Abstract
Progressive fibrosis is a complication of many chronic diseases, and collectively, organ fibrosis is the leading cause of death in the United States. Fibrosis is characterized by accumulation of activated fibroblasts and excessive deposition of extracellular matrix proteins, especially type I collagen. Extensive research has supported a role for matrix signaling in propagating fibrosis, but type I collagen itself is often considered an end product of fibrosis rather than an important regulator of continued collagen deposition. Type I collagen can activate several cell surface receptors, including α2β1 integrin and discoidin domain receptor 2 (DDR2). We have previously shown that mice deficient in type I collagen have reduced activation of DDR2 and reduced accumulation of activated myofibroblasts. In the present study, we found that DDR2-null mice are protected from fibrosis. Surprisingly, DDR2-null fibroblasts have a normal and possibly exaggerated activation response to transforming growth factor-β and do not have diminished proliferation compared with wild-type fibroblasts. DDR2-null fibroblasts are significantly more prone to apoptosis, in vitro and in vivo, than wild-type fibroblasts, supporting a paradigm in which fibroblast resistance to apoptosis is critical for progression of fibrosis. We have identified a novel molecular mechanism by which DDR2 can promote the activation of a PDK1 (3-phosphoinositide dependent protein kinase-1)/Akt survival pathway, and we have found that inhibition of PDK1 can augment fibroblast apoptosis. Furthermore, our studies demonstrate that DDR2 expression is heavily skewed to mesenchymal cells compared with epithelial cells and that idiopathic pulmonary fibrosis cells and tissue demonstrate increased activation of DDR2 and PDK1. Collectively, these findings identify a promising target for fibrosis therapy.
Keywords: fibrosis, collagen, apoptosis, discoidin domain receptor 2
Progressive fibrosis is a devastating consequence of a wide variety insults, affecting nearly every organ system and collectively accounting for over 45% of deaths in the developed world (1, 2). Fibrosis in the lung can result from an acute injury, chronic inflammation, or primary diseases such as idiopathic pulmonary fibrosis (IPF). Regardless of the cause, fibrosis can lead to a devastating clinical course owing to a lack of good therapeutic treatment (3–5).
Pulmonary fibrosis is characterized by accumulation of activated fibroblasts and deposition of fibrotic extracellular matrix (ECM) proteins (3, 4). Progressive fibrosis is believed to be the result of a dysregulated repair mechanism in response to injury or some other inciting event, which leads to excessive and continued deposition of fibrotic matrix proteins (1). Activated fibroblasts are believed to accumulate in the fibrotic tissue through increased proliferation, differentiation into the activated phenotype, and resistance to apoptosis. In addition to direct deposition of ECM proteins, especially type I collagen, activated fibroblasts secrete a number of profibrotic signaling ligands that lead to paracrine activation of neighboring cells (6).
Although primarily regarded as the scaffolding protein of fibrotic matrix, type I collagen can initiate cell signaling through activation of a number of cell surface receptors with significant influence on cell behavior (7–9). Type I collagen signaling has been shown to be upregulated in cancer progression, and inhibition of collagen I synthesis can lead to decreased tumor invasion. In the lung, type I collagen has been shown to be upregulated quickly after acute injury, both in human patients and in animal models, implicating a role for collagen I signaling as a regulator of progressive fibrosis rather than serving solely as the end product of fibrosis. We have previously shown that selective deletion of collagen I from alveolar epithelial cells surprisingly led to robust protection from fibrosis (10). We found that early production of collagen activated collagen receptors in vivo, which promoted further fibroblast activation, potentially establishing an important positive feedback loop in progressive fibrosis (10, 11). Thus, type I collagen is both an important physical component of the fibrotic matrix and a potential profibrotic signaling molecule.
Collagens can signal through several different families of cell surface receptors, including several integrins, especially α2β1 integrin, and the discoidin domain receptors (DDRs) (7, 8, 12). The DDRs are receptor tyrosine kinases that are activated upon binding to collagens. DDR1 primarily binds type IV collagen and has already been implicated in mediating the fibrotic response (13). Fibrosis is characterized by increased production of fibrillar collagens, especially type I collagen, but a role for type I collagen signaling during progressive fibrosis has not been well characterized. DDR2 is the primary receptor for fibrillar collagens, including type I and type III collagen (7, 8, 12). There is increased recognition of an important role of DDR2 in a variety of malignancies, leading to interest in generating DDR2-specific inhibitors (14). The role of DDR2 in fibrosis is mixed. Mice deficient in DDR2 have an increased propensity for liver fibrosis (15), but knockdown of DDR2 reduced angiotensin-induced cardiac fibrosis (16). Authors of a recent report found attenuated lung fibrosis in mice deficient in DDR2; however, the molecular mechanisms involved in DDR2 regulation of fibrosis remain poorly explored (17). Most of the research has focused on DDR2-mediated fibroblast activation. Notably, nintedanib, one of the approved therapies for IPF, is a nonspecific tyrosine kinase inhibitor with known activity against DDR2 (18). Several other tyrosine kinase inhibitors that are known to block DDR2 have also been shown to attenuate experimental fibrosis (19).
In the present study, we explored the mechanisms by which collagen signaling through DDR2 regulates fibroblast behavior during lung fibrosis. We found that DDR2 is a major regulator of fibroblast apoptosis through activation of a 3-phosphoinositide dependent protein kinase-1 (PDK1)/Akt pathway. In addition, we found that DDR2 expression is skewed to a mesenchymal population and that IPF fibroblasts demonstrate upregulation of this pathway, offering a potentially attractive target for fibrosis therapy (20, 21).
Methods
Reagents
Phosphorylated DDR2 (phospho-DDR2; Y740) antibody and α2β1 integrin inhibitor TC-I15 were purchased from R&D Systems. Phospho-tyrosine 9-PDK1, DDR2, and integrin-α2 antibodies were obtained from Abcam. Collagen, type I, alpha 1 (Col1a1) antibody was purchased from Thermo Fisher Scientific. Hydroxyproline reagent and cycloheximide (CHX), as well as α-smooth muscle actin (ACTA2), SMA-FITC, β-actin, and FLAG M2 antibodies, were obtained from Sigma-Aldrich. AKT, phospho-AKT (Ser473), phospho-AKT (Thr308), extracellular signal-regulated kinase 1/2 (ERK1/2), phospho-ERK1/2 (T202/Y204), Src, phospho-Src, XIAP (X-linked inhibitor of apoptosis protein), GAPDH, phospho-SMAD3, phospho-Tyr, and phospho-Tyr-horseradish peroxidase antibodies were purchased from Cell Signaling Technology. SMAD2/3 primary antibody and horseradish peroxidase–conjugated secondary antibodies were obtained from Santa Cruz Biotechnology. Phospho-tyrosine 376-PDK1 antibody was purchased from Signalway Antibody. Phospho-SMAD2 antibody, phosphatase inhibitor cocktail, and bleomycin were purchased from MilliporeSigma. [3H]thymidine was purchased from GE Healthcare PerkinElmer. Collagen I and antimouse CD95 were obtained from BD Biosciences. Protein A– and protein G–conjugated agarose beads and the In Situ Cell Death Detection Kit (TMR red) were obtained from Roche. Caspase-Glo 3/7 reagent was purchased from Promega. IncuCyte Caspase-3/7 Apoptosis Assay Reagent was obtained from Essen BioScience. PDK1 inhibitors BX-795 and BX-912 were purchased from Selleckchem. Phosphatase inhibitor cocktail and bleomycin were purchased from MilliporeSigma. FLAG-tagged PDK1 cloned into a retroviral expression vector was purchased from Addgene.
