Graphical abstract
Keywords: Phytochemicals, Flavonoids, Glucosinolates, Food processing, Digestion
Highlights
-
•
A process for incorporating phytochemical-rich vegetables into potato snacks was developed.
-
•
Highest phytochemical concentrations were achieved using freeze-dried vegetables.
-
•
There was excellent retention of flavonoids and glucosinolates in the final snack product.
-
•
Bioavailability of phytochemicals from the snack was similar to that of cooked vegetables.
Abstract
The aim was to incorporate vegetables containing the phytochemicals quercetin, apigenin, glucoraphanin and carotenoids into a processed potato-based snack and assess their bioaccessibility and bioavailability. Three different processing routes were tested for incorporation and retention of phytochemicals in snacks using individually quick frozen or freeze-dried vegetables. No significant differences in the uptake or transport of quercetin or apigenin between a vegetable mix or snacks were observed using the CaCo-2 transwell model. Simulated in vitro digestions predicted a substantial release of quercetin and apigenin, some release of glucoraphanin but none for carotenes from either the snack or equivalent steamed vegetables. In humans, there were no significant differences in the bioavailability of quercetin, apigenin or glucoraphanin from the snack or equivalent steamed vegetables. We have shown that significant quantities of freeze-dried vegetables can be incorporated into snacks with good retention of phytochemicals and with similar bioavailability to equivalent steamed vegetables.
1. Introduction
Increasing the consumption of fruit and vegetables is a key preventative strategy for chronic diseases such as cardiovascular disease and cancer (Aune et al., 2017, National Health and Medical Research Council, 2013, Wang et al., 2014, World Health Organization, 2009). The benefits of a plant-based diet may in part be due to the numerous small secondary metabolites or phytochemicals that are ubiquitous in fruits and vegetables and have been associated with reduced risk of cardiovascular disease, stroke, arthritis, inflammatory bowel diseases, and some types of cancers (Hollman et al., 1999, Hu, 2003, Luo et al., 2008).
Plant foods contain phytochemicals, relatively small compounds that are synthesised to perform specific functions such as provide colour/attraction, act as anti-feedants, or protect macromolecules and cell machinery from stresses such as from UV light. When consumed by humans, phytochemicals have been shown to possess a number of biological activities including antioxidant, anti-inflammatory and vasoactive properties (Seifried, Anderson, Fisher, & Milner, 2007). This has been shown in numerous randomised controlled trials (RCTs) specifically related to commonly consumed phytochemicals such as polyphenols (including flavonoids), sulphur-containing compounds (such as glucosinolates and their breakdown products, isothiocyanates) and fat-soluble terpene-derived compounds (such as carotenoids). Specifically, these studies have shown significant improvements in biomarkers of disease risk including endothelial function, blood pressure and circulating lipoproteins in relation to cardiovascular disease risk (Armah et al., 2015, Cheng et al., 2017, Hooper et al., 2008, Wallace et al., 2016), macular pigment optical density in relation to visual function (Stringham & Stringham, 2016) and blood flow and cognitive performance in relation to brain health (Lamport et al., 2016, Rafnsson et al., 2013).
However, despite the supporting evidence for the benefits of increasing fruit and vegetable intake, and the phytochemicals within, the majority of the population in affluent and developing countries still do not consume the recommended target of five or more portions (≥400 g) of fruit and vegetables per day. For example, only 12% of adults in the European Union achieve this recommended target, with geographical differences observed from a low of 4% in Romania and Bulgaria to a high of 25% in Denmark and the Netherlands (OECD/EU, 2016). It is therefore important that new approaches are found to increase fruit and vegetable consumption (Hall et al., 2009, Guenther et al., 2013, U.S. Department of Agriculture and U.S. Department of Agriculture and USDA, 2013, and Rekhy & McConchie, 2014). At the same time, increasing awareness of the problems derived from inadequate diet translates into rising demand from consumers and pressure from policy makers for healthier alternatives to traditional products.
An alternative/complementary approach to improve diets via the consumption of more fruits and vegetables is to improve the nutritional quality of foods that are widely consumed in large quantities. Examples of potential candidates for this strategy include staple carbohydrate sources such as potatoes, pasta and rice, and products made from these. Savoury snack food products currently have a global market value of approximately 146 billion US dollars (2016) forecasted to be $151bn in 2017. Potato-based snacks account for a large proportion of the savoury snack market ($33.6 bn in 2016, forecasted to be $34.7 bn in 2017, whilst vegetable, pulse and bread chips were valued at $2.9 bn in 2016 and forecasted $3.2 bn in 2017, Euromonitor International research on Packaged Food, 20-Dec-2017). Despite a number of changes in the recipes and processing of potato-based snacks to improve their nutritional properties, including reductions in salt content, replacement of saturated cooking fats with unsaturated fats, and development of baked snacks as healthier alternatives to deep fried products, the majority of potato-based snacks provide mostly digestible (high glycaemic index) starch and few phytochemicals and vitamins. The incorporation of phytochemical-rich, low calorie plant foods such as vegetables and herbs could improve the nutritional value and potential health benefits of food products like potato-based. However, it is important to assess the influence of processing and manufacturing factors like temperature, pressure, moisture and mixing on the bioavailability and bioaccessiblity of these phytochemicals in the final product as they can be destroyed or rendered them non-bioavailable while being incorporated into food products (Manach et al., 2004, Riaz et al., 2009) critically affecting the stability of the components and their capacity to provide health benefits (Manach et al., 2004, Rothwell et al., 2015).
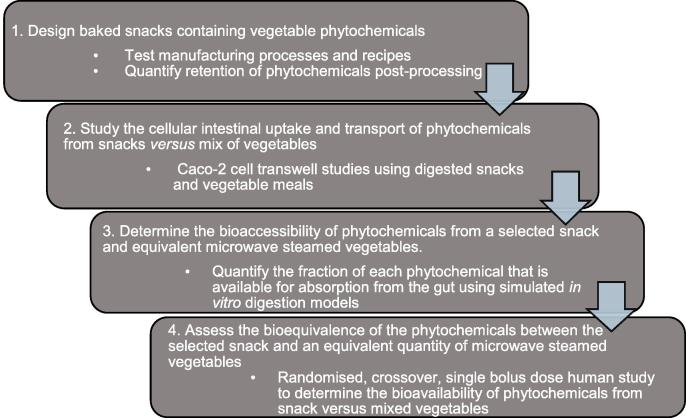
In this work, we describe an innovative way of incorporating vegetables into an existing popular potato-based snack food as a potentially promising route for increasing vegetable consumption. Four vegetables were selected as rich sources of different types of phytochemicals with potential health benefits (carrots providing carotenes, broccoli providing glucoraphanin/sulphoraphane, onion providing quercetin, parsley providing apigenin). We performed a step-wise series of in vitro and in vivo experiments to assess the impact of processing on phytochemical retention (the fraction of incorporated phytochemicals retained after processing), bioaccessibility (fraction available for absorption from the gut after consumption) and bioavailability (amount absorbed and reaching the peripheral circulation), and the effects of food matrix on intestinal uptake and transport of quercetin and apigenin (Fig. 1).
