Abstract
Evidence exists that mitochondrial content and/or function is reduced in muscle of aging individuals. The purposes of this study were to investigate the contribution of outer membrane protein import and assembly processes to this decline and to determine whether the assembly process could adapt to chronic contractile activity (CCA). Tom40 assembly into the translocases of the outer membrane (TOM complex) was measured in subsarcolemmal mitochondria obtained from young (6 mo old) and aged (36 mo old) Fischer 344 × Brown Norway animals. While the initial import of Tom40 did not differ between young and aged animals, its subsequent assembly into the final ∼380 kDa complex was 2.2-fold higher (P < 0.05) in mitochondria from aged compared with young animals. This was associated with a higher abundance of Tom22, a protein vital for the assembly process. CCA induced a greater initial import and subsequent assembly of Tom40 in mitochondria from young animals, resulting in a CCA-induced 75% increase (P < 0.05) in Tom40 within mitochondria. This effect of CCA was attenuated in mitochondria from old animals. These data suggest that the import and assembly of proteins into the outer membrane do not contribute to reduced mitochondrial content or function in aged animals. Indeed, the greater assembly rate in mitochondria from aged animals may be a compensatory mechanism attempting to offset any decrements in mitochondrial content or function within aged muscle. Our data also indicate the potential of CCA to contribute to increased mitochondrial biogenesis in muscle through changes in the outer membrane import and assembly pathway.
Keywords: protein import, protein complex assembly, mitochondrial biogenesis
aging involves the progressive accumulation of cellular alterations, leading to a greater susceptibility of the body to environmental challenges and disease (30). Tissues that possess a high energy demand such as brain, skeletal muscle, and heart are more susceptible to the effects of aging (42, 56). A hallmark feature of the aging process in skeletal muscle is sarcopenia, involving the degenerative loss of muscle mass and strength (34). While this decrement is believed to be due to a combination of muscle atrophy and fiber loss (33, 35), the upstream events responsible for these alterations remain largely unknown.
In an effort to gain insight into the molecular mechanisms that mediate sarcopenia and result in the aging phenotype, several studies have focused on mitochondria and the key processes that are regulated by this organelle. Mitochondria are the major site of reactive oxygen species (ROS) production within the cell. Elevated levels of ROS within mitochondria lead to an increased oxidative stress and damage to cellular components such as DNA, proteins, and lipids (4, 24, 49). The involvement of mitochondrial damage in aging has now been fortified by studies in both animals (44, 57) and humans (12, 47, 51) showing an accelerated accumulation of mtDNA defects in skeletal muscle from aged compared with young individuals. These changes are associated with a decline in mitochondrial content (10), respiratory capacity (58), and enzyme activity (3, 20).
Mitochondrial biogenesis and the expansion of the mitochondrial reticulum relies heavily on the incorporation of hundreds of proteins into the organelle, the majority of which are encoded in the nucleus and then imported into mitochondrial subcompartments (21). Mitochondrial protein import is mediated by two multisubunit complexes, referred to as the translocases of the outer membrane (TOM complex) and the translocases of the inner membrane (TIM complex). The majority of precursor proteins traverse the outer membrane via a highly stable general import pore consisting of the main channel-forming protein Tom40, Tom22, and the smaller TOM proteins, Tom5, Tom6, and Tom7 (7, 41).
The dynamic nature of the TOM channel has been illustrated recently with the characterization of the import and assembly of the Tom40 precursor protein into the TOM complex in yeast (43, 60). Tom40 is a β-barrel protein that follows a unique pathway of insertion into the outer membrane through a number of assembly intermediates. In the cytosol, the Tom40 precursor protein is targeted to the Tom20 and Tom70 receptors via heat shock protein (Hsp)70 and Hsp90 chaperone proteins. The precursor protein then crosses the TOM channel and is inserted by small TIM proteins from within the intermembrane space into a ∼250 kDa intermediate I complex which is primarily composed of the sorting and assembly machinery (SAM) complex (23, 60, 61). The precursor is then assembled into a ∼120 kDa intermediate II complex, before its final incorporation into the ∼380 kDa TOM complex. Preexisting TOM and TIM proteins as well as the SAM complex are required for Tom40 import and assembly into the outer membrane (5, 7). Although the biogenesis of Tom40 has been well investigated in yeast and plants (15, 59, 60), few studies (25, 26) have examined the assembly kinetics of the TOM channel within mammalian cells.
Previous research conducted in our laboratory has shown that the mitochondrial protein import pathway is a metabolic sensor that can adapt to perturbations in the energy status of a cell by modifying the rate of precursor protein import into the matrix compartment of the organelle (13, 52). Using electrical stimulation-induced chronic contractile activity (CCA), a marked increase was observed in the import of several matrix-destined proteins including mitochondrial transcription factor A (Tfam) and malate dehydrogenase (18, 52). These alterations are partly mediated by the upregulation of protein import machinery components and are associated with increased levels of cytochrome-c oxidase activity and ATP production (52). With regard to aging, Craig and Hood (14) demonstrated an increased rate of import into the matrix of mitochondria isolated from cardiac muscle of aged animals, when compared with their younger counterparts. However, to date, no study has examined the effect of aging or contractile activity on the import and assembly of proteins into the mitochondrial outer membrane in skeletal muscle.
To investigate the effects of aging on Tom40 import and assembly in skeletal muscle, we used Fischer 344 × Brown Norway F1-hybrid (F344BN) rats, which are known to have a high resistance to age-related pathologies and a relatively longer life span when compared with other strains (19). While there is considerable evidence to suggest that mitochondrial oxidative enzyme activities and mitochondrial content are reduced in this aging model (10, 20, 29), these findings have not been corroborated in all studies (37). These discrepancies between studies may be due to 1) the age of the animals, 2) muscle and fiber type differences, and/or 3) the methods used to measure mitochondrial content. Nonetheless, alterations in respiratory function, ROS production, and protein release have all been documented in mitochondria from aging muscle (10). Thus, our study focused on the premise that mitochondrial content and/or function is reduced in the F344BN aging model and that alterations in the protein import and assembly pathway contribute to these mitochondrial-associated changes observed in aging muscle.