Mice
DDR2-null mice (slie/slie) were previously described (22) and were purchased from The Jackson Laboratory. Floxed Col1a1 mice were previously described (10). Six- to eight-week-old wild-type (WT) and DDR2-null mice were injured with 40 μl of 2 U/kg of bleomycin dissolved in saline versus saline alone by oropharyngeal aspiration as previously described (23). Mice were killed at the specified time points after treatment in each respective experiment and analyzed as described. All mice were maintained in a specific pathogen–free environment until the time of mice were killed. All animal experiments were approved by the animal care and use committee at the University of Michigan.
BAL and Lung Myeloid Cell Isolation
For BAL, mice were killed and lavaged with 1 ml of PBS. Total cells were quantified by counting with a hemocytometer. For cell differential calculation, cells were fixed, cytofuged onto glass slides, stained with hematoxylin and eosin, and visualized by light microscopy. Lung myeloid cells were isolated as previously described (24, 25). Briefly, lungs were removed from killed mice, minced, and digested with collagenase and dispase. Erythrocytes were lysed, and the myeloid cells were isolated by filtration and centrifugation in Percoll.
Hydroxyproline Assay
Whole-lung hydroxyproline was measured as previously described (26, 27). Mice were killed at the specified time points in each experiment. Whole lung was harvested, homogenized in water, and baked in 12N hydrochloric acid at 120°C overnight. Samples were then neutralized with citrate buffer and incubated in chloramine-T solution at room temperature. Ehrlich’s solution was added before incubation at 65°C. The absorbance at 540 nm was measured, and the hydroxyproline concentration was quantified against a standard.
Histology
Lung section trichrome staining was previously described (10). Briefly, whole lungs harvested from killed mice were fixed by inflation with formaldehyde to a pressure of 25 cm H2O. Lungs were then embedded in paraffin. Sections were stained with Masson’s trichrome by the McClinchey Histology Lab.
Immunofluorescence Staining
Lungs were perfused with PBS, inflated and embedded in optimal cutting temperature compound, and frozen in a dry ice and alcohol bath. Ten-micrometer lung sections were then stained as previously described (10). Briefly, lung sections were fixed and permeabilized with methanol and blocked with 5% goat serum and 1% BSA in PBS. The slides were stained with primary antibody overnight at 4°C. After washing, slides were stained with appropriate secondary antibody and for TUNEL by using the In Situ Cell Death Detection Kit (TMR red). Slides were mounted in Molecular Probes ProLong Gold mountant containing DAPI (Thermo Fisher Scientific). Lung sections were visualized on an Olympus BX-51 fluorescence microscope, and images were captured with an Olympus DP-70 camera and analyzed with DP controller software version 3.1.1.267 (Olympus). Quantification of costained cells was completed in a blinded fashion.
Mouse and Human Lung Fibroblast Isolation and Culture
Primary murine lung fibroblasts were isolated and cultured as previously described (10). Ten- to 24-week-old mice were killed, and whole lungs were removed after perfusion and lavage with PBS. Whole-lung samples were finely minced and incubated in Dulbecco’s modified Eagle medium with 10% FBS, penicillin, and streptomycin until fibroblasts adhered to the plate. Human lung fibroblasts were isolated from IPF and normal patients as previously described (28) and cultured in Dulbecco’s modified Eagle medium with 10% FBS, penicillin, streptomycin, and amphotericin B. All cells were cultured in a 37°C incubator supplemented with 5% CO2.
For cell expression experiments, equal numbers of cells were seeded on tissue culture plates in serum-containing media and allowed to adhere overnight. Cells were rinsed and serum starved for 24 hours. Cells were then treated with transforming growth factor (TGF)-β (4 ng/ml) or vehicle control for the time points indicated. Some cells were treated with TC-I15 (2.5 μM) versus vehicle control for 1 hour, followed by treatment with TGF-β.
Retroviral and Lentiviral Infection of Cells
Murine DDR2 was cloned into a retroviral expression vector (pWZL-blast) by PCR and standard cloning techniques. Retroviral supernatants were generated using Phoenix-E cells as previously described (29). Briefly, 1 day after seeding, primary WT or DDR2-null murine lung fibroblasts were treated with retroviral supernatants to overexpress DDR2 or FLAG-PDK1 (vs. vehicle control) as indicated. After 2 days, the media were replaced, and cells were treated and analyzed as indicated. For DDR2 shRNA, lentivirus was generated by the University of Michigan Vector Core using RNA interference vectors purchased from Open BioSystems. One day after seeding, primary WT murine fibroblasts were treated with DDR2 shRNA lentivirus (5 plaque-forming units per cell) or scrambled control lentivirus. After 2 days, the media were replaced, and cells were treated and analyzed as described previously (30).
IB and Immunoprecipitation
IB and immunoprecipitation of samples were performed as previously described (10). Immunoblots are representative of a minimum of three separate experiments.
Cell Proliferation Assays
Cellular proliferation was measured by the [3H]thymidine incorporation method as previously described (30). Briefly, equal numbers of stimulated cells were incubated with the addition of 5 μl of 0.1 mCi/ml [3H]thymidine per well in a 96-well plate overnight. Sixteen hours after addition of [3H]thymidine, cells were harvested for incorporated [3H]thymidine using scintillation fluid in a β-scintillation counter.
Cell Death Assays
Equal numbers of WT and DDR2-null cells were seeded on 24-well plates in serum-containing media. The next day, the cells were washed and covered with serum-free media. After an additional 24 hours, cells were treated with vehicle control or Fas-activating antibody (0.5 μg/ml) and CHX (5 μg/ml) to induce apoptosis. Some cells were pretreated for 1 hour with PDK1 inhibitor BX-795 (1 nM) or BX-912 (2 nM) before addition of Fas-activating antibody or CHX. After the time points indicated, cell death was assessed by caspase 3/7 activity using the Caspase-Glo 3/7 Assay (Promega) per the product protocol and as previously described (23). Briefly, treated cells were washed with PBS and lysed in radioimmunoprecipitation assay buffer. Active caspase 3/7 activity was captured using a Veritas Microplate Luminometer (Promega), normalized to protein concentration per sample, and expressed as fold differences in relative luminescence. Cell death by caspase 3/7 activity was also captured using the IncuCyte Live Cell Analysis System by incubating treated cells with IncuCyte Caspase-3/7 Apoptosis Assay Reagent. At least four images were captured per well in a 96-well plate. Discrete fluorescent apoptotic cells were counted using the IncuCyte basic analyzer and expressed as fold differences in relative numbers over time.
