Abstract
The value of testosterone replacement therapy (TRT) for older men is currently a topic of intense debate. While US testosterone prescriptions have tripled in the past decade (9), debate continues over the risks and benefits of TRT. TRT is currently prescribed for older men with either low serum testosterone (T) or low T plus accompanying symptoms of hypogonadism. The normal range for serum testosterone is 300 to 1,000 ng/dl. Serum T ≤ 300 ng/dl is considered to be low, and T ≤ 250 is considered to be frank hypogonadism. Most experts support TRT for older men with frank hypogonadism and symptoms. Treatment for men who simply have low T remains somewhat controversial. TRT is most frequently administered by intramuscular (im) injection of long-acting T esters or transdermally via patch or gel preparations and infrequently via oral administration. TRT produces a number of established benefits in hypogonadal men, including increased muscle mass and strength, decreased fat mass, increased bone mineral density, and improved sexual function, and in some cases those benefits are dose dependent. For example, doses of TRT administered by im injection are typically higher than those administered transdermally, which results in greater musculoskeletal benefits. TRT also produces known risks including development of polycythemia (Hct >50) in 6% of those treated, decrease in HDL, breast tenderness and enlargement, prostate enlargement, increases in serum PSA, and prostate-related events and may cause suppression of the hypothalamic-pituitary-gonadal axis. Importantly, TRT does not increase the risk of prostate cancer. Putative risks include edema and worsening of sleep apnea. Several recent reports have also indicated that TRT may produce cardiovascular (CV) risks, while others report no risk or even benefit. To address the potential CV risks of TRT, we have recently reported via meta-analysis that oral TRT increases CV risk and suggested that the CV risk profile for im TRT may be better than that for oral or transdermal TRT.
Keywords: testosterone, muscle, bone, cardiovascular risk
herein, we review the literature, which indicates that intramuscularly injected testosterone replacement therapy (TRT) produces greater musculoskeletal benefits and lower cardiovascular risk compared with transdermal TRT. TRT also produces risks of polycythemia, prostate enlargement, and suppression of the hypothalamic-pituitary-gonadal axis. The effects of injection vs. transdermal administration on these risks are unknown. We also review the literature discussing the use of 5α-reductase inhibitors as a promising means of improving the safety profile of TRT.
Definition of Hypogonadism
The Endocrine Society recommends TRT for men with androgen deficiency, defined as low serum T with consistent symptoms and signs of hypogonadism (5), including decreased sexual function, loss of axillary and pubic hair, low bone mineral density, loss of motivation, mood, or concentration, and loss of muscle strength and work capacity. In older men, hypogonadism is commonly defined as a serum total T concentration of ≤300 ng/dl (i.e., below the normal range) or ≤250 ng/dl (i.e., frank hypogonadism). Both groups may benefit from TRT. Determination of serum T should be based on the average of two blood samples drawn before 10:00 AM. Kaufman and Vermeulen (45) have reviewed the literature and reported that ∼20% of men over the age of 60 yr have a serum total T concentration of ≤320 ng/dl. Similarly, we (19) have reported that 24% of men over 60 yr have a serum total T of ≤300 ng/dl. Additionally, hypogonadism is sometimes defined as low free T or low bioavailable T (bioT). In this regard, a small fraction of circulating T is unbound while the bulk is either loosely bound to albumin or very tightly bound to steroid hormone-binding globulin (SHBG). BioT is the sum of the free and albumin-bound T and represents the most physiologically important T fraction. BioT is the fraction able to cross cell membranes and bind to androgen receptors (ARs). An increase in SHBG due to aging (85) and traumatic events such as spinal cord injury (11) will result in reduced bioT. Total T assays are available to most clinicians, whereas free T and bioT assays are less available. However, serum bioT may be estimated from serum total T, SHBG, and albumin by using one of several empirical formulas (57, 86). The Endocrine Society recommends that treatment be individualized, including a determination of whether the patient has primary hypogonadism (primary deficit lies within the testes) or secondary hypogonadism (primary deficit lies outside of the testes) (13). When T-enanthate or T-cypionate are injected, one should aim for a serum total T concentration of 400–700 ng/dl at 1 wk after the most recent injection.