Fig. 1.
Overview of the steps followed to design a snack which incorporated freeze-dried vegetables and asses its bioequivalence with an equivalent quantity of steamed vegetables.
2. Materials and methods
2.1. Processing and incorporation of phytochemicals in a baked snack product
All the snacks were manufactured on pilot scale processing equipment. To allow a direct comparison, the vegetables were sourced from the same harvest as a “single time point supply”, carrot, parsley (both harvested in September 2012) and onion (harvested in June 2012) were sourced in fresh form while broccoli was sourced as whole frozen florets from “Waitrose” (Leicester, UK, May 2013). After harvesting, the vegetables (carrots, onion, parsley) were peeled, rough cut, washed, diced to desired size (10 mm onion, 10 mm carrot, and parsley in 6 mm chop), re-washed, de-watered, frozen rapidly and packed, this manufacturing process is known as instant quick frozen (IQF). Subsequently, a proportion of these IQF vegetables were freeze-dried across a 48-hour cycle by European Freeze Dry Ltd. (Lancashire, U.K.). Notably, IQF material (again, from the same harvest) was also retained to be used later in the study as a comparator meal of “minimally processed vegetables” in the in vitro gastric model and the human trial.
The targeted phytochemical enrichment was set based on previous studies at a level considered a priori to be safe but also quantifiable (after consumption) in both blood and urine samples (Goltz et al., 2013, Meyer et al., 2006, Mullen et al., 2006, Saha et al., 2012). In accordance with previous data, a 5 mg target dose for glucoraphanin was considered suitable for blood and urine analysis, however both quercetin and apigenin required higher amounts for plasma analysis than urine analysis i.e. 25 mg versus 5 mg for quercetin and 15 mg versus 10 mg apigenin respectively. β-carotene was only possible to be quantifed in plasma and a target dose of 15 mg was set. To achieve these targets, the vegetable/herb content was initially proportioned in the products as follows: 10.5% broccoli, 27.6% onion, 56.9% carrot and 5% parsley.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jff.2018.07.035.
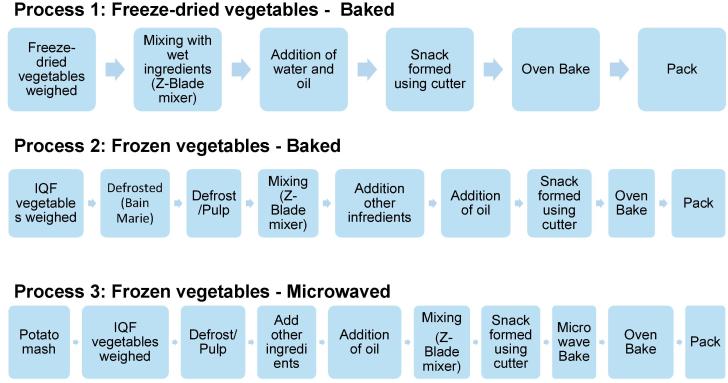
For preparation of snacks, three different processing routes were tested and recipes varied as necessary. Each processing route and individual steps had validated tolerance ranges to ensure samples were within control limits throughout the production process. The 3 processing routes investigated were: 1 – a “freeze-dried-baked” product produced by incorporating the three freeze-dried vegetables and IQF parsley with the other ingredients and baking the dough shapes in a hot air oven to produce a snack product, 2 – a “frozen-baked” product that used IQF vegetables and IQF parsley which were defrosted and mixed with the other ingredients before baking, and 3 – a “frozen-microwaved” product that was prepared by making a dough using IQF vegetables and IQF parsley in the same way as the “frozen-baked” product, but the dough shapes were microwave cooked and oven dried to finish the bake (see Fig. 2). For detailed processing information see Supplementary Material A.1 and Table A.1.
Fig. 2.
Processing steps in the preparation of snacks products.
Supplementary Material.
2.2. Preparation of a minimally processed “vegetable meal” comparator in the bioaccessibility and bioavailability experiments
Microwave steaming was considered a priori to be the most preservative cooking method for phytochemicals. Thus, a 461 g meal of frozen IQF vegetables (carrots, onion, parsley) and frozen broccoli florets (v/v of 10.5% broccoli, 27.6% onion, 56.9% carrot and 5% parsley) was prepared, along with 20 g water, in a microwave steamer for 9.5 min (800 Watt oven) and used as a “vegetable meal” comparator in the gastric-duodenal digestion model experiment and the human study. The weight and relative ratio of vegetables to each other was equivalent to the wet weight of freeze-dried vegetables contained in the 75 g baked snack food test meal. To address differences in meal mass and fluid balance between the snack food product and the vegetable meal that may affect the absorption, distribution, metabolism, and excretion (ADME) of the phytochemicals, variable amounts of bottled water (Buxton, Derbyshire, UK) were added to the snack product and vegetable meal for the digestion model studies, and consumed with the snack meal by the participants in the human study. Finally, the baked snack meal consisted of 75 g snack food +500 g water (total of 575 g), whilst the 461 g vegetable meal was provided with 20 g water (for microwaving, with the leached condensate retained and consumed) and 94 g bottled water (total of 575 g).
2.3. Determination of phytochemicals in food products and human samples
2.3.1. Determination of phytochemicals in food products
The extraction and quantification of glucoraphanin in broccoli, dough and snacks was performed according to the methods described in Saha et al., 2012, and flavonoids in onions, parsley, dough and snacks according to the method of Price, Bacon, & Rhodes, 1997. As apigenin was found to be not stable in the samples analysed, the corresponding identified metabolites were included in the analysis as described by Hostetler, Riedl, & Schwartz, 2013. The extraction and quantification of carotenoids in carrots, dough and snacks was based on the method reported by Hart & Scot, 1995. For further details see supplementary information A.2.
2.3.2. Determination of phytochemicals in biological samples
An LCMS method was developed to measure quercetin, apigenin, carotenoids and glucoraphanin concentrations in urine and serum samples. In addition to the aglycones, glucuronides of apigenin, isorhamnetin and quercetin were measured, as well as apigenin and quercetin-glucoside and quercetin-sulfate. Urine samples were prepared using liquid-liquid extraction, and serum samples were pooled per treatment and prepared using solid phase extraction (SPE). LCMS was performed using an Agilent 1200 series HPLC and a QTrap 3200 linear ion trap mass spectrometer (ABSciex, Warrington, UK). The chromatographic methods differed slightly between the flavonoids and sulforaphane and are detailed in supplementary information A.3. Carotenoids levels were assessed in serum samples as described previously (Leung et al., 2009) and in supplementary information A.3. Phytochemical concentration data for the biological samples obtained after consumption of each of the two meals were statistically evaluated using a paired t-test (SAS, version 9.3, SAS Institute, Inc., Cary, NC, USA).
