Abstract
Previously, our group demonstrated that recombinant porcine oviductin (pOVGP1) binds to the zona pellucida (ZP) of in vitro-matured (IVM) porcine oocytes with a positive effect on in vitro fertilization (IVF). The fact that pOVGP1 was detected inside IVM oocytes suggested that this protein had a biological role during embryo development. The aim of this study was to evaluate the effects of pOVGP1 on bovine in vitro embryo development. We applied 10 or 50 µg/ml of pOVGP1 during IVF, embryonic in vitro culture (IVC), or both, to evaluate cleavage and embryo development. Blastocyst quality was assessed by analyzing the expression of important developmental genes and the survival rates after vitrification/warming. pOVGP1 was detected in the ZP, perivitelline space, and plasma membrane of blastocysts. No significant differences (P > 0.05) were found in cleavage or blastocyst yield when 10 or 50 µg/ml of pOVGP1 was used during IVF or IVC. However, when 50 µg/ml pOVGP1 was used during IVF + IVC, the number of blastocysts obtained was half that obtained with the control and 10 µg/ml pOVGP1 groups. The survival rates after vitrification/warming of expanded blastocysts cultured with pOVGP1 showed no significant differences between groups (P > 0.05). The use of pOVGP1 during IVF, IVC, or both, increased the relative abundance of mRNA of DSC2, ATF4, AQP3, and DNMT3A, the marker-genes of embryo quality. In conclusion, the use of pOVGP1 during bovine embryo in vitro culture does not affect embryo developmental rates but produces embryos of better quality in terms of the relative abundance of specific genes.
Keywords: Embryo development, Embryo quality, OVGP1, Oviduct
The oviduct is the female organ in which fertilization and early embryo development take place. During the first 3–4 days of development, the embryo remains in the oviduct where the conceptus is exposed to the oviductal fluid (OF). A 16-cell stage bovine embryo then enters the uterus. Although the embryo in its early stages of development does not need contact with the maternal tract to regulate its own cell division and differentiation, the reproductive tract, and its secretions, have important roles in providing the optimal environment for embryo development [1].
Preimplantation embryos are able to develop in vitro and to produce normal offspring after embryo transfer. However, the development of preimplantation mammalian embryos in vitro is compromised compared with those grown in vivo. Studies comparing bovine oocyte maturation, fertilization, and embryo culture in vivo versus in vitro have shown that the post-fertilization environment determines blastocyst quality, measured in terms of cryotolerance [2, 3] and relative transcript abundance [4]. Deprivation of some in vivo-produced maternal factors could be responsible for impaired in vitro development and decreased viability [4], as well as some pathological alterations associated with in vitro-produced embryos [5, 6]. Therefore, oviductal secretions are thought to be key factors for improving embryo quality during in vitro embryo production in the context of assisted reproductive techniques.
The OF is composed of simple and complex carbohydrates, ions, lipids, phospholipids, and proteins [7]. Individual oviductal secretions have an effect on oocyte and sperm function [8], and the possible role of proteins such as oviductin (presently termed as OVGP1), osteopontin, glycodelins, and lactoferrin on gamete interaction has been described [9]. Proteomic analysis of the OF and gene expression analysis of oviductal cells pointed to OVGP1 and its codifying gene as the main protein/gene in the oviduct in quantitative terms, whose abundance increases at estrus [10, 11]. OVGP1 has particularly been detected in the perivitelline space in fertilized eggs, where it is endocytosed by the blastomeres of 2-cell, 4-cell, and 8-cell embryos, as well as blastocysts, in hamster [12, 13]. This suggests that in hamster, OVGP1 could play a role in supporting the growth and differentiation of the embryo. Indeed, the positive effect of recombinant OVGP1 on embryo development has been described in mammalian species such as goat [14] and domestic cat [15]. Furthermore, antibodies against the C-terminal peptide of rabbit OVGP1 inhibit early embryo development in mouse [16].
It is clear that a study of the oviductal environment, especially the effect of OVGP1, is crucial to further our understanding of the underlying regulatory mechanisms controlling embryo development. Most functional studies in ovine, porcine, and bovine species have used native OVGP1 purified from OF or complete OF [17,18,19,20]. However, OF undergoes continuous renewal in the oviduct in vivo as the reproductive tract modifies its activity to provide the optimal environment for the gametes and embryos at each step of the reproductive process [1]. As a result, the amount of proteins is modified by the oviduct according to each embryo-stage or the different phases of the estrous cycle, potentially hindering a successful transfer to in vitro conditions. Moreover, as native OVGP1 is difficult to obtain in sufficient amounts, recombinant OVGP1 represents a reasonable alternative.
Previously, we have shown that recombinant porcine OVGP1 (pOVGP1) has a positive effect on porcine fertilization efficiency, when it is added to the IVM or IVF medium and we attribute an order-specific role in modulating sperm binding, penetration, and fertilization, to the C-terminus of OVGP1 [21]. This allows heterologous OVGP1 proteins (porcine and bovine) sharing the same conservative regions at their C-terminal regions (Supplementary Fig. 1: online only) [21] to exert the same positive effect on IVF [22]. However, to our knowledge, the effects of recombinant OVGP1 on bovine embryo development have not yet been studied. Based on the amino acid sequence, porcine (Q28990) and bovine (Q28042) OVGP1 are 78% homologous and, more importantly, both proteins share the same C-terminal regions which are involved in the specific ZP-binding patterns that remodel the ZP matrix and allow protein endocytosis [21].
In vitro bovine embryo production is already well established and is used on a global scale for the cheap production of calves. The bovine species is an economically important livestock animal and investigations into cow reproduction have recently generated renewed interest due to its importance as a model animal in transgenesis and stem cell research. Therefore, the main objective of the present study was to analyze in vitro the effects of purified recombinant pOVGP1 supplementation on bovine embryo development and embryo quality. For that purpose, we used two different concentrations of pOVGP1 (10 and 50 µg/ml) during the period when, in vivo, the embryo is still in the oviduct: IVF (from day 0 to day 1) (D0–D1), early IVC (from day 1 to day 3.5) (D1–D3.5), or both (from day 0 to day 3.5) (D0–D3.5).
Materials and Methods
Unless otherwise stated, all chemicals were purchased from Sigma Aldrich Quimica S.A Company (Madrid, Spain).
Recombinant OVGP1 protein production
Recombinant pOVGP1 was expressed in the Human Embryonic Kidney (HEK 293T) cell line and purified as described previously [21]. HEK 293T cells were grown in Corning® Roller Bottles (Sigma-Aldrich, St. Louis, MO, USA) (37°C, 5% CO2, and 95% humidity) for 48–72 h to 80–90% confluence in DMEM medium (Dulbecco’s Eagle Medium, Thermo Fisher Scientific, Rockford, IL, USA) supplemented with 10% fetal bovine serum and 4 mM glutamine (both from Gibco BRL-Life Technologies, Gaithersburg, MD, USA). Cells were transfected using polyethylenimine (PEI, Sigma-Aldrich) to express pOVGP1. Conditional media were harvested at 72 h after transfection and adjusted with a buffer resulting in a final composition of 10 mM imidazole, 20 mM HEPES, and 150 mM NaCl, pH 7.8, and incubated with Ni-NTA Superflow (Qiagen, Hilden, Germany) overnight at 4ºC. The nickel beads were washed and the proteins were eluted with a 500 mM imidazole, 20 mM HEPES, 150 mM NaCl, pH 7.8 buffer. A buffer exchange was made by dialysis to obtain the protein in a 150 mM Tris HCl, 200 mM NaCl, 10% glycerol buffer. The authenticity of pOVGP1 was verified as described previously [21].
Immunoblotting
Purified protein was separated by SDS-PAGE and transferred to PVDF membranes, which were probed with Penta-His mouse monoclonal antibody (Qiagen) and visualized by chemiluminescence. Proteins were also stained with Simply BlueTM Safe Stain (Invitrogen, Carlsbad, CA) after SDS-PAGE.
