Abstract
The intimate association of cumulus cells with one another and with the oocyte is important for regulating oocyte meiotic arrest and resumption. The objective of this study was to determine the effects of heat stress on cumulus cell communication and functions that may be related to accelerated oocyte meiosis during early maturation. Bovine cumulus-oocyte complexes underwent in vitro maturation for up to 6 h at thermoneutral control (38.5°C) or elevated (40.0, 41.0 or 42.0°C) temperatures. Gap junction communication between the cumulus cells and the oocyte was assessed using the fluorescent dye calcein after 4 h of in vitro maturation. Dye transfer was reduced in cumulus-oocyte complexes matured at 41.0°C or 42.0°C; transfer at 40.0°C was similar to control (P < 0.0001). Subsequent staining of oocytes with Hoechst revealed that oocytes matured at 41.0 or 42.0°C contained chromatin at more advanced stages of condensation. Maturation of cumulus-oocyte complexes at elevated temperatures reduced levels of active 5’ adenosine monophosphate activated kinase (P = 0.03). Heat stress exposure had no effect on active extracellular-regulated kinase 1/2 in oocytes (P = 0.67), associated cumulus cells (P = 0.60) or intact cumulus-oocyte complexes (P = 0.44). Heat-induced increases in progesterone production by cumulus-oocyte complexes were detected during the first 6 h of maturation (P = 0.001). Heat-induced alterations in gap junction communication and other cumulus-cell functions likely cooperate to accelerate bovine oocyte meiotic progression.
Keywords: Cumulus cell, Gap junction, Heat stress, Meiotic maturation, Oocyte
Oocyte maturation is a critical developmental period for acquiring competence to progress to embryonic stages after fertilization. Exposure of the cumulus-oocyte complex to heat stress during the first half of meiotic maturation decreases rates of development to the blastocyst stage after fertilization (in vivo hyperthermia, [1]; in vitro heat stress, [2,3,4,5] ). Some of the influences of heat stress on oocyte competence may in part relate to heat-induced hastening of meiotic progression.
In the bovine, heat stress during the first 12 h of in vitro maturation (hIVM) accelerated progression to metaphase-I and metaphase II, with more oocytes completing meiotic progression at 16 and 18 h compared with oocytes matured only at a thermoneutral control temperature [6]. Heat-induced hastening of meiotic maturation is not without consequence because subsequent efforts by Schrock et al. [7] demonstrated that earlier fertilization of heat-stressed bovine oocytes improved blastocyst development rates. In 2015, Hooper and others [8] demonstrated that heat-induced hastening of meiotic maturation is detectable as early as 4 hIVM. Specifically, more heat-stressed bovine oocytes underwent germinal vesicle breakdown compared to control counterparts; this difference was more prominent by 6 hIVM. The possibility for heat stress to hasten the onset of maturation has been observed in other species. Baumgartner and Chrisman [9] noted an increase in the bicellular presentation of oocytes obtained from hyperthermic murine females (i.e., one cell presumed to be the first polar body, the other being the oocyte). Kim et al. [10] reported that an in vitro heat stress exposure accelerated germinal vesicle breakdown in murine oocytes.
The control of oocyte meiotic arrest and resumption is largely mediated by the cumulus cells. The oocyte is most susceptible to heat stress during the first 12 h of maturation [1, 2], when the surrounding cumulus cells are intimately associated with one another and the oocyte through intercellular gap junctions [11,12,13]. A reduction in gap junction permeability occurs around the time of oocyte resumption of meiosis in the bovine [13,14,15]. In mice, Norris and others [16] demonstrated LH-induced closure of gap junctions in cumulus cells reduces cGMP content in the oocyte. Gap junction communication is partly regulated by kinases, either directly through phosphorylation by extracellular signal regulated kinase 1/2 (ERK1/2) [17, 18] or indirectly through control of protein synthesis by 5’ adenosine monophosphate activated kinase (AMPK; [19]). ERK1/2 and AMPK may also mediate other cumulus cell functions during oocyte maturation, including progesterone production [20, 21]. In turn, progesterone regulates levels of connexin 43, the primary cumulus-derived gap junction protein [22, 23]. Furthermore, increases in cumulus-derived progesterone during early maturation may accelerate germinal vesicle breakdown and meiotic progression in the bovine oocyte [24, 25].
Taken together, we hypothesized that heat stress exposure during early maturation may alter cumulus cell communication and function to hasten onset of meiotic progression. To that end, we evaluated the effects of heat stress on gap junction function and chromatin configuration in the maturing bovine cumulus-oocyte complex. To further evaluate gap junction communication we also examined the effect of heat stress on cGMP content in oocytes and their surrounding cumulus cells. We also investigated the impact of heat stress on activation of ERK1/2 and AMPK as well as the production of progesterone during early maturation.
Materials and Methods
Collection and in vitro maturation of cumulus-oocyte complexes
Unless otherwise noted, reagents and chemicals were procured from MilliporeSigma (St. Louis, MO, USA). Oocytes were collected from abattoir-derived bovine ovaries as described previously [3]. Oocyte maturation medium (OMM) and HEPES-TALP were prepared as described by Rispoli et al. [26]. Cumulus-oocyte complexes (COCs) were placed into OMM in polystyrene tubes (Sarstedt AG and Co., Nümbrecht, Germany) and matured at 38.5°C or at an elevated temperature observed in heat-stressed cows [1, 27,28,29,30,31]. Care was taken to ensure that only COCs with similarly sized cumulus cell vestments were selected.