The specific purposes of this study were 1) to examine Tom40 import and assembly in mitochondria from aged skeletal muscle and 2) to determine whether aged muscle has the same capacity to adapt to CCA as muscle from young animals. We hypothesized that Tom40 import and assembly would be reduced in aged muscle and that this may contribute to the alteration in mitochondrial function that is typically observed within aged animals (10, 36). Furthermore, we hypothesized that the Tom40 import and assembly process would be adaptable to CCA in both young and aged animals but that this adaptation would be attenuated in muscle from aged animals.
MATERIALS AND METHODS
Animals and surgery.
All experiments were conducted after approval by the York University Animal Care Committee, in accordance with Canadian Council of Animal Care guidelines. Male F344BN rats (National Institute on Aging, Bethesda, MD) were studied at both 6 mo of age (young) and 35–38 mo of age (referred to in this study as either aged or old). The procedure as outlined previously (32) was followed for the implantation of electrodes. Briefly, animals were anesthetized with a ketamine-xylazine mix. An incision was made at the abdominal musculature, and the stimulator was placed in the peritoneal cavity of the animal. The electrodes were then passed subcutaneously from the abdominal cavity to the left hindlimb and sutured on either side of the peroneal nerve that innervates the tibialis anterior and the extensor digitorum longus muscle. All incisions were sutured, and sterile ampicillin (Penbritin, Ayerst, Montreal, QC, Canada) was applied to prevent infection. The animals were then allowed to recover for 7 days before commencement of the stimulation protocol.
Stimulation protocol to induce CCA.
Animals were allowed 1 wk to recover from surgery and were stimulated (10 Hz, 0.1-ms duration) for 3 h/day for 7 days, as done previously (52, 55). After the indicated number of days of stimulation, the animals were anesthetized and the tibialis anterior and the extensor digitorum longus muscles were removed from the stimulated and the contralateral limb that served as a control. The tibialis anterior muscle was immediately placed in cold buffer for mitochondrial isolation, and the extensor digitorum longus muscle was used for whole muscle protein analyses.
Mitochondrial isolations.
Subsarcolemmal mitochondria were isolated from both young and aged animals by differential centrifugation following a brief polytron homogenization from whole rat tibialis anterior and extensor digitorum longus muscle, as described previously (11, 54). We focused on the import process in subsarcolemmal mitochondria because it is well established that this subfraction responds to chronic muscle use more readily than the intermyofibrillar subfraction (31, 52). Mitochondria were then resuspended in a buffer containing 10 mM HEPES, 0.25 M sucrose, 2.5 mM potassium phosphate dibasic, 10 mM succinate, 0.21 mM ADP, and 1 mM dithiothreitol (pH 7.4), and protein concentrations were measured using a Bradford assay (9).
In vitro synthesis of precursor proteins.
Full-length Tom40 cDNA (pGEM4Z/hTom40, from Dr. M. T. Ryan, La Trobe University, Melbourne, Australia) was isolated using an alkaline lysis DNA preparation method and DNA linearized using BamHI. The plasmid was resuspended in Tris-EDTA (pH 7.8) to a final concentration of 0.8 μg/μl. Transcription reactions were performed with SP6 RNA polymerase (Promega, Fisher Canada) for 90 min at 40°C (54). Tom40 mRNA was isolated by phenol extraction followed by ethanol precipitation, and final concentrations were adjusted to 2.8 μg/μl. In vitro translation was performed at 30°C for 30 min using cell-free rabbit reticulocyte lysate (Promega, Fisher Canada) in the presence of [35S]methionine (Perkin Elmer, Canada).
Import of precursor proteins into isolated mitochondrial subfractions.
Isolated mitochondria and lysate containing the translated radiolabeled precursor protein were allowed to equilibrate separately at 30°C for 10 min. The two samples were then combined and further incubated at 30°C for various time points. For standard import reactions using SDS-PAGE, 25 μg of mitochondria and 12 μl of reticulocyte lysate were used. In contrast, for determining Tom40 assembly by blue native-PAGE (BN-PAGE), 65 μg of mitochondria and 31.2 μl of lysate were used. Mitochondria were recovered by centrifugation (16,000 g) through 600 μl of 20% sucrose in 0.1 M potassium chloride, 2 mM magnesium chloride, and 20 mM HEPES (pH 7.4) at 4°C for 15 min. For Tom40 import, mitochondrial pellets were resuspended in freshly prepared 0.1 M sodium carbonate (Na2CO3; pH 11.5) and incubated on ice for 30 min. For standard import reactions, mitochondrial pellets were then resuspended in 0.6 M sorbital, and 20 mM HEPES-KOH (pH 7.4), and equal volumes of sample buffer [10% (vol/vol) glycine, 80 mM SDS, 62.5 mM Tris·HCl, pH 6.8, 5% (vol/vol) 2-mercaptoethanol, and 5% (vol/vol) dye] were added. Samples were denatured for 5 min and electrophoresed through a 12% SDS-polyacrylamide gel. Following electrophoresis, gels were treated for 5 min in boiling 5% trichloroacetic acid, followed by a 30-s wash in distilled water, 5 min in 10 mM Tris base (pH 9.0), and 30 min in 1 M sodium salicylate. Gels were subsequently dried with a vacuum gel dryer (model 583; Bio-Rad). Radiolabeled precursor proteins were detected using phosphorimaging (Pharos FX; Bio-Rad) and quantified using Quantity 1 software.
BN-PAGE.