Gene Expression Analysis
qRT-PCR was performed as previously described (10). Briefly, RNA was isolated from lung tissue and cells with TRIzol reagent (Life Technologies) per the manufacturer’s protocol. Reverse transcription was performed with the SuperScript III First-Strand Synthesis Kit (Life Technologies), and RT-PCR was performed using the Power SYBR Green PCR Master Mix Kit (Life Technologies) and the ABI PRISM 7000 Sequence Detection System (Life Technologies). Relative expression of genes was normalized to β-actin and GAPDH as previously described (30) using the following primers: α-actin forward: 5′-CCGTGAAAAGATGACCCAGATC-3′, β-actin reverse: 5′-CACAGCCTGGATGGCTACGT-3′; GAPDH forward: 5′-AACTTTGGCATTGTGGAAGG-3′, GAPDH reverse: 5′-ACACATTGGGGGTAGGAACA-3′; COL1A1 forward: 5′-GCCAAGAAGACAAACTTT-3′, COL1A1 reverse: 5′-GGCCTTGGAAACCTTGTGGAC-3′; COL1A2 forward: 5′-GGAGGGAACGGTCCACGAT-3′, COL1A2 reverse: 5′-GAGTCCGCGTATCCACAA-3′; COL3A1 forward: 5′-CAAGGTCTTCCTGGTCAGCCT-3′, COL3A1 reverse: 5′-TGCCACGAGATCCATCTC-3′; ACTA2 forward: 5′-AGAGACTCTCTTCCAGCCATC-3′, ACTA2 reverse: 5′-GACGTTGTTAGCATAGAGATC-3′; TGFβ1 forward: 5′-ATCCTGTCCAAACTAAGGCTCG-3′, TGFβ1 reverse: 5′-ACCTCTTTAGCATAGTAGTCCGC-3′; IL1β forward: 5′-TGTTCTTTGAAGTTGACGGAC-3′, IL1β reverse: 5′-GATACTGCCTGCCTGAAGCTC-3′; CCL2 forward: 5-TTCTGGGCCTGCTGTTCACAG-3′, CCL2 reverse: 5′-CTACTCATTGGGATCATCTTGC-3′; CCL7 forward: 5′- TGCTTTCAGCATCCAAGTGTG-3′, CCL7 reverse: 5′-GGACACCGACTACTGGTGATC-3′; CCL12 forward: 5′-TAGCTACCACCATCAGTCCTC-3′, CCL12 reverse; 5′-GGGACACTGGCTGCTTGTGAT3′; TNFα forward: 5′-CCAAAGGGATGAG AAGTTCCC-3′, TNFα reverse: 5′-GCTCCTCCACTTGGTGGTTTG-3′.
Statistical Analysis
Data are expressed as means, and error bars indicate the SEM. For evaluation of group differences, the two-tailed Student’s t test was used. A P value less than 0.05 was accepted as significant.
Results
Mice Deficient in DDR2 Are Protected from Fibrosis
We have previously shown that mice deficient in type I collagen expression have decreased fibrosis and decreased numbers of active fibroblasts, suggesting an important role for collagen signaling in propagating progressive fibrosis (10). We first examined the relative expression of two of the best-described collagen I receptors, α2β1 and DDR2, in alveolar epithelial cells and fibroblasts (Figure 1A). DDR2 was expressed almost exclusively on fibroblasts as compared with alveolar epithelial cells, consistent with prior reports (7, 8, 18). Integrin α2β1 was expressed in both cell types, but in higher amounts in fibroblasts. We have previously shown increased activation and total concentrations of DDR2 in mouse lungs after bleomycin-induced injury (10). We therefore focused on DDR2 as a potential mediator of collagen-induced fibroblast activation. To determine if DDR2 regulated the fibrotic response, DDR2-null and WT mice were injured with bleomycin. After 21 days, DDR2 mice had no increase in fibrosis as determined in trichrome-stained lung sections or by hydroxyproline assay, indicating that collagen signaling through the DDR2 receptor regulates subsequent collagen deposition and fibrosis (Figure 1; see also Figure E1 in the data supplement). Notably, DDR2 is not highly expressed on myeloid cells, and the absence of DDR2 did not affect myeloid cell recruitment or expression of several inflammatory and profibrotic cytokines and chemokines (Figure E2).
Figure 1.
Discoidin domain receptor 2 (DDR2) regulates lung fibrosis. (A) Amounts of collagen type I receptors DDR2 and α2 integrin in primary murine alveolar epithelial cells (AECs) and primary murine lung fibroblasts were determined by IB. (B) Hydroxyproline assay of total lungs 21 days after intratracheal saline or bleomycin in DDR2-null or littermate control mice. DDR2-null mice have less fibrosis after bleomycin than control mice treated with bleomycin (n = 6–10 per group). *P < 0.05. (C and D) Trichrome stain of wild-type (WT) (C) and DDR2-null (D) lung sections (20 × ) 21 days after injury with intratracheal bleomycin. DDR2-null mice are protected from bleomycin-induced fibrosis compared with control. Scale bars: 200 μm.
DDR2 Does Not Regulates Fibroblast Activation or Proliferation
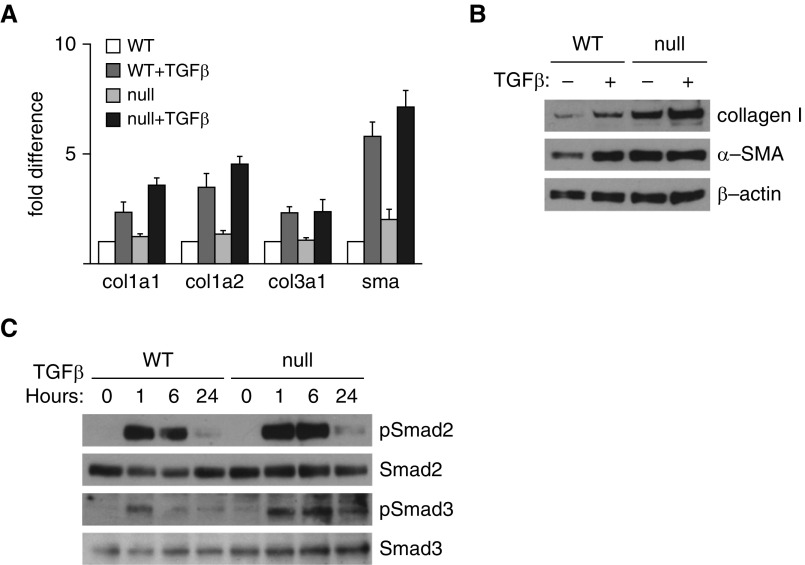
We have previously shown that fibroblasts with deletion of Col1a1 have reduced expression of fibroblast activation markers, including Col1a2, Col3a1, and ACTA2 (10). DDR2 is highly expressed on fibroblasts that accumulate during fibrosis and are believed to be the primary fibrotic effector cells. To determine if DDR2 regulates fibroblast activation, WT and DDR2-null primary lung fibroblasts were isolated and treated with TGF-β. After 24 hours, cells were analyzed by qPCR and IB for several well-described markers of fibroblast activation, including type I collagen and ACTA2. Contrary to our expectations, DDR2-null fibroblasts did not have decreased concentrations of fibroblast activation markers and indeed showed a trend toward increased concentrations of ACTA2 and collagen I (Figures 2A and 2B). We also found no differences in TGF-β–induced activation of Smad2 and Smad3 (Figure 2C).
Figure 2.