Confocal microscopy
Bovine presumptive zygotes and embryos supplemented with pOVGP1 during IVF, IVC, or both processes, at two different concentrations of pOVGP1 (10 and 50 µg/ml), were analyzed by confocal microscopy to detect the presence of the protein.
After treatment, presumptive zygotes (D1) and embryos (D3.5 and D9) were washed and fixed with 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS. Half of the D3.5 embryos and all of the D1 presumptive zygotes and D9 embryos were permeabilized using TritonTM X-100 at 1% in PBS for 5 min (Sigma-Aldrich) to enable detection of pOVGP1. Half of the D3.5 embryos were left unpermeabilized in order to check whether pOVGP1 had bound to the blastomere membranes. D3.5 embryos were stained for 15 min in 1% Hoechst in PBS. Permeabilized and non-permeabilized presumptive zygotes and embryos were stained with Penta-His mouse monoclonal antibody diluted 1:100 in PBS. Stained embryos were mounted on a microscope slide. Samples were analyzed with a DM IRE2 confocal microscope (True Confocal Scanner TCS-SP2, Leica Microsystems, Barcelona, Spain).
Oocyte collection and IVM
Immature cumulus oocyte complexes (COCs) were obtained by aspirating follicles (2–8 mm) from ovaries of heifers collected at a commercial abattoir. COCs were matured for 24 h in 500 µl of TCM 199 supplemented with 10% (v/v) fetal calf serum (FCS) and 10 ng/ml epidermal growth factor in a four-well dish, in groups of 50 COCs per well at 38.5ºC, under an atmosphere of 5% CO2 in air with maximum humidity.
Sperm preparation and IVF
Frozen semen from a single Asturian Valley bull, was thawed at 37ºC in a water bath for 1 min and centrifuged for 5 min at 290 × g through a gradient of 1 ml of 40% and 1 ml of 80% Bovipure, according to the manufacturer’s specification (Nidacon Laboratories AB, Göthenborg, Sweden). The sperm pellet was isolated and washed in 3 ml of Boviwash by centrifugation at 290 × g for 5 min. The pellet was resuspended in the remaining 300 µl of Boviwash. Sperm concentration was determined and adjusted to a final concentration of 1 × 106 sperm/ml for IVF. Gametes were co-incubated for 18–22 h in droplets of 100 µl of fertilization medium (Tyrode’s medium with 25 mM bicarbonate, 22 mM Na lactate, 1 mM Na-pyruvate, and 6 mg/ml fatty acid-free bovine serum albumin (BSA) supplemented with 10 mg/ml heparin sodium salt, Calbiochem, San Diego, CA, USA), in groups of 50 COCs per droplet under an atmosphere of 5% CO2 in air with maximum humidity at 38.5ºC. Depending on the experimental group (Fig. 1), the fertilization medium was supplemented with 10 or 50 µg/ml of recombinant pOVGP1.
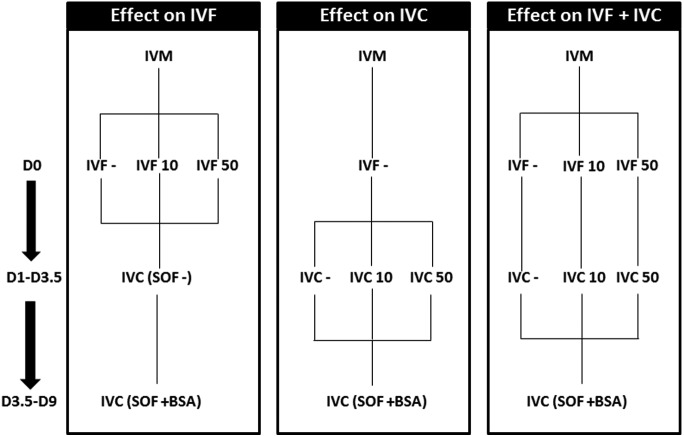
Fig. 1.
Experimental design. The effect of 10 and 50 µg/ml of pOVGP1 supplementation was evaluated at three levels: during IVF supplementation (D0–D1) (Control, n = 141; IVF 10, n = 189; IVF 50, n = 151), during IVC (D1–D3.5) (Control, n = 141, IVC 10, n = 147; IVC 50, n = 150), and during IVF and IVC (D0–D3.5) (Control, n =141, IVF+IVC 10, n = 194; IVF+IVC 50, n = 201). On the left, the numbers of days post-insemination that the COCs or zygotes were kept in each treatment are indicated. Three replicates were carried out.
In vitro culture (IVC) of presumptive zygotes
At approximately 20 h post-insemination (p.i.), presumptive zygotes were denuded of cumulus cells by vortexing for 3 min and then cultured in groups of 25 in droplets of 25 µl of culture medium (Synthetic Oviduct Fluid-SOF) [23] with 4.2 mM sodium lactate, 0.73 mM sodium pyruvate, 30 µl/ml BME amino acids, 10 µl/ml MEM amino acids, 1 µg/ml phenol-red, under mineral oil at 38.5ºC and under an atmosphere of 5% CO2, 5% O2, and 90% N2. Depending on the experimental group (Fig. 1), SOF was supplemented with 10 or 50 µg/ml of pOVGP1 until D3.5. Then, from D3.5 until D9, all embryos were cultured in SOF supplemented with 3 mg/ml of BSA.
Assessment of embryo development and quality
Embryo development: Cleavage rate was recorded at Day 2 (48 h p.i.) and cumulative blastocyst yield was evaluated at Days 7, 8, and 9 p.i.
Embryo quality assessed in terms of cryotolerance: The ability of the produced embryos to survive after vitrification was used as an indicator of embryo quality. BSA was used as a control as this implies a delay in embryo development compared with embryos cultured with FCS, resulting in a lower proportion of blastocysts on D7 and an increase on D8 (Tables 1, 2, 3). For that reason, D7 and D8 expanded blastocysts were vitrified following the two-step protocol described previously [2, 24], using a Cryoloop device (Hampton Research, Aliso Viejo, CA, USA). Briefly, the blastocysts were first exposed to Holding Medium (HM; TCM 199 supplemented with 10% (v/v) FCS) with 7.5% ethylene glycol and 7.5% dimethyl sulfoxide (DMSO) for 3 min. In the second step, the embryos were exposed to HM with 16.5% ethylene glycol, 16.5% DMSO, and 0.5 M sucrose for 20–25 sec, directly transferred to the cryoloop, and plunged into liquid nitrogen. The blastocysts were then warmed in two steps, being first exposed to HM with 0.25 M sucrose for 5 min, followed by an incubation in HM with 0.15 M sucrose for 5 min. Finally, the blastocysts were left in HM for 5 min before they were put in 25-ml droplets of SOF with 5% FCS. The definition of embryo survival was based on re-expansion of the blastocoel, its maintenance for 24, 48, and 72 h, and its ability to hatch from the ZP.
Table 1. The effects of supplementation with 10 or 50 µg/ml of pOVGP1 during in vitro fertilization on embryo development (D0–D1) (three replicates).
| Groups | N | Cleavage, n Mean ± S.E.M. (%) |
Blastocyst Yield |
||
|---|---|---|---|---|---|
| D7, n Mean ± S.E.M. (%) |
D8, n Mean ± S.E.M. (%) |
D9, n Mean ± S.E.M. (%) |
|||
| Control | 160 | 131 (82.45 ± 4.27) | 29 (19.32 ± 3.41) | 35 (22.69 ± 2.66) | 39 (25.72 ± 4.05) |
| IVF 10 | 237 | 186 (77.89 ± 4.40) | 34 (14.23 ± 0.73) | 48 (19.93 ± 2.26) | 62 (26.08 ± 0.99) |
| IVF 50 | 182 | 147 (81.62 ± 3.15) | 18 (10.79 ± 3.35) | 33 (19.34 ± 4.48) | 42 (24.51 ± 5.13) |
N = total number of presumptive zygotes placed in culture. One way ANOVA (P > 0.05).