2.4. Intestinal uptake and transport studies using Caco-2 cells
The Caco-2/TC7 cell model was used to assess if changes in the recipe and the way of processing the snack would affect the rate of intestinal absorption and the trans-epithelial transport of the flavonoid phytochemicals. First, snack products generated by the different processing routes were subjected to a simplified simulated stomach and small intestinal digestion which was allowed to go to completion and centrifuged to generate clear supernatants. Next it was shown that none of these digestates were toxic to Caco-2/TC7 cells treated with the digestates for 2 h at the doses tested (see supplementary information section A.4). Finally, a 1:1 mixture of digestate:DMEM media was used to determine cellular uptake and transport.
Samples of “freeze-dried-baked” snacks prepared with the initial ratio of vegetables and a set of mixed vegetables incorporating freeze-dried vegetables broccoli, onion and parsley mixed at the same ratio as the snacks underwent a simplified simulated digestion similar to that used by Aherne, Jiwan, Daly, and O’Brien (2009) but including amylase before being applied to Caco-2/TC7 cells.
The total uptake of quercetin or apigenin by the cells was quantified as the sum of metabolites in the incubation medium, metabolites located in the cells and aglycone in the cells. The rate of uptake by the cells was calculated as:
The transport of quercetin and apigenin across small intestinal enterocytes, the major site for their absorption in humans, was determined by the apparent permeability coefficient (Papp), a measure of the efflux of a compound across the Caco-2/TC7 monolayer defined by the equation:
where dQ/dt is the rate of accumulation in the basolateral chamber, Co is the initial concentration of the compound of interest, and A is the area of the cell monolayer. Papp was calculated by taking the total polyphenol content in the basolateral chamber (total metabolites + aglycone).
Details of the in vitro digestion, cell culture, toxicity testing, uptake and transport experiments as well as LC-MS conditions are provided in the supplementary information section A.4.
2.5. In vitro gastric-duodenal digestion
The bioaccessibility of phytochemicals in the 75 g baked snack food and the 461 g “vegetable meal” comparator was assessed in vitro using oral, gastric and duodenal digestion models (Minekus et al., 2014, Wickham et al., 2012) to determine the amounts of phytochemicals released from the food matrix to the liquid phase and thus made accessible for absorption in the gastrointestinal tract over time. This experiment consisted of (1) an oral phase that included mastication and salivary fluid addition, (2) a 36-minute gastric phase in the presence of priming acid and gastric enzymes, and (3) incubation of 239 g baked snack food and 251 g “vegetable meal” comparator (both weighed post-oral phase) in the duodenal phase at 37 °C and 170 rpm shaking conditions for 8 h, in the presence of 30 ml and 32 ml simulated bile solution with 91 ml and 95 ml pancreatic enzyme solution respectively. Further details of the gastric-duodenal method and justification for use are presented in the supplementary information A.5.
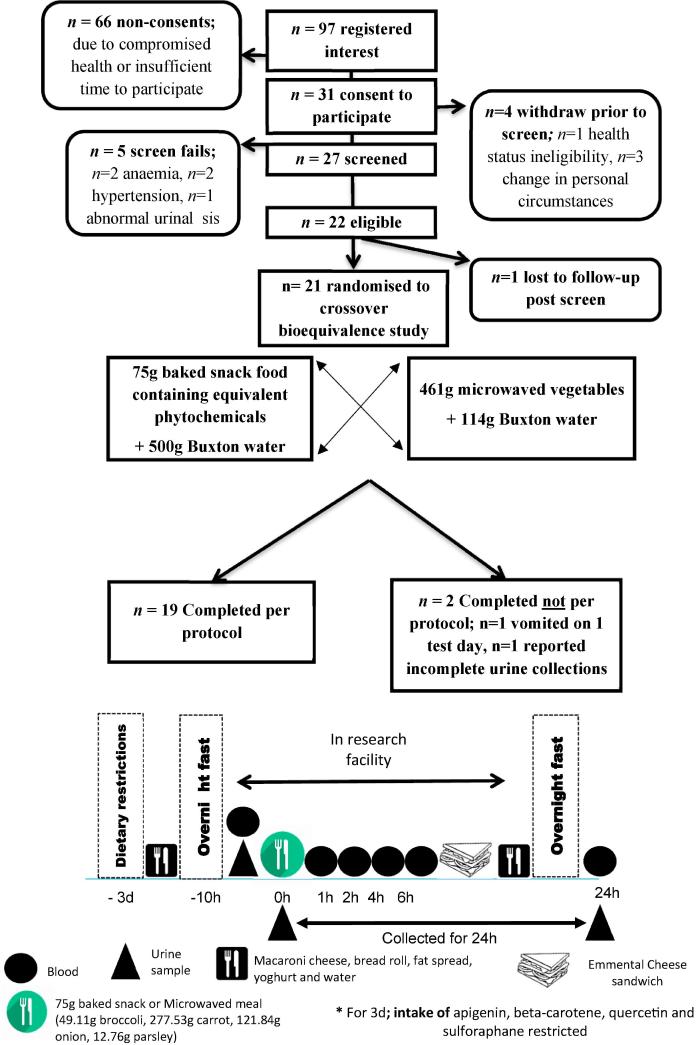
2.6. Human study
After National Research Ethics Committee approval, the study was conducted between July and December 2014 at the Clinical Research and Trials facility, (University of East Anglia, United Kingdom). Healthy adults aged ≥18 years, who were non-smokers with no significant pre-existing ill health were screened to take part in this study, with suitability judged by the study research nurses and clinician. Exclusion criteria included: Pregnant or breastfeeding women, current smokers (or ex-smokers ceasing within 3-months), existing or significant past history of diabetes, cancer, hepatic, renal or digestive conditions, prescription of ADME affecting medications, and intake of dietary supplements containing quercetin, apigenin, isothiocyaniates and carotenes. Participants gave written consent before enrolment and the study, which was registered at www.clinicaltrials.gov (NCT02231502), followed the principles of the Declaration of Helsinki.
The study was conducted using an un-blinded, cross-over design where participants experienced both treatments in random order. The primary aim was to determine the comparative bioavailability (i.e. bioequivalence) of quercetin, apigenin, isothiocyaniates, carotenes and their metabolites, after the consumption of either 75 g of the baked snack food, or a matched comparator “vegetable meal” (containing the equivalent wet weight of vegetables). Test meals were consumed on separate days, with ≥7 days observed between each intervention meal. For pre-menopausal participants, each study day was initiated during the follicular phase of their menstrual cycle. For 3 days prior to each intervention assessment day, participants were instructed to restrict the intake of quercetin, apigenin, glucoraphanin and carotenoid rich foods.