Study one: Gap junction communication, chromatin configuration, and cGMP content of heat-stressed cumulus-oocyte complexes
Gap junction communication between oocytes and associated cumulus cells was assessed as described previously [13] in COCs (35 per 500 µl phenol red-free OMM) matured at 38.5, 40.0, 41.0 or 42.0°C for 4 h. Maturation time was selected based on previous efforts in our laboratory documenting that heat-induced hastening of meiotic maturation occurs as early as 4 hIVM [8]. Elevated temperatures were selected based on rectal temperatures recorded in cows experiencing moderate (40 to 41°C; [27,28,29,30]) or severe (42°C; [1, 31]) hyperthermia. A separate subset of COCs were cultured at 38.5°C in the presence of the gap junction inhibitor carbenoxolone (Alfa Aesar, Tewksbury, MA, USA) at a concentration (243 µM) previously shown to be effective [13]. After 4 hIVM, COCs were incubated with 5 µM calcein acetoxymethyl (calcein-AM; Cayman Chemicals, Ann Arbor, MI, USA) in phenol red-free OMM for 15 min at 38.5°C. COCs were then washed and incubated in calcein-AM-free, phenol red-free OMM for 30 min to allow cleavage of the acetoxymethyl group and transfer of the fluorescent calcein molecule into the oocyte. Phenol red-free OMM was used to prevent non-specific cleavage of the calcein-AM molecule [13]. Oocytes were denuded of surrounding cumulus by vortexing for 5 min in HEPES-TALP. Fluorescence (excitation 450–490 nm; emission > 515 nm) was measured using a Nikon Eclipse TE300 inverted microscope and the NIS Elements BR imaging software (version 3.0; Nikon Instruments, Melville, NY, USA). This study was replicated across six different days of COC collection (n = 360 to 393 total COCs/group).
After measuring fluorescence, oocytes were fixed in 3% paraformaldehyde in Dulbecco’s phosphate buffered saline (DPBS) for 15 min and then stained with 0.5 µg/ml Hoechst 33342 (~6 h after placement into OMM). Chromatin configuration was evaluated with fluorescence (excitation 330–380 nm; emission > 420 nm) using a Nikon Eclipse TE300 inverted microscope for three different days of COC collection (n = 74 to 135 total oocytes/group) and scored based on previously defined criteria [14]. Those with a diffuse chromatin taking up approximately 20−30% of the oocytes surface were assigned a score of one, oocytes with mostly diffuse chromatin with a few condensed foci beginning to form were assigned a score of two, oocytes with chromatin condensed into clumps were assigned a score of three, oocytes with a single mass of condensed chromatin were assigned a score of four, and oocytes at prometaphase-I, where individual chromosomes were apparent, were assigned a score of five.
To further evaluate gap junction communication we examined the effect of heat stress on cGMP content in oocytes and their surrounding cumulus cells separately. Intact COCs (55 to 60 per 500 µl OMM) were cultured at 38.5 or 41.0°C for 4 h. Thereafter, oocytes were denuded of associated cumulus cells by vortexing for 5 min in HEPES-TALP. Disassociated cumulus cells were washed with DPBS and collected after centrifugation at 300 × g for 10 min. Denuded oocytes that were not lysed and completely free of cumulus cells were collected (50 oocytes/pool as per [32]). Cumulus cells and cumulus-free oocytes were lysed as separate pools in 40 µl 0.1 N HCl before being stored at −80°C. Samples were acetylated and intracellular cGMP concentrations were determined in triplicate in denuded oocytes and associated cumulus cells using a commercially available cGMP ELISA kit (Cayman Chemicals) according to the manufacturer’s instructions. Intra-assay coefficient of variation was 10.3%. This study was replicated using nine different COC pools across three different days of collection (n = 450 total oocytes or cumulus masses/treatment group).
Study two: Levels of protein kinases ERK1/2 and AMPK in heat-stressed cumulus-oocyte complexes
To determine levels of active ERK1/2 (phosphorylated at Thr185/Tyr187), total ERK1/2, and active AMPK (phosphorylated at Thr172), COCs (55 to 60 per 3 ml OMM) were matured at 38.5, 40.0 or 41.0°C for 4 or 6 hIVM (a priori test of hypothesis using a 3 × 2 factorial treatment arrangement). Maturation times of 4 and 6 hIVM were chosen based on previous efforts of Hooper et al. [8] reporting heat-induced differences in meiotic progression in heat-stressed oocytes. Omission of a maturation temperature (i.e., 42°C) was required to ensure that COCs were processed in a timely manner for assessing kinase activity after maturation for 4 or 6 h. This temperature is severe and is seldom observed in heat-stressed cows. When interactions were not significant, main effect means for maturation time and temperature were reported. Kinase levels were also examined in immature COCs when possible (i.e., after collection but before placement into OMM, 0 hIVM) as a reference starting point. In instances where a surplus of COCs were available on a given collection day (n = 5), separate pools of COCs were also matured for 24 h at 38.5°C to evaluate the effect of maturation time on activity of AMPK and ERK1/2 under thermoneutral conditions.