Modes of TRT and Doses Delivered
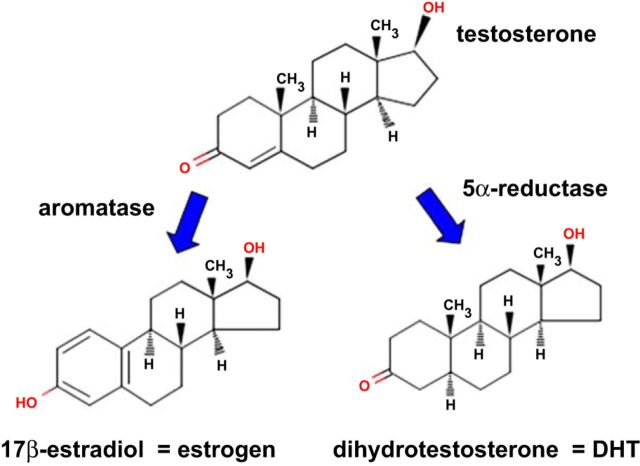
T use in the United States increased more than threefold between 2000 and 2011 (36). The most common modes of T administration are patch and gel preparations and im injection of long-acting T esters. Transdermal doses are typically intended to be replacement doses, with the patch being administered at a doses of 5–10 mg T/day and gel administration involving a somewhat greater amount of T due to low absorption (Table 1). Injection of T esters typically delivers higher amounts, 50–400 mg every 2–4 wk. Esters such as T-enanthate or T-cypionate have virtually no aqueous solubility and remain in the muscle depot until muscle esterase activity releases T, which can then enter the circulation. Administered T may be converted to estradiol via the action of aromatase or to dihydrotestosterone (DHT) by the action of 5α-reductase (Fig. 1), both of which exert biological actions at estrogen receptor and ARs, respectively. As such, T may be considered both a hormone (because it binds to ARs) and a prohormone for the synthesis of estradiol and DHT. Interestingly, the mode of TRT administration appears to alter the metabolism of T, as evidenced by our recent meta-analysis (18) reporting that transdermal T (patch and gel) elevated serum DHT 5.46-fold, whereas im-injected T elevates serum DHT only 2.2-fold. This surprising phenomenon occurs despite the fact that transdermal and im TRT elevated serum T to a roughly similar degree and may be explained by relatively high expression of 5α-reductase in skin (40) vs. lower expression in skeletal muscle (93).
Table 1.
Common modes of testosterone (T) administration
| Mode | Trade Names | Dosing Interval | Dose |
|---|---|---|---|
| i.m. Injection of T-cypionate or T-enanthate | Depot-Testosterone® Delatestryl® | Weekly or biweekly | 50–400 mg T/every 2–4 wk |
| Patch administration of T | Androderm® | Daily | 28–35 mg T/wk |
| Gel administration of T | Androgel® | Daily | 140–280 mg T/wk (low absorption) |
| Oral administration of T-undecanoate | Andriol® | Daily | 840–1,120 mg T/wk (low absorption, 1st pass) |
Fig. 1.
Testosterone may act directly at androgen receptors or indirectly following conversion to estradiol or dihydrotestosterone (DHT).
Effect of TRT on Muscle, Bone, and Body Composition
Age is accompanied by a progressive decline in muscle mass with a decrease in both the number and diameter of muscle fibers, especially type II (fast) fibers (59). Fiber loss with aging is secondary to a loss in motor neurons (81). TRT increases muscle mass and strength by increasing the cross-sectional area of both type I and type II fibers in a dose-dependent manner (70). At low replacement doses, T administration produces muscle protein accretion mainly by preventing protein degradation (30), which is mediated, at least in part, by an increased phosphorylation-induced inactivation of FOXO3a (89). At higher doses, T also stimulates muscle protein synthesis via a number of mechanisms that include proliferation of muscle satellite cells and donation of their nuclei to the myofibril (71), elevation of muscle IGF-I (69), and activation of the Akt/4 mammalian target of rapamycin (mTOR) pathway (90). Binding of T to ARs present in mesenchymal pluripotent cells causes translocation of β-catenin to the nucleus, causing them to differentiate into the myocyte lineage rather than the adipocyte lineage (68). Preservation of lower body muscular strength is an important factor in maintaining independence in older individuals. Resistance training produces substantial strength increases in the elderly. For example, Villanueva et al. (88) found that, in a group of healthy older men, resistance training for 12 wk increased 1-RM leg strength by 100%. By contrast, TRT produces smaller gains but does so independently of exercise. In general, the response to lower doses of TRT administered transdermally may be considered modest, whereas the effects on injected TRT are moderate. Transdermal TRT to older men often increases lean mass; however, most studies do not report increases in strength (20, 48). A 2006 meta-analysis by Ottenbacher et al. (60), assessing 11 RCTs of TRT in older men, reported that injected TRT produced a “moderate” increase in muscle strength, whereas the effects of transdermal and oral TRT were much less. We surveyed 10, mostly more recent, RCTs of TRT in older men lasting 12 wk or more and reporting 1-RM strength as an outcome. None of the four transdermal studies (10, 37, 47, 58) reported a significant increase in 1-RM strength. In contrast, all six of the im injection studies (19, 22, 30, 64, 75, 78) reported significantly increased strength. We administered a moderately high dose of 125 mg/wk T-enanthate im to older hypogonadal men and observed increases of 8–14% in 1-RM strength over a 12-mo period, with most of the improvement occurring during the first 3 mo (19). Storer et al. (75) administered a higher dose of 300 mg T/wk im to young eugonadal men for 5 mo and reported that 1-RM leg press strength was increased 23% and leg press power by 39%. The benefits with even higher doses of im T (300–600 mg/wk) are even more substantial (upward of 20% increase in 1-RM strength in older men over 20 wk), but the number of adverse events observed at these doses precludes their clinical implementation (14). Thus, injected TRT has value for increasing muscle strength in older men, especially because not all patients have the means or ability to exercise. In addition, hypogonadism may reduce both the response to and the motivation for resistance exercise.