Table 2. The effects of supplementation with 10 or 50 µg/ml of pOVGP1 during early in vitro culture on embryo development (D1–D3.5) (three replicates).
| Groups | N | Cleavage, n Mean ± S.E.M. (%) |
Blastocyst Yield |
||
|---|---|---|---|---|---|
| D7, n Mean ± S.E.M. (%) |
D8, n Mean ± S.E.M. (%) |
D9, n Mean ± S.E.M. (%) |
|||
| Control | 160 | 131 (82.45 ± 4.27) | 29 (19.32 ± 3.41) | 35 (22.69 ± 2.66) | 39 (25.72 ± 4.05) |
| IVC 10 | 174 | 146 (83.61 ± 1.69) | 26 (15.28 ± 3.15) | 34 (19.74 ± 1.90) | 37 (21.32 ± 1.16) |
| IVC 50 | 153 | 128 (84.01 ± 3.93) | 24 (15.78 ± 4.97) | 37 (24.48 ± 4.09) | 45 (29.53 ± 2.28) |
N = total number of presumptive zygotes placed in culture. One way ANOVA (P > 0.05).
Table 3. The effects of supplementation with 10 or 50 µg/ml of pOVGP1 during in vitro fertilization and early embryo culture on embryo development (D0–D3.5) (three replicates).
| Groups | N | Cleavage, n Mean ± S.E.M. (%) |
Blastocyst Yield |
||
|---|---|---|---|---|---|
| D7, n Mean ± S.E.M. (%) |
D8, n Mean ± S.E.M. (%) |
D9, n Mean ± S.E.M. (%) |
|||
| Control | 160 | 131 (82.45 ± 4.27) | 29 (19.32 ± 3.41) a | 35 (22.69 ± 2.66) a | 39 (25.72 ± 4.05) a |
| IVF + IVC 10 | 194 | 170 (85.52 ± 7.26) | 32 (17.18 ± 2.97) a | 42 (22.13 ± 1.73) a | 52 (27.21 ± 1.73) a |
| IVF + IVC 50 | 202 | 160 (79.70 ± 1.66) | 15 (7.56 ± 0.56) b | 22 (11.14 ± 0.82) b | 27 (13.28 ± 0.34) b |
N = total number of presumptive zygotes placed in culture. Different letters (a, b) in the same column indicate differences between groups (One way ANOVA, P < 0.05).
Embryo quality assessed in terms of gene expression pattern: As another indicator of embryo quality, genes related to glucose metabolism, epigenetics, cell-to-cell communication, endoplasmic reticulum homeostasis, and aquaporins were studied. Gene expression was analyzed in three pools of 10 expanded blastocysts recovered from D7-D8 for each experimental group.
Poly (A) RNA was extracted using a Dynabeads® mRNA DIRECT™ Micro Kit (Ambion®, Thermo Fisher Scientific, Oslo, Norway) following the manufacturer’s instructions with minor modifications [25]. After a 10-min incubation in lysis buffer with Dynabeads, the poly (A) RNA attached to the Dynabeads was extracted with a magnet and washed twice with washing buffer A and washing buffer B. The RNA was then eluted with Tris-HCl. Immediately after extraction, the reverse transcription (RT) reaction was carried out following the manufacturer’s instructions (Epicentre Technologies, Madison, WI, USA) using poly (T) primers, random primers, and MMLV High Performance Reverse Transcriptase enzyme in a total volume of 40 µl, to prime the RT reaction and to produce cDNA. The tubes were heated at 70ºC for 5 min to denature the secondary RNA structure and the RT mix was then completed with the addition of 50 units of reverse transcriptase. The reactions were then incubated at 25ºC for 10 min to favor the annealing of random primers, followed by 37ºC for 60 min to allow the RT of RNA, and finally 85ºC for 5 min to denature the enzyme.
All primers were designed using Primer-BLAST software (www.ncbi.nlm.nih.gov/tools/primersblast/) to span exon-exon boundaries when possible. All qPCR reactions were carried out in duplicate on a Rotor-GeneTM 6000 Real Time Cycler (Corbett Research, Sydney, Australia) by adding a 2 µl aliquot of each sample to the PCR mix (GoTaq® qPCR Master Mix, Promega, Madison, WI, USA) containing the specific primers selected to amplify activating transcription factor 4 (ATF4), DNA-damage-inducible transcript 3 (DDIT3), DNA (cytosine-5-)-methyltransferase 3 alpha (DNMT3A), lysine (K)-specific demethylase 1A (KDM1A), desmocollin 2 (DSC2), gap junction protein, alpha 1 (GJA1, formerly CX43), insulin-like growth factor 2 receptor (IGF2R), aquaporin 3 (AQP3), and solute carrier family 2 (facilitated glucose transporter) member 1 (SCL2A1, formerly GLUT1). Primer sequences and the approximate sizes of the amplified fragments of all transcripts are shown in Supplementary Table 1 (online only). Cycling conditions were 94ºC for 3 min followed by 35 cycles of 94ºC for 15 sec, 56ºC for 30 sec, 72ºC for 10 sec, and 10 sec of fluorescence acquisition. Each pair of primers was tested to achieve efficiencies close to 1 and the comparative cycle threshold (CT) method was used to quantify expression levels, as described by Schmittgen et al. in 2008 [26]. To avoid primer dimer artifacts, fluorescence was acquired in each cycle at a temperature higher than the melting temperature of the primer dimers (specific for each product, 80 to 86°C). The threshold cycle, or the cycle during the log-linear phase of the reaction, at which fluorescence increased above background was determined for each sample. The ΔCT value was determined by subtracting the endogenous control (an average of H2AZ and ACTB) CT value for each sample from each gene CT value of the sample. Calculation of ΔΔCT involved using the highest sample ΔCT value (i.e., the sample with the lowest target expression) as a constant to subtract from all other ΔCT sample values. Fold-changes in the relative gene expression of the target were determined using the equation 2–ΔΔCT.
Experimental design
Based on previous studies [14, 15], two different concentrations of pOVGP1 were used in vitro: 10 and 50 µg/ml. The developmental capacity and quality of the blastocysts obtained was assessed after the supplementation with pOVGP1 in three different steps: during IVF (D0-D1), early IVC (D1-D3.5), or during IVF and early IVC (D0-D3.5) (Fig. 1). In all experiments, embryo development was assessed at D2 and D7-D9. D7 and D8 embryos were used for gene expression analysis. Three replicates were carried out, after which a further four replicates were carried out, but only using the group supplemented with pOVGP1 during early IVC (D1-D3.5). The embryos obtained at D7 and D8 were vitrified/warmed to evaluate their survival rate.
The chosen species to test recombinant porcine OVGP1 was bovine because the embryo production system is more optimized than in porcine species. In addition, based on the amino acid sequence, porcine (Q28990) and bovine (Q28042) OVGP1 are 78% homologous and, more importantly, both proteins share the same C-terminal regions (Supplementary Fig. 1), which are involved in the specific ZP-binding patterns to remodel the ZP matrix and allow protein endocytosis [21]. These data suggest that pOVGP1 would be able to bind to the bovine ZP to be then endocytosed.
The effect of supplementation with pOVGP1 during IVF on embryo development (D0–D1)
After IVM, COCs were fertilized in three groups: without supplementation (Control; n = 160), supplemented with 10 µg/ml of pOVGP1 (IVF 10; n = 237), and supplemented with 50 µg/ml of pOVGP1 (IVF 50; n = 182). The presumptive zygotes were then cultured in SOF supplemented with 3 mg/ml of BSA until D7–D9. Presumptive zygotes were fixed (n = 15) at D1.
The effect of supplementation with pOVGP1 during early IVC on embryo development (D1–D3.5)
Presumptive zygotes were cultured in SOF without supplementation (Control; n = 160), supplemented with 10 µg/ml (IVC 10; n = 174), or 50 µg/ml of pOVGP1 (IVC 50; n = 153) until D3.5, when all groups were cultured in SOF supplemented with 3 mg/ml BSA until D7–D9. At D3.5 embryos were fixed (n = 56).