Active ERK1/2 and total ERK1/2 were determined in oocytes (n = 30 of the original pool of 55 to 60 total) and their associated cumulus cells using a Milliplex MAP phosphorylated/total ERK1/2 duplex kit (MilliporeSigma) as described by Chouzouris et al. [33]. After the indicated maturation period, oocytes were denuded of associated cumulus cells by vortexing for 5 min in HEPES-TALP. Cumulus cells were washed with DPBS, centrifuged at 300 × g for 10 min, and cell pellets were stored at –80°C. Oocytes that were completely free of cumulus cells and were not lysed were stored at –80°C. Oocytes and cumulus cells were lysed by incubating in lysis buffer [33] supplemented with 1 mM phenylmethylsulfonyl fluoride for 10 min on ice, sonicating for 1 min at 100% amplitude, and then a 10 min incubation on ice. Cell lysates were centrifuged at 10,000 × g for 10 min at 4°C to remove debris. Total protein concentrations in the cumulus cell lysate were determined using the BradfordRED (Expedeon, San Diego, CA, USA) or FluoroProfile (MilliporeSigma) kits against a bovine serum albumin standard curve, according to manufacturer’s instructions. The proportion of total ERK1/2 that was active was determined in duplicate cumulus cell (equivalent 6.25 μg total protein/well) and oocyte (equivalent 15 oocytes/well) lysates using the Milliplex MAP kit according to the manufacturer’s instructions. Median fluorescence intensity was determined using a Luminex 200 (Luminex, Austin, TX, USA). Wells that did not reach a minimum of 35 bead counts (manufacturer’s recommendation) or that had a bead coefficient of variation > 200% [34, 35] were excluded from the analysis. Intra-assay coefficient of variation for active and total ERK1/2 were 22.0 and 8.2%, respectively, consistent with previously published studies using a magnetic bead approach [36, 37]. Effort to examine active ERK1/2 and total ERK1/2 was replicated across eleven different days of COC collection (n = 330 total oocytes or cumulus masses/group). Outcomes of these efforts prompted us to conduct a follow-on study to examine the extent to which separating the oocyte from its cumulus cells by vortexing influenced the proportion of total ERK1/2 that was active in intact COCs cultured for 4 h at 38.5 or 41.0°C. This effort was replicated using five different COC pools on a single day of collection (n = 150 total COCs/treatment group).
Active AMPK levels were determined in intact COCs (20 COCs out of the original pool of 55 to 60 total) using a commercial ELISA kit (Cell Signaling Technology, Danvers, MA, USA). To this end, COCs were lysed with the provided lysis buffer supplemented with 1 mM phenylmethylsulfonyl fluoride and total protein concentrations were determined as described above. Levels of active AMPK phosphorylated at Thr172 were determined in COC cell lysates in duplicate at 37°C as per manufacturer’s instructions. Absorbance (450 nm) was measured using a Synergy H1 microplate reader (BioTek Instruments, Burlington, VT, USA). Intra-assay coefficient of variation was 4.1%. Absorbance was corrected for relative protein amount. This study was replicated across ten different days of COC collection (n = 200 total COCs/treatment group).
Study three: Cumulus-derived progesterone production by heat-stressed cumulus-oocyte complexes
The amount of progesterone released into the conditioned maturation medium after culture of COCs (30 to 40 per 500 µl OMM) at 38.5, 41.0 or 42.0°C for 1, 2, 3, 4, 5 or 6 hIVM. Effort to include earlier maturation times in this study allowed for assessing when levels in the conditioned medium become detectable. Priority for examining consequences of 41°C was based on previous efforts reporting heat-induced increases in progesterone during the latter part of maturation [38, 39]. Effort to examine consequences of a more severe heat stress (42°C) was prioritized over examining consequences of 40.0°C to increase the likelihood of detecting heat-induced differences during times when progesterone production by the cumulus is low. A double antibody 125I radioimmunoassay progesterone assay (MP Biomedicals, Costa Mesa, CA, USA) was used according to the manufacturer’s instructions. The assay sensitivity was 0.2 ng/ml, intra-assay coefficient of variation was 5.1%, and the inter-assay coefficient of variation was 5.9%. This study was replicated across nine different days of COC collection (n = 296 total COCs/treatment group).
Statistical analyses
Data were analyzed using generalized linear mixed models (PROC GLIMMIX, SAS 9.4, SAS Institute, Cary, NC, USA) as a randomized block design, blocking on replicate (i.e., day of COC collection). For study one, the fixed effect included in statistical model was IVM temperature. The ERK1/2 data were log-transformed to achieve normality before analysis; fixed effects included in the model for this variable and AMPK included IVM temperature, IVM time and respective interaction. Relevant for study three, fixed effects in the model included IVM temperature, IVM time and respective interaction. A P-value < 0.05 was considered significant. In instances where interactions were not significant (P > 0.05), main effect means were reported. Treatment differences were determined using F-protected least significant differences and are reported as least squares means ± standard error of the mean (SEM).
Results
Study one: Gap junction communication, chromatin configuration, and cGMP content of heat-stressed cumulus-oocyte complexes
Exposure of COCs to heat stress for the first 4 hIVM reduced transfer of the calcein dye (P < 0.001; Fig. 1). Impaired gap junction communication was noted in COCs matured at 41.0 or 42.0°C, whereas dye transfer in COCs matured at 40.0°C was similar to the thermoneutral control. Exposure to a known gap junction inhibitor, carbenoxolone, also reduced dye transfer compared to the control (P < 0.0001; Fig. 1). Heat-induced changes in gap junction communication were coincident with differences in chromatin configuration (Table 1). Mean oocyte chromatin configuration scores were higher in COCs matured at 41.0 or 42.0°C or with carbenoxolone compared to those matured at 38.5 or 40.0°C (P < 0.0001). In other words, oocytes exposed to temperatures ≥ 41.0°C had progressed further through meiosis compared to oocytes matured at 38.5 or 40.0°C.