DDR2 does not regulate lung fibroblast activation. (A) Real-time qPCR of primary lung fibroblasts from control (WT) and DDR2-null (null) mice with or without treatment with transforming growth factor (TGF)-β. Loss of DDR2 does not significantly alter expression of collagen, type I, alpha 1 (Col1a1), Col1a2, Col3a1, and ACTA2 (sma) (n = 4). (B) IB for collagen I and ACTA2 (α-SMA) of primary lung fibroblasts from WT and null mice. Presence of DDR2 did not influence fibroblast production of collagen I and α-SMA. (C) IB of primary lung fibroblasts from WT and null mice after treatments with TGF-β at 0, 1, 6, and 24 hours. Phospho-Smad2 (pSmad2), Smad2, phospho-Smad3 (pSmad3), and Smad3 are shown. The presence of DDR2 does not influence Smad phosphorylation.
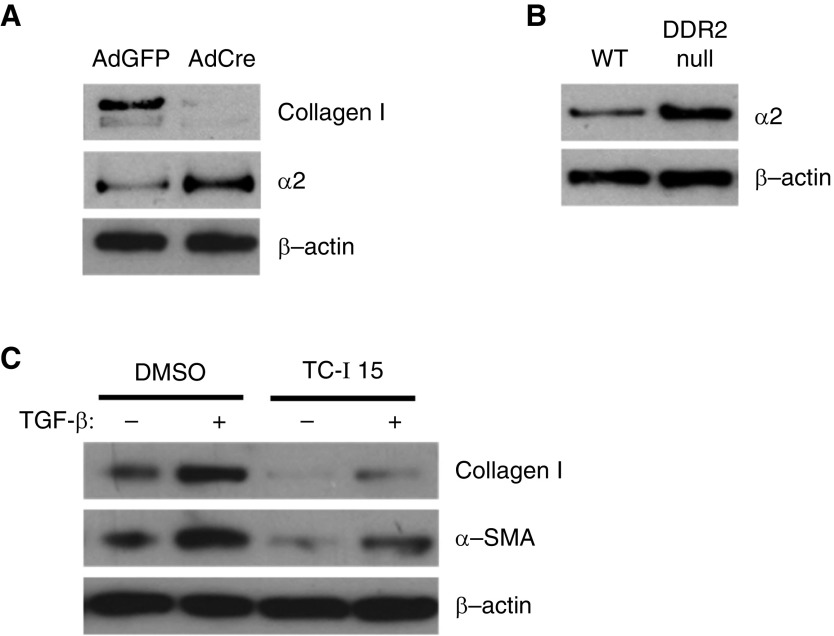
To determine if collagen I/DDR2 signaling regulates expression of the other major fibroblast fibrillar collagen receptor, fibroblasts from floxed type I collagen mice treated with adenovirus expressing Cre recombinase or control adenovirus were analyzed for expression of α2 integrin. Indeed, fibroblasts deficient in Col1a1 had markedly increased expression of α2 integrin (Figure 3A). Similarly, DDR2-null fibroblasts had increased expression of α2 integrin, suggesting that collagen I/DDR2 signaling negatively regulates expression of α2 integrin and that higher expression of α2 integrin might regulate the increased expression of profibrotic markers (Figure 3B). WT fibroblasts were treated with TGF-β1 with or without pretreatment with an α2 integrin–specific inhibitor, TC-I15 (2.5 μM). Indeed, fibroblasts treated with TC-I15 had reduced expression of type I collagen and ACTA2 (Figure 3C). These results demonstrate one aspect by which type I collagen signaling regulates accumulation of activated fibroblasts, but it does not clarify the mechanism by which DDR2-null mice are protected from fibrosis.
Figure 3.
Collagen I/DDR2 signaling regulates expression of α2 integrin. (A) Lung fibroblasts from floxed Col1a1 were treated with an adenovirus expressing Cre recombinase (AdCre) or a control adenovirus expressing GFP (AdGFP). After 1 week, cells were analyzed from expression of collagen I and α2 integrin by IB. (B) Primary lung fibroblasts from WT and DDR2-null mice were analyzed from expression of α2 integrin by IB. (C) WT primary lung fibroblasts were treated with or without TGF-β and an α2 integrin inhibitor, TC-I15, and analyzed for expression of collagen I and ACTA2 (α-SMA) by IB.
Fibroblast proliferation has been implicated in the progression of fibrosis. We cultured WT and DDR2-null fibroblasts in serum-free or 10% serum-containing conditions and measured proliferation with the incorporation of [3H]thymidine. Again, contrary to our expectations, the presence of DDR2 did not influence fibroblast proliferation (Figure 4A).
Figure 4.
DDR2 regulates fibroblast apoptosis but not proliferation. (A) Cellular proliferation assessed by [3H]thymidine incorporation for 16 hours in WT and DDR2-null primary lung fibroblasts under serum-free or 10% serum-containing media measured by counts per minute (CPM) in a scintillation counter. DDR2 does not regulate lung fibroblast proliferation (n = 4). (B) Activation of caspase 3/7 using a fluorescent reagent and the IncuCyte System was trended over time in WT or DDR2-null primary lung fibroblasts cultured with and without a Fas-activating antibody and cycloheximide (Fas/CHX). Values are expressed as numbers of fluorescent cells per image field (n = 4). (C) Activation of caspase 3/7 in WT or DDR2-null primary lung fibroblasts upon induction of apoptosis with Fas/CHX was measured by using the Caspase-Glo luciferase-based assay 24 hours after treatment with Fas/CHX. Values are expressed as fold differences in relative luminescence units (n = 5). *P < 0.01 compared with WT fibroblasts.
DDR2 Regulates Fibroblast Apoptosis
Fibroblast resistance to apoptosis has emerged as a critical regulator of fibrosis. WT and DDR2-null fibroblasts were treated with CHX and a Fas-activating antibody (or vehicle control) to induce robust apoptosis. To track the induction of apoptosis over time, WT and DDR2-null fibroblasts were cultured with a reagent that fluoresces when cleaved by caspase 3/7. Cells were imaged over time using the IncuCyte system, and numbers of fluorescent cells were quantified over time. Greater induction of apoptosis in the DDR2-null fibroblasts was apparent within 20 hours after addition of Fas/CHX (Figure 4B). To confirm increased activation of caspase 3/7 in DDR2-null fibroblasts, WT and DDR2-null cells were treated with Fas/CHX as before, then lysed after 24 hours. Caspase 3/7 activity was measured in cell lysates using a luciferase-based Caspase-Glo system. As expected, DDR2-null fibroblasts demonstrated a more robust activation of caspase 3/7 than WT fibroblasts.
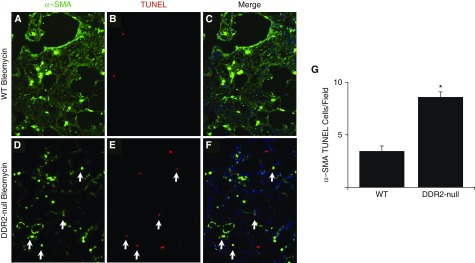
To determine if DDR2 contributes to fibroblast survival in vivo, WT and DDR2-null mice were treated with bleomycin. After 14 days, lung sections were stained for TUNEL and counterstained with ACTA2 to mark the active myofibroblasts. Consistent with the in vitro data, the numbers of TUNEL-positive myofibroblasts in bleomycin-treated DDR2-null lungs was significantly greater than bleomycin-injured WT lungs (Figure 5).