The effect on embryo development of supplementation with pOVGP1 during IVF and early IVC (D0–D3.5)
The matured COCs were fertilized in three groups: without supplementation (Control; n = 160), supplemented with 10 µg of pOVGP1 during IVF and early IVC (IVF+IVC 10; n = 194), or 50 µg of pOVGP1 during IVF and early IVC (IVF+IVC 50; n = 202) until D3.5 when all groups were cultured in SOF supplemented with 3 mg/ml BSA until D7–D9. At Day 9 embryos were fixed (n = 21).
Statistical analysis
All statistical analyses were carried out using the SigmaStat software package (Jandel Scientific, San Rafael, CA, USA). Cleavage rate, blastocyst yield, embryo survival after vitrification, and relative mRNA abundance were analyzed using one-way ANOVA. Tukey’s post-hoc test was used after one-way ANOVA. Values were considered significantly different when P was lower than 0.05.
Results
Recombinant pOVGP1 binds to the ZP of presumptive bovine zygotes and early embryos
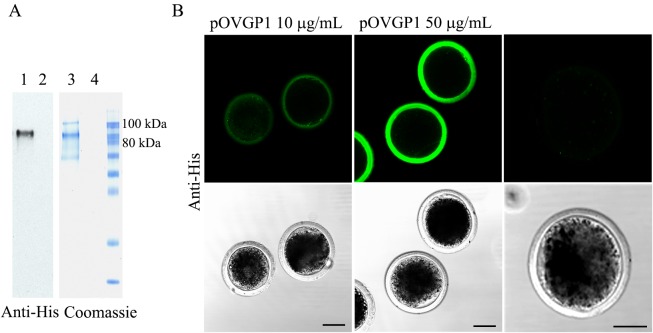
As expected, bright immunofluorescence was detected through the entire thickness of the ZP, when bovine mature oocytes were incubated in IVF medium containing purified pOVGP1 (Fig. 2A), displaying the highest intensity at 50 µg/ml (Fig. 2B). In contrast, the fluorescent ZP-signal on D9 blastocysts was only detected when IVC medium was supplemented with 50 µg/ml of pOVGP1 (Fig. 3). This observation confirms that OVGP1-ZP binding is reversible [22], but the protein remains attached to the ZP when IVC is supplemented with 50 µg/ml of pOVGP1, even though the medium lacks the recombinant protein from Day 3.5 post-insemination.
Fig. 2.
pOVGP1 expressed by mammalian cells binds to ZP of bovine presumptive zygotes. A. pOVGP1 was expressed in HEK 293T cells, purified, separated by SDS-PAGE, and analyzed by immunoblot using a monoclonal antibody against the His tag and Coomassie blue stain. Bands of ~90 kDa correspond to reduced pOVGP1 (lanes 1 and 3) were detected. Lanes 2 and 4 correspond to the negative control. B. Bovine presumptive zygotes incubated in IVF medium containing pOVGP1 at 10 or 50 µg/ml and in IVF medium without pOVGP1 were fixed and imaged by confocal fluorescence microscopy using a monoclonal antibody against the His tag (n = 15). Bright immunofluorescence was detected throughout the entire thickness of the ZP. The intensity was higher in the group supplemented with 50 µg/ml of pOVGP1 than in the 10 µg/ml group. No fluorescent signal was found in the untreated group. Scale bars = 50 µm.
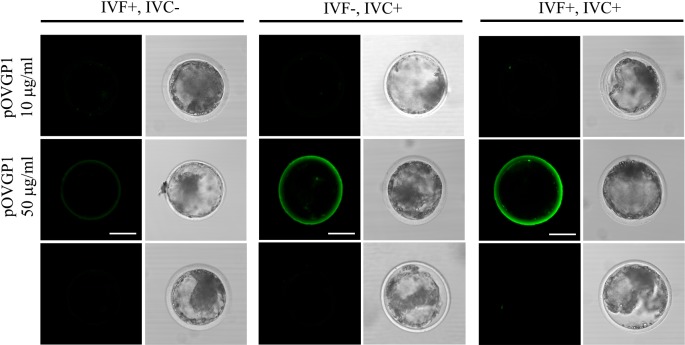
Fig. 3.
pOVGP1 is detected in the ZP of D9 blastocyst when IVC medium is supplemented with 50 µg/ml of pOVGP1. On Day 9, blastocysts were recovered, fixed, and imaged by confocal fluorescence microscopy using a monoclonal antibody against the His tag (n = 21). Bright immunofluorescence was only detected in the ZP of the blastocysts when IVC medium was supplemented with 50 µg/ml of pOVGP1. No fluorescent signal was found in the groups supplemented with 10 µg/ml of pOVGP1, or in the untreated group. Scale bars = 50 µm.
Bovine D3.5 embryos cultured in 50 µg/ml of pOVGP1 showed specific labeling in the perivitelline space and plasma membrane between blastomeres when they were fixed and unpermeabilized. When embryos were permeabilized, a strong signal was detected within the blastomere cytoplasm (Fig. 4).
Fig. 4.
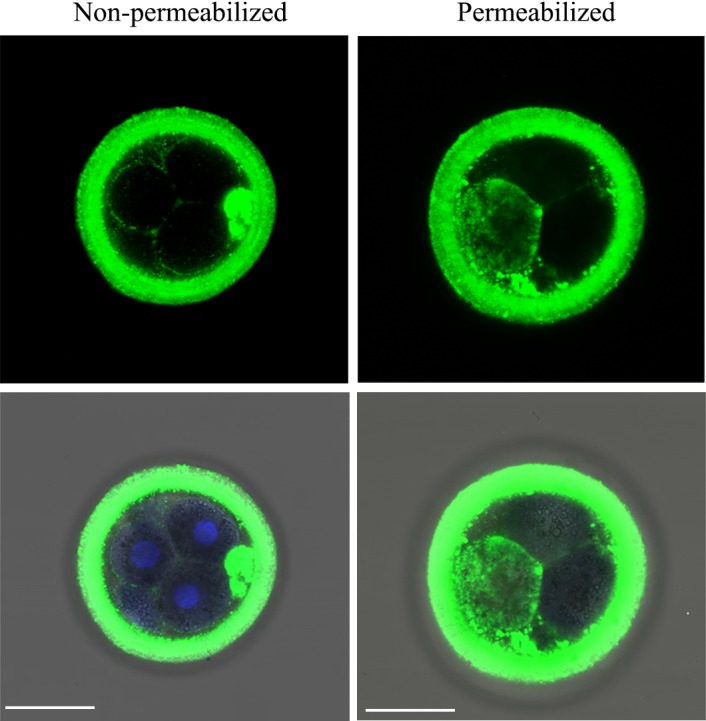
pOVGP1 was bound to the ZP and in the perivitelline space, plasma membrane, and cytoplasm of bovine D3.5 embryos. Upper panels: Bovine D3.5 embryos cultured in IVC medium containing 50 µg/ml of pOVGP1 were fixed and a half of them were permeabilized. The embryos were imaged by confocal fluorescence microscopy using a monoclonal antibody against the His tag (n = 56). Specific label was detected in the ZP, perivitelline space, and plasma membrane between blastomeres in unpermeabilized embryos. We also detected bright immunofluorescence within the blastomere cytoplasm when embryos were permeabilized. Lower panels: Blue signal corresponding to nucleic acid stained with Hoechst is shown merged with the immunofluorescent green signal and the bright field image. Scale bars = 50 µm.
The effects of pOVGP1 on in vitro embryo development
Supplementation with pOVGP1 during IVF did not have any effect on the cleavage rate or blastocyst yield at Days 7, 8, or 9. In addition, no dose-dependent effect was observed. The cleavage rate ranged from 77.89 to 82.45%, while the blastocyst rate at D9 ranged from 24.51 to 26.08% (Table 1, P > 0.05)
When pOVGP1 was added during early IVC (D1–D3.5), the supplementation had no effect on the cleavage rate or blastocyst yield (Table 2). The cleavage and blastocyst rates were similar in all groups, ranging from 82.45 to 84.01% and from 21.32 to 29.53%, respectively (P > 0.05). These results were confirmed when four more replicates were carried out to evaluate the ability of the blastocyst to survive vitrification (data not included).