For analysis of [35S]hTom40 import and assembly into the TOM complex, samples were prepared as previously described (26, 48). Briefly, following centrifugation of the sample through a sucrose gradient, the pellet was washed in 50 μl of import buffer (250 mM sucrose, 5 mM magnesium acetate, 80 mM potassium acetate, 10 mM sodium succinate, 1 mM dithiothreitol, 0.1 mM ADP, and 20 mM HEPES-KOH, pH 7.4) and centrifuged at 10,000 g for 5 min. The supernate was removed and the pellet resuspended in 50 μl of digitonin buffer [1% (wt/vol), 50 mM NaCl, 10% (vol/vol) glycerol, and 20 mM BisTris, pH 7.0] and incubated on ice for 10 min. The samples were then centrifuged at 16,000 g for 5 min, and the supernate was transferred to a new tube. Sample buffer [5% (wt/vol) Coomassie Brilliant Blue G-250, 500 mM ϵ-amino-n-caproic acid, and 160 mM BisTris, pH 7.0] was added to the supernate, and the samples were subjected to 5–13% BN-PAGE (46, 48) and electrophoresed overnight at 23 V. The following day, the gels were destained (50% methanol, 10% acetic acid) and fixed [7% methanol, 7% acetic acid, 1% (vol/vol) glycerol]. The gels were then dried with a vacuum gel dryer and imaged as described above.
Immunoblotting.
Isolated mitochondria were used for Western blotting analyses as previously described (53). Briefly, protein samples were separated by gel electrophoresis on 8–15% polyacrylamide gels and transferred to nitrocellulose membranes. Blots were blocked for 1 h in 5% skim milk in 1X Tris-buffered saline-Tween 20-Tris · HCl (TBST, pH 7.4) and probed with the appropriate primary antibody (1:500 for Tom40 and 1:1,000 for Tom22 and porin). Blots were then incubated with anti-rabbit secondary antibody conjugated with horseradish peroxidase at a dilution of 1:3,000 (Tom40) and anti-mouse secondary at a dilution of 1:1,000 (Tom22, porin). Blots were washed with TBST (3 X 5 min), and proteins subsequently visualized with an enhanced chemiluminescence kit (Santa Cruz) and quantified with SigmaScan software (Jandel Scientific, San Rafael, CA). Immunoblotting of porin was used to normalize for the amount of protein loaded. The staining intensity of the blue native gels was used to correct for loading of assembly experiments.
Antibodies.
For immunoblotting experiments, the Tom40 antibody was provided by Dr. M. T. Ryan (La Trobe University, Melbourne, Australia). Antibodies were obtained from Sigma (Tom22) and MitoSciences (porin).
Statistical analysis.
Data are presented as means ± SE and were analyzed with Student's t-test or two-way ANOVA between young and aged animals, where appropriate. A main effect of time or age would indicate that there was a significantly higher import and/or assembly over time, or with age when compared with young animals. Differences were considered statistically significant if P < 0.05.
RESULTS
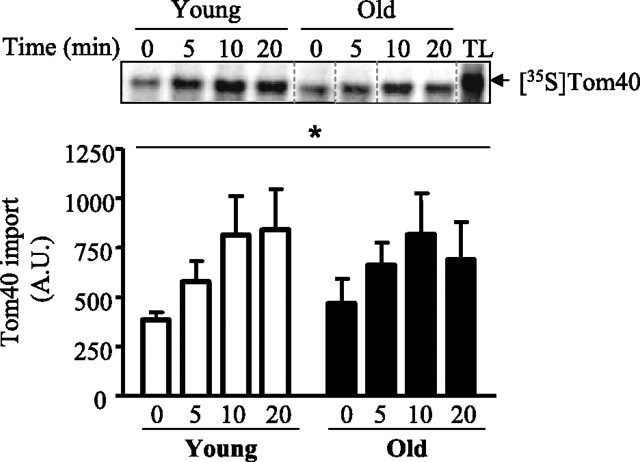
To examine the import of Tom40 in mitochondria, radiolabeled Tom40 was incubated with isolated mitochondria for various time points and subjected to SDS-PAGE. Our results indicate a time-dependent increase (P < 0.05) in the amount of Tom40 imported in mitochondria from both young and old animals (Fig. 1).
Fig. 1.
Import of Tom40 in skeletal muscle mitochondria of young and aged animals. Top: representative autoradiogram of Tom40 import in mitochondria isolated from skeletal muscle of young and aged animals. Lanes represent a 20-min time course of Tom40 import. Dashed lines indicate the lanes that have been realigned in a single gel. TL, 5 μl of translated product not incubated with mitochondria. Bottom: graphical representation of Tom40 import (n = 6). Data are expressed as arbitrary units (AU). *P < 0.05, main effect of time on protein import.
To directly monitor the assembly of Tom40 into the outer membrane, BN-PAGE analysis was performed. The incorporation of Tom40 into its assembly intermediates increased over time with a sequential pattern of insertion into a ∼230 kDa intermediate I complex (labeled “I”), followed by a ∼120 kDa intermediate II complex (labeled “II”), before its final incorporation into a ∼380 kDa TOM complex (labeled “TOM”; Fig. 2, B and C). This pattern of assembly is similar to previous data which fully characterized Tom40 import and assembly kinetics in isolated skeletal muscle mitochondria (Joseph AM and Hood DA, unpublished observation). We found that the assembly pattern differed between the two groups, with old animals (Fig. 2C) exhibiting a more rapid progression of the Tom40 precursor protein into the outer membrane when compared with mitochondria from young animals (Fig. 2B). By the end of the import reaction, Tom40 assembly into the TOM complex was more than twofold greater (P < 0.05; 46% of the total Tom40 intermediates) in mitochondria from old animals, compared with mitochondria from young animals (21%).
Fig. 2.
