Abstract
Previous reports demonstrate that the microbiome impacts allergic airway responses, including airway hyperresponsiveness, a characteristic feature of asthma. Here we examined the role of the microbiome in pulmonary responses to a nonallergic asthma trigger, ozone. We depleted the microbiota of conventional mice with either a single antibiotic (ampicillin, metronidazole, neomycin, or vancomycin) or a cocktail of all four antibiotics given via the drinking water. Mice were then exposed to room air or ozone. In air-exposed mice, airway responsiveness did not differ between antibiotic- and control water–treated mice. Ozone caused airway hyperresponsiveness, the magnitude of which was decreased in antibiotic cocktail–treated mice versus water-treated mice. Except for neomycin, single antibiotics had effects similar to those observed with the cocktail. Compared with conventional mice, germ-free mice also had attenuated airway responsiveness after ozone. 16S ribosomal RNA gene sequencing of fecal DNA to characterize the gut microbiome indicated that bacterial genera that were decreased in mice with reduced ozone-induced airway hyperresponsiveness after antibiotic treatment were short-chain fatty acid producers. Serum analysis indicated reduced concentrations of the short-chain fatty acid propionate in cocktail-treated mice but not in neomycin-treated mice. Dietary enrichment with pectin, which increased serum short-chain fatty acids, also augmented ozone-induced airway hyperresponsiveness. Furthermore, propionate supplementation of the drinking water augmented ozone-induced airway hyperresponsiveness in conventional mice. Our data indicate that the microbiome contributes to ozone-induced airway hyperresponsiveness, likely via its ability to produce short-chain fatty acids.
Keywords: airway responsiveness, antibiotics, germ-free mice, neutrophil, 16S rRNA gene sequencing
Clinical Relevance
This study demonstrates a role for the gut microbiome in pulmonary responses to ozone, an asthma trigger. The data indicate that the ability of the microbiome to produce short-chain fatty acids likely contributes to this role. A better understanding of the relationship between the microbiome and asthma could lead to new diagnostic biomarkers for asthma and novel therapeutics, including probiotics and prebiotics.
There is increasing evidence that the microbiome plays an important role in asthma (1–3). Patients with asthma harbor different microbes in their lungs and sputum compared with healthy humans (3, 4). In addition, children whose guts are colonized with Helicobacter pylori are 40% less likely to have childhood-onset asthma than children who are not colonized (5). Prenatal and postnatal uses of antibiotics in humans have been associated with an increased risk of asthma development (6), and oral ingestion of various strains of Lactobacillus and ingestion of bacterial products have also been shown to impact allergic pulmonary inflammation (7). In mice, disruption of the microbiota by antibiotic treatment leads to abnormal allergic airway responses, including increased levels of eosinophils, mast cells, IL-5, IL-13, γ-IFN, IgE, and mucus-secreting cells (8). Similarly, germ-free (GF) mice have augmented allergic airway responses compared with mice raised in conventional specific-pathogen-free (SPF) facilities (9). Taken together, these data support the hypothesis that the microbiome shapes allergic airway responses. However, it is unknown how the microbiome impacts pulmonary responses to nonallergic asthma triggers.
The air pollutant ozone (O3) is a major public health concern worldwide. O3 exposure causes dyspnea, cough, decreased lung function, susceptibility to lung infections, pulmonary inflammation, and increased asthma exacerbations (10–12). Indeed, the number of hospital admissions for asthma increases on days after high ambient O3 concentrations (13). O3 causes airway epithelial cell injury, induces production of inflammatory cytokines and chemokines, and causes neutrophil recruitment (14). Importantly, O3 also causes airway hyperresponsiveness (AHR) (15), a canonical feature of asthma.
Our purpose in this study was to examine the hypothesis that the microbiome contributes to O3-induced AHR. To do so, we compared O3-induced AHR in SPF versus GF mice, and in control mice versus mice treated with either individual antibiotics (ampicillin, metronidazole, neomycin, and vancomycin) or the combination of these antibiotics. O3-induced AHR was attenuated in GF mice, in mice treated with the antibiotic cocktail, and in mice treated with all individual antibiotics except neomycin. 16S ribosomal RNA (rRNA) gene sequencing of fecal DNA indicated that bacterial genera that were associated with O3-induced AHR were short-chain fatty acid (SCFA) producers. SCFAs are primarily produced by anaerobic gut bacterial fermentation of fiber, and have been implicated in the role of the microbiome in various diseases, including allergic asthma, insulin sensitivity, and colitis (16–19). Serum analysis indicated reduced concentrations of the SCFA propionate in mice with reduced O3-induced AHR. Furthermore, diets high in fermentable fiber that also resulted in increased serum SCFAs and propionate supplementation of the drinking water both augmented O3-induced AHR. Our data suggest that the microbiome is required for O3-induced AHR, likely via its ability to produce SCFAs.