Supplementation with pOVGP1 during fertilization and culture was detrimental to embryo development at the higher dose. Thus, although the cleavage rate was similar in the control, IVF+IVC 10, and IVF+IVC 50 groups (82.45 ± 4.27, 85.52 ± 7.26, and 79.70 ± 1.66%, respectively) the blastocyst rate at D7, D8, and D9 was much lower in the IVF+IVC 50 group compared with the control and IVF+IVC 10 groups (13.28 ± 0.34 vs. 25.72 ± 4.05 and 27.21 ± 1.73% at D9, respectively; P < 0.05) (Table 3).
pOVGP1 during early IVC does not affect embryo survival after vitrification
No significant differences were observed on survival or hatching rate when D7 or D8 expanded blastocysts were vitrified, so the results presented in Table 4 include D7 and D8 blastocysts. Compared with the control, the use of 10 or 50 µg/ml of pOVGP1 during early embryo development did not have any significant effect on embryo survival at any time point measured (72 h: 76.77 ± 9.67 vs. 79.70 ± 7.16 vs. 57.05 ± 10.77, respectively, P > 0.05) nor on the hatching rate after vitrification/warming (63.71 ± 10.19 vs. 63.23 ± 4.98 vs. 60.00 ± 9.44, respectively, P > 0.05) (Table 4).
Table 4. In vitro survival after vitrification and warming of D7+D8 expanded blastocyst that were cultured with 10 or 50 µg/ml of pOVGP1 during early in vitro culture (D1–D3.5) (four replicates).
| Groups | N | Embryo survival after, n (Mean % ± S.E.M.) |
Hatching rate, n (Mean % ± S.E.M.) |
||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| Control | 44 | 35 (84.39 ± 6.94) | 34 (80.82 ± 8.49) | 32 (76.77 ± 9.67) | 26 (63.71 ± 10.19) |
| IVC 10 | 49 | 41 (82.88 ± 7.08) | 41 (82.88 ± 7.08) | 39 (79.70 ± 7.16) | 31 (63.23 ± 4.98) |
| IVC 50 | 36 | 27 (80.46 ± 8.25) | 22 (70.68 ± 12.44) | 19 (57.05 ± 10.77) | 21 (60.00 ± 9.44) |
N = total number of expanded D7 and D8 blastocysts vitrified and warmed. One way ANOVA, P > 0.05.
pOVGP1 affects specific genes related to embryo quality
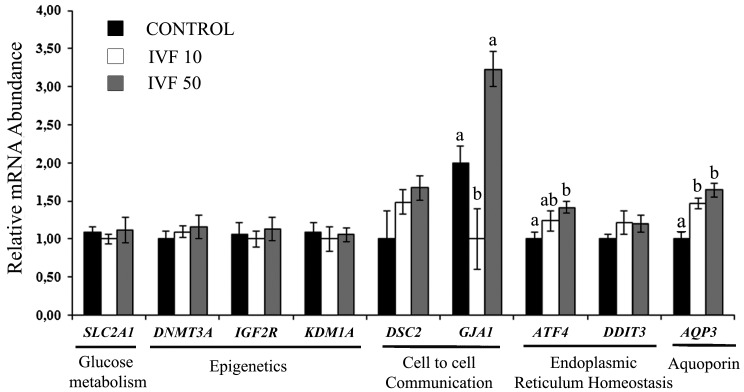
Supplementation with pOVGP1 during IVF significantly increased the expression of AQP3, at both 10 and 50 µg/ml, compared with the control (P < 0.05). ATF4, a gene involved in endoplasmic reticulum homeostasis, was upregulated in the IVF 50 group (P < 0.05), while the effect in the IVF 10 group only showed borderline significance (P = 0.054) compared with the control. In the cell-to-cell communication category, GJA1 was downregulated in the IVF 10 group and upregulated in the IVF 50 group compared with the control (P < 0.05) (Fig. 5).
Fig. 5.
Relative mRNA abundance (normalized against H2AZ and ACTB) of selected genes for the assessment of blastocyst quality when COCs were cultured with pOVGP1 during IVF (D0–D1). For each transcript, bars with different superscripts differ significantly (P < 0.05).
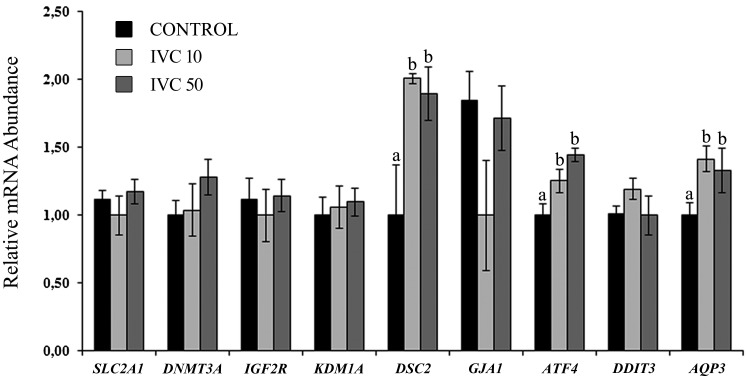
In IVF supplementation, the use of pOVGP1 during IVC showed similar differences in the gene expression of AQP3. In this case, ATF4 was upregulated in both pOVGP1 concentrations compared with the control (P < 0.05). In addition, during IVC supplementation, DSC2, which corresponds to the cell-to-cell communication category, was upregulated in both groups supplemented with pOVGP1 compared with the control (P < 0.05) (Fig. 6).
Fig. 6.
Relative mRNA abundance (normalized against H2AZ and ACTB) of selected genes for the assessment of blastocyst quality when presumptive zygotes were cultured with pOVGP1 during early IVC (D1–D3.5). For each transcript, bars with different superscripts differ significantly (P < 0.05).
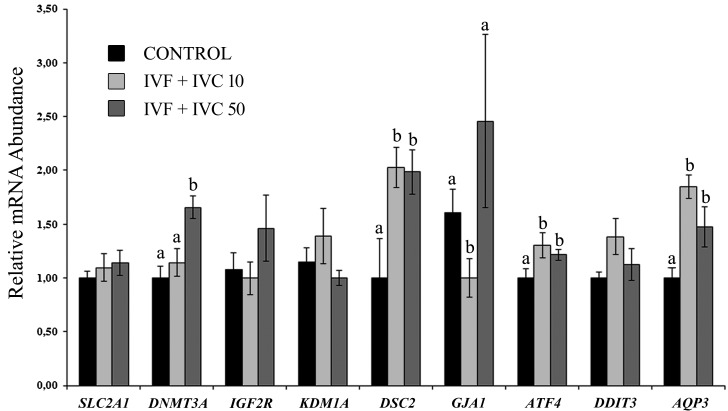
With regards to AQP3, the use of pOVGP1 during IVF+IVC showed a similar gene expression pattern to that observed after individual supplementation during IVF or IVC. DSC2 presented the same distribution to that observed after supplementation during IVC, suggesting that the strongest effect on this gene is when pOVGP1 is used during early embryo culture. In addition, GJA1 showed similar expression to IVF supplementations, being downregulated in the IVF+IVC 10 group compared with the control but not showing differences in the IVF+IVC 50 group. ATF4 was also upregulated when both doses of pOVGP1 were used compared with the control group, as occurred with supplementation during IVC. Furthermore, one gene related with epigenetics, DNMT3A, was upregulated in the IVF+IVC 50 group compared with the other groups (P < 0.05; Fig. 7).
Fig. 7.
Relative mRNA abundance (normalized against H2AZ and ACTB) of selected genes for the assessment of blastocyst quality when COCs and presumptive zygotes were cultured with pOVGP1 during IVF and early IVC, respectively (D0–D3.5). For each transcript, bars with different superscripts differ significantly (P < 0.05).