Fig. 1.
Effect of maturation of bovine cumulus-oocyte complexes for 4 h at either 38.5, 40.0, 41.0 or 42.0°C on gap junction communication as determined by the transfer of the fluorescent dye calcein from cumulus cells into the oocyte. Groups that do not share a letter are significantly different (P = 0.001). A separate subset of cumulus-oocyte complexes were maturated at 38.5°C in the presence of the gap junction inhibitor carbenoxolone (CBX).
Table 1. Chromatin configuration in control and heat-stressed oocytes after assessment of gap junction function.
Heat stress of COCs for the first 4 hIVM did not alter intracellular cGMP levels in denuded oocytes (0.527 ± 0.04 vs. 0.502 ± 0.04 fmol/oocyte at 38.5 and 41.0°C, respectively; P = 0.68) or their associated cumulus cells (1.663 ± 0.445 vs. 1.578 ± 0.920 fmol/cumulus at 38.5 and 41.0°C, respectively; P = 0.43).
Study two: Levels of protein kinases ERK1/2 and AMPK in heat-stressed cumulus-oocyte complexes
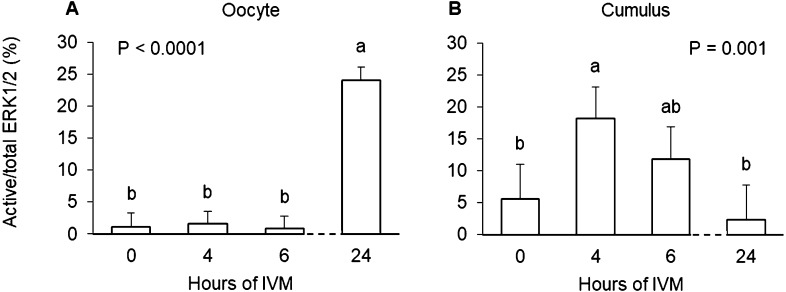
The proportion of total ERK1/2 that was active was highest in oocytes matured at 38.5°C for 24 h (P < 0.0001) compared to those matured for 6 h or less (Fig. 2A). Culture of intact COCs at an elevated temperature of 40 or 41°C for 4 to 6 h did not impact levels of active ERK1/2 in the oocyte (P = 0.67; Table 2).
Fig. 2.
The proportion of total ERK1/2 that was active in bovine oocytes (panel A) and associated cumulus cells (panel B) after in vitro maturation (IVM) of cumulus-oocyte complexes at 38.5°C for 0, 4, 6 or 24 h. Groups that do not share a letter are significantly different.
Table 2. Effects of in vitro maturation time (hIVM), temperature and hIVM × temperature interaction on the activation of extracellular-regulated kinase 1/2 (ERK1/2) in oocyte and associated cumulus cells.
| Active/total ERK1/2 (%) |
|||
|---|---|---|---|
| Oocytes | Cumulus cells | ||
| hIVM 1 | |||
| 4 | 0.75 ± 0.51 | 10.63 ± 3.39 a | |
| 6 | 0.58 ± 0.52 | 8.15 ± 3.38 b | |
| Temperature (°C) 2 | |||
| 38.5 | 0.67 ± 0.52 | 10.23 ± 3.42 | |
| 40.0 | 0.76 ± 0.52 | 8.74 ± 3.47 | |
| 41.0 | 0.57 ± 0.52 | 9.21 ± 3.44 | |
| hIVM × Temperature | |||
| 4 / 38.5°C | 0.91 ± 0.54 | 12.82 ± 3.57 | |
| 4 / 40.0°C | 0.72 ± 0.53 | 8.53 ± 3.61 | |
| 4 / 41.0°C | 0.63 ± 0.52 | 10.55 ± 3.61 | |
| 6 / 38.5°C | 0.43 ± 0.54 | 7.64 ± 3.57 | |
| 6 / 40.0°C | 0.81 ± 0.53 | 8.95 ± 3.65 | |
| 6 / 41.0°C | 0.50 ± 0.58 | 7.87 ± 3.58 | |
| hIVM P value | 0.92 | 0.046 | |
| Temperature P value | 0.67 | 0.60 | |
| hIVM × Temperature P value | 0.07 | 0.18 | |
1 Main effect of time (4 or 6 h) averaged across temperatures. 2 Main effect of temperature (38.5, 40.0 or 41.0°C) averaged across maturation times. a–b Means with different superscripts within a column differ (P < 0.05).
When evaluating the cumulus enveloping immature vs. matured oocytes (i.e., 0 vs. 24 hIVM), no differences were evident in the proportion of total ERK1/2 that was active (P = 0.02; Fig. 2B). Interestingly, the proportion of total ERK1/2 that was active was lower at 6 vs. 4 h (P = 0.046; Table 2). Relevant for examining the main effect of temperature, culture of intact COCs for 4 to 6 h at an elevated temperature of 40 or 41°C did not impact levels of active ERK1/2 in the surrounding cumulus (P = 0.60; Table 2). Lack of an effect heat stress to impact active ERK1/2 was also noted when levels were examined in intact COCs at 4 hIVM (23.46 ± 1.43 vs. 25.20% ± 1.43 at 38.5 and 41.0°C, respectively; P = 0.44).