Figure 5.
DDR2 regulates fibroblast apoptosis in vivo. Fourteen days after bleomycin injury, lung sections from WT (A–C) and DDR2-null (D–F) mice were stained for ACTA2 (α-SMA; green, A and D) and TUNEL (red; B and E), and images were merged (C and F). Compared with WT lungs, the DDR2-null lung sections had more TUNEL-positive myofibroblasts (G) (n = 4). *P < 0.05 compared with WT. Several cells co-positive for ACTA2 and TUNEL are indicated (arrows).
DDR2 Regulates Fibroblast Apoptosis through a PDK1/Akt Pathway
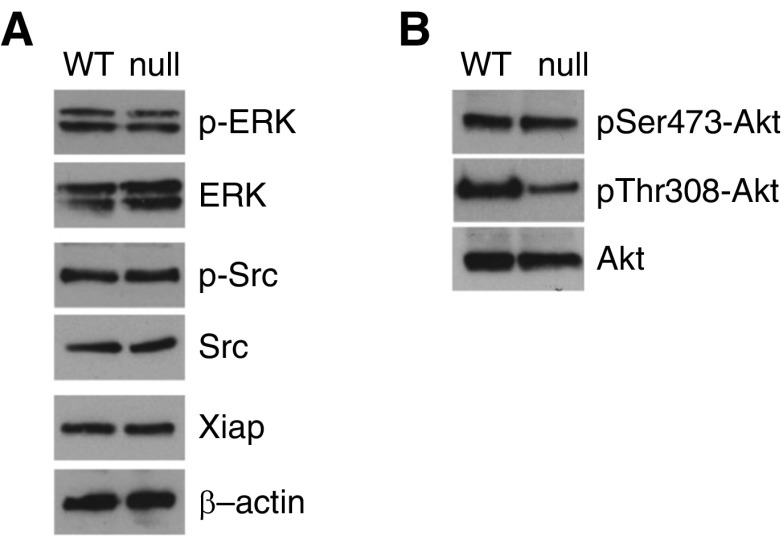
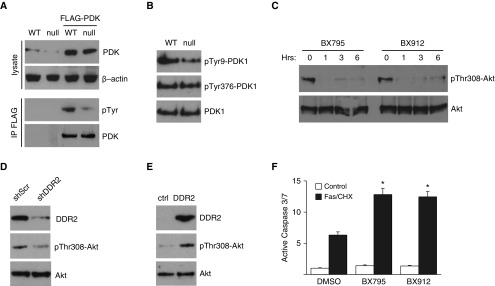
We next interrogated several well-described pathways involved in fibroblast survival that might predispose DDR2-null cells to greater apoptosis. Some of these pathways, most notably Src signaling, have been linked to DDR2 activation as well as fibroblast survival (19). However, we found no differences between WT and DDR2-null fibroblasts in concentrations of total or phospho-Src. We recently reported that XIAP is a major regulator of fibroblast survival in the context of lung fibrosis (31, 32), but again we found no differences between WT and DDR2-null fibroblasts (Figure 6A). Erk and Akt signaling are among the best-studied survival pathways. WT and DDR2 fibroblasts had no differences in Erk phosphorylation. Akt phosphorylation at serine 473 (Ser473) and threonine 308 (Thr308) lead to Akt activation and increased survival in several cell types. We found no difference in pSer473-Akt; however, DDR2-null fibroblasts had marked reduction in pThr308-Akt, suggesting that decreased Akt activation may be linked to the observed increased apoptosis (Figure 6B).
Figure 6.
DDR2 regulates fibroblast Akt pathway. (A) IB for phospho-extracellular signal-regulated kinase (p-ERK), ERK, phospho-Src (p-Src), Src, and XIAP (X-linked inhibitor of apoptosis protein) in primary lung fibroblasts isolated from DDR2-null (null) and littermate control (WT) mice. (B) IB for phospho-Ser473-Akt (pSer473-Akt), phospho-Thr308-Akt (pThr308-Akt), and total Akt in primary lung fibroblasts isolated from null and WT mice.
PDK1 is the best-described kinase in phosphorylating Akt at Thr308, and PDK1 activity is known to be regulated by tyrosine phosphorylation (33). DDR2 has not previously been described to regulate PDK activity. To determine if the presence of DDR2 regulates tyrosine phosphorylation of PDK1, we used a FLAG-tagged PDK1 vector. WT and DDR2-null fibroblasts were treated with retrovirus-encoding control vector or FLAG-PDK1. FLAG-PDK1 was immunoprecipitated, and the amounts of tyrosine phosphorylation were determined by IB for phospho-tyrosine. DDR2-null fibroblasts exhibited markedly decreased concentrations of phospho-tyrosine PDK1 (Figure 7A). We next obtained antibodies specific for PDK1 phosphorylation at PDK1’s two major regulatory tyrosine residues, tyrosine 9 (Tyr9) and tyrosine 376 (Tyr376). Consistent with the FLAG-PDK1 overexpression experiment, IB of WT and DDR2-null fibroblasts demonstrated reduced numbers of pTyr9-PDK1 in DDR2 fibroblasts compared with WT fibroblasts (Figure 7B).
Figure 7.
DDR2 regulates fibroblast survival through 3-phosphoinositide dependent protein kinase-1 (PDK1)/Akt. (A) Lysate from WT and DDR2-null (null) lung fibroblasts overexpressing FLAG-PDK1 were immunoprecipitated using a FLAG antibody and immunoblotted for phospho-tyrosine. Null cells have less tyrosine phosphorylation of FLAG-PDK1. (B) Null cells have less phospho-Tyr9-PDK1 (pTyr9-PDK1) and equal concentrations of phospho-Tyr376-PDK1 (pTyr376-PDK1) and total PDK1 compared with WT primary lung fibroblasts assessed by IB. (C) IB for pThr308-Akt and total Akt in WT primary lung fibroblasts treated with two small-molecule inhibitors of PDK1 (BX795 and BX912) for 0, 1, 3, and 6 hours. (D) IB of WT lung fibroblasts treated with lentivirus-mediated shRNA to DDR2 (shDDR2) or scrambled control (shScr) for DDR2 and pThr308-Akt. (E) DDR2-null lung fibroblasts treated with retrovirus-mediated overexpression of DDR2 (DDR2) or empty control retrovirus (ctrl) and immunoblotted for DDR2 and pThr308-Akt. (F) Activation of caspase 3/7 in WT primary lung fibroblasts upon induction of apoptosis by treatment with a Fas-activating antibody and cycloheximide (Fas/CHX) with or without BX795 and BX912. Caspase 3/7 activity was significantly higher in lung fibroblasts with additional inhibition of PDK1 than with Fas/CHX induction of apoptosis alone. Values are expressed as fold differences in relative luminescence units (n = 6). *P < 0.01 compared with treatment with Fas/CHX and vehicle control.