Discussion
It has been shown that co-culturing embryos with oviductal cells or the in vivo culture of embryos in oviducts of recipient animals increases the success of development and mimics the pattern of transcript abundance of some genes observed in in vivo derived embryos [27, 28]. Therefore, it seems reasonable to include secreted oviductal components in the in vitro culture medium. Many studies have tested the OF effect on early development [19, 20, 24]. However, in such studies, the complete OF is usually recovered by aspiration from whole oviducts so that all the components are mixed in the same fluid, though a gradient has been observed in the biosynthetic activity of the functional oviductal segments [29]. In addition, there are also variations in the components of the OF during the estrous cycle [30]. Thus, the components of OF should be added (i) gradually to the in vitro system to be able to study their involvement and influence in early development and (ii) at the appropriate time. In the present study, pOVGP1 was added during IVF and early IVC, the time period when the embryo remains in the bovine oviduct in vivo. Recently, we showed that pOVGP1 added during IVF remodels porcine ZP, leading to a significant increase in monospermy rates compared with the control, without affecting penetration rates and resulting in a general increase in the final output of the IVF system [21]. In an attempt to elucidate the role of OVGP1 in the early development, we describe here for the first time the application of recombinant pOVGP1 to an in vitro culture system to produce bovine embryos. Similarly, as we showed previously in porcine IVF [21], during the present study pOVGP1 was bound to the ZP of presumptive bovine zygotes, when protein was added to the IVF medium. However, the ZP signal disappeared after 9 days of incubation in OVGP1-free medium or medium with a low concentration of OVGP1 in the IVC, suggesting reversible and dose-dependent OVGP1-ZP binding [22]. The presence of OVGP1 in multivesicular-like bodies in IVM oocytes [21] and in blastomeres of developing embryos [12] could explain the influence of OVGP1 in the early stages of development, as has been reported for many species [16,17,18, 31, 32]. In accordance with these data, we report that D3.5 embryos cultured with recombinant pOVGP1 showed specific labeling in the plasma membrane, perivitelline space, and inside blastomeres, confirming protein activity beyond the ZP of bovine embryos. Together, these data confirm that the C-terminal region of OVGP1 regulates its activity and accounts for the reproductive role of OVGP1 in different mammalian orders [21]. Therefore, OVGP1 proteins containing same C-terminal region (Supplementary Fig. 1) could be used in heterologous systems.
A beneficial effect of purified native OVGP1 on the cleavage rate and number of embryos that reach the blastocyst stage was demonstrated in goat when the protein was used at 10 µg/ml during IVF and IVC. However, using higher concentrations of OVGP1 (50 or 100 µg/ml) had an inhibitory effect on embryo production [14]. Indeed, in the porcine system, treatment with OVGP1 (10 µg/ml) during preincubation/IVF increased the number of oocytes reaching the blastocyst stage, while OVGP1 added during both IVF (10 µg/ml) and IVC (50 or 100 µg/ml), tended to diminish the above effect [18]. In the present study, the use of pOVGP1 during IVF or IVC during bovine in vitro embryo production maintained the number of oocytes reaching the blastocyst stage compared to control, while pOVGP1 at a higher dose (50 µg/ml) added during both IVF and IVC, had a detrimental effect on embryo production. Similarly, when the IVC medium was supplemented with high concentrations (5, 10, or 25%) of complete bovine OF, a negative effect on blastocyst development was observed [24]. In the present study, the use of pOVGP1 did not improve embryo production in any of the experimental groups (IVF, IVC, or IVF+IVC). This is consistent with a published study in which recombinant cat OVGP1 was used in IVF, with no significant effects on the cleavage or blastocyst rates [15]. Furthermore, in vivo studies by null mutation of the OVGP1 glycoprotein have shown that OVGP1 is dispensable for the process of fertilization, at least in mice [33].
All these data reflect the importance of establishing the optimal protein dose in in vitro assays. Histochemical and immunocytochemical studies have demonstrated regional differences in the localization of materials and proteins in the oviductal epithelium [34]. Besides, the differing presence of proteins within the three functional oviductal segments (infundibulum, ampulla, and isthmus) is probably related with their individual functions [29]. The segmented microenvironment in the oviduct is likely to ensure the optimal protein doses necessary for fertilization and early embryo development in vivo. Consequently, a detailed protein analysis of the oviductal content from the different segments is necessary to optimize the concentration of OVGP1 for culture conditions [35] .
The culture environment during embryo development has an effect on embryo quality in terms of gene expression [2, 36, 37]. Our results show the overexpression of AQP3, a gene coding for aquaporin 3, in all treatments with pOVGP1. Aquaporins are proteins that form membrane channels which facilitate rapid and passive movement of water [38, 39], whose presence has been detected in mouse oocytes and human embryos [40]. They play a crucial role in cellular homeostasis and transport of cryopreserved oocytes and mouse blastocysts [41,42,43]. Therefore, the upregulation of this gene improves the survival of mouse oocytes and embryos following cryopreservation, as well as regulating apoptosis in the same embryos [44,45,46,47]. The downregulation of this gene is associated with low fertilization rates [48] and inhibits embryonic development in 2-cell mouse embryos [40], suggesting an important role in embryonic development. In mice, the knockout of Aqp3 leads to a deterioration in the formation of epidermal cells [49]. Despite these reports, this was not confirmed under our experimental conditions. Regardless of AQP3 overexpression in blastocysts produced from mature oocytes and early embryos treated with either concentration of pOVGP1, their cryotolerance was not enhanced. However, it cannot be ruled out that pOVGP1 may be responsible for distinct embryo qualitative differences not associated with cryotolerance, but essential for implantation and/or fetal development.
Our results also point to a significant upregulation of the gene encoding transcription factor 4 (ATF4) when either concentration of pOVGP1 was used during IVC. This factor has been described as being critical for normal cell proliferation, especially during peak proliferation, and is required in fetal hematopoiesis, while its absence results in severe fetal anemia [50]. In mouse embryos, Atf4 deficiency produces a severe defect in the formation of the lens [51].
Another gene showing differences after treatment with both doses of pOVGP1 during IVC is DSC2 (desmocollin 2), a member of the superfamily of cadherins. DSC2 is a cell adhesion protein present in desmosomes [52], where it is involved in the formation of desmosomal junctions, which stabilize the expansion of the blastocyst [53, 54]. Desmosomal junctions play an important role in integrity and signaling activity in tissues [55]. DSC2 upregulation has been described in the morula and blastocyst stage [56] and it has been observed that the relative abundance of mRNA is significantly greater in high quality blastocysts than in those of low quality [57]. The expression of this gene affects embryo quality, and could be used as a marker in IVC. In our study, both 10 and 50 µg/ml of pOVGP1 used during IVC upregulated DSC2, indicating that supplementation of bovine embryos with pOVGP1 during early embryonic culture favors the production of high quality embryos.
In the present study, the experimental group that produced the fewest embryos was IVF+IVC 50. The embryos obtained in this group overexpressed a gene related with epigenetic embryo markers, DNMT3A (DNA methyltransferase 3 alpha). The methyltransferase family (DNMTs) mediates the establishment and maintenance of dynamic patterns of DNA methylation in the genome. DNA methylation is an epigenetic event of great importance in gene regulation and for maintaining an optimal DNA methylation pattern during oocyte maturation. This is essential for the viability and development of embryos [58]. DNMT3A encodes a methyltransferase that has been identified as acting as a de novo DNA methyltransferase [59], establishing both maternal and paternal imprints [60, 61]. The expression of DNMT3A is affected by in vitro culture [62], as well as by serum supplementation [24].
Our results showed that the expression pattern of the Gap junction protein alpha 1 (GJA1) was contrary for both doses during IVF, i.e., 10 µg/ml of pOVGP1 downregulated and 50 µg/ml upregulated its expression. The use of recombinant feline OVGP1 during IVF increased the expression of GJA1 in cat blastocysts [15]. In the bovine system, GJA1 was expressed to a significantly higher extent in in vivo-cultured embryos from D4 onward, compared with those cultured in vitro [63], suggesting its importance in embryo development.