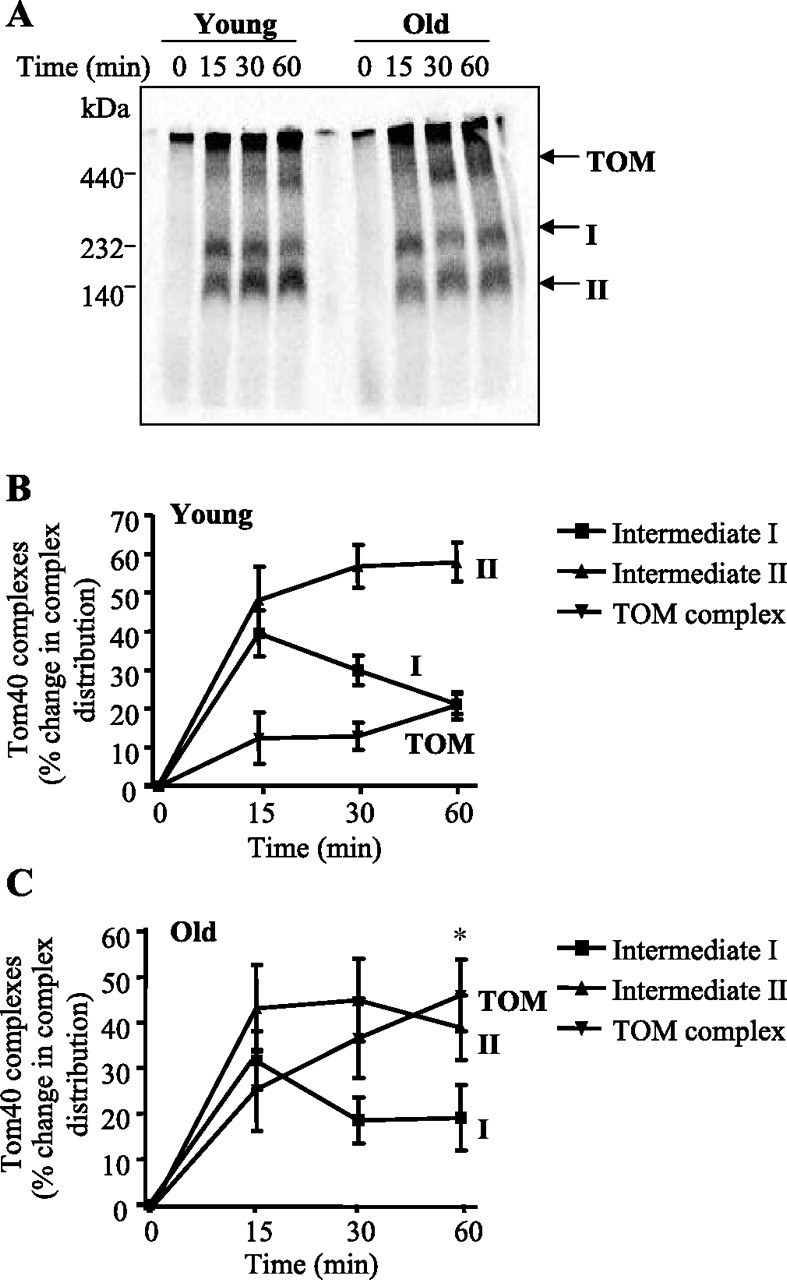
Tom40 assembly into the outer membrane in mitochondria isolated from muscle of young and old animals. A: representative autoradiogram of Tom40 precursor protein import in mitochondria isolated from young and old animals for 0, 15, 30, and 60 min. Solubilized mitochondria were reisolated with digitonin and loaded on a 5–13% blue native (BN) gel. B and C: summary of multiple experiments (n = 7) illustrating the sequential import of Tom40 into intermediate I (I), intermediate II (II), and the translocases of the outer membrane (TOM) over 60 min, measured in both young (B) and old (C) animals. Values are expressed as a percentage change in the distribution of [35S]hTom40 into each intermediate complex. *P < 0.05, effect of age on the final incorporation into the TOM complex at 60 min.
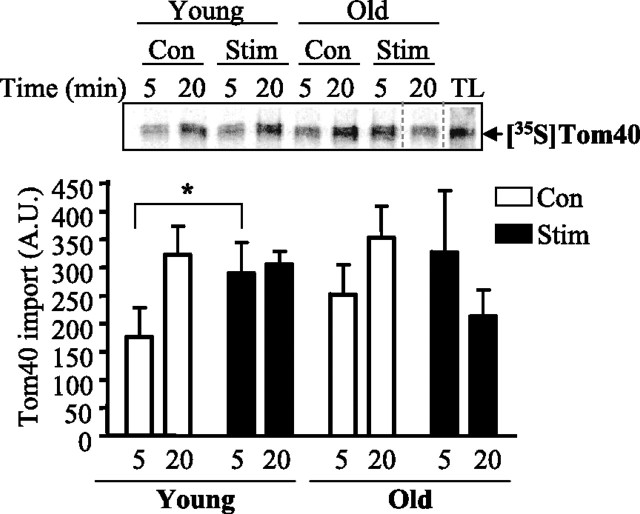
It has previously been shown that protein import into mitochondria can be enhanced with the use of a stimulus such as CCA (18, 52). Indeed, in young animals, CCA resulted in a greater (P < 0.05) amount of Tom40 import into mitochondria at 5 min in the stimulated, when compared with control muscle (Fig. 3). This effect was not evident in mitochondria isolated from old muscle. The CCA effect was time dependent, since the acceleration of protein import was no longer evident at 20 min of the import reaction in either young or old animals.
Fig. 3.
Import of [35S]hTom40 precursor in isolated mitochondria following 7 days of chronic contractile activity (CCA). Top: representative autoradiogram of Tom40 import in mitochondria from control (Con) and stimulated (Stim) skeletal muscle of young and aged animals for 5 and 20 min. The dashed line indicates that the right portion of the gel has been realigned to allow for a better visual comparison of the lanes between the two experimental groups in a single gel. Bottom: graphical representation of Tom40 import (n = 6). Data are expressed as arbitrary units. *P < 0.05, Stim vs. Con.