After an overnight fast (≥10 h), a fasted blood sample and spot urine were collected at the clinical facility. Study meals were then consumed within a targeted 15 min, in the presence of a research nurse who monitored compliance and full consumption. Bioavailability was determined by measuring phytochemical concentrations in urine collected over a 24 h postprandial period, and blood samples collected +1, +2, +4, +6 and +24 h after each treatment intake (see Fig. 3). Urine samples produced at the clinical facility (0–6 h postprandial) were collected as single aliquots (for each pass), with a combined ‘overnight’ pass collected outside of the research facility (>6 to 24 h). Compliance to overnight urine collection was self-reported by the participants. All biological samples were immediately processed before being stored at −80 °C until future analysis. For further details see supplementary information A.6.
Fig. 3.
Design of the Human Study.
3. Results
3.1. Processing routes and incorporation of phytochemicals
The incorporation of phytochemicals in a final snack product following the three manufacturing processes studied is presented in Table 1. “Freeze-dried-baked” snacks contained the highest content of phytochemicals for all the phytochemicals studied. “Frozen-microwaved” snacks had reasonable concentrations of phytochemicals, but the concentrations of phytochemicals in “frozen-baked” products were considerably lower. In addition to having the highest levels of phytochemicals in the final snack, “freeze-dried-baked” snacks would probably deliver a sufficient dose of most of the phytochemicals to allow the bioavailability to be assessed (i.e. sufficient quantities absorbed to be quantified in human samples) and was therefore selected as the processing method for further evaluation. The ratio of vegetables used for the comparison of cooking processes was broccoli:onion:carrot:parsley = 6:1.8:4.6:1.
Table 1.
Phytochemicals in freeze-dried-baked snacks, frozen baked snacks and frozen microwaved snacks using a ratio of vegetables broccoli:onion:carrot:parsley = 6:1.8:4.6:1.
| Final product (mg/100 g dry matter) |
||||
|---|---|---|---|---|
| Broccoli (glucoraphanin) | Oniona (quercetin) | Carrots (β-carotene) | Parsleyb (apigenin) | |
| Freeze-dried-baked | 54.1 ± 3.2 | 27.5 ± 1.8 | 10.3 ± 0.3 | 138.9 ± 4.2 |
| Frozen-baked | 15.2 ± 1.9 | 3.9 ± 0.11 | 2.0 ± 0.01 | 22.1 ± 1.4 |
| Frozen-microwaved | 19.1 ± 1.7 | 11.4 ± 0.2 | 5.7 ± 0.47 | 61.3 ± 1.6 |
Total mg quercetin glucosides.
Total mg aglycone apigenin.
Using the selected “freeze-dried-baked” process, several ratios of vegetable dry powders were tested in order to increase the concentration of those phytochemicals that were considered insufficient to allow quantification of their bioavailability upon consumption by humans. Most phytochemicals, with the exception of β-carotene in carrots, are present in the final snack product at concentrations largely consistent with the relative quantity of the vegetable source that had been incorporated into the dough obtained after mixing all the ingredients (Table 2). For example, when preparing a snack using a ratio broccoli:onion:carrot:parsley = 2.1:5.5:11.4:1 the amount of glucoraphanin, quercetin and apigenin found in the dough were 19.7 ± 0.5, 39.5 ± 3.6 and 38.3 ± 11.6 mg per 100 g dry matter, while the amounts in the final product were 16.9 ± 0.4, 44.1 ± 1.3 and 40.7 ± 4.9 mg per 100 g dry matter, indicating that only small changes of −14%, +12% and +6%, respectively occurred during the baking process. The apparently higher concentrations of quercetin and apigenin in the snack product compared to the dough might have been due to within-batch variation in the distribution of phytochemicals in the dough, or more likely due to increased extractability of the flavonoids caused by the baking process.
Table 2.
Phytochemicals measured in dough and in “freeze-dried-baked” snack product using different ratios of vegetables.
| (mg/100 g dry matter) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Broccoli | Onion | Carrot | Parsley | |||||
| (Glucoraphanin) |
(Quercetin)a |
(β-Carotene) |
(Apigenin)b |
|||||
| Dough | Final Product | Dough | Final Product | Dough | Final Product | Dough | Final Product | |
| broccoli:onion:carrot:parsley 6:1.8:4.6:1 | 66.1 ± 4.3 | 54.1 ± 3.2 | 27.6 ± 1.0 | 27.5 ± 1.8 | 10.9 | 10.3 ± 0.3 | 139.7 ± 6.2 | 138.9 ± 4.2 |
| broccoli:onion:carrot:parsley 7.4:18.8:39.1:1 | 21.7 ± 1.8 | 22.4 ± 2.5 | 43.9 ± 1.7 | 49.4 ± 1.6 | 23.8 ± 0.7 | 10.3 ± 0.9 | 12.1 ± 1.0 | 13.0 ± 1.5 |
| broccoli:onion:carrot:parsley 3.5:9.2:19.6:1 | 18.6 ± 0.9 | 17.3 ± 0.8 | 36.1 ± 4.1 | 47.2 ± 2.1 | 28.5 ± 1.0 | 10.4 ± 1.5 | 14.3 ± 4.1 | 19.1 ± 0.2 |
| broccoli:onion:carrot:parsley 2.1:5.5:11.4:1 | 19.7 ± 0.5 | 16.9 ± 0.4 | 39.5 ± 3.6 | 44.1 ± 1.3 | 24.0 ± 0.7 | 10.1 ± 0.6 | 38.3 ± 11.6 | 40.7 ± 4.9 |
| 100% Carrot only | n/a | n/a | n/a | n/a | 42.8 ± 3.7 | 13.6 ± 2.8 | n/a | n/a |
Total quercetin glycosides.
Total apigenin aglycones.
There were however substantial losses in the amount of β-carotene found in the final snack compared to the amounts measured in the dough. The final products obtained using several ratios of vegetables that contained 34–100% (w/w) of carrot revealed losses after processing of between 6 and 68% (w/w) of β-carotene respectively. The highest amount measured in the final product was 13.6 mg of β-carotene/100 g dry matter, obtained from a dough that contained only carrots as vegetables, with 42.8 mg of β-carotene/100 g dry matter in the dough giving a yield of 31.8% (w/w) in the final product. Surprisingly, there appeared to be no direct relationship between the amount of β-carotene incorporated into the dough and the amount measured in the final product, with increasing proportions of carrots/β-carotenes giving little or no increase in β-carotene in the final product. These data appear to show that above a threshold value of around 10–13 mg per 100 g dry matter, β-carotene is lost during the baking process.