In conclusion, the use of pOVGP1 at an appropriate dose during IVC of bovine embryos does not affect embryo development, but produces embryos of better quality in terms of the relative abundance of specific quality-related genes. In vitro bovine embryo production is already well established, but it is necessary to further study embryo development in order to analyze the effects of assisted reproductive technologies (ART) in bovine and other species. Recently, it has been shown that DNA methylation and gene expression changes derived from ART can be decreased by using reproductive fluids [64] as OF, highlighting the importance of searching for strategies to mitigate the adverse effects on the health of ART-derived offspring.
Conflict of Interests
The authors declare no competing financial interests.
Supplementary
Acknowledgments
The authors thank the Spanish Association of breeders of selected cattle of the Asturian Valley breed (ASEAVA) for providing the semen used in this work. Special thanks are also extended to the slaughterhouse of “Transformación Ganadera de Leganés”; “Matadero Madrid Norte S.A.”; “Matadero Municipal Colmenar Viejo” of Madrid, Spain for giving us access to collect the biological material (bovine ovaries) used in this study.
The research was supported by the Spanish Ministry of Economy and Competitiveness (DR: AGL2015-70140-R; AGA: AGL2015-66145-R) and FEDER (AGL2015-70159-P) and the Fundación Séneca (19357/PI/14).
References
- 1.Buhi WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 2002; 123: 355–362. [DOI] [PubMed] [Google Scholar]
- 2.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61: 234–248. [DOI] [PubMed] [Google Scholar]
- 3.Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, De La Fuente J, Boland MP, Lonergan P. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod 2003; 68: 236–243. [DOI] [PubMed] [Google Scholar]
- 4.Rizos D, Lonergan P, Boland MP, Arroyo-García R, Pintado B, de la Fuente J, Gutiérrez-Adán A. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol Reprod 2002; 66: 589–595. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Gonzalez R, Moreira P, Bilbao A, Jiménez A, Pérez-Crespo M, Ramírez MA, Rodríguez De Fonseca F, Pintado B, Gutiérrez-Adán A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci USA 2004; 101: 5880–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Gonzalez R, Ramirez MA, Bilbao A, De Fonseca FR, Gutiérrez-Adán A. Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects. Mol Reprod Dev 2007; 74: 1149–1156. [DOI] [PubMed] [Google Scholar]
- 7.Avilés M, Gutiérrez-Adán A, Coy P. Oviductal secretions: will they be key factors for the future ARTs? Mol Hum Reprod 2010; 16: 896–906. [DOI] [PubMed] [Google Scholar]
- 8.Mondéjar I, Martínez-Martínez I, Avilés M, Coy P. Identification of potential oviductal factors responsible for zona pellucida hardening and monospermy during fertilization in mammals. Biol Reprod 2013; 89: 67. [DOI] [PubMed] [Google Scholar]
- 9.Coy P, Yanagimachi R. The common and species-specific roles of oviductal proteins in mammalian fertilization and embryo development. Bioscience 2015; 65: 973–984. [Google Scholar]
- 10.Soleilhavoup C, Riou C, Tsikis G, Labas V, Harichaux G, Kohnke P, Reynaud K, de Graaf SP, Gerard N, Druart X. Proteomes of the female genital tract during the oestrous cycle. Mol Cell Proteomics 2016; 15: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauersachs S, Rehfeld S, Ulbrich SE, Mallok S, Prelle K, Wenigerkind H, Einspanier R, Blum H, Wolf E. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J Mol Endocrinol 2004; 32: 449–466. [DOI] [PubMed] [Google Scholar]
- 12.Kan FWK, Roux E, Bleau G. Immunolocalization of oviductin in endocytic compartments in the blastomeres of developing embryos in the golden hamster. Biol Reprod 1993; 48: 77–88. [DOI] [PubMed] [Google Scholar]
- 13.Kan FWK, Roux E. Elaboration of an oviductin by the oviductal epithelium in relation to embryo development as visualized by immunocytochemistry. Microsc Res Tech 1995; 31: 478–487. [DOI] [PubMed] [Google Scholar]
- 14.Pradeep MA, Jagadeesh J, De AK, Kaushik JK, Malakar D, Kumar S, Dang AK, Das SK, Mohanty AK. Purification, sequence characterization and effect of goat oviduct-specific glycoprotein on in vitro embryo development. Theriogenology 2011; 75: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 15.Hribal R, Hachen A, Jewgenow K, Zahmel J, Fernandez-Gonzalez L, Braun BC. The influence of recombinant feline oviductin on different aspects of domestic cat (Felis catus) IVF and embryo quality. Theriogenology 2014; 82: 742–749. [DOI] [PubMed] [Google Scholar]
- 16.Yong P, Gu Z, Luo JP, Wang JR, Tso JK. Antibodies against the C-terminal peptide of rabbit oviductin inhibit mouse early embryo development to pass 2-cell stage. Cell Res 2002; 12: 69–78. [DOI] [PubMed] [Google Scholar]
- 17.Hill JL, Walker SK, Brown GH, Nancarrow CD. The effects of an ovine oviductal estrus-associated glycoprotein on early embryo development. Theriogenology 1996; 46: 1367–1377. [Google Scholar]
- 18.Kouba AJ, Abeydeera LR, Alvarez IM, Day BN, Buhi WC. Effects of the porcine oviduct-specific glycoprotein on fertilization, polyspermy, and embryonic development in vitro. Biol Reprod 2000; 63: 242–250. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd RE, Romar R, Matás C, Gutiérrez-Adán A, Holt WV, Coy P. Effects of oviductal fluid on the development, quality, and gene expression of porcine blastocysts produced in vitro. Reproduction 2009; 137: 679–687. [DOI] [PubMed] [Google Scholar]
- 20.Cebrian-Serrano A, Salvador I, García-Roselló E, Pericuesta E, Pérez-Cerezales S, Gutiérrez-Adán A, Coy P, Silvestre MA. Effect of the bovine oviductal fluid on in vitro fertilization, development and gene expression of in vitro-produced bovine blastocysts. Reprod Domest Anim 2013; 48: 331–338. [DOI] [PubMed] [Google Scholar]
- 21.Algarra B, Han L, Soriano-Úbeda C, Avilés M, Coy P, Jovine L, Jiménez-Movilla M. The C-terminal region of OVGP1 remodels the zona pellucida and modifies fertility parameters. Sci Rep 2016; 6: 32556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coy P, Cánovas S, Mondéjar I, Saavedra MD, Romar R, Grullón L, Matás C, Avilés M. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc Natl Acad Sci USA 2008; 105: 15809–15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999; 52: 683–700. [DOI] [PubMed] [Google Scholar]
- 24.Lopera-Vasquez R, Hamdi M, Maillo V, Lloreda V, Coy P, Gutiérrez-Adán A, Bermejo-Alvarez P, Rizos D. Effect of bovine oviductal fluid on development and quality of bovine embryos produced in vitro. Reprod Fertil Dev 2017; 29: 621–629. [DOI] [PubMed] [Google Scholar]
- 25.Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutiérrez-Adán A. Epigenetic differences between male and female bovine blastocysts produced in vitro. Physiol Genomics 2008; 32: 264–272. [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 27.Xu JS, Chan STH, Lee WWM, Lee KF, Yeung WSB. Differential growth, cell proliferation, and apoptosis of mouse embryo in various culture media and in coculture. Mol Reprod Dev 2004; 68: 72–80. [DOI] [PubMed] [Google Scholar]
- 28.Rizos D, Pintado B, de la Fuente J, Lonergan P, Gutiérrez-Adán A. Development and pattern of mRNA relative abundance of bovine embryos cultured in the isolated mouse oviduct in organ culture. Mol Reprod Dev 2007; 74: 716–723. [DOI] [PubMed] [Google Scholar]