Effect of TRT on Bone
Men over the age of 65 yr are subject to an increased incidence of osteoporosis and to increased falls and fractures ultimately contributing to increased mortality (15). In older men, low serum T is associated with osteopenia (49) and increased fracture risk (55). T administration increases bone mineral density (BMD), mainly by suppressing bone resorption (12, 16). TRT may increase BMD in men by a direct AR-mediated effect of T or by an indirect action requiring conversion to estradiol (94). In this regard, low serum estradiol is more strongly associated with osteopenia in older men than is low serum T (49). Interestingly, the indirect effects of T may be more important than the direct effects, as evidenced by the work of Falahati-Nini et al. (28), who observed elevated blood markers of bone resorption following combined administration, to older men, of a GnRH agonist and an aromatase inhibitor to inhibit production of T and estradiol, respectively. Subsequent administration of transdermal estradiol alone suppressed markers of bone resorption, while transdermal T alone did not, whereas full suppression of bone resorption occurred only with combined T plus estradiol administration. Whereas bone protection in response to replacement doses of T requires aromatization, bone protection resulting from high doses of androgen does not appear to.
We have reported that im administration of either T or its nonaromatizable analog trenbolone can completely prevent orchiectomy-induced bone loss in skeletally mature rats (54) and that coadministration of the aromatase inhibitor anastrozole does not inhibit the effects of T and trenbolone (12).
In older men, T increases BMD in regions that have a large component of cancellous bone, such as the lumbar spine and hip (84). These increases are important because they occur at sites where fractures frequently occur in the elderly. We have reported that older men receiving 125 mg T-enanthate/wk im for 1 yr exhibited a 4% increase in lumbar spine BMD and a 2% increase in hip BMD over 12 mo (19). Amory et al. (7) reported continued BMD improvement through 36 mo of im TRT, with lumbar spine BMD increasing 10% and hip BMD increasing 3% compared with baseline. Interestingly, in a 2006 meta-analysis of eight TRT trials in older hypogonadal men, Tracz et al. (84) found that im TRT produced a significant 8% increase in lumbar spine BMD and a nonsignificant 4% in hip BMD, whereas transdermal TRT produced no increases in BMD. We (92) have also reported that, in rats, T-enanthate prevents orchiectomy-induced loss of bone mechanical strength. The large clinical trials that are needed to assess fracture risk following TRT have not yet been conducted.
Adverse Effects of TRT
Established risks of TRT.
Meta-analysis has confirmed three adverse events (AEs) resulting from TRT (21, 29, 34): 1) polycythemia occurring in ∼6% of participants, 2) an increased number of prostate-related events, and 3) a small reduction in HDL-cholesterol. Prostate events consist of the combined incidence of elevated PSA, prostate biopsy necessitated by results of digital rectal exam, increased urinary symptoms, and prostate cancer. Meta-analysis by Calof et al. (21) reported no evidence that T administration increases prostate cancer (odds ratio = 1.09 with no trend toward significance), when considered as an independent outcome. More recently, a 15-yr retrospective study of 150,000 men by Kaplan and Hu (43) found that TRT was not associated with prostate cancer. In addition, TRT inhibits endogenous T production and suppresses the hypothalamic-pituitary-gonadal axis. Endogenous T production may not resume or may be diminished following cessation of TRT. The relative effects of im-injected vs. transdermal TRT on prostate enlargement, polycythemia, and suppression of the hypothalamic-pituitary-gonadal axis are unknown.
In addition, some risks depend specifically on the mode of TRT administration (13). Injected TRT may cause pain or bleeding at the site of injection and should not be given to men receiving anticoagulants. Patches may cause skin reactions, and gel may result in transfer of T to a partner.
Less common and putative risks of TRT.
T may also cause edema, breast tenderness, and gynecomastia (79), effects that are thought to result from elevation of estradiol subsequent to TRT. Because adipose tissue is the principal site of systemic aromatization (conversion of T to estradiol), TRT is often contraindicated for men with a BMI of 30 or more.
Causing or worsening of sleep apnea is frequently listed among potential AEs of TRT, based primarily on case reports in the literature. Meta-analysis of 19 clinical trials through 2005 by Calof et al. (21) showed no significant increase in obstructive sleep apnea with TRT; however, the studies were not conducted with polysomnography. The one study using this technique was performed by Hoyos et al. (39), who treated middle-aged men who had severe obstructive sleep apnea with T. T mildly worsened sleep-disordered breathing after 7 wk of treatment, but not after 18 wk.
Possible CV risks of TRT.
Recently, four reports have caused concern regarding the potential CV risks of T administration. Basaria et al. (10), in their randomized controlled trial of T gel administration, reported a greater number of CV AEs in treated vs. placebo, resulting in cessation of the trial. However, it should be noted that the trial was not designed to assess prespecified CV surrogate outcomes or clinical end points. Furthermore, the increased CV-related adverse events were considered as a composite end point including events of varying severity and mechanisms. Vigen et al. (87), in a retrospective study of men with low T and angina who also had undergone angiography, reported a higher CV risk in those who subsequently received T than in those who did not. The methodology of that paper was criticized in numerous letters to the editor of JAMA. Another observational study, by Finkle et al. (31), reported a greater risk of myocardial infarction in men who had received a prescription for T. Finally, Xu et al. (91) published a 2013 meta-analysis of CV adverse events in 27 randomized controlled trials (RCTs) of TRT published through December of 2012 and reported a statistically significant odds ratio (OR) of 1.54, indicating that participants receiving TRT were 54% more likely to develop CV AEs. As a result of those studies, the FDA (4), VA (1), and Endocrine Society (2) have all issued advisories calling for more research on potential TRT-related CV risks. The latest FDA Drug Safety Communication, issued March 2015, will require manufacturers of approved T products to conduct clinical trials assessing risks of heart attack and stroke (3).