3.2. Intestinal uptake and transport of quercetin and apigenin
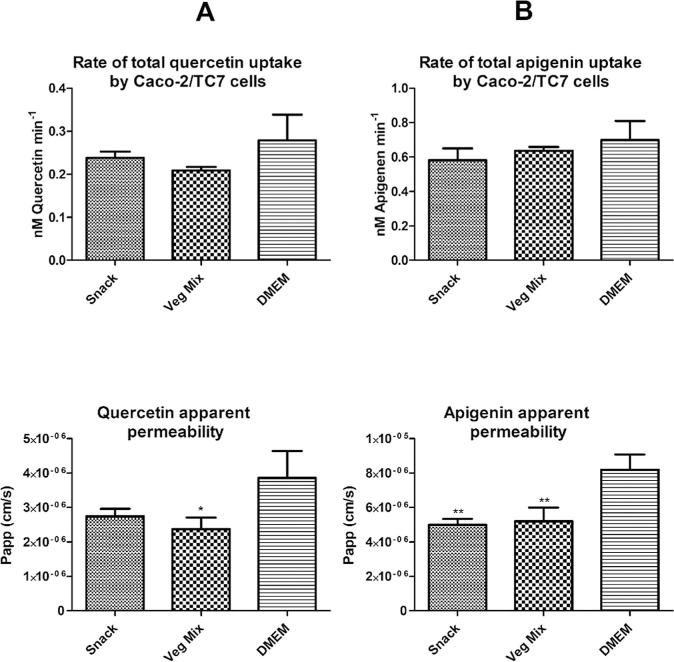
Using the Caco-2 model, it was shown that no significant differences (all p > 0.05) in the rate of uptake of either quercetin or apigenin were seen between the snack product and the matched vegetable mix digests (Fig. 4). However, the rate of apigenin uptake by Caco-2/TC7 cells was significantly higher compared to quercetin for all the digestates and for the DMEM-only control (Fig. 4B versus Fig. 4A top panels, p < 0.05).
Fig. 4.
Rates of uptake and transport (Papp) by Caco-2/TC7 cells for quercetin (A) and apigenin (B) from a digested “freeze-dried-baked” snack and a digested “comparator vegetable mix” compared to an equivalent DMEM buffer control containing 40 µM apigenin or quercetin. Data are mean ± SD (n = 3). Significance (*p < 0.05, **p < 0.01, ***p < 0.005) are for comparisons with the DMEM buffer control.
In terms of transport, the Papp values for quercetin and apigenin from both the snack product and the vegetable mix were significantly lower than for the media-only matrix, showing a negative effect of the food matrices on transport (p < 0.05). However, there were no significant differences in Papp values for quercetin or apigenin between the snack and vegetable mix matrices. Consistent with the higher rate of uptake of apigenin compared to quercetin, the Papp for apigenin was higher than that for quercetin (Fig. 4B versus Fig. 4A bottom panels, p < 0.05).
3.3. Phytochemicals in test meals
A comparison of phytochemicals present in 75 g of the “freeze-dried-baked” snack meal and in an equivalent amount of vegetables (461 g) in a microwave-steamed meal (“vegetable meal” comparator), confirmed that both meals contained similar doses of phytochemicals and at levels predicted to be detectable in blood and urine samples after their consumption, with the exception of carotenes (Table 3).
Table 3.
Comparison of mg of phytochemicals present in “freeze-dried-baked” snack product (75 g) and “vegetable meal” comparator (461 g).
| Glucoraphanin (mg) | Quercetin (mg) | Beta-Carotene (mg) | Apigenin (mg) | |
|---|---|---|---|---|
| Snack | 12.7 ± 0.4 | 33.1 ± 1.3 | 7.6 ± 0.6 | 30.5 ± 4.9 |
| Cooked veg. meal | 12.6 ± 1.3 | 30.9 ± 0.9 | 23.5 ± 3.6 | 48.2 ± 3.5 |
3.4. In vitro gastric-duodenal digestion
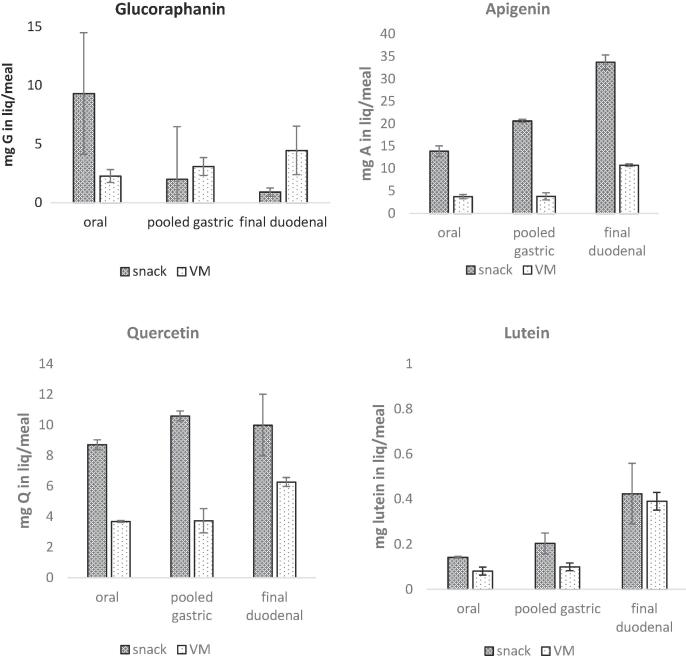
At the end of a simulated gastro-duodenal in vitro digestion there was a substantial release of quercetin and apigenin to the liquid phase. The snack meal released 10.0 ± 2.0 mg of quercetin and 33.7 ± 1.6 mg of apigenin while the steamed “vegetable meal” comparator made available 6.3 ± 0.3 mg of quercetin and 10.7 ± 0.3 mg of apigenin. Glucoraphanin was also released from both food matrices but in higher amounts from the steamed “vegetable meal” comparator compared to the snack meal (4.5 ± 2.1 mg versus 0.9 ± 0.3 mg).
The in vitro digestion predicted a very limited release of carotenoids during gastrointestinal digestion for both snack and vegetable meals, with only around 0.4 mg of lutein and no detectable β-carotene present in the liquid phase. Fig. 5 shows the amount of phytochemicals measured in the liquid phase at the end of each simulated phase.
Fig. 5.
Amount of glucoraphanin, quercetin, apigenin and carotenoids (lutein) measured in the liquid phase for samples taken at the end of the main stages during simulated in vitro digestion of a snack meal (snack) and a cooked vegetable meal comparator (VM). Each value is the average of duplicate measurements. Oral = sample after simulated oral phase, Pooled gastric = pool of timed samples from the simulated gastric phase, Duodenal = Sample taken at the end of the simulated duodenal digestion.