- 29.Buhi WC, Alvarez IM, Kouba AJ. Secreted proteins of the oviduct. Cells Tissues Organs 2000; 166: 165–179. [DOI] [PubMed] [Google Scholar]
- 30.Seytanoglu A, Georgiou AS, Sostaric E, Watson PF, Holt WV, Fazeli A. Oviductal cell proteome alterations during the reproductive cycle in pigs. J Proteome Res 2008; 7: 2825–2833. [DOI] [PubMed] [Google Scholar]
- 31.McCauley TC, Buhi WC, Wu GM, Mao J, Caamano JN, Didion BA, Day BN. Oviduct-specific glycoprotein modulates sperm-zona binding and improves efficiency of porcine fertilization in vitro. Biol Reprod 2003; 69: 828–834. [DOI] [PubMed] [Google Scholar]
- 32.Martus NS, Verhage HG, Mavrogianis PA, Thibodeaux JK. Enhancement of bovine oocyte fertilization in vitro with a bovine oviductal specific glycoprotein. J Reprod Fertil 1998; 113: 323–329. [DOI] [PubMed] [Google Scholar]
- 33.Araki Y, Nohara M, Yoshida-Komiya H, Kuramochi T, Ito M, Hoshi H, Shinkai Y, Sendai Y. Effect of a null mutation of the oviduct-specific glycoprotein gene on mouse fertilization. Biochem J 2003; 374: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe H. The mammalian oviductal epithelium: regional variations in cytological and functional aspects of the oviductal secretory cells. Histol Histopathol 1996; 11: 743–768. [PubMed] [Google Scholar]
- 35.Desantis S, Zizza S, Accogli G, Acone F, Rossi R, Resta L. Morphometric and ultrastructural features of the mare oviduct epithelium during oestrus. Theriogenology 2011; 75: 671–678. [DOI] [PubMed] [Google Scholar]
- 36.Wrenzycki C, Herrmann D, Niemann H. Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology 2007; 68(Suppl 1): S77–S83. [DOI] [PubMed] [Google Scholar]
- 37.Komiya H, Onuma T, Hiroi M, Araki Y. In situ localization of messenger ribonucleic acid for an oviduct-specific glycoprotein during various hormonal conditions in the golden hamster. Biol Reprod 1996; 55: 1107–1118. [DOI] [PubMed] [Google Scholar]
- 38.Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 2003; 555: 72–78. [DOI] [PubMed] [Google Scholar]
- 39.Benga G. The first discovered water channel protein, later called aquaporin 1: molecular characteristics, functions and medical implications. Mol Aspects Med 2012; 33: 518–534. [DOI] [PubMed] [Google Scholar]
- 40.Xiong Y, Tan Y-J, Xiong Y-M, Huang Y-T, Hu X-L, Lu Y-C, Ye YH, Wang TT, Zhang D, Jin F, Huang HF, Sheng JZ. Expression of aquaporins in human embryos and potential role of AQP3 and AQP7 in preimplantation mouse embryo development. Cell Physiol Biochem 2013; 31: 649–658. [DOI] [PubMed] [Google Scholar]
- 41.Edashige K, Tanaka M, Ichimaru N, Ota S, Yazawa K, Higashino Y, Sakamoto M, Yamaji Y, Kuwano T, Valdez DM, Jr, Kleinhans FW, Kasai M. Channel-dependent permeation of water and glycerol in mouse morulae. Biol Reprod 2006; 74: 625–632. [DOI] [PubMed] [Google Scholar]
- 42.Edashige K, Yamaji Y, Kleinhans FW, Kasai M. Artificial expression of aquaporin-3 improves the survival of mouse oocytes after cryopreservation. Biol Reprod 2003; 68: 87–94. [DOI] [PubMed] [Google Scholar]
- 43.Seki S, Edashige K, Wada S, Mazur P. Effect of the expression of aquaporins 1 and 3 in mouse oocytes and compacted eight-cell embryos on the nucleation temperature for intracellular ice formation. Reproduction 2011; 142: 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edashige K, Ohta S, Tanaka M, Kuwano T, Valdez DM, Jr, Hara T, Jin B, Takahashi S, Seki S, Koshimoto C, Kasai M. The role of aquaporin 3 in the movement of water and cryoprotectants in mouse morulae. Biol Reprod 2007; 77: 365–375. [DOI] [PubMed] [Google Scholar]
- 45.Bell CE, Larivière NMK, Watson PH, Watson AJ. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating Aquaporin 3 and 9 expression and localization, as well as embryonic apoptosis. Hum Reprod 2009; 24: 1373–1386. [DOI] [PubMed] [Google Scholar]
- 46.Kuzmany A, Havlicek V, Wrenzycki C, Wilkening S, Brem G, Besenfelder U. Expression of mRNA, before and after freezing, in bovine blastocysts cultured under different conditions. Theriogenology 2011; 75: 482–494. [DOI] [PubMed] [Google Scholar]
- 47.Yamaji Y, Seki S, Matsukawa K, Koshimoto C, Kasai M, Edashige K. Developmental ability of vitrified mouse oocytes expressing water channels. J Reprod Dev 2011; 57: 403–408. [DOI] [PubMed] [Google Scholar]
- 48.Meng Q-X, Gao H-J, Xu C-M, Dong M-Y, Sheng X, Sheng J-Z, Huang HF. Reduced expression and function of aquaporin-3 in mouse metaphase-II oocytes induced by controlled ovarian hyperstimulation were associated with subsequent low fertilization rate. Cell Physiol Biochem 2008; 21: 123–128. [DOI] [PubMed] [Google Scholar]
- 49.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med (Berl) 2008; 86: 221–231. [DOI] [PubMed] [Google Scholar]
- 50.Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood 2002; 99: 736–745. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Tsujimura T, Takeda K, Sugihara A, Maekawa A, Terada N, Yoshida N, Akira S. Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes Cells 1998; 3: 801–810. [DOI] [PubMed] [Google Scholar]
- 52.Buxton RS, Cowin P, Franke WW, Garrod DR, Green KJ, King IA, Koch PJ, Magee AI, Rees DA, Stanley JR, Steinberg MS. Nomenclature of the desmosomal cadherins. J Cell Biol 1993; 121: 481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleming TP, Garrod DR, Elsmore AJ. Desmosome biogenesis in the mouse preimplantation embryo. Development 1991; 112: 527–539. [DOI] [PubMed] [Google Scholar]
- 54.Collins JE, Lorimer JE, Garrod DR, Pidsley SC, Buxton RS, Fleming TP. Regulation of desmocollin transcription in mouse preimplantation embryos. Development 1995; 121: 743–753. [DOI] [PubMed] [Google Scholar]
- 55.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol 2000; 1: 208–216. [DOI] [PubMed] [Google Scholar]
- 56.Bloor DJ, Metcalfe AD, Rutherford A, Brison DR, Kimber SJ. Expression of cell adhesion molecules during human preimplantation embryo development. Mol Hum Reprod 2002; 8: 237–245. [DOI] [PubMed] [Google Scholar]
- 57.Sathanawongs A, Nganvongpanit K, Mekchay S. Expression patterns of cell adhesion molecules in bovine preimplantation embryos cultured in vitro. Wetchasan Sattawaphaet 2012; 42: 455–461. [Google Scholar]
- 58.Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev 2014; 28: 812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet 1998; 19: 219–220. [DOI] [PubMed] [Google Scholar]
- 60.Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004; 429: 900–903. [DOI] [PubMed] [Google Scholar]
- 61.Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M, Sasaki H. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet 2007; 16: 2272–2280. [DOI] [PubMed] [Google Scholar]
- 62.Drallmeyer S, Muller K, Hadeler KG, Korsawe K, Niemann H, Wrenzycki C. Localization of DNMT1 and DNMT3a protein in preimplantation bovine embryos derived in vivo and in vitro. Reprod Domest Anim 2008; 43Suppl: 8–9.18638100 [Google Scholar]
- 63.Lonergan P, Rizos D, Gutierrez-Adán A, Moreira PM, Pintado B, de la Fuente J, Boland MP. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 2003; 69: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 64.Cánovas S, Ivanova E, Romar R, García-Martínez S, Soriano-Úbeda C, García-Vázquez FA, Saadeh H, Andrews S, Kelsey G, Coy P. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. eLife 2017; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.