To our knowledge, PDK1 has not previously been reported to be involved in fibroblast survival. To confirm that PDK1-mediated Akt phosphorylation at Thr308 regulates fibroblast apoptosis, WT lung fibroblasts were treated with two different PDK1 inhibitors. PDK1 inhibition demonstrated rapid and sustained reduction in pThr308-Akt compared with fibroblasts treated with vehicle-alone control (Figure 7C). Consistent with DDR2 regulation of Akt phosphorylation, compared with DDR2-null fibroblasts treated with control retrovirus, WT lung fibroblasts in which expression of DDR2 was knocked down by shRNA had reduced amounts of pThr308-Akt (Figure 7D), and DDR2-null lung fibroblasts with retrovirus-mediated overexpression of DDR2 had increased amounts of pThr308-Akt (Figure 7E). Next, WT lung fibroblasts were treated with PDK1 inhibitors or vehicle control and stimulated with Fas/CHX to induce apoptosis. As before, Fas/CHX induced robust activation of caspase 3/7; however, the amount of caspase activation was much greater in the cells treated with the PDK1 inhibitors (Figure 7F).
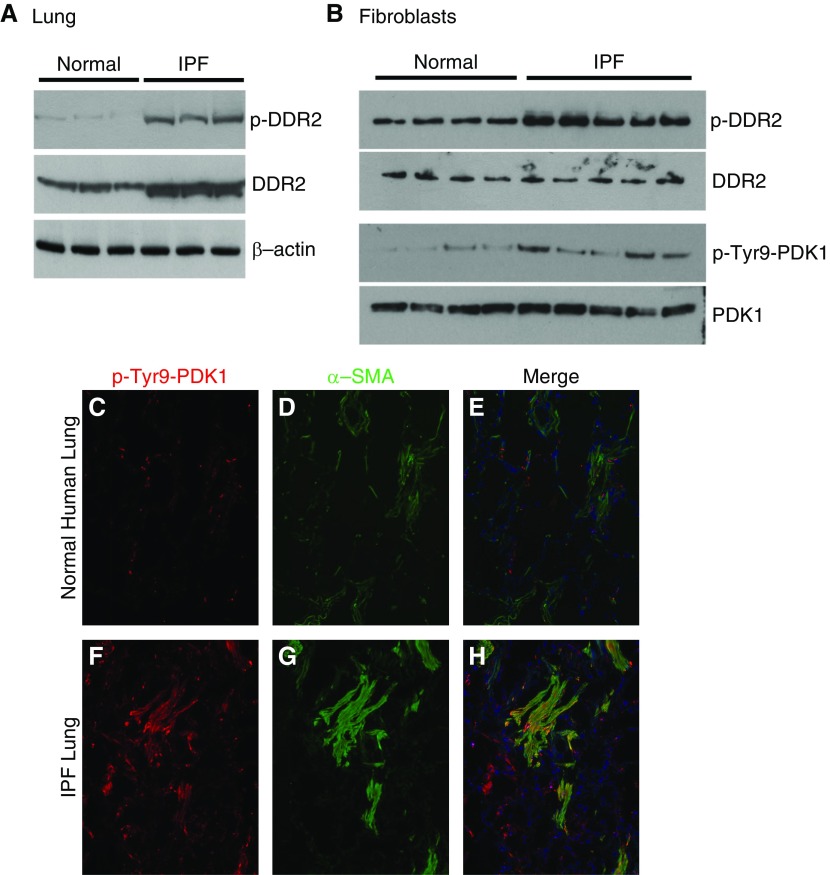
Human Fibroblasts Have Increased Activation of DDR2 and PDK
IPF lungs are characterized in part by the accumulation of fibroblasts and type I collagen. Because DDR2 is known to be highly expressed by fibroblasts and phosphorylated in response to type I collagen, we confirmed that IPF lungs demonstrate increased concentrations of DDR2 and pDDR2 (Figure 8A). Compared with normal human lung fibroblasts, IPF fibroblasts also have greater collagen production and are resistant to apoptosis. To assess whether IPF fibroblasts exhibit greater activation of DDR2 and PDK1, we used primary human lung fibroblasts isolated from IPF lungs or normal human lungs. Consistent with the murine data, IPF fibroblasts exhibited increased concentrations of p-DDR2 and pTyr9-PDK1 (Figure 8B). Finally, frozen optimal cutting temperature compound–embedded lung tissue from IPF and normal human lungs were stained for pTyr9-PDK1 and ACTA2. As expected, IPF lungs demonstrated increased staining for pTyr9-PDK1, primarily within ACTA2-positive myofibroblasts (Figure 8). Collectively, these results suggest that collagen I signaling mediated through pDDR2/pTyr9-PDK1/pThr308-Akt contributes to increased fibroblast survival in progressive lung fibrosis.
Figure 8.
Idiopathic pulmonary fibrosis (IPF) fibroblasts have greater concentrations of phosphorylated DDR2 (pDDR2) and phosphorylated PDK1 (pPDK1). (A) IPF and normal human lung tissues were analyzed by IB for pDDR2 and DDR2. (B) IPF and normal human primary lung fibroblasts were analyzed by IB for pDDR2, DDR2, pTyr9-PKD1, and PDK1. (C–H) Normal human (C–E) and IPF (F–H) lungs were stained for pTyr9-PDK1 (red; C and F) and ACTA2 (α-SMA) (green; D and G), and the images were merged (E and H).
Discussion
Our results support a model in which fibroblast resistance to apoptosis is a major driver of sustained and progressive fibrosis (31, 32). The current paradigm suggests that in normal wound healing after injury, there is measured activation of fibroblasts with appropriate scar formation and disappearance of activated myofibroblasts through apoptosis or de-differentiation. In contrast, in fibrosis, there is exuberant activation of fibroblasts and resistance to apoptosis, leading to exaggerated and progressive fibrosis. The fibrosis itself can propagate this sustained fibrotic response (34, 35). Prior research has focused primarily on the mechanical properties of the fibrotic matrix leading to activation of intracellular pathways such as focal adhesion kinase (FAK) and Rho, as well as to greater fibroblast activation (36–38). We have recently shown that resistance to fibroblast apoptosis may actually be the more significant component of this progressive fibrotic response (31, 32). In support of this model, we find that DDR2-null fibroblasts actually have a tendency toward greater activation, even though DDR2-null mice have a significantly attenuated fibrosis.
The role of signaling through the ECM has been well studied as a regulator of fibrosis (4, 38). Prior studies have focused primarily on provisional matrix proteins such as fibronectin (39, 40). The role of collagen signaling during progressive fibrosis is not well studied. There is clearly upregulation of fibrillar collagens, including type I, type III, and others. The role of type IV collagen is less clear, although one of its main receptors, DDR1, has been shown to regulate lung fibrosis (13). Type I collagen is the most abundant protein in the fibrotic matrix, and we and others have found that collagen I expression is induced early after injury, suggesting that collagen signaling might have a role in regulating continued collagen expression and the development of fibrosis (10). Like many matrix proteins, collagen can activate a number of integrins, especially α2β1 (41, 42). Collagen is fairly unique among matrix proteins in its ability to activate a receptor tyrosine kinase (8, 43). Our results suggest that these receptors have differing roles in regulating the cell response to type I collagen, with DDR2 regulating cell survival and α2β1 regulating myofibroblast activation. It is noteworthy that deletion of DDR2 or type I collagen led to increased amounts of α2 integrin, which may complicate attempts to target this pathway for therapy. Consistent with collagen I–mediated inhibition of α2 integrin expression, authors of a recent report found reduced amounts of α2 integrin among IPF fibroblasts (44). Notably, there is growing evidence for matrix/integrin signaling having a key role in translating matrix mechanotransduction signaling to cell behavior, including fibroblast activation and survival through well-known integrin intracellular signaling pathways involving FAK, Rho, and myocardin-related transcription factor (45). DDR2 signaling may involve an independent survival mechanism in response to early collagen production that might precede formation of a mature dense collagen matrix. Although our interest in DDR2 signaling arose from our prior studies of type I collagen deletion (10), DDR2 can be activated by a number of collagens, including type III collagen, which is also upregulated during fibrosis (46). The extent to which type I and other collagens promote fibroblast DDR2 activation during fibrosis is not well characterized.
