experimental group treated with si-lncRNA XLOC_006390. Similar role of miR-7 was found in the regulation of SET8 in pancreatic cancer cells as that of lncRNA XLOC_006390 in cervical cancer cells [49]. We thus speculated that the abundance of miRNA might vary in different organs and tissues, for miR-122 is highly expressed in liver. In addition, non-coding RNAs (lncRNA and miRNA) are involved in the regulation of a specific biological process, whereas a type of non-coding RNA participates in the modulation of distinct processes. Our previous study illustrated the effect of lncRNA XLOC_006390 on cervical cancer proliferation through the regulation of SET8. However, in this study, we showed that lncRNA XLOC_006390 could affect cervical cancer tumorigenesis by modulating NRP2, EYA2, and PKM2; of which, the sponge of lncRNA XLOC_006390 to miR-331-3p and miR-338-3p played a key role. However, future studies focusing on the clinical value of lncRNA XLOC_006390 within in vivo model are required to elaborate the mechanism systematically.
Our study showed that XLOC_006390 may serve as a ceRNA and reversely regulates the expression of miR-331-3p and miR-338-3p, thus facilitating cervical cancer tumorigenesis and metastasis. Difference in expression level of XLOC_006390 in cervical cancer could be considered as the new molecular marker of cervical cancer progression and diagnosis and provide novel insights for the treatment of cervical cancer.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.X., W.Y.
- Data curation: L.X., W.Y.
- Formal analysis: L.X., W.Y.
- Investigation: L.X., W.Y.
- Project administration: L.X., W.Y.
- Software: L.X.
- Validation: W.Y.
- Visualization: W.Y.
- Writing - original draft: L.X., W.Y.
- Writing - review & editing: W.Y.
SUPPLEMENTARY MATERIALS
Target genes of miR-331-3p and miR-338-3p involving in cancer tumorigenesis and metastasis
Cell invasion and migration capacities were detected by Transwell assay and wound healing assay, respectively. (A) Cell invasion and migration capacities after SiHa and Caski cells were transfected with miR-331-3p or miR-338-3p mimics. (B) Cell invasion and migration capacities after XLOC_006390 siRNA was co-transfected with miR-331-3p inhibitor in SiHa and Caski cells. (C) Cell invasion and migration capacities after XLOC_006390 siRNA was co-transfected with miR-338-3p inhibitor in SiHa and Caski cells.
Western blotting of miR-331-3p target gene NRP2 and miR-338-3p target genes PKM2 and EYA2 in SiHa and Caski cells. (A, B) NRP2, PKM2, and EYA2 in the XLOC_006390 knockdown SiHa and Caski cells. (C) NRP2, PKM2, and EYA2 protein level in SiHa and Caski cells after up-regulations of miR-331-3p and miR-338-3p. (D) NRP2 protein level in SiHa and Caski cells after inhibition of miR-331-3p. (E) PKM2 and EYA2 protein level in SiHa and Caski cells after inhibition of miR-338-3p.
References
- 1.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther. 2016;10:1885–1895. doi: 10.2147/DDDT.S106412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front Genet. 2014;5:8. doi: 10.3389/fgene.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14:42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Sun H, Wang H. Long noncoding RNAs in DNA methylation: new players stepping into the old game. Cell Biosci. 2016;6:45. doi: 10.1186/s13578-016-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Leary VB, Hain S, Maugg D, Smida J, Azimzadeh O, Tapio S, et al. Long non-coding RNA PARTICLE bridges histone and DNA methylation. Sci Rep. 2017;7:1790. doi: 10.1038/s41598-017-01875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han P, Chang CP. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015;12:1094–1098. doi: 10.1080/15476286.2015.1063770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, et al. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15–25. doi: 10.1097/00006676-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Li Z, Zheng S, Zhou Y, Zhao L, Ye H, et al. Expression profile of long non-coding RNAs in pancreatic cancer and their clinical significance as biomarkers. Oncotarget. 2015;6:35684–35698. doi: 10.18632/oncotarget.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luan X, Wang Y. Long non-coding RNA XLOC_006390 promotes cervical cancer proliferation and metastasis through the regulation of SET domain containing 8. Oncol Rep. 2017;38:159–166. doi: 10.3892/or.2017.5663. [DOI] [PubMed] [Google Scholar]