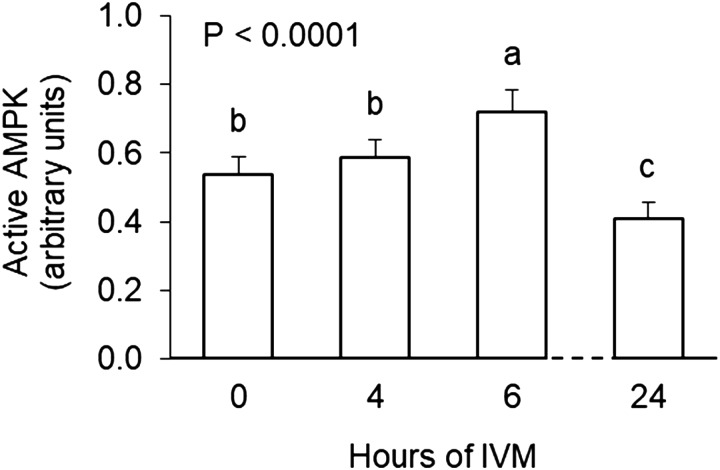
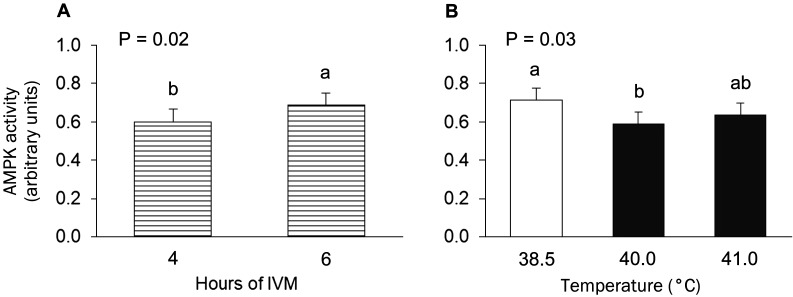
Active AMPK levels in intact COCs were lower after 24 h of maturation at 38.5°C compared to 0 hIVM (P < 0.0001; Fig. 3). However, during the early part of maturation, active AMPK was higher at 6 vs. 4 hIVM (P < 0.02; Figs. 3 and 4A). Culture of intact COCs at an elevated temperature during the first 6 h reduced levels of active AMPK (P = 0.03; Fig. 4B). Maturation at 40.0°C decreased active AMPK levels (P = 0.008); exposure to 41.0°C resulted in levels that were intermediate to those obtained for COCs matured at 38.5 or 40.0°C (P = 0.08).
Fig. 3.
Active AMPK in bovine cumulus-oocyte complexes after in vitro maturation (IVM) at 38.5°C for 0, 4, 6 or 24 h Groups that do not share a letter are significantly different (P < 0.0001).
Fig. 4.
Main effects of maturation time (4 or 6 h of in vitro maturation (IVM) averaged across temperatures; panel A) and maturation temperature (38.5, 40.0 or 41.0°C averaged across maturation times; panel B) on active AMPK levels in bovine cumulus-oocyte complexes. Groups that do not share a letter are significantly different.
Study three: Cumulus-derived progesterone production by heat-stressed cumulus-oocyte complexes
Progesterone levels in the conditioned maturation medium increased progressively during the first 6 hIVM (P < 0.0001; Fig. 5A). Exposure of COCs to an elevated temperature of 41 or 42°C increased progesterone production (P = 0.001; Fig. 5B). Heat-induced increases in progesterone did not differ depending on time of maturation (hIVM × Temperature interaction, P = 0.80).
Fig. 5.
Main effects of maturation time (1 to 6 h of in vitro maturation (IVM) averaged across temperatures; panel A) and maturation temperature (38.5, 41.0 or 42.0°C averaged across maturation times; panel B) on progesterone released into conditioned medium from bovine cumulus-oocyte complexes. Groups that do not share a letter are significantly different.
Discussion
The findings presented herein identify novel cumulus cell components/pathways affected by heat stress exposure that may explain, in part, heat-induced hastening of the onset of meiotic progression reported in study one and previously by others. To this end, accelerated condensation of chromatin was coincident with heat-induced reductions in gap junction communication. Heat stress exposure during this early time period also reduced AMPK activity in the intact COC and heightened progesterone release into the maturation medium.
Intercellular communication through gap junctions is a key pathway by which the cumulus cells regulate oocyte meiotic arrest and resumption [13, 14]. In the current study, exposure to an elevated temperature during early maturation impaired gap junction communication, which was coincident with accelerated oocyte chromatin condensation. Heat-induced changes in chromatin status are likely the result of heat-induced changes in cumulus cell communication because treatment of COCs with the gap junction inhibitor carbenoxolone markedly reduced gap junction transfer (~90%) and also stimulated condensation of maternal chromatin. This is consistent with prior studies on bovine oocytes that demonstrated gap junction connectivity is highly correlated to chromatin status; efforts to uncouple gap junction channels with heptanol stimulated germinal vesicle breakdown [14, 15]. Although heat stress exposure for 4 h reduced gap junction transfer of calcein by approximately 50%, cGMP content was not impacted in oocytes or surrounding cumulus cells. In retrospect this finding is not surprising because Xi et al. [40] reported that treating bovine COCs with a different gap junction inhibitor (octanol) did not alter cGMP content in the oocyte or cumulus. This same study showed that the bovine oocyte may have an intrinsic ability to produce cGMP suggesting that, unlike what occurs in the mouse [16], transfer of cGMP from the cumulus may not be entirely required to maintain meiotic arrest.