Methods
Animals
All protocols were approved by the Harvard Medical Area Standing Committee on Animals. Male C57BL/6 mice were used and were 10 weeks old at the time of exposure. Further details regarding these animals can be found in the data supplement.
Protocol
Four experimental protocols were performed. In the first protocol, mice were given a cocktail of antibiotics (AMNV [ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L, and vancomycin 0.5 g/L]) by addition to the drinking water for 2 weeks. Sucralose (8 g/L) was added for taste. This type of antibiotic treatment protocol has been reported to result in a marked reduction in the gut bacteria load as assessed DNA concentration in fecal pellets (20). Control mice were given regular drinking water with sucralose. Additional mice were treated with each antibiotic given individually for 2 weeks before O3 exposure and evaluation. After 2 weeks of treatment, the mice were exposed to room air or to O3 (2 ppm for 3 h). The mice were anesthetized for measurements of pulmonary mechanics and airway responsiveness to inhaled aerosolized methacholine (1–100 mg/ml) 24 hours after exposure. After these measurements, the mice were killed, blood was collected for the preparation of serum, and BAL was performed. Fecal samples were collected from each mouse 1 day before exposure. In the second protocol, GF mice were administered the same cocktail of antibiotics or control water for 2 weeks inside isolator cages. They were then taken out of the isolators, immediately exposed to O3, and evaluated 24 hours later. GF mice were compared with SPF mice that had also been exposed to O3. In the third protocol, mice were placed on diets in which 30% of the calories derived from fiber, either in the form of pectin or in the form of cellulose. The diets were obtained from Research Diets Inc. After 3 days the mice were exposed and evaluated as described above. In the fourth protocol, mice were given sodium propionate (200 mM) in the drinking water for 3 days before O3 exposure. Control mice were given saline (50 mM) in the drinking water, as described previously (16). In all protocols, treatment (antibiotics, propionate, or diet) was continued in the period between exposure and evaluation.
The methods used for O3 exposure, measurements of airway responsiveness, BAL, 16S rRNA gene sequencing and analysis, and measurement of SCFAs are detailed in the data supplement.
Statistical Analysis
Except for microbial community analysis (see the data supplement), the significance of differences between groups was assessed using ANOVA (for SCFAs) or factorial ANOVA combined with Fisher’s least significant difference post hoc analysis (Statistica Software) using treatment and exposure as main effects. Student’s t test was used for the GF mice and the exogenous propionate experiment. A P value < 0.05 (two-tailed) was considered significant. All values are expressed as mean ± SEM.
Results
Treatment with an Antibiotic Cocktail Reduces Responses to O3
To deplete the microbiome, mice were administered an antimicrobial cocktail (AMNV) by addition to the drinking water for 2 weeks. Treatment with the AMNV cocktail did not affect body weight (26.8 ± 0.7 g vs. 27.8 ± 0.6 g). After exposure to room air, airway responsiveness to methacholine did not differ between mice given regular drinking water and those treated with the AMNV cocktail (Figure 1A), except at the PBS dose (see Figure E1 for an expanded scale of the responses at the lowest doses of methacholine for this and other figures displaying airway responsiveness data). In O3-exposed water-treated mice, O3 exposure resulted in increased airway responsiveness, as assessed by changes in respiratory system resistance (RRS) (Figure 1A) or respiratory system elastance (ERS) (Figure E2). O3 also increased BAL neutrophils and macrophages and BAL protein, a marker of damage to the alveolar/capillary barrier (21) (Figures 1A–1D), consistent with previous reports (21–23). Compared with water-treated mice, O3-exposed AMNV-treated mice had significantly reduced airway responsiveness when assessed using RRS (Figure 1A), but not ERS (Figure E2), suggesting that the effect of antibiotics was not mediated at the level of changes in airway closure. AMNV treatment also resulted in lower BAL neutrophils (Figure 1B), but did not impact O3-induced increases in BAL macrophages or protein (Figures 1C and 1D). Together, these findings indicated that the microbiome contributes to the development of AHR and neutrophilic inflammation in the lung after O3 exposure, without affecting lung barrier injury.