3.5. Human bioavailability study
As shown in Fig. 3, 19 participants completed the bioequivalence feeding study per protocol. Participants were predominantly female (n = 18 of study population, of which n = 10 were premenopausal), and on average 42 years (age range 20–68 yr), within the healthy weight range (23.4 kg/m2 ± 2.9 SD) and normotensive at screening (systolic and diastolic blood pressure of 129/79 mm Hg (±14 and ±7 SD respectively). Dietary intakes, assessed by food frequency questionnaire (FFQ) prior to the study, suggested typical UK intakes for a predominantly female population i.e. 1804 Kcal, 34.4%, 47.8% and 18.1% food energy (FE) from fat, carbohydrate and protein respectively. Notably, reported fruit and vegetables intakes were higher than anticipated (746 g/d) which may reflect health-conscious nature of those recruited to the study, or the reporting of socially desirable intakes. All participants were approved for enrolment by the study clinical advisor (a general practitioner (GP)) and no serious adverse events were reported.
Between the snack and the “vegetable meal”, only isorhamnetin-3-glucuronide levels significantly differed (p = 0.02), with higher concentrations found in 24 h urine collections after the comparator “vegetable meal” (Table 4), similarly, sulforaphane-N-acetyl-cysteine levels approached significance (p = 0.06). Conversely, urinary levels of apigenin, glucoraphanin, quercetin and their metabolites, were not significantly different between the baked snack product and the comparator “vegetable meal” (all p > 0.05). This suggests general bioequivalence of the two test meals, albeit that concentrations were almost exclusively higher following the comparator “vegetable meal” (i.e. expressed as a nmol per mg intake comparison of phytochemical levels in 24 h urine, the baked snack product was on average 30.6% lower than the comparator vegetable meal; Table 4). Despite frequent collections throughout the 24 h period, serum concentrations of the flavonoids and isothiocyanates were below the limit of detection (data not shown).
Table 4.
24 h urinary excretion of quercetin, apigenin and glucoraphanin metabolites expressed in nmol per mg intake of the corresponding compound. Statistical difference tested between vegetable based food product and minimally processed “vegetable meal” comparator using paired t-test, P < 0.05. A = apigenin, Q = quercetin, IR = isorhamnetin (3-O-methylquercetin), Glc = glucoside, GlcA = glucuronide, NAC = n-acetyl-cysteine.
| Vegetable meal | Snack | |||
|---|---|---|---|---|
| (nmol/mg intake) | (nmol/mg intake) | Difference (absolute) | P-value | |
| Apigenin (A) | 3.55 ± 4.6 | 3.37 ± 6.6 | 0.19 | 0.89 |
| A-7-Glc | 0.21 ± 0.3 | 0.15 ± 0.4 | 0.05 | 0.37 |
| A-7-GlcA | 5.33 ± 6.6 | 6.32 ± 8.1 | 0.99 | 0.55 |
| Isorhamnetin (IR) | 0.44 ± 0.6 | 0.33 ± 0.4 | 0.11 | 0.19 |
| IR-3-GlcA | 2.67 ± 2.7 | 1.60 ± 2.0 | 1.07 | 0.02 |
| Quercetin (Q) | 0.72 ± 1.3 | 0.34 ± 0.7 | 0.38 | 0.12 |
| Quercetin-3-GlcA | 4.40 ± 2.5 | 4.17 ± 3.3 | 0.23 | 0.75 |
| Quercetin-3-Glc | 0.07 ± 0.1 | 0.04 ± 0.1 | 0.03 | 0.45 |
| Quercetin-3-sulfate | 3.36 ± 1.8 | 2.58 ± 1.9 | 0.79 | 0.10 |
| Sulforaphane | 43.72 ± 44.2 | 36.25 ± 27.9 | 7.47 | 0.42 |
| Sulforaphane-NAC | 508.54 ± 450.9 | 272.17 ± 280.8 | 236.4 | 0.06 |
| Sulforaphane-NAC | 508.54 ± 450.9 | 272.17 ± 280.8 | 236.4 | 0.06 |
Notably, wide variations in serum β-carotene levels were observed in baseline samples, inter-individual variations ranged from 13.6 to 447.9 nmol/L, and 24.7 to 516.1 nmol/L respectively, after intake of both meals. Likewise, the mean intra-individual variation was 47.5% (min. to max. of 11.6–136.9%, between the 2 assessment visits). This was observed despite 3 days of restricted dietary intake (i.e. restrictions for glucaraphanin, quercetin, apigenin and carotenoid intake) and the provision of a standardised low flavonoid/carotenoid meal, suggesting likely carryover from endogenous fat stores, which reflect habitual carotenoid intakes. After correcting for baseline level (via subtraction), the AUC over 24 hr for absolute serum circulatory levels of carotenoids (nmol/L) were not significantly different between the test foods (i.e. baked snack product and the comparator “vegetable meal”) and this remained the case when further adjusting the analyses to account for minor differences in the amount of carotenoids in the test meals (nmol/L/mg intake, p > 0.05).
4. Discussion
The overall aims of this work was to develop a process that allowed incorporation of bioactive-rich vegetables into a low fat baked snack with a high degree of retention of the bioactives in the final snack, and to compare the bioavailability of the bioactives between the snack matrix and an equivalent dose of minimally processed vegetables. Here we report that potato-based snacks prepared using freeze-dried vegetables had a much higher vegetable dry matter incorporation rate than frozen-baked and frozen-microwaved snacks that incorporated high water content IQF vegetables. It was subsequently demonstrated that, with the exception of carotenes, incorporation of freeze-dried vegetables allowed the production of pre-cook doughs containing substantial amounts of phytochemicals and their retention following an oven bake process. When comparing the freeze-dried baked snack to a vegetable meal comparator, in vitro simulated gastric and duodenal digestions predicted high bioaccessibility of quercetin and apigenin, moderate bioaccessibility of glucoraphanin but poor bioaccessibility for carotenes. In vitro intestinal epithelial cell uptake and transport studies gave similar results for the freeze-dried-baked snack and microwave-steamed vegetable comparator matrices. The bioavailability of quercetin, apigenin and glucoraphanin was validated in the human study, as well as the bioequivalence between 75 g of a baked snack product meal and a comparator meal of 461 g of microwaved vegetables corresponding to 5.75 equivalent portions of vegetables (at 80 g per portion) containing broccoli, onion, carrots and parsley.