Permeability of gap junctions in the COC is primarily regulated by ERK1/2-dependent phosphorylation in the mouse [18]. However, species-specific differences likely exist because despite having observed heat-induced reductions in gap junction transfer by approximately 50%, a corresponding change in active ERK1/2 in bovine cumulus cells was not detected in our second study. Heat stress exposure and carbenoxolone addition in our first study elicited similar oocyte phenotypes (i.e., reduced gap junction communication and earlier changes in chromatin) suggesting heat stress may be perturbing gap junction transfer and/or meiotic progression in a similar manner. The mechanism of action for glycyrrhetinic acid derivatives, such as carbenoxolone, appears to be through the activation of protein kinases (e.g., ERK1/2, CamKII and/or PKA) which in turn may phosphorylate connexin subunits of gap junctions to decrease communication (reviewed by [41]). In other species and cell types, hyperthermia induces phosphorylation of connexin 43, however the protein kinase responsible for mediating heat stress effects differ depending on the cellular context [42,43,44]. Heat stress may be affecting gap junction communication through any number of kinases in the COC because connexins have multiple phosphorylation sites responding to numerous different kinases, including protein kinase A, protein kinase B, protein kinase C, and EGFR tyrosine kinase to name a few (reviewed by [45]).
Lack of an effect of heat stress exposure on ERK1/2 activation at the temperatures tested was initially surprising. Mild heat shock exposure has been shown to activate ERK1/2 in other cell types [46] and premature activation of MAPK activity in bovine oocytes had been shown to accelerate germinal vesicle breakdown and reduce the time required to reach metaphase I [47]. Our initial efforts examining levels in the oocyte and their surrounding cumulus after denuding by vortex resulted in different values than those for an equivalent number of intact COCs (proportion of active ERK1/2 ranged from 23 to 25% in intact COCs, 8 to 13% in cumulus cells, and 0.4 to 0.9% in denuded oocytes). Thus, additional effort was taken to examine consequences of heat stress exposure in intact COCs. Regardless of whether COCs were intact or not, heat stress exposure in the manner applied in our studies had no detectable effect on ERK1/2.
Related to the intact COC, a heat stress exposure of 40°C during the first part of maturation in study two reduced levels of AMPK in COCs by 17%, whereas 41°C exposure reduced levels by 11%. Because heat stress mediates AMPK inhibition in other cell types [48] and a decline in AMPK activity has been associated with accelerated meiotic progression in bovine oocytes [21] it was tempting to speculate that heat-induced changes described herein may be contributing to chromatin changes (study one and previously reported [8]). However, heat-induced hastening of chromatin changes described herein were only noted when maturation temperatures were ≥ 41°C. Furthermore, heat-induced reductions in AMPK were most prominent after maturation of COCs at 40°C; effects after 41°C exposure were subtle.
Heat-induced increases in progesterone production during maturation have been described previously by our laboratory [38, 39]. Efforts of study three extend these findings and document an effect of heat stress exposure to increase progesterone production during the early period of maturation. Although consequences are not yet understood, bovine oocytes express genomic and non-genomic progesterone receptors [49]. Others have shown that supplementation of culture medium with progesterone stimulates germinal vesicle breakdown [25] and meiotic progression [24]. While species differences do exist, progesterone supplementation accelerated meiosis in porcine oocytes [50] and other non-mammalian species (reviewed in [51]). Therefore, we cannot preclude the possible role of progesterone in the heat-induced hastening of chromatin changes in the bovine oocyte after resumption of meiosis. In support of this, Shimada and Terada [22] reported that suppression of progesterone production in porcine COCs using aminoglutethimide was sufficient to almost entirely prevent germinal vesicle breakdown; inhibitory effects on germinal vesicle breakdown were overcome by additional progesterone.
Heightened release of progesterone into the medium by heat-stressed COCs is likely due to enhanced synthesis. Previous efforts of our laboratory noted elevated expression levels of CYP11A1 and HSD3B1 [38]. Heat-induced increases in progesterone production by the COC were likely not related to changes in ERK1/2 despite the reported role of ERK1/2 in progesterone synthesis [20]. Regardless of the underlying mechanism, elevated progesterone production by heat-stressed COCs document that cumulus cells are “luteinizing” earlier, which may be influencing dissociation of the gap junction connections [22]. Preliminary results from our laboratory indicate that carbenoxolone treatment effective for reducing gap junction communication by > 90%, increased progesterone production by COCs (N = 2 replicates; 40.2 vs. 84.8 and 93.3 vs. 176.5 pg progesterone/COC for control and carbenoxolone, respectively). This is suggestive that heat-induced alterations in the interconnected cumulus may precede or contribute to changes in progesterone production. Increased progesterone production is associated with reduced AMPK activity in bovine COCs [21] and both components are influential on meiotic resumption. Nonetheless, our findings highlight the progress of our studies towards identifying putative components problematic in the heat-stressed oocyte during the early maturation.
Acknowledgments
This project was supported in part by Agriculture and Food Research Initiative Competitive Grant no. 2016-67015-24899 from the USDA National Institute of Food and Agriculture, the state of Tennessee through UT AgResearch, East Tennessee Research and Education Center, the Department of Animal Science, and the USDA National Institute of Food and Agriculture, Hatch Project No. 227701. The authors wish to thank the UTIA Genomics Hub for the use and technical assistance of Sujata Agarwal with the Luminex (xMAP) technology, Dr Ky Pohler for critical review of manuscript, and Gessica Franco for running some of our progesterone samples. A special thanks is also extended to James and Pam Brantley for their generous contributions to our research efforts.