Figure 1.
An antibiotic cocktail attenuates O3-induced airway hyperresponsiveness (AHR) and neutrophil recruitment. Mice were treated with a cocktail of four antibiotics via their drinking water (ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L, vancomycin 0.5 g/L [AMNV]). Sucralose (8 g/L) was added for taste. Control mice were given regular drinking water with sucralose. Shown are (A) airway responsiveness to methacholine; (B) BAL neutrophils; (C) BAL macrophages; (D) BAL protein, a marker of lung barrier injury; and (E) BAL leukotriene B4 (LTB4) 24 hours after exposure to air or O3 (2 ppm for 3 h). Results are mean ± SE of data from n = 6–8 per group. *P < 0.05 compared with air-exposed mice given the same treatment; #P < 0.05 compared with mice with control drinking water with the same exposure. RRS = respiratory system resistance.
The cytokines IL-17A and osteopontin have each been reported to play a role in O3-induced AHR in mice (22–24). IL-33–induced activation of innate lymphoid cells type 2 has also been reported to contribute (25, 26). Compared with air, O3 caused a significant increase in BAL concentrations of IL-17A, osteopontin, IL-33, and IL-5, a marker of innate lymphoid cell type 2 activation (Figures E3A–E3D). However, concentrations of these cytokines did not differ between water-treated and antibiotic-treated mice.
We recently reported that gastrin-releasing peptide (GRP) also contributes to O3-induced AHR (24). Named for its ability to promote gastrin release in the stomach, GRP also affects the release of other gastrointestinal hormones (27), and has effects on gastrointestinal motility (28). Importantly, GRP also causes airway smooth muscle contraction (29). Consequently, we also measured GRP in BAL fluid of mice treated with antibiotics or control water (Figure E3E). BAL levels of GRP were higher in O3-exposed mice than in air-exposed mice, as previously described (24), but there was no impact of antibiotic treatment on BAL GRP.
Several other BAL cytokines and chemokines, including granulocyte colony stimulating factor (G-CSF), eotaxin, IL-6, IP-10, leukemia inhibitory factor (LIF), monocyte chemoattractant protein 1 (MCP-1), keratinocyte chemoattractant (KC), macrophage inhibitory protein 2 (MIP-2), and macrophage inhibitory factor 1 α (MIP-1α symbol) were elevated in O3- versus air-exposed mice, but none were reduced by antibiotic treatment (Figure E4). O3 exposure also caused a small but significant increase in BAL LTB4 with O3 exposure (Figure 1E). Importantly, there was a significant reduction in BAL LTB4 in mice treated with antibiotics (Figure 1E).
GF Mice Have Attenuated Responses to O3
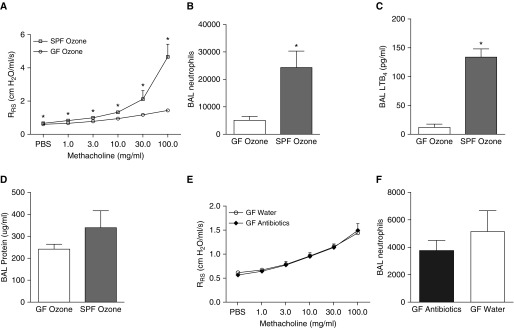
To confirm that it was indeed microbial perturbation and not an off-target effect of the antibiotic treatment that dampened responses to O3 (Figures 1A and 1B), we next compared the responses of GF and SPF mice exposed to O3. Only O3-exposed mice were used in experiments on GF mice because antibiotic treatment had no effect in air-exposed mice (Figure 1A). The body weights of SPF and GF mice were not different (data not shown). Compared with SPF mice, GF mice had reduced airway responsiveness after O3 exposure (Figure 2A). BAL neutrophils and BAL LTB4, but not BAL protein, were also significantly reduced in O3-exposed GF versus SPF mice (Figures 2B–2D). To further evaluate the possibility of nonspecific effects from the AMNV cocktail, we compared GF mice treated with drinking water with other GF mice that were given the AMNV antibiotic cocktail for 2 weeks before O3 exposure. Airway responsiveness and BAL neutrophils did not differ between water-treated and antibiotic-treated GF mice exposed to O3 (Figures 2E and 2F), nor was there any difference in body weight (25.5 ± 0.6 g vs. 26.8 ± 0.7 g). Together, these findings indicate that a decrease in the microbial burden, whether by antibiotic treatment or by GF conditions, results in an attenuation of the response to O3