The assembly pattern of Tom40 into its intermediate complexes was then assessed by BN-PAGE. Similar to the results shown in Fig. 2, the assembly of the Tom40 precursor protein into the outer membrane begins with its incorporation into the intermediate I complex, followed by its integration into the intermediate II complex, before its final assembly into the mature TOM complex which is detected at 90 min of import (Fig. 4, A–C). In young animals, CCA elicited a 75% (P < 0.05) increase in the amount of Tom40 assembled into the final TOM complex in mitochondria from stimulated, when compared with nonstimulated muscle (Fig. 4A). In contrast, in old animals (Fig. 4B) an overall 65% (P < 0.05) increase was observed in stimulated muscle when compared with control muscle, suggesting that CCA can further enhance the import and assembly of Tom40 into the TOM complex regardless of age.
Fig. 4.
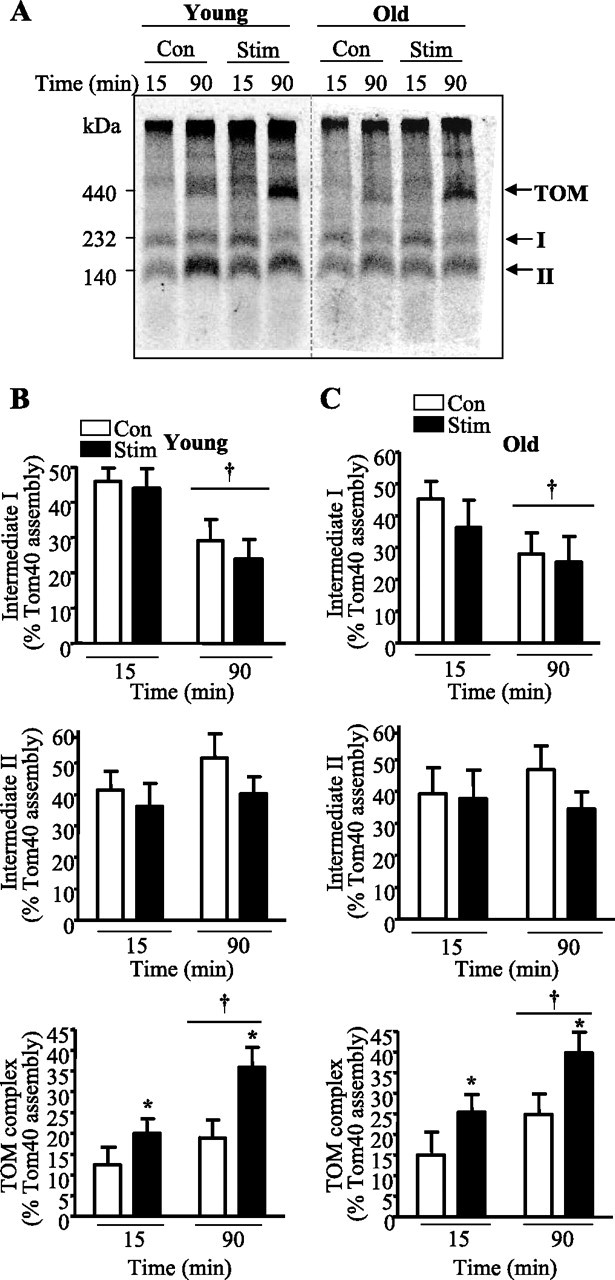
CCA-induced changes in Tom40 assembly in isolated mitochondria of young and aged animals. A: representative autoradiogram of Tom40 precursor protein import in mitochondria isolated from control and stimulated muscle of young and aged animals. Radiolabeled Tom40 was imported into mitochondria for 15 and 90 min. Mitochondria were reisolated with digitonin and loaded on a 5–13% blue native gel. Dashed lines indicate the lanes that have been realigned in a single gel. B and C: summary of the data (n = 7–8) where Tom40 incorporation into intermediate I, intermediate II, and the TOM complex was measured in both young (B) and aged (C) animals. †P < 0.05, 15 vs. 90 min; *P < 0.05, Stim vs. Con.
To further explore potential regulatory factors of the import and assembly process, we examined the levels of Tom22, which is an essential protein required for Tom40 import and assembly. Tom22 protein levels were ninefold (P < 0.05) higher in mitochondria from old compared with young animals, and this was associated with a 60% (P < 0.05) greater level of Tom40 protein (Fig. 5). In response to CCA, we found that Tom22 levels were induced by threefold (P < 0.05) in mitochondria from young animals (Fig. 5) and that this corresponded to a 65% (P < 0.05) increase in Tom40 protein within this subfraction. In old animals, CCA had no effect on Tom22 or Tom40 protein levels (Fig. 5). These results point to a parallelism between changes in Tom22 expression and the level of Tom40 within skeletal muscle mitochondria.
Fig. 5.