The “freeze-dried-baked” snack was manufactured reaching targets of phytochemicals set a priori as likely to be identifiable in our in vitro and in vivo bioavailability studies. Surprisingly, it was confirmed that β-carotene content in the finished product reached a threshold of around 10–13 mg per 100 g dry matter, with increasing proportions of carrots giving little or no increase in β-carotene content. It is well known that carotenoids are susceptible to losses during processing (and storage); the unsaturated nature of the molecules makes them particularly susceptible to oxidation and isomerisation. However, we are not aware of any reports showing an upper limit to the concentration of β-carotene that can be achieved in composite foods such as the potato starch and vegetable-based snacks described here; this appears to be a newly described phenomena. There is an extensive literature describing the effects of numerous processing methods and storage conditions on carotenoids including for β-carotene in carrots (e.g. see Rodriguez-Amaya, 2003 for a review). We included both cis- and trans-isomers in our analyses. The susceptibility of carotenoids to losses caused by oxidation increases in the presence of oxygen, certain enzymes, metals, oxidants and via exposure to light, and can be increased if processing conditions are more severe. However, it is not clear why the absolute loss of β-carotene increases beyond a threshold level such that any additional β-carotene from carrot that is included is lost during processing. It suggests that this particular food matrix has a fixed capacity to protect β-carotene from processing-induced losses, at around 10 mg β-carotene (100 g dry weight baked snack product)−1. There is a report describing the development of maize-based β-carotene-rich product but these contained β-carotene at levels below 10 mg (100 g dry weight)-1 (Bhavani and Kamini, 1998).
No significant differences in the transport or rate of uptake of either quercetin or apigenin were seen, using a Caco-2 cell model, between the product and the matched vegetable mix digests. However, although the uptake was not different between the two matrices, the transport of metabolites was inhibited by both the snack product and vegetable mix digests when compared to the cell culture medium alone. These data are in keeping with the food matrix affecting the basolateral efflux of the flavonoid aglycones and phase-2 conjugates. Transport of metabolites of apigenin showed a different pattern to that of quercetin likely indicating the involvement of different transporter proteins. These transporters are inhibited to a similar degree in the product and vegetable mix digests, which may reflect an effect of the digestion process on the transport proteins involved in apigenin-metabolites transport, although the mechanism is not clear.
Also, the rate of apigenin uptake by Caco-2/TC7 cells was significantly higher compared to quercetin for the two digestates and for the DMEM-only control. This almost certainly reflects the higher lipophilicity of apigenin compared to quercetin (Rothwell, Day, & Morgan, 2005) which allows apigenin to diffuse more freely across the cellular plasma membrane and to accumulate to higher concentrations in the cell interior.
The simulated in vitro digestion predicted that there was a significant release of quercetin and apigenin from both the snack and microwaved “vegetable meal” comparator. The amount of glucoraphanin released from the vegetable meal was however higher than when it was incorporated in a processed snack (4.5 and 0.9 mg respectively). Carotenes, on the other hand, were not detected in the bioaccessible aqueous phase, which is most likely due to the absence in the meals of oil required to transfer carotenoids from the food matrix to the aqueous phase (e.g. in mixed micelles) (see Kopec and Failla, 2018). The inclusion of a minimal amount of fat in the snack product could make the snack food carotenes significantly more bioavailable (see Lakshminarayana and Baskaran, 2013 for a review), but the incorporation of more carotenoids in a processed and manufactured snack product remains a challenge.
In the human study we showed general agreement with the in vitro bioaccesibility (Caco-2) and bioavailability (duodenal gastric model) experiments, confirming bioequivalence in relation to excreted phytochemical content in 24 h urine samples between the 75 g baked snack product and a comparator meal of 461 g of microwaved vegetables. Whilst almost all phytochemicals and their metabolites were found in higher proportions following the comparator vegetable meal (on average, 30.6% greater levels in 24 h urine), it is important to recognise that microwaving the comparator vegetable meal was considered a priori to be the cooking method most likely to retain bioactive compounds. As many consumers cook vegetables less sympathetically, for example by boiling in water (which leads to substantial losses via leaching), it is likely that our approach has over-emphasized the likely difference between the baked snack product and some other methods of cooking vegetables.
There were some limitations in our human study and product design such as a study population almost exclusively female (86% of study group), and an insufficient dietary carotenoid restriction period (3 days abstinence) to reduce the impact of endogenous stores on baseline β-carotene levels. However, accepting these limitations, our results suggest that utilizing freeze-dried vegetable materials allows the production of snacks containing substantial amounts of phytochemicals found in vegetables and could provide a means to further increase the intake of phytochemical compounds in the consumer diet. If the cost effectiveness of technologies such as freeze drying is further improved, or if cheaper drying processes were used, it may become feasible to market baked snack products that contain significant quantities of vegetables. There are numerous alternative methods of drying vegetables and each one will have different effects on the retention of bioactive phytochemicals such as flavonoids and carotenoids (McSweeney and Seetharaman, 2015) and potentially also on their subsequent bioaccessbility and bioavailability. More studies are required to determine the physiological importance of delivering the phytochemical compounds at the levels observed, especially following sustained intakes.
5. Conclusions
To our knowledge, this is the first report describing the incorporation of significant quantities of vegetables into potato-based snacks with high retention of the phytochemicals and providing evidence from a proof-of-concept bioequivalence human study that the phytochemicals are similarly bioavailable compared to those in an equivalent quantity of microwave-steamed vegetables. We have demonstrated that incorporation of freeze-dried vegetables allows the production of snacks containing substantial amounts of phytochemicals with excellent retention of quercetin, apigenin and glucoraphanin. Further, we have shown that there was no significant difference in the bioavailability of these three bioactives between the snack and an equivalent mix of microwave steamed vegetables, which was predicted by the in vitro assessments. There is a need for the development of low-cost vegetable drying processes that allow high retention of bioactives and can be used in the production of healthier snacks.
Author declarations of interest
NPM, SS, MP, MSW, WJH, VvdV, PJC have no conflicts of interest to declare. MS and JB are employees of PepsiCo. PepsiCo part funded the research reported here and is the assignee for a patent application that relates to the research reported here, and MS, JB, PAK and DJH are proposed inventors on this patent application.
Ethical statement
The following statement has been included in the manuscript:
Participants gave written consent before enrolment and the study, which was registered at www.clinicaltrials.gov (NCT02231502), followed the principles of the Declaration of Helsinki.
Acknowledgements
This research was supported by a grant from Innovate UK (Grant number 101120) with additional support from PepsiCo Inc and from the Biotechnology and Biological Sciences Research Council (UK) through an Institute Strategic Programme Grant (‘Food and Health’, Grant No: BB/J004545/1) to the Quadram Institute Bioscience (formerly Institute of Food Research). The carotenoid analysis in human samples was performed under sub-contractor arrangements by Dr Georg Leitz and Dr Anthony Oxley (both Newcastle University). The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of PepsiCo Inc.