References
- 1.Putney D, Mullins S, Thatcher W, Drost M, Gross T. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci 1989; 19: 37–51. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JL, Hansen PJ. Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocytes. Biol Reprod 1996; 55: 341–346. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JL, Payton RR, Godkin JD, Saxton AM, Schrick FN, Edwards JL. Retinol improves development of bovine oocytes compromised by heat stress during maturation. J Dairy Sci 2004; 87: 2449–2454. [DOI] [PubMed] [Google Scholar]
- 4.Zhandi M, Towhidi A, Nasr-Esfahani MH, Eftekhari-Yazdi P, Zare-Shahneh A. Unexpected detrimental effect of Insulin like growth factor-1 on bovine oocyte developmental competence under heat stress. J Assist Reprod Genet 2009; 26: 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascari IJ, Alves NG, Jasmin J, Lima RR, Quintão CCR, Oberlender G, Moraes EA, Camargo LSA. Addition of insulin-like growth factor I to the maturation medium of bovine oocytes subjected to heat shock: effects on the production of reactive oxygen species, mitochondrial activity and oocyte competence. Domest Anim Endocrinol 2017; 60: 50–60. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JL, Saxton AM, Lawrence JL, Payton RR, Dunlap JR. Exposure to a physiologically relevant elevated temperature hastens in vitro maturation in bovine oocytes. J Dairy Sci 2005; 88: 4326–4333. [DOI] [PubMed] [Google Scholar]
- 7.Schrock GE, Saxton AM, Schrick FN, Edwards JL. Early in vitro fertilization improves development of bovine ova heat stressed during in vitro maturation. J Dairy Sci 2007; 90: 4297–4303. [DOI] [PubMed] [Google Scholar]
- 8.Hooper LM, Payton RR, Rispoli LA, Saxton AM, Edwards JL. Impact of heat stress on germinal vesicle breakdown and lipolytic changes during in vitro maturation of bovine oocytes. J Reprod Dev 2015; 61: 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgartner AP, Chrisman CL. Ovum morphology after hyperthermic stress during meiotic maturation and ovulation in the mouse. J Reprod Fertil 1981; 61: 91–96. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Geum D, Khang I, Park YM, Kang BM, Lee KA, Kim K. Expression pattern of HSP25 in mouse preimplantation embryo: heat shock responses during oocyte maturation. Mol Reprod Dev 2002; 61: 3–13. [DOI] [PubMed] [Google Scholar]
- 11.Hyttel P. Bovine cumulus-oocyte disconnection in vitro. Anat Embryol (Berl) 1987; 176: 41–44. [DOI] [PubMed] [Google Scholar]
- 12.Hyttel P, Xu KP, Smith S, Greve T. Ultrastructure of in-vitro oocyte maturation in cattle. J Reprod Fertil 1986; 78: 615–625. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod 2004; 70: 548–556. [DOI] [PubMed] [Google Scholar]
- 14.Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Mol Reprod Dev 2007; 74: 740–749. [DOI] [PubMed] [Google Scholar]
- 15.Luciano AM, Franciosi F, Modina SC, Lodde V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s). Biol Reprod 2011; 85: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 16.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009; 136: 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimada M, Terada T. Phosphorylation of connexin-43, gap junctional protein, in cumulus cells is regulated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase during in vitro meiotic resumption in porcine follicular oocytes. J Mamm Ova Res 1999; 16: 37–42. [Google Scholar]
- 18.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 2008; 135: 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiquet N, Sasseville M, Laforest M, Guillemette C, Gilchrist RB, Richard FJ. Activation of 5′ adenosine monophosphate-activated protein kinase blocks cumulus cell expansion through inhibition of protein synthesis during in vitro maturation in Swine. Biol Reprod 2014; 91: 51. [DOI] [PubMed] [Google Scholar]
- 20.Su YQ, Nyegaard M, Overgaard MT, Qiao J, Giudice LC. Participation of mitogen-activated protein kinase in luteinizing hormone-induced differential regulation of steroidogenesis and steroidogenic gene expression in mural and cumulus granulosa cells of mouse preovulatory follicles. Biol Reprod 2006; 75: 859–867. [DOI] [PubMed] [Google Scholar]
- 21.Tosca L, Uzbekova S, Chabrolle C, Dupont J. Possible role of 5'AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod 2007; 77: 452–465. [DOI] [PubMed] [Google Scholar]
- 22.Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: a requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod 2002; 8: 612–618. [DOI] [PubMed] [Google Scholar]
- 23.Shimada M, Yamashita Y, Ito J, Okazaki T, Kawahata K, Nishibori M. Expression of two progesterone receptor isoforms in cumulus cells and their roles during meiotic resumption of porcine oocytes. J Mol Endocrinol 2004; 33: 209–225. [DOI] [PubMed] [Google Scholar]
- 24.Sirotkin AV. Involvement of steroid hormones in bovine oocytes maturation in vitro. J Steroid Biochem Mol Biol 1992; 41: 855–858. [DOI] [PubMed] [Google Scholar]
- 25.Siqueira LC, Barreta MH, Gasperin B, Bohrer R, Santos JT, Buratini J, Jr, Oliveira JF, Gonçalves PB. Angiotensin II, progesterone, and prostaglandins are sequential steps in the pathway to bovine oocyte nuclear maturation. Theriogenology 2012; 77: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 26.Rispoli LA, Lawrence JL, Payton RR, Saxton AM, Schrock GE, Schrick FN, Middlebrooks BW, Dunlap JR, Parrish JJ, Edwards JL. Disparate consequences of heat stress exposure during meiotic maturation: embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction 2011; 142: 831–843. [DOI] [PubMed] [Google Scholar]