A unique attractive feature of DDR2 is its extremely skewed high expression on mesenchymal cells and limited expression on other cell types, including epithelial cells and myeloid cells. Attempts to target survival and activation pathways have been challenging, owing to the commonality of these pathways among multiple cell types. For example, imatinib was shown to be a potent inhibitor of fibroblast activation and survival through inhibition of platelet-derived growth factor receptor and Abl, but it was later shown to influence epithelial cell survival as well (47–49). Similarly, we recently reported that inhibition of FAK signaling, which has been proposed as another potential therapeutic target in fibrosis, can also influence epithelial cell survival, potentially leading to sustained injury and fibrosis (26, 50). DDR2 expression is not limited to fibroblasts and mesenchymal cells. DDR2 expression on myeloid cells has been reported (51, 52), although we found very low expression on myeloid cells isolated from WT murine lungs (Figure E2). Recently, mutations in DDR2 have been linked to a subset of squamous cell lung cancers, and DDR2 signaling has been linked to epithelial–mesenchymal transition in other malignancies (53, 54). It is possible that DDR2 expression can be induced in alveolar epithelial cells; however, this population is likely to be profibrotic as well.
On the basis of lung cancer findings, there has been a concerted effort to develop DDR2-specific inhibitors (21). Indeed, many tyrosine kinase inhibitors are fairly nonspecific. As noted above, imatinib has activity against Abl, platelet-derived growth factor receptor, and many other tyrosine kinases. It is worth noting that dasatinib, which has been shown to block fibrosis in vivo, has significant activity against DDR2 (55, 56). Furthermore, nintedanib, which is approved therapy for IPF, is a fairly nonspecific tyrosine kinase inhibitor with activity against DDR2 (18, 57).
Although DDR1 and DDR2 have previously been studied in different fibrosis models, the results have been mixed, and the direct downstream targets are not well described (13, 15, 17, 58). These mixed results may be due to the complexity of the signaling networks that intersect with the DDRs. As noted above, deletion of DDR2 or collagen led to increased amounts of α2 integrin, which may lead to greater myofibroblast activation. Indeed, other studies have found a complex regulation of collagen receptor signaling influencing the expression and function of other collagen receptors and other intracellular signaling pathways (7, 8, 59, 60). Our findings also link collagen/DDR2 signaling to a signaling network that intersects with other pathways implicated in fibrosis. We found that the presence of DDR2 signaling promoted PDK phosphorylation at tyrosine 9. Because DDR2 is a well-described tyrosine kinase, it is possible that PDK is a direct downstream target of DDR2. Alternatively, DDR2 is well known to interact with a number of other kinases, and PDK phosphorylation may be indirectly regulated by DDR2 (43, 61). More detailed in vitro studies are underway to help distinguish between these possibilities. The role of different tyrosine sites in regulating PDK activity at remains controversial, with some reports suggesting a more prominent role for tyrosine 376 and others suggesting an important role for tyrosine 9 (62–64). It may be that regulation of PDK activity is dependent on different cell types or other cell culture conditions. Inhibition of Akt has also been shown to promote myofibroblast apoptosis and block fibrosis (35, 65, 66). Akt can be activated at two main sites: Ser473, which is primarily regulated by mTOR, and Thr308, which is regulated primarily by PDK1 (33). Notably, mTOR inhibition is actively being pursued as a potential therapy for fibrosis (67), and it is possible that dual inhibition of Akt with PDK1 inhibition will augment the effect.
In summary, we found that collagen/DDR2 signaling can activate the Akt prosurvival pathway through activation of PDK1, leading to fibroblast survival and progressive fibrosis. DDR2 expression is skewed to the mesenchymal cell population, making it an attractive target for therapy.
Footnotes
Supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grants HL108904 (K.K.K.) and HL141195 (J.C.H.).
Author Contributions: Conception and design: S.J., J.Y., J.C.H., E.S.W., and K.K.K.; analysis and interpretation: S.J., M.A., J.Y., J.C.H., and K.K.K.; and drafting of the manuscript: S.J., M.A., J.C.H., E.S.W., and K.K.K.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0419OC on April 13, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thannickal VJ. Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S23. doi: 10.1186/1755-1536-5-S1-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, et al. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol. 2014;184:1643–1651. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 6.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 7.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 8.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, LaFemina MJ, Frank JA, et al. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol. 2013;183:1559–1570. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. Am J Physiol Lung Cell Mol Physiol. 2014;306:L786–L796. doi: 10.1152/ajplung.00243.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;35:2871–2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174:420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Lu X, Ren X, Ding K. Small molecule discoidin domain receptor kinase inhibitors and potential medical applications. J Med Chem. 2015;58:3287–3301. doi: 10.1021/jm5012319. [DOI] [PubMed] [Google Scholar]
- 15.Olaso E, Arteta B, Benedicto A, Crende O, Friedman SL. Loss of discoidin domain receptor 2 promotes hepatic fibrosis after chronic carbon tetrachloride through altered paracrine interactions between hepatic stellate cells and liver-associated macrophages. Am J Pathol. 2011;179:2894–2904. doi: 10.1016/j.ajpath.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George M, Vijayakumar A, Dhanesh SB, James J, Shivakumar K. Molecular basis and functional significance of angiotensin II-induced increase in discoidin domain receptor 2 gene expression in cardiac fibroblasts. J Mol Cell Cardiol. 2016;90:59–69. doi: 10.1016/j.yjmcc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Bian H, Bu X, Zhang S, Zhang P, Yu J, et al. Targeting of discoidin domain receptor 2 (DDR2) prevents myofibroblast activation and neovessel formation during pulmonary fibrosis. Mol Ther. 2016;24:1734–1744. doi: 10.1038/mt.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz-Castro PA, Shaw D, Jarai G. Discoidin domain receptor signaling and pharmacological inhibitors. In: Fridman R, Huang PH, editors. Discoidin domain receptors in health and disease. New York: Springer; 2016. pp. 217–238. [Google Scholar]
- 19.von Mässenhausen A, Sanders C, Brägelmann J, Konantz M, Queisser A, Vogel W, et al. Targeting DDR2 in head and neck squamous cell carcinoma with dasatinib. Int J Cancer. 2016;139:2359–2369. doi: 10.1002/ijc.30279. [DOI] [PubMed] [Google Scholar]
- 20.DeLeon-Pennell KY. May the fibrosis be with you: is discoidin domain receptor 2 the receptor we have been looking for? J Mol Cell Cardiol. 2016;91:201–203. doi: 10.1016/j.yjmcc.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Richters A, Nguyen HD, Phan T, Simard JR, Grütter C, Engel J, et al. Identification of type II and III DDR2 inhibitors. J Med Chem. 2014;57:4252–4262. doi: 10.1021/jm500167q. [DOI] [PubMed] [Google Scholar]