References
- Aherne S.A., Jiwan M.A., Daly T., O’Brien N.M. Geographical location has greater impact on carotenoid content and bioaccessibility from tomatoes than variety'. Plant Foods For Human Nutrition. 2009;64:250–256. doi: 10.1007/s11130-009-0136-x. [DOI] [PubMed] [Google Scholar]
- Armah C.N., Derdemezis C., Traka M.H. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Molecular Nutrition and Food Research. 2015;59(5):918–926. doi: 10.1002/mnfr.201400863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N., Norat T.…Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology. 2017;46(3):1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavani K.N., Kamini D. Development and acceptability of a ready-to-eat beta-carotene rich, maize based supplementary product. Plants for Human Nutrition. 1998;52(3):271–278. doi: 10.1023/a:1007997832407. [DOI] [PubMed] [Google Scholar]
- Cheng D., Wang R., Wang C. Mung bean (Phaseolus radiatus L.) polyphenol extract attenuates aluminum-induced cardiotoxicity through an ROS-triggered Ca2+/JNK/NF-kappa B signaling pathway in rats. Food and Function. 2017;8(2):851–859. doi: 10.1039/c6fo01817c. [DOI] [PubMed] [Google Scholar]
- Euromonitor International research report on Packaged Food (20-Dec-2017) <http://www.euromonitor.com/packaged-food>.
- Goltz S.R., Sapper T.N., Failla M.L., Campbell W.W., Ferruzzi M.G. Carotenoid bioavailability from raw vegetables and a moderate amount of oil in human subjects is greatest when the majority of daily vegetables are consumed at one meal. Nutrition Research. 2013;33(5):358–366. doi: 10.1016/j.nutres.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Guenther P.M., Casavale K.O., Reedy J., Kirkpatrick S.I., Hiza H.A.B., Kuczynski K.J. Update of the healthy eating Index: HEI-2010. Journal of the Academy of Nutrition and Dietetics. 2013;113(4):569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.N., Moore S., Harper S.B., Lynch J.W. Global variability in fruit and vegetable consumption. Amer. J. Prev. Med. 2009;36(5):402–409. doi: 10.1016/j.amepre.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Hart D.J., Scot K.H. Development and evaluation of an HPLC method for the analysis of carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chemistry. 1995;54:101–111. [Google Scholar]
- Hollman P.C., Feskens E.J., Katan M.B. Tea flavonols in cardiovascular disease and cancer epidemiology. Proceedings of the Society for Experimental Biology and Medicine. 1999;220:198–202. doi: 10.1046/j.1525-1373.1999.d01-33.x. [DOI] [PubMed] [Google Scholar]
- Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A.…Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2008;88(1):38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- Hostetler Gregory L., Riedl Ken M., Schwartz Steven J. Effects of food formulation and thermal processing on flavones in celery and chamomile. Food Chemistry. 2013;141(2):1406–1411. doi: 10.1016/j.foodchem.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F.B. Plant-based food and prevention of cardiovascular disease: An overview. American Journal of Clinical Nutrition. 2003;78:S544–S551. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- Kopec R.E., Failla M.L. Recent in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. Journal of Food Composition and Analysis. 2018;68:16–30. [Google Scholar]
- Lakshminarayana R., Baskaran V. Influence of olive oil on the bioavailability of carotenoids. European Journal of Lipid Science and Technology. 2013;115(10):1085–1093. [Google Scholar]
- Lamport D.J., Lawton C.L., Merat N. Concord grape juice, cognitive function, and driving performance: A 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. American Journal of Clinical Nutrition. 2016;103(3):775–783. doi: 10.3945/ajcn.115.114553. [DOI] [PubMed] [Google Scholar]
- Leung W.C., Hessel S., Meplan C., Flint J., Oberhauser V., Tourniaire F.…Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB Journal. 2009;23(4):1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- Luo H., Jiang B.H., King S.M., Chen Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutrition and Cancer. 2008;60(6):800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- McSweeney M., Seetharaman K. State of Polyphenols in the Drying Process of Fruits and Vegetables. Critical Reviews in Food Science and Nutrition. 2015;55(5):660–669. doi: 10.1080/10408398.2012.670673. [DOI] [PubMed] [Google Scholar]
- Meyer H., Bolarinwa A., Wolfram G., Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Annals of Nutrition and Metabolism. 2006;50(3):167–172. doi: 10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C.…Brodkorb A. A standardised static in vitro digestion method suitable for food – An international consensus. Food and Function. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Mullen W., Edwards C.A., Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. British Journal of Nutrition. 2006;96(1):107–116. doi: 10.1079/bjn20061809. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council, Australian dietary guidelines, (2013).
- OECD/EU Fruit and vegetable consumption among adults, in Health at a Glance: Europe 2016: State of Health in the EU Cycle, (2016) OECD Publishing, Paris.
- Price K.R., Bacon J.R., Rhodes M.J.C. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa) Journal of Agricultural and Food Chemistry. 1997;45(3):938–942. [Google Scholar]
- Rafnsson S.B., Dilis V., Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: A systematic review of population-based cohort studies. European Journal of Nutrition. 2013;52(6):1553–1567. doi: 10.1007/s00394-013-0541-7. [DOI] [PubMed] [Google Scholar]
- Rekhy R., McConchie R. Promoting consumption of fruit and vegetables for better health. Have campaigns delivered on the goals? Appetite. 2014;79:113–123. doi: 10.1016/j.appet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Riaz M., Asif M., Ali R. Stability of vitamins during extrusion. Critical Reviews in Food Science and Nutrition. 2009;49(4):361–368. doi: 10.1080/10408390802067290. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya D.B. Food carotenoids: Analysis, composition and a lterations during storage and processing of foods. Forum of Nutrition. 2003;56:35–37. [PubMed] [Google Scholar]
- Rothwell J.A., Day A.J., Morgan M.R.A. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. Journal of Agriculture and Food Chemistry. 2005;53(11):4355–4360. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- Rothwell J.A., Medina-Remo A., Perez-Jimenez J., Neveu V., Knaze V., Slimani N., Scalbert A. Effects of food processing on polyphenol contents: A systematic analysis using Phenol-Explorer data. Molecular Nutrition & Food Research. 2015;59:160–170. doi: 10.1002/mnfr.201400494. [DOI] [PubMed] [Google Scholar]
- Saha S., Hollands W., Teucher B., Needs P.W., Narbad A., Ortori C.A. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Molecular Nutrition & Food Research. 2012;56(12):1906–1916. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- Seifried H.E., Anderson D.E., Fisher E.I., Milner J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. Journal of Nutritional Biochemistry. 2007;18(9):567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Stringham J.M., Stringham N.T. Serum and retinal responses to three different doses of macular carotenoids over 12 weeks of supplementation. Experimental Eye Research. 2016;151:1–8. doi: 10.1016/j.exer.2016.07.005. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services (USDA) 7th ed. U.S. Government Printing Office; Washington DC: 2013. Dietary guidelines for Americans. [Google Scholar]
- Wallace T.C., Slavin M., Frankenfeld C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. 2016;8(1):32–45. doi: 10.3390/nu8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ouyang Y., Liu J. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ-British Medical Journal. 2014;349 doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham M.F., Faulks R.M., Mann J., Mandalary G. The design, operation, and application of a dynamic gastric model. Dissolution Technologies. 2012:15–22. [Google Scholar]
- World Health Organization (2009). Global health risks: Mortality and burden of disease attributable to selected major risks, (Copenhagen).