- 27.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 2012; 20: 249–259. [DOI] [PubMed] [Google Scholar]
- 28.Rivera RM, Hansen PJ. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 2001; 121: 107–115. [PubMed] [Google Scholar]
- 29.Ferreira RM, Ayres H, Chiaratti MR, Ferraz ML, Araújo AB, Rodrigues CA, Watanabe YF, Vireque AA, Joaquim DC, Smith LC, Meirelles FV, Baruselli PS. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. J Dairy Sci 2011; 94: 2383–2392. [DOI] [PubMed] [Google Scholar]
- 30.Dikmen S, Wang XZ, Ortega MS, Cole JB, Null DJ, Hansen PJ. Single nucleotide polymorphisms associated with thermoregulation in lactating dairy cows exposed to heat stress. J Anim Breed Genet 2015; 132: 409–419. [DOI] [PubMed] [Google Scholar]
- 31.Putney DJ, Drost M, Thatcher WW. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between Days 1 to 7 post insemination. Theriogenology 1988; 30: 195–209. [DOI] [PubMed] [Google Scholar]
- 32.De Cesaro MP, Macedo MP, Santos JT, Rosa PR, Ludke CA, Rissi VB, Gasperin BG, Gonçalves PB. Natriuretic peptides stimulate oocyte meiotic resumption in bovine. Anim Reprod Sci 2015; 159: 52–59. [DOI] [PubMed] [Google Scholar]
- 33.Chouzouris TM, Dovolou E, Krania F, Pappas IS, Dafopoulos K, Messinis IE, Anifandis G, Amiridis GS. Effects of ghrelin on activation of Akt1 and ERK1/2 pathways during in vitro maturation of bovine oocytes. Zygote 2017; 25: 183–189. [DOI] [PubMed] [Google Scholar]
- 34.Zhi W, Sharma A, Purohit S, Miller E, Bode B, Anderson SW, Reed JC, Steed RD, Steed L, Hopkins D, She JX. Discovery and validation of serum protein changes in type 1 diabetes patients using high throughput two dimensional liquid chromatography-mass spectrometry and immunoassays. Mol Cell Proteomics 2011; 10: 012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purohit S, Sharma A, Hopkins D, Steed L, Bode B, Anderson SW, Reed JC, Steed RD, Yang T, She JX. Large-scale discovery and validation studies demonstrate significant reductions in circulating levels of IL8, IL-1Ra, MCP-1, and MIP-1beta in patients with type 1 diabetes. J Clin Endocrinol Metab 2015; 100: E1179–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Defawe OD, Fong Y, Vasilyeva E, Pickett M, Carter DK, Gabriel E, Rerks-Ngarm S, Nitayaphan S, Frahm N, McElrath MJ, De Rosa SC. Optimization and qualification of a multiplex bead array to assess cytokine and chemokine production by vaccine-specific cells. J Immunol Methods 2012; 382: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staples E, Ingram RJ, Atherton JC, Robinson K. Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. J Immunol Methods 2013; 394: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rispoli LA, Payton RR, Gondro C, Saxton AM, Nagle KA, Jenkins BW, Schrick FN, Edwards JL. Heat stress effects on the cumulus cells surrounding the bovine oocyte during maturation: altered matrix metallopeptidase 9 and progesterone production. Reproduction 2013; 146: 193–207. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin MR, Rispoli LA, Payton RR, Saxton AM, Edwards JL. Developmental consequences of supplementing with matrix metallopeptidase-9 during in vitro maturation of heat-stressed bovine oocytes. J Reprod Dev 2016; 62: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi G, An L, Jia Z, Tan K, Zhang J, Wang Z, Zhang C, Miao K, Wu Z, Tian J. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology 2018; 106: 198–209. [DOI] [PubMed] [Google Scholar]
- 41.Salameh A, Dhein S. Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim Biophys Acta 2005; 1719: 36–58. [DOI] [PubMed] [Google Scholar]
- 42.Azzam EI, de Toledo SM, Little JB. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res 2003; 63: 7128–7135. [PubMed] [Google Scholar]
- 43.Hamada N, Kodama S, Suzuki K, Watanabe M. Gap junctional intercellular communication and cellular response to heat stress. Carcinogenesis 2003; 24: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 44.Antanavičiūtė I, Mildažienė V, Stankevičius E, Herdegen T, Skeberdis VA. Hyperthermia differently affects connexin43 expression and gap junction permeability in skeletal myoblasts and HeLa cells. Mediators Inflamm 2014; 2014: 748290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pogoda K, Kameritsch P, Retamal MA, Vega JL. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: a revision. BMC Cell Biol 2016; 17(Suppl 1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci 2005; 62: 10–23. [DOI] [PubMed] [Google Scholar]
- 47.Fissore RA, He CL, Vande Woude GF. Potential role of mitogen-activated protein kinase during meiosis resumption in bovine oocytes. Biol Reprod 1996; 55: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Yu Q, Chen J, Deng B, Qian L, Le Y. PP2A mediated AMPK inhibition promotes HSP70 expression in heat shock response. PLoS One 2010; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aparicio IM, Garcia-Herreros M, O’Shea LC, Hensey C, Lonergan P, Fair T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol Reprod 2011; 84: 910–921. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita Y, Shimada M, Okazaki T, Maeda T, Terada T. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol Reprod 2003; 68: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 51.Liang CG, Su YQ, Fan HY, Schatten H, Sun QY. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol 2007; 21: 2037–2055. [DOI] [PubMed] [Google Scholar]