We have recently performed the largest meta-analysis of RCTs to date (including 35 RCTs of TRT lasting 12 wk or more, reporting CV adverse events and published through May of 2014) evaluating TRT-related CV risks (18) and using guidelines for analysis of low-frequency events (95). Our main finding was that the form of T (18) administration influenced its CV risk profile (Table 2). Specifically, we reported the following new and significant findings. Because Xu et al. did not use statistical techniques suited to analysis of low-frequency events, and because some recent studies have reported fewer TRT-induced CV AEs, the estimate of risk for CV AEs is revised downward (RR = 1.28, nonsignificant). Oral TRT produces significant risk for CV AEs (RR = 2.20, P = 0.015). Transdermal (patch or gel) TRT produces a nonsignificant directional trend toward CV risk (RR = 1.27), and im TRT produces a nonsignificant directional trend toward CV protection (RR = 0.66). Transdermal and oral TRT cause greater elevation of serum DHT (but not T) compared with injected TRT (Table 1). Serum DHT concentrations following transdermal and oral TRT correspond to the concentrations that have been linked to CV disease and mortality in observational studies (66).
Table 2.
Risk of CV events in placebo-controlled randomized clinical trials published through May 2014
| Author, Year | TRT Mode | TRT Events/Subjects | Placebo Events/Subjects | Relative Risk |
|---|---|---|---|---|
| Basaria, 2010 (10) | Gel | 25/106 | 5/103 | 4.86 |
| Brockenbrough, 2006 (61) | Gel | 9/19 | 9/21 | 1.11 |
| Glintborg, 2013 (32) | Gel | 0/20 | 0/18 | 0.90 |
| Kaufman, 2011 (44) | Gel | 11/234 | 0/40 | 4.01 |
| Kenny, 2010 (47) | Gel | 14/69 | 19/62 | 0.66 |
| Marin, 1993 (53) | Gel | 1/11 | 0/10 | 2.75 |
| Spitzer, 2012 (73) | Gel | 4/70 | 2/70 | 2.00 |
| Srinivas-Shankar, 2010 (74) | Gel | 5/138 | 2/136 | 2.46 |
| Hildreth, 2013 (37) | Gel | 3/96 | 10/47 | 0.15 |
| Jones, 2011 (41) | Gel | 1/108 | 2/212 | 0.52 |
| All gel studies | 1.22 | |||
| English, 2000 (27) | Patch | 2/25 | 025 | 5.00 |
| Malkin, 2006 (52) | Patch | 4/37 | 4/39 | 1.05 |
| Merza, 2006 (56) | Patch | 0/20 | 1/19 | 0.32 |
| Snyder, 2001 (72) | Patch | 9/54 | 5/54 | 1.8 |
| Nair, 2006 (58) | Patch | 7/27 | 6/31 | 1.34 |
| All patch studies | 1.46 | |||
| Amory, 2004 (7) | Injection | 1/24 | 0/24 | 3.00 |
| Aversa, 2010 (8) | Injection | 0/40 | 1/10 | 0.09 |
| Caminiti, 2009 (22) | Injection | 2/35 | 1/35 | 2.00 |
| Hackett, 2014 (33) | Injection | 1/97 | 0/102 | 3.15 |
| Hall, 1996 (35) | Injection | 0/17 | 2/18 | 0.21 |
| Ho, 2012 (38) | Injection | 1/60 | 1/60 | 1.00 |
| Hoyos, 2012 (39) | Injection | 1/33 | 0/34 | 3.09 |
| Kalinchenko, 2010 (42) | Injection | 0/113 | 2/71 | 0.13 |
| Kenny, 2004 (46) | Injection | 0/6 | 1/5 | 0.29 |
| Sih, 1997 (67) | Injection | 1/17 | 1/15 | 0.88 |
| Svartberg, 2008 (78) | Injection | 1/19 | 0/19 | 3.00 |
| Svartberg, 2004 (77) | Injection | 0/15 | 1/14 | 0.31 |
| Borst, 2014 (19) | Injection | 0/31 | 1/29 | 0.31 |
| Tan, 2013 (80) | Injection | 2/56 | 2/58 | 1.04 |
| Sheffield-Moore, 2011 (64) | Injection | 0/8 | 0/8 | 1.00 |
| Ferrando, 2002 (30) | Injection | 0/7 | 0/5 | 0.75 |
| All injection studies | 0.66 | |||
| Chapman, 2009 (23) | Oral | 1/11 | 1/12 | 1.09 |
| Emmelot-Vonk, 2008 (26) | Oral | 8/120 | 3/117 | 2.60 |
| Legros, 2009 (50) | Oral | 1/237 | 0/79 | 1.01 |
| Copenhagen, 1986 (6) | Oral | 16/134 | 5/87 | 2.08 |
| All oral studies | 2.20 |
Cardiovascular (CV) risk varies by route of Tadministration. Oral T replacement therapy (TRT) produces significant risk, and gel and patch TRT produce possible risk,whereas im-injected TRT produces possible benefit (18).