- 22.Kano K, Marín de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, et al. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol. 2008;22:1866–1880. doi: 10.1210/me.2007-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheaton AK, Velikoff M, Agarwal M, Loo TT, Horowitz JC, Sisson TH, et al. The vitronectin RGD motif regulates TGF-β-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1206–L1217. doi: 10.1152/ajplung.00424.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osterholzer JJ, Christensen PJ, Lama V, Horowitz JC, Hattori N, Subbotina N, et al. PAI-1 promotes the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury. J Pathol. 2012;228:170–180. doi: 10.1002/path.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, et al. Implicating exudate macrophages and Ly-6Chigh monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheaton AK, Agarwal M, Jia S, Kim KK. Lung epithelial cell focal adhesion kinase signaling inhibits lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2017;312:L722–L730. doi: 10.1152/ajplung.00478.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, et al. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol. 2005;174:5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 28.Huang SK, Scruggs AM, McEachin RC, White ES, Peters-Golden M. Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS One. 2014;9:e107055. doi: 10.1371/journal.pone.0107055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, et al. Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Velikoff M, Agarwal M, Disayabutr S, Wolters PJ, Kim KK. Overexpression of inhibitor of DNA-binding 2 attenuates pulmonary fibrosis through regulation of c-Abl and Twist. Am J Pathol. 2015;185:1001–1011. doi: 10.1016/j.ajpath.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajayi IO, Sisson TH, Higgins PD, Booth AJ, Sagana RL, Huang SK, et al. X-linked inhibitor of apoptosis regulates lung fibroblast resistance to Fas-mediated apoptosis. Am J Respir Cell Mol Biol. 2013;49:86–95. doi: 10.1165/rcmb.2012-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley SL, Sisson TH, Wheaton AK, Kim KK, Wilke CA, Ajayi IO, et al. Targeting inhibitor of apoptosis proteins protects from bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol. 2016;54:482–492. doi: 10.1165/rcmb.2015-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horowitz JC, Martinez FJ, Thannickal VJ. Mesenchymal cell fate and phenotypes in the pathogenesis of emphysema. COPD. 2009;6:201–210. doi: 10.1080/15412550902905953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, et al. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinz B. Matrix mechanics and regulation of the fibroblast phenotype. Periodontol 2000. 2013;63:14–28. doi: 10.1111/prd.12030. [DOI] [PubMed] [Google Scholar]
- 39.Fontana L, Chen Y, Prijatelj P, Sakai T, Fässler R, Sakai LY, et al. Fibronectin is required for integrin αvβ6-mediated activation of latent TGF-β complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 40.Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to α4β7 integrin receptor and MAPK/Erk1/2-dependent signaling. FASEB J. 2010;24:4503–4512. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 42.Staatz WD, Walsh JJ, Pexton T, Santoro SA. The α2β1 integrin cell surface collagen receptor binds to the α1(I)-CB3 peptide of collagen. J Biol Chem. 1990;265:4778–4781. [PubMed] [Google Scholar]
- 43.Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, et al. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288:7430–7437. doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia H, Seeman J, Hong J, Hergert P, Bodem V, Jessurun J, et al. Low α2β1 integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am J Pathol. 2012;181:222–233. doi: 10.1016/j.ajpath.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol. 2015;185:969–986. doi: 10.1016/j.ajpath.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahzeidi S, Mulier B, de Crombrugghe B, Jeffery PK, McAnulty RJ, Laurent GJ. Enhanced type III collagen gene expression during bleomycin induced lung fibrosis. Thorax. 1993;48:622–628. doi: 10.1136/thx.48.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR Imatinib-IPF Study Investigators. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 49.Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007;321:35–44. doi: 10.1124/jpet.106.113407. [DOI] [PubMed] [Google Scholar]
- 50.Lagares D, Busnadiego O, García-Fernández RA, Kapoor M, Liu S, Carter DE, et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653–1664. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afonso PV, McCann CP, Kapnick SM, Parent CA. Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood. 2013;121:1644–1650. doi: 10.1182/blood-2012-08-451575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poudel B, Yoon DS, Lee JH, Lee YM, Kim DK. Collagen I enhances functional activities of human monocyte-derived dendritic cells via discoidin domain receptor 2. Cell Immunol. 2012;278:95–102. doi: 10.1016/j.cellimm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira Cruz F, Horta LFB, de Albuquerque Maia L, Lopes-Pacheco M, da Silva AB, Morales MM, et al. Dasatinib reduces lung inflammation and fibrosis in acute experimental silicosis. PLoS One. 2016;11:e0147005. doi: 10.1371/journal.pone.0147005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yilmaz O, Oztay F, Kayalar O. Dasatinib attenuated bleomycin-induced pulmonary fibrosis in mice. Growth Factors. 2015;33:366–375. doi: 10.3109/08977194.2015.1109511. [DOI] [PubMed] [Google Scholar]
- 57.Vukin E, Greenberg R, Auerbach M, Chang L, Scotten M, Tenney-Soeiro R, et al. Use of simulation-based education: a national survey of pediatric clerkship directors. Acad Pediatr. 2014;14:369–374. doi: 10.1016/j.acap.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Suratt BT, Ubags NDJ, Rastogi D, Tantisira KG, Marsland BJ, Petrache I, et al. Allergy, Immunology, and Inflammation Assembly. An official American Thoracic Society workshop report: Obesity and metabolism. An emerging frontier in lung health and disease. Ann Am Thorac Soc. 2017;14:1050–1059. doi: 10.1513/AnnalsATS.201703-263WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz PA, Jarai G. Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem. 2011;286:12912–12923. doi: 10.1074/jbc.M110.143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu H, Bihan D, Chang F, Huang PH, Farndale RW, Leitinger B. Discoidin domain receptors promote α1β1- and α2β1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS One. 2012;7:e52209. doi: 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, et al. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 63.Yang KJ, Shin S, Piao L, Shin E, Li Y, Park KA, et al. Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem. 2008;283:1480–1491. doi: 10.1074/jbc.M706361200. [DOI] [PubMed] [Google Scholar]
- 64.Park J, Hill MM, Hess D, Brazil DP, Hofsteenge J, Hemmings BA. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J Biol Chem. 2001;276:37459–37471. doi: 10.1074/jbc.M105916200. [DOI] [PubMed] [Google Scholar]
- 65.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffith BE, Flamini V, DeAnda A, Scotten L. Simulating the dynamics of an aortic valve prosthesis in a pulse duplicator: numerical methods and initial experience. J Med Device. 2013;7:0409121–0409122. doi: 10.1115/1.4025768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax. 2016;71:701–711. doi: 10.1136/thoraxjnl-2015-207429. [DOI] [PMC free article] [PubMed] [Google Scholar]