- 14.Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, et al. DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res. 2016;44:D231–D238. doi: 10.1093/nar/gkv1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Zhan M, Chen T, Chen W, Zhang Y, Xu S, et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep. 2017;7:43109. doi: 10.1038/srep43109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia CW, Sun Y, Zhang TT, Lu ZH, Chen J. Effects of miR-125a-5p on cell proliferation, apoptosis and cell cycle of pancreatic cancer cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:415–421. doi: 10.3881/j.issn.1000-503X.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Shen Z, Qin X, Yan M, Li R, Chen G, Zhang J, et al. Cancer-associated fibroblasts promote cancer cell growth through a miR-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget. 2017;8:1290–1303. doi: 10.18632/oncotarget.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi C, Xu X. MiR-670-5p induces cell proliferation in hepatocellular carcinoma by targeting PROX1. Biomed Pharmacother. 2016;77:20–26. doi: 10.1016/j.biopha.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Luo H, Li X, Tian X, Peng B, Liu S, et al. miR-331-3p functions as an oncogene by targeting ST7L in pancreatic cancer. Carcinogenesis. 2018;39:1006–1015. doi: 10.1093/carcin/bgy074. [DOI] [PubMed] [Google Scholar]
- 20.Sun F, Yu M, Yu J, Liu Z, Zhou X, Liu Y, et al. miR-338-3p functions as a tumor suppressor in gastric cancer by targeting PTP1B. Cell Death Dis. 2018;9:522. doi: 10.1038/s41419-018-0611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao D, Sui Y, Zheng X. MiR-331-3p inhibits proliferation and promotes apoptosis by targeting HER2 through the PI3K/Akt and ERK1/2 pathways in colorectal cancer. Oncol Rep. 2016;35:1075–1082. doi: 10.3892/or.2015.4450. [DOI] [PubMed] [Google Scholar]
- 22.Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem. 2009;284:24696–24704. doi: 10.1074/jbc.M109.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii T, Shimada K, Asano A, Tatsumi Y, Yamaguchi N, Yamazaki M, et al. MicroRNA-331-3p suppresses cervical cancer cell proliferation and E6/E7 expression by targeting NRP2. Int J Mol Sci. 2016;17:1351. doi: 10.3390/ijms17081351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epis MR, Giles KM, Beveridge DJ, Richardson KL, Candy PA, Stuart LM, et al. miR-331-3p and Aurora Kinase inhibitor II co-treatment suppresses prostate cancer tumorigenesis and progression. Oncotarget. 2017;8:55116–55134. doi: 10.18632/oncotarget.18664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Zhang J, Xiong D, Wang D, Wu T, Huang A, et al. Hsa-miR-331-3p inhibits VHL expression by directly targeting its mRNA 3′-UTR in HCC cell lines. Acta Biochim Pol. 2015;62:77–82. doi: 10.18388/abp.2014_779. [DOI] [PubMed] [Google Scholar]
- 26.Chang RM, Yang H, Fang F, Xu JF, Yang LY. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology. 2014;60:1251–1263. doi: 10.1002/hep.27221. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Chen J, Wang D, Peng H, Tan X, Xiong D, et al. Upregulated in hepatitis B virus-associated hepatocellular carcinoma cells, miR-331-3p promotes proliferation of hepatocellular carcinoma cells by targeting ING5. Oncotarget. 2015;6:38093–38106. doi: 10.18632/oncotarget.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X, Guo L, Ji J, Zhang J, Zhang J, Chen X, et al. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun. 2010;398:1–6. doi: 10.1016/j.bbrc.2010.05.082. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Pan M, Han L, Lu H, Hao X, Dong Q. miR-338-3p suppresses neuroblastoma proliferation, invasion and migration through targeting PREX2a. FEBS Lett. 2013;587:3729–3737. doi: 10.1016/j.febslet.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 30.Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma H, et al. MiR-338-3p inhibits epithelial-mesenchymal transition in gastric cancer cells by targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget. 2015;6:15222–15234. doi: 10.18632/oncotarget.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu X, Tan D, Hou Z, Hu Z, Liu G. miR-338-3p is down-regulated by hepatitis B virus X and inhibits cell proliferation by targeting the 3′-UTR region of CyclinD1. Int J Mol Sci. 2012;13:8514–8539. doi: 10.3390/ijms13078514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Zhao L, Fang Q, Sun J, Zhang S, Zhan C, et al. MiR-338-3p inhibits hepatocarcinoma cells and sensitizes these cells to sorafenib by targeting hypoxia-induced factor 1α. PLoS One. 2014;9:e115565. doi: 10.1371/journal.pone.0115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Shao G, Lin X, Liu Y, Yang Z. MiR-338-3p inhibits the growth and invasion of non-small cell lung cancer cells by targeting IRS2. Am J Cancer Res. 2017;7:53–63. [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JT, Yao KH, Hua L, Zhang LP, Wang CY, Zhang JJ. MiR-338-3p inhibits the proliferation and migration of gastric cancer cells by targeting ADAM17. Int J Clin Exp Pathol. 2015;8:10922–10928. [PMC free article] [PubMed] [Google Scholar]
- 35.Shang C, Hong Y, Guo Y, Xue YX. Mir-338-3p inhibits malignant biological behaviors of glioma cells by targeting MACC1 gene. Med Sci Monit. 2016;22:710–716. doi: 10.12659/MSM.897055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Shi B, Chen J, Hu L, Zhao C. MiR-338-3p targets pyruvate kinase M2 and affects cell proliferation and metabolism of ovarian cancer. Am J Transl Res. 2016;8:3266–3273. [PMC free article] [PubMed] [Google Scholar]
- 37.Han J, Li J, Tang K, Zhang H, Guo B, Hou N, et al. miR-338-3p confers 5-fluorouracil resistance in p53 mutant colon cancer cells by targeting the mammalian target of rapamycin. Exp Cell Res. 2017;360:328–336. doi: 10.1016/j.yexcr.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Liang Y, Xu X, Wang T, Li Y, You W, Fu J, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8:e2928. doi: 10.1038/cddis.2017.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun NX, Ye C, Zhao Q, Zhang Q, Xu C, Wang SB, et al. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One. 2014;9:e100340. doi: 10.1371/journal.pone.0100340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Jin X, Chen X, Hu Y, Ying F, Zou R, Lin F, et al. LncRNA-TCONS_00026907 is involved in the progression and prognosis of cervical cancer through inhibiting miR-143-5p. Cancer Med. 2017;6:1409–1423. doi: 10.1002/cam4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frixa T, Donzelli S, Blandino G. Oncogenic microRNAs: key players in malignant transformation. Cancers (Basel) 2015;7:2466–2485. doi: 10.3390/cancers7040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YK, Xi WY, Xi RX, Li JY, Li Q, Gao YE. MicroRNA-494 promotes cervical cancer proliferation through the regulation of PTEN. Oncol Rep. 2015;33:2393–2401. doi: 10.3892/or.2015.3821. [DOI] [PubMed] [Google Scholar]
- 43.Park S, Eom K, Kim J, Bang H, Wang HY, Ahn S, et al. MiR-9, miR-21, and miR-155 as potential biomarkers for HPV positive and negative cervical cancer. BMC Cancer. 2017;17:658. doi: 10.1186/s12885-017-3642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang T, Wong HK, Gu W, Yu MY, To KF, Wang CC, et al. MicroRNA-182 plays an onco-miRNA role in cervical cancer. Gynecol Oncol. 2013;129:199–208. doi: 10.1016/j.ygyno.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 45.Xie H, Lee L, Scicluna P, Kavak E, Larsson C, Sandberg R, et al. Novel functions and targets of miR-944 in human cervical cancer cells. Int J Cancer. 2015;136:E230–E241. doi: 10.1002/ijc.29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su K, Wang CF, Zhang Y, Cai YJ, Zhang YY, Zhao Q. miR-940 upregulation contributes to human cervical cancer progression through p27 and PTEN inhibition. Int J Oncol. 2017;50:1211–1220. doi: 10.3892/ijo.2017.3897. [DOI] [PubMed] [Google Scholar]
- 47.Hao Z, Yang J, Wang C, Li Y, Zhang Y, Dong X, et al. MicroRNA-7 inhibits metastasis and invasion through targeting focal adhesion kinase in cervical cancer. Int J Clin Exp Med. 2015;8:480–487. [PMC free article] [PubMed] [Google Scholar]
- 48.Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ, Xu B. Long noncoding RNA PVT1 facilitates cervical cancer progression via negative regulating of miR-424. Oncol Res. 2017;25:1391–1398. doi: 10.3727/096504017X14881559833562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma J, Fang B, Zeng F, Pang H, Zhang J, Shi Y, et al. Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol Lett. 2014;231:82–91. doi: 10.1016/j.toxlet.2014.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Target genes of miR-331-3p and miR-338-3p involving in cancer tumorigenesis and metastasis
Cell invasion and migration capacities were detected by Transwell assay and wound healing assay, respectively. (A) Cell invasion and migration capacities after SiHa and Caski cells were transfected with miR-331-3p or miR-338-3p mimics. (B) Cell invasion and migration capacities after XLOC_006390 siRNA was co-transfected with miR-331-3p inhibitor in SiHa and Caski cells. (C) Cell invasion and migration capacities after XLOC_006390 siRNA was co-transfected with miR-338-3p inhibitor in SiHa and Caski cells.
Western blotting of miR-331-3p target gene NRP2 and miR-338-3p target genes PKM2 and EYA2 in SiHa and Caski cells. (A, B) NRP2, PKM2, and EYA2 in the XLOC_006390 knockdown SiHa and Caski cells. (C) NRP2, PKM2, and EYA2 protein level in SiHa and Caski cells after up-regulations of miR-331-3p and miR-338-3p. (D) NRP2 protein level in SiHa and Caski cells after inhibition of miR-331-3p. (E) PKM2 and EYA2 protein level in SiHa and Caski cells after inhibition of miR-338-3p.