Corona et al. (25) have published a new meta-analysis of 75 studies, assessing the CV risks of TRT, using less stringent inclusion criteria than our study and that of Xu et al. Corona et al. found no risk of all CV events (odds ratio = 1.01) or of serious CV events (odds ratio = 1.07).
Possible CV Benefits of T.
It is well described that low serum T is associated with poor CV outcomes, including coronary artery disease, heart failure, and stroke (82). In particular, Shores et al. (66) have reported that low serum T in men is associated with increased incidence of CV disease and increased all-cause mortality. In addition, several studies directly demonstrate CV benefits of TRT. For example, English et al. (27) have shown that, in men with stable angina, low-dose TRT (5 mg/day by patch) for 12 wk caused a significant 17% increase in time to the development of ischemic EKG changes (i.e., 1-mm ST segment depression) during treadmill exercise testing. Stout et al. (76) have shown that TRT in men with chronic heart failure improves exercise performance (increased V̇o2max). Toma et al. (83) published a meta-analysis of four studies showing that TRT improved exercise capacity in heart failure patients. TRT-induced CV benefits may derive from the properties of T as a coronary dilator. Chou et al. (24) reported that, in dogs, injection of T into the coronary circulation caused vasodilation and increased coronary blood flow, and we (17) have reported that T-enanthate improves recovery of aortic flow, cardiac work, and left ventricular developed pressure in an orchiectomized male rat model of global ischemia/reperfusion.
Role of DHT in Responses to T
As previously discussed, 5α-reductase enzyme actively converts T to DHT in a local tissue-specific manner, which locally increases its action. As such, it remains biologically and clinically important to evaluate the role of 5α-reductase in mediating the effects of TRT, especially given that DHT binds to ARs with approximately three times the affinity of T (93) and that DHT may mediate several of the AEs resulting from TRT. 5α-Reductase exists in three isoforms (types I, II, and III), with skeletal muscle expressing types I and III, but not II (93). We have shown that, in older hypogonadal men, finasteride, a specific inhibitor of 5α-reductase type II, did not block T-induced increases in lean mass or muscle strength (19). Similarly, Bhasin et al. (14a) have shown in young men that dutasteride, a dual inhibitor of 5α-reductase types I and II, also does not inhibit T-induced increases in lean mass and 1-RM strength. In addition, both our laboratory (19) and others (7) have shown that DHT is not required for the T-induced increase in BMD or hematocrit (62). Macukat et al. (51) have shown that dutasteride administration does not decrease BMD in older men. Taken together, these studies suggest that conversion of T to DHT is not required for the effects of TRT on muscle, bone, or hematocrit. In contrast, some evidence suggests that DHT may cause the putative risks resulting from TRT. As discussed above, the higher circulating DHT levels, obtained with transdermal as opposed to injected T, may be responsible for the trend for increased CV risk with transdermal TRT. In observational studies, Shores et al. have shown that shown that high DHT is associated with increased cardiovascular events (66) and increased incident ischemic stroke (65). Interestingly, Zwadlo et al. (96) have shown that cardiac expression of 5α-reductase is markedly increased in humans with heart failure and in a mouse aortic constriction model of heart failure. In a mouse model, treatment with finasteride attenuated cardiac hypertrophy and improved left ventricular function. Similarly, Rubio-Gayosso et al. (63) reported that T administration protected against cardiac ischemia/reperfusion (I/R) injury in male rats and that inhibition of 5α-reductase reduced I/R injury in both orchiectomized and intact rats, whereas DHT administration worsened I/R damage. Taken together, this evidence suggests that conversion of T to DHT may underlie CV AEs resulting from TRT.
Combination Therapy of T Plus a 5α-Reductase Inhibitor
The observation that DHT may mediate several AEs resulting from TRT but is not required for musculoskeletal benefits results in the proposed addition of finasteride or dutasteride to improve the safety of TRT. In our study of one year of im TRT in older men, T alone caused a 40% increase in prostate volume, whereas T plus finasteride produced the same musculoskeletal benefits as T alone but with no prostate enlargement (19). Similarly, Amory et al. (7) reported that T plus finasteride produced less prostate enlargement than did T alone and did not inhibit T-induced increases in BMD, although T-induced increases in muscle strength were not observed in that study. In a trial of 18,882 men, Thompson et al. (81) demonstrated that finasteride reduced the incidence of all prostate cancer by 30% while increasing the incidence of high-grade prostate cancer by 17%. However, that study may have underestimated the benefit of finasteride because finasteride shrinks the prostate, making detection of prostate cancer easier. Taken together, these findings indicate that coadministration of finasteride with T may increase its safety without sacrificing benefits.