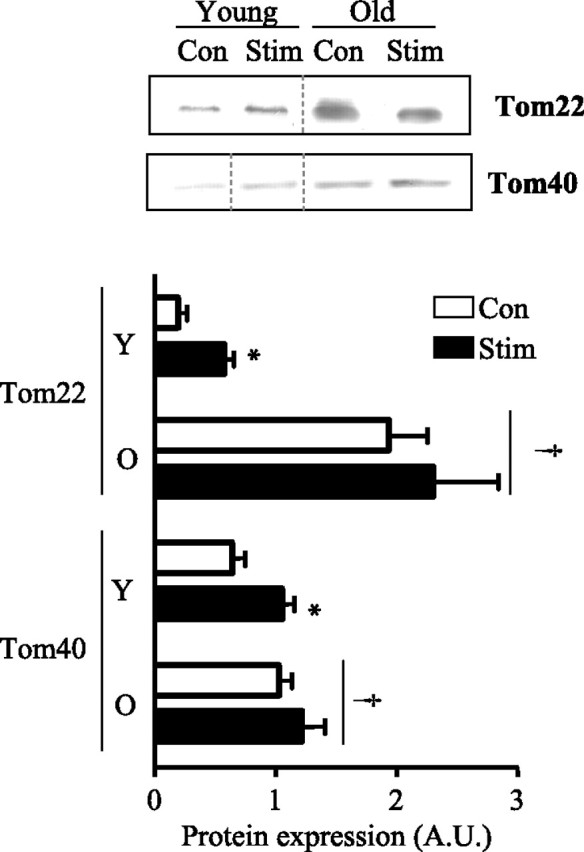
Altered levels of Tom22 and Tom40 following CCA in young (Y) and aged (O) animals. Top: representative Western blots of Tom22 and Tom40 in stimulated and nonstimulated mitochondria from young and aged animals. Dashed lines indicate that lanes from the gel have been excised and the lanes from a single gel reordered to show a representative image. Bottom: graphical representation of multiple experiments (n = 5–10). Values were corrected for porin and are expressed as arbitrary units. †P < 0.05, main effect of age; *P < 0.05, Stim vs. Con.
DISCUSSION
The aging process that occurs in skeletal muscle is a classic example of the consequence of continued exposure to environmental stresses and the cumulative damage to cellular processes that occur over time. Aging in skeletal muscle manifests as sarcopenia, a process that involves several key features, including the accumulation of mtDNA mutations, increased ROS production, and a greater incidence of apoptosis (4, 16, 24, 38, 49). To date, the molecular mechanisms by which mitochondrial dysregulation contributes to aging, sarcopenia, and a decreased life span remain unclear. It has been proposed that factors responsible for maintaining mitochondrial integrity, and hence mitochondrial biogenesis, are altered in aged skeletal muscle. In particular, mitochondrial biogenesis largely depends on several key processes, including the transcription of nuclear-encoded genes, the translation of genes within the cytosol, and the targeting and import of these newly synthesized precursor proteins into mitochondrial subcompartments. Previous studies have primarily focused on the upstream events of this pathway, examining signaling pathways and alterations in gene transcript levels, with little attention given to the importance of mitochondrial protein import in the aging process. Thus, given the decrements observed in mitochondrial enzyme activity, function, and/or and content within aged muscle (10, 36), we wanted to determine whether alterations in the import and multisubunit complex assembly could contribute to the overall mitochondrial phenotype that is associated with aging.
Tom40 is the main component of the outer membrane TOM complex, the channel through which precursor proteins enter mitochondria. While no differences were found in the initial import of Tom40 into the outer membrane, our results indicate that endogenous levels of Tom40 protein were higher in mitochondria from old, compared with young animals. The higher levels of Tom40 within mitochondria from aged animals may be due to several factors, including the initial import of Tom40 into the outer membrane, or the assembly rate at which Tom40 precursor proteins are incorporated into intermediate complexes. Although we observed no differences between young and old animals with respect to the early stages of the import process, we found that skeletal muscle mitochondria from aged animals had higher levels of Tom40 assembled into the TOM complex. The greater incorporation of Tom40 in aged animals was associated with a higher abundance of Tom22 in these cells. Tom22 has been reported to play several roles in the biogenesis of Tom40, one of which involves the final step in the incorporation of Tom40 from intermediate II into the TOM complex (25, 40). This is supported by the finding that overexpression of Tom22 in HeLa cells leads to a greater assembly of Tom40 into the TOM complex (25). Thus, it appears that higher levels of Tom22 within aged skeletal muscle may be responsible, in part, for the greater assembly of Tom40 precursor proteins, and in turn the greater abundance of Tom40 in mitochondria from aged animals. Alternatively, this greater amount of Tom40 in mitochondria from aged animals may be related to a progressive decline in protease activity within mitochondria. Although the specific protease responsible for Tom40 degradation is not known, the accumulation of oxidatively damaged mitochondrial proteins has been directly associated with a reduced expression and activity of the Lon protease in skeletal muscle from aged animals (8). However, specific degradation assays identifying proteolytic enzymes that target Tom40 molecules in young and aged animals must be performed to support these findings.
Interestingly, an enhanced rate of precursor protein import into mitochondria has also been demonstrated in cells depleted of mtDNA (rho−), as well as in patient cells harboring mtDNA mutations (28, 45). Thus, it appears that in situations where there is a disruption in the homeostatic energy status of the cell and a mitochondrial stress signal is activated, the import and assembly into mitochondria are accelerated in a compensatory effort to meet the energy requirements of the cell. Evidence for a retrograde signaling pathway between the mitochondria and nucleus has been shown in numerous models of genetic and metabolic stress (2, 6), and this study suggests that these pathways may also be evident in aged muscle.
Given that sarcopenia is associated with decrements in mitochondrial function, it has been proposed that therapeutic interventions that have the potential to induce mitochondrial biogenesis may have beneficial effects on the aging phenotype. CCA has been shown to improve multiple biochemical and functional parameters in muscle such as oxidative enzyme capacity and mitochondrial content (1, 17, 22). Similar adaptations to CCA have also been reported in aged animals, including increased mitochondrial content, respiratory activity, and a reduced susceptibility to apoptosis (39, 50), although the response is attenuated in muscle from aged compared with young animals (36). In muscle from healthy, young individuals, these CCA-induced changes are partly due to the adaptability of the protein import machinery components allowing for the accelerated rate of precursor proteins into the organelle (52). However, whether these adaptations occur in muscle from aged animals has remained largely unknown.
In the present study, we found that the effect of CCA on Tom40 import and assembly was more pronounced in muscle from young animals. These changes were associated with increased expression of both Tom22 and Tom40 in young animals. The fact that muscle from aged animals did not exhibit similar adaptations in these protein import machinery components suggests that the mechanisms regulating the Tom40 assembly process may differ with age. This is even more likely the case for Tom40 since its biogenesis into the TOM complex is dependent on several different import machinery complexes (e.g., Tim8/13, Tim9/12, SAM). Future work is needed to examine the expression of these complexes to determine their importance in CCA-induced adaptations.
Mitochondrial protein import plays a vital role in the assembly of the organelle and in the CCA-induced adaptation of the mitochondrial phenotype that occurs in skeletal muscle. In this report, we show that following the initial stage of import, the assembly of Tom40 in skeletal muscle mitochondria from aged animals is not impaired but is actually enhanced. This may serve as a compensatory cellular mechanism to offset decrements in the transcriptional drive for mRNA synthesis evident in aged skeletal muscle (10). Furthermore, the dynamic nature of the protein import pathway in response to CCA in skeletal muscle indicates the potential of regularly performed contractile activity to regulate mitochondrial biogenesis and improve mitochondrial function in aged skeletal muscle. We believe that a thorough understanding of the molecular mechanisms governing the age-related decline in mitochondrial content and/or function will provide crucial information for the development of possible interventions (including exercise) that may offset or delay age-associated diseases, and improve the quality of life for the aging population.
GRANTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research. A.-M. Joseph was a recipient of an Ontario Graduate Scholarship. V. Ljubicic is a recipient of a Heart and Stroke award. D. A. Hood holds a Canada Research Chair in Cell Physiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Dr. M. T. Ryan (La Trobe University, Melbourne, Australia) for providing the pGEM4Z/hTom40 DNA.
REFERENCES
- 1. Adhihetty PJ , Ljubicic V , Hood DA. Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am J Physiol Endocrinol Metab : E748–E755, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Amuthan G , Biswas G , Zhang SY , Klein-Szanto A , Vijayasarathy C , Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J : 1910–1920, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker DJ , Betik AC , Krause DJ , Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J Gerontol A Biol Sci Med Sci : 675–684, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Balaban RS , Nemoto S , Finkel T. Mitochondria, oxidants, and aging. Cell : 483–495, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Becker T , Vogtle FN , Stojanovski D , Meisinger C. Sorting and assembly of mitochondrial outer membrane proteins. Biochim Biophys Acta : 557–563, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Biswas G , Adebanjo OA , Freedman BD , Anandatheerthavarada HK , Vijayasarathy C , Zaidi M , Kotlikoff M , Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J : 522–533, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolender N , Sickmann A , Wagner R , Meisinger C , Pfanner N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep : 42–49, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bota DA , Van RH , Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett : 103–106, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem : 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 10. Chabi B , Ljubicic V , Menzies KJ , Huang JH , Saleem A , Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell : 2–12, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Cogswell AM , Stevens RJ , Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol Cell Physiol : C383–C389, 1993. [DOI] [PubMed] [Google Scholar]
- 12. Corral-Debrinski M , Horton T , Lott MT , Shoffner JM , Beal MF , Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet : 324–329, 1992. [DOI] [PubMed] [Google Scholar]
- 13. Craig EE , Chesley A , Hood DA. Thyroid hormone modifies mitochondrial phenotype by increasing protein import without altering degradation. Am J Physiol Cell Physiol : C1508–C1515, 1998. [DOI] [PubMed] [Google Scholar]
- 14. Craig EE , Hood DA. Influence of aging on protein import into cardiac mitochondria. Am J Physiol Heart Circ Physiol : H2983–H2988, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Dekker PJ , Martin F , Maarse AC , Bomer U , Muller H , Guiard B , Meijer M , Rassow J , Pfanner N. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J : 5408–5419, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dirks A , Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol : R519–R527, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Freyssenet D. Energy sensing and regulation of gene expression in skeletal muscle. J Appl Physiol : 529–540, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Gordon JW , Rungi AA , Inagaki H , Hood DA. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol : 389–396, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Greenlund LJ , Nair KS. Sarcopenia–consequences, mechanisms, and potential therapies. Mech Ageing Dev : 287–299, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Hagen JL , Krause DJ , Baker DJ , Fu MH , Tarnopolsky MA , Hepple RT. Skeletal muscle aging in F344BN F1-hybrid rats: I. Mitochondrial dysfunction contributes to the age-associated reduction in VO2max. J Gerontol A Biol Sci Med Sci : 1099–1110, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol : 1137–1157, 2001. [DOI] [PubMed] [Google Scholar]
- 22. Hood DA , Irrcher I , Ljubicic V , Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol : 2265–2275, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Hoppins SC , Nargang FE. The Tim8-Tim13 complex of Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J Biol Chem : 12396–12405, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Huang TT , Carlson EJ , Gillespie AM , Shi Y , Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J Gerontol A Biol Sci Med Sci : B5–B9, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Humphries AD , Streimann IC , Stojanovski D , Johnston AJ , Yano M , Hoogenraad NJ , Ryan MT. Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem : 11535–11543, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Johnston AJ , Hoogenraad J , Dougan DA , Truscott KN , Yano M , Mori M , Hoogenraad NJ , Ryan MT. Insertion and assembly of human tom7 into the preprotein translocase complex of the outer mitochondrial membrane. J Biol Chem : 42197–42204, 2002. [DOI] [PubMed] [Google Scholar]
- 28. Joseph AM , Rungi AA , Robinson BH , Hood DA. Compensatory responses of protein import and transcription factor expression in mitochondrial DNA defects. Am J Physiol Cell Physiol : C867–C875, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Kerner J , Turkaly PJ , Minkler PE , Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am J Physiol Endocrinol Metab : E1054–E1062, 2001. [DOI] [PubMed] [Google Scholar]
- 30. Kirkwood TB. Understanding the odd science of aging. Cell : 437–447, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Krieger DA , Tate CA , Millin-Wood J , Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol : 23–28, 1980. [DOI] [PubMed] [Google Scholar]
- 32. Lanmuller H , Ashley Z , Unger E , Sutherland H , Reichel M , Russold M , Jarvis J , Mayr W , Salmons S. Implantable device for long-term electrical stimulation of denervated muscles in rabbits. Med Biol Eng Comput : 535–540, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Larsson L , Edstrom L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci : 69–89, 1986. [DOI] [PubMed] [Google Scholar]
- 34. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 35. Lexell J , Taylor CC , Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci : 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 36. Ljubicic V , Joseph AM , Adhihetty PJ , Huang JH , Saleem A , Uguccioni G , Hood DA. Molecular basis for an attenuated mitochondrial adaptive plasticity in aged skeletal muscle. Aging : 818–830, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathieu-Costello O , Ju Y , Trejo-Morales M , Cui L. Greater capillary-fiber interface per fiber mitochondrial volume in skeletal muscles of old rats. J Appl Physiol : 281–289, 2005. [DOI] [PubMed] [Google Scholar]
- 38. McKenzie D , Bua E , McKiernan S , Cao Z , Aiken JM. Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur J Biochem : 2010–2015, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Menshikova EV , Ritov VB , Fairfull L , Ferrell RE , Kelley DE , Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci : 534–540, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Model K , Meisinger C , Prinz T , Wiedemann N , Truscott KN , Pfanner N , Ryan MT. Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat Struct Biol : 361–370, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Mokranjac D , Neupert W. Protein import into isolated mitochondria. Methods Mol Biol : 277–286, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Muller-Hocker J. Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J Neurol Sci : 14–21, 1990. [DOI] [PubMed] [Google Scholar]
- 43. Paschen SA , Waizenegger T , Stan T , Preuss M , Cyrklaff M , Hell K , Rapaport D , Neupert W. Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature : 862–866, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Piko L , Hougham AJ , Bulpitt KJ. Studies of sequence heterogeneity of mitochondrial DNA from rat and mouse tissues: evidence for an increased frequency of deletions/additions with aging. Mech Ageing Dev : 279–293, 1988. [DOI] [PubMed] [Google Scholar]
- 45. Rungi AA , Primeau A , Nunes CL , Gordon JW , Robinson BH , Hood DA. Events upstream of mitochondrial protein import limit the oxidative capacity of fibroblasts in multiple mitochondrial disease. Biochim Biophys Acta : 146–154, 2002. [DOI] [PubMed] [Google Scholar]
- 46. Ryan MT , Voos W , Pfanner N. Assaying protein import into mitochondria. Methods Cell Biol : 189–215, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Sato W , Tanaka M , Ohno K , Yamamoto T , Takada G , Ozawa T. Multiple populations of deleted mitochondrial DNA detected by a novel gene amplification method. Biochem Biophys Res Commun : 664–672, 1989. [DOI] [PubMed] [Google Scholar]
- 48. Schagger H , von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem : 223–231, 1991. [DOI] [PubMed] [Google Scholar]
- 49. Schriner SE , Linford NJ , Martin GM , Treuting P , Ogburn CE , Emond M , Coskun PE , Ladiges W , Wolf N , Van RH , Wallace DC , Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science : 1909–1911, 2005. [DOI] [PubMed] [Google Scholar]
- 50. Song W , Kwak HB , Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal : 517–528, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Soong NW , Hinton DR , Cortopassi G , Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat Genet : 318–323, 1992. [DOI] [PubMed] [Google Scholar]
- 52. Takahashi M , Chesley A , Freyssenet D , Hood DA. Contractile activity-induced adaptations in the mitochondrial protein import system. Am J Physiol Cell Physiol : C1380–C1387, 1998. [DOI] [PubMed] [Google Scholar]
- 53. Takahashi M , Hood DA. Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle. J Appl Physiol : 934–941, 1993. [DOI] [PubMed] [Google Scholar]
- 54. Takahashi M , Hood DA. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. Differential import regulation in distinct subcellular regions. J Biol Chem : 27285–27291, 1996. [DOI] [PubMed] [Google Scholar]
- 55. Takahashi M , McCurdy DT , Essig DA , Hood DA. delta-Aminolaevulinate synthase expression in muscle after contractions and recovery. Biochem J : 219–223, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taylor RW , Barron MJ , Borthwick GM , Gospel A , Chinnery PF , Samuels DC , Taylor GA , Plusa SM , Needham SJ , Greaves LC , Kirkwood TB , Turnbull DM. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest : 1351–1360, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trifunovic A , Hansson A , Wredenberg A , Rovio AT , Dufour E , Khvorostov I , Spelbrink JN , Wibom R , Jacobs HT , Larsson NG. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci : 17993–17998, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trounce I , Byrne E , Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet : 637–639, 1989. [DOI] [PubMed] [Google Scholar]
- 59. Truscott KN , Pfanner N , Voos W. Transport of proteins into mitochondria. Rev Physiol Biochem Pharmacol : 81–136, 2001. [DOI] [PubMed] [Google Scholar]
- 60. Wiedemann N , Kozjak V , Chacinska A , Schonfisch B , Rospert S , Ryan MT , Pfanner N , Meisinger C. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature : 565–571, 2003. [DOI] [PubMed] [Google Scholar]
- 61. Wiedemann N , Truscott KN , Pfannschmidt S , Guiard B , Meisinger C , Pfanner N. Biogenesis of the protein import channel Tom40 of the mitochondrial outer membrane: intermembrane space components are involved in an early stage of the assembly pathway. J Biol Chem : 18188–18194, 2004. [DOI] [PubMed] [Google Scholar]