Summary and Recommendations
For treatment of older hypogonadal men, there are advantages to administering TRT by injection rather than transdermally or orally. First, the musculoskeletal benefits are greater, due to the higher doses administered im vs. transdermally. Second, although the doses are higher, im TRT may not pose the same CV risks that result from transdermal TRT. A possible explanation for the latter phenomenon is that transdermal T causes greater elevation of serum DHT, due to significant expression of 5α-reductase in skin, but not in muscle. Meta-analysis of existing randomized placebo-controlled trials is, to date, insufficient to definitively assess the CV effects of TRT. However, existing data exhibit trends indicting 1) that TRT may not accelerate underlying early-stage prostate cancer, 2) that transdermal TRT may cause CV risk, and 3) that im-injected TRT may cause CV benefit. In addition, several studies demonstrate that in older hypogonadal men the combination of im T plus finasteride produces musculoskeletal benefits without the prostate enlargement that results from T alone (7, 19). Finasteride produces relatively few adverse events and may also produce cardiovascular benefits and/or reduce prostate cancer by reducing DHT. Although further research is needed, it appears at this time that im-injected T plus finasteride may be both the safest and the most effective treatment for older hypogonadal men.
GRANTS
This work was supported by a Department of Veterans Affairs Merit Award to S. E. Borst.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.E.B. and J.F.Y. conception and design of research; S.E.B. prepared figures; S.E.B. and J.F.Y. drafted manuscript; S.E.B. and J.F.Y. edited and revised manuscript; J.F.Y. approved final version of manuscript.
REFERENCES
- 1.Anonymous. Department of Veterans Affairs VHA Pharmacy Benefits Management Advisory panel: Testosterone Products and Cardiovascular Safety. http://pmb.va.gov/PMB/vacenterformmedicationsfety/nationalpmbbulletin/Testosterone_Products_and_Caridiovascular_Safety_NATIONAL_PBM_BULLETIN_02.pdf.
- 2.Anonymous. Endocrine Society Statement on The Risk of Cardiovascular Events in Men Receiving Testosterone Therapy. http://endocrine.org/membership/email-newsletters/endocrine-insider/2014/february-20-2014/society-statement-risk-of-cardiovascular-events-in-men-receiving-testosterone-therapy-available.
- 3.Anonymous. FDA cautions about using testosterone for low testosterone due to aging; requires labeling change to inform of possible increased risk or heart attack or stroke with use. FDA Drug Safety Communication, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Testosterone Products: Drug Safety Communication—FDA Investigating Risk of Cardiovascular Events. http://www.fda.gov/safety/medwatch/safetyinfomation/safetyalertsforhumanmedicalproducts/ucm384225.htm.
- 5.Anonymous. Testosterone Therapy in Adult Men with Androgen Deficiency Syndromes: an Endocrine Society Clinical Practice Guideline. http://www.endocrine.org/∼/media/endosociety/Files/Publications/clinical%20Practice%20Guidelines/FINAL-Androgens-in-Men-Standalone.pdf. [DOI] [PubMed]
- 6.Anonymous. Testosterone treatment of men with alcoholic cirrhosis: a double-blind study. The Copenhagen Study Group for Liver Diseases. Hepatology : 807–813, 1986. [PubMed] [Google Scholar]
- 7.Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab : 503–510, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, Lenzi A, Spera G. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med : 3495–3503, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med : 1465–1466, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med : 109–122, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med : 32–39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck DT, Yarrow JF, Beggs LA, Otzel DM, Ye F, Conover CF, Miller JR, Balaez A, Combs SM, Leeper AM, Williams AA, Lachacz SA, Zheng N, Wronski TJ, Borst SE. Influence of aromatase inhibition on the bone-protective effects of testosterone. J Bone Miner Res : 2405–2413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM, and Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab : 2536–2559, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab : 678–688, 2005. [DOI] [PubMed] [Google Scholar]
- 14a.Bhasin S, Travison TG, Storer TW, Lakshman K, Kaushik M, Mazer NA, Ngyuen AH, Davda MN, Jara H, Aakil A, Anderson S, Knapp PE, Hanka S, Mohammed N, Daou P, Miciek R, Ulloor J, Zhang A, Brooks B, Orwoll K, Hede-Brierley L, Eder R, Elmi A, Bhasin G, Collins L, Singh R, Basaria S. Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA : 931–939, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliuc D, Nguyen ND, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporosis Int 2015. [DOI] [PubMed] [Google Scholar]
- 16.Borst SE, Conover CF, Carter CS, Gregory CM, Marzetti E, Leeuwenburgh C, Vandenborne K, Wronski TJ. Anabolic effects of testosterone are preserved during inhibition of 5α-reductase. Am J Physiol Endocrinol Metab : E507–E514, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Borst SE, Quindry JC, Yarrow JF, Conover CF, Powers SK. Testosterone administration induces protection against global myocardial ischemia. Horm Metab Res : 122–129, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Borst SE, Shuster JJ, Zou B, Ye F, Jia H, Wokhlu A, Yarrow JF. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med : 211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA, Braith RW, Beck DT, Martin JS, Morrow M, Roessner S, Beggs LA, McCoy SC, Cannady DF 2nd, Shuster JJ. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab : E433–E442, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab : 5649–5657, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci : 1451–1457, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, Mammi C, Piepoli M, Fini M, Rosano GM. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol : 919–927, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Chapman IM, Visvanathan R, Hammond AJ, Morley JE, Field JB, Tai K, Belobrajdic DP, Chen RY, Horowitz M. Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. Am J Clin Nutr : 880–889, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation : 2614–2619, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, Maggi M. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Exp Opin Drug Safety : 1327–1351, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA : 39–52, 2008. [DOI] [PubMed] [Google Scholar]
- 27.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation : 1906–1911, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest : 1553–1560, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab : 2560–2575, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab : E601–E607, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLos One : e85805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glintborg D, Christensen LL, Kvorning T, Larsen R, Brixen K, Hougaard DM, Richelsen B, Bruun JM, Andersen M. Strength training and testosterone treatment have opposing effects on migration inhibitor factor levels in ageing men. Med Inf (Lond) : 539156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P, Saghir A, and Blast Study Group. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract : 203–215, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Bolona ER, Sideras K, Uraga MV, Erwin PJ, Montori VM. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc : 29–39, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Hall GM, Larbre JP, Spector TD, Perry LA,. and Da Silva JA. A randomized trial of testosterone therapy in males with rheumatoid arthritis. Br J Rheumatol : 568–573, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust : 548–551, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, Nakamura T, Wolfe P, Kohrt WM, Ruscin JM, Kittelson J, Cress ME, Ballard R, Schwartz RS. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab : 1891–1900, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho CC, Tong SF, Low WY, Ng CJ, Khoo EM, Lee VK, Zainuddin ZM, Tan HM. A randomized, double-blind, placebo-controlled trial on the effect of long-acting testosterone treatment as assessed by the Aging Male Symptoms scale. BJU Int : 260–265, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Hoyos CM, Killick R, Yee BJ, Grunstein RR, Liu PY. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol : 599–607, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol : 168–171, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, Morales AM, Volterrani M, Yellowlees A, Howell JD, Channer KS, Investigators T. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care : 828–837, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol : 602–612, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan AL, Hu JC. Use of testosterone replacement therapy in the United States and its effect on subsequent prostate cancer outcomes. Urology : 321–326, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman JM, Miller MG, Garwin JL, Fitzpatrick S, McWhirter C, Brennan JJ. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med : 2079–2089, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev : 833–876, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci : 75–78, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, McGee D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc : 1134–1143, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci : M266–M272, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Laurent M, Gielen E, Claessens F, Boonen S, Vanderschueren D. Osteoporosis in older men: recent advances in pathophysiology and treatment. Best Pract Res Clin Endocrinol Metab : 527–539, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Legros JJ, Meuleman EJ, Elbers JM, Geurts TB, Kaspers MJ, Bouloux PM, Study I. Oral testosterone replacement in symptomatic late-onset hypogonadism: effects on rating scales and general safety in a randomized, placebo-controlled study. Eur J Endocrinol : 821–831, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Macukat IR, Spanjol J, Orlic ZC, Butorac MZ, Marinovic M, Cupic DF. The effect of 5alpha-reductase inhibition with finasteride and dutasteride on bone mineral density in older men with benign prostatic hyperplasia. Coll Anthropol : 835–839, 2014. [PubMed] [Google Scholar]
- 52.Malkin CJ, Pugh PJ, West JN, van Beek E, Jones TH, Channer KS. Testosterone therapy in men with heart failure: A double blind placebo controlled trial. Heart : A20–A21, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Marin P, Holmang S, Gustafsson C, Jonsson L, Kvist H, Elander A, Eldh J, Sjostrom L, Holm G, Bjorntorp P. Androgen treatment of abdominally obese men. Obes Res : 245–251, 1993. [DOI] [PubMed] [Google Scholar]
- 54.McCoy SC, Yarrow JF, Conover CF, Borsa PA, Tillman MD, Conrad BP, Pingel JE, Wronski TJ, Johnson SE, Kristinsson HG, Ye F, Borst SE. 17β-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone : 667–673, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, Meikle AW, Center JR, Eisman JA, Seibel MJ. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med : 47–54, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Merza Z, Blumsohn A, Mah PM, Meads DM, McKenna SP, Wylie K, Eastell R, Wu F, Ross RJ. Double-blind placebo-controlled study of testosterone patch therapy on bone turnover in men with borderline hypogonadism. Int J Androl : 381–391, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Morris PD, Malkin CJ, Channer KS, Jones TH. A mathematical comparison of techniques to predict biologically available testosterone in a cohort of 1072 men. Eur J Endocrinol : 241–249, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med : 1647–1659, 2006. [DOI] [PubMed] [Google Scholar]
- 59.O'Connell MD, Wu FC. Androgen effects on skeletal muscle: implications for the development and management of frailty. Asian J Androl : 203–212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc : 1666–1673, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Page ST, Amory JK, Anawalt BD, Irwig MS, Brockenbrough AT, Matsumoto AM, Bremner WJ. Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab : 4374–4380, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab : 1502–1510, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Rubio-Gayosso I, Ramirez-Sanchez I, Ita-Islas I, Ortiz-Vilchis P, Gutierrez-Salmean G, Meaney A, Palma I, Olivares I, Garcia R, Meaney E, Ceballos G. Testosterone metabolites mediate its effects on myocardial damage induced by ischemia/reperfusion in male Wistar rats. Steroids : 362–369, 2013. [DOI] [PubMed] [Google Scholar]
- 64.Sheffield-Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon-Jones D, Durham WJ, Grady JJ, Urban RJ. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab : E1831–1837, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shores MM, Arnold AM, Biggs ML, Longstreth WT Jr, Smith NL, Kizer JR, Cappola AR, Hirsch CH, Marck BT, Matsumoto AM. Testosterone and dihydrotestosterone and incident ischaemic stroke in men in the Cardiovascular Health Study. Clin Endocrinol : 746–753, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shores MM, Biggs ML, Arnold AM, Smith NL, Longstreth WT Jr, Kizer JR, Hirsch CH, Cappola AR, Matsumoto AM. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab : 2061–2068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sih R, Morley JE, Kaiser FE, Perry HM 3rd Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab : 1661–1667, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology : 5081–5088, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology : 1259–1268, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab : E154–E164, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab : 3024–3033, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Snyder PJ, Peachey H, Berlin JA, Rader D, Usher D, Loh L, Hannoush P, Dlewati A, Holmes JH, Santanna J, Strom BL. Effect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of age. Am J Med : 255–260, 2001. [DOI] [PubMed] [Google Scholar]
- 73.Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, Mazer NA, Knapp PE, Hanka S, Lakshman KM, Ulloor J, Zhang A, Orwoll K, Eder R, Collins L, Mohammed N, Rosen RC, DeRogatis L, Bhasin S. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med : 681–691, 2012. [DOI] [PubMed] [Google Scholar]
- 74.Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab : 639–650, 2010. [DOI] [PubMed] [Google Scholar]
- 75.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab : 1478–1485, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Stout M, Tew GA, Doll H, Zwierska I, Woodroofe N, Channer KS, Saxton JM. Testosterone therapy during exercise rehabilitation in male patients with chronic heart failure who have low testosterone status: a double-blind randomized controlled feasibility study. Am Heart J : 893–901, 2012. [DOI] [PubMed] [Google Scholar]
- 77.Svartberg J, Aasebo U, Hjalmarsen A, Sundsfjord J, Jorde R. Testosterone treatment improves body composition and sexual function in men with COPD, in a 6-month randomized controlled trial. Respir Med : 906–913, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res : 378–387, 2008. [DOI] [PubMed] [Google Scholar]
- 79.Tan RS, Culberson JW. An integrative review on current evidence of testosterone replacement therapy for the andropause. Maturitas : 15–27, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Tan WS, Low WY, Ng CJ, Tan WK, Tong SF, Ho C, Khoo EM, Lee G, Lee BC, Lee V, Tan HM. Efficacy and safety of long-acting intramuscular testosterone undecanoate in aging men: a randomised controlled study. BJU Int : 1130–1140, 2013. [DOI] [PubMed] [Google Scholar]
- 81.Thompson IM Jr, Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, Lucia MS, Ford LG. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med : 603–610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tirabassi G, Biagioli A, Balercia G. Bone benefits of testosterone replacement therapy in male hypogonadism. Panminerva Medica : 151–163, 2014. [PubMed] [Google Scholar]
- 83.Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, Armstrong PW, Ezekowitz JA. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail : 315–321, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Tracz MJ, Sideras K, Bolona ER, Haddad RM, Kennedy CC, Uraga MV, Caples SM, Erwin PJ, Montori VM. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab : 2011–2016, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab : 1821–1826, 1996. [DOI] [PubMed] [Google Scholar]
- 86.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab : 3666–3672, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA : 1829–1836, 2013. [DOI] [PubMed] [Google Scholar]
- 88.Villanueva MG, He J, Schroeder ET. Periodized resistance training with and without supplementation improve body composition and performance in older men. Eur J Appl Physiol : 891–905, 2014. [DOI] [PubMed] [Google Scholar]
- 89.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cellular Endocrinol : 174–186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Y, Bauman WA, Blitzer RD, Cardozo C. Testosterone-induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun : 679–683, 2010. [DOI] [PubMed] [Google Scholar]
- 91.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med : 108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, Borst SE. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab : E1213–E1222, 2008. [DOI] [PubMed] [Google Scholar]
- 93.Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med Sci Sports Exerc : 818–826, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yarrow JF, Wronski TJ, Borst SE. Testosterone and adult male bone: are actions of 5-alpha reductase and aromatase required? Exerc Sport Sci Rev 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou B, Jin B, Koch GG, Zhou H, Borst SE, Menon S, Shuster JJ. On model selections for repeated measurement data in clinical studies. Stat Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zwadlo C, Schmidtmann E, Szaroszyk M, Kattih B, Froese N, Hinz H, Schmitto JD, Widder J, Batkai S, Bahre H, Kaever V, Thum T, Bauersachs J, Heineke J. Anti-androgenic therapy with finasteride attenuates cardiac hypertrophy and left ventricular dysfunction. Circulation 2015. [DOI] [PubMed] [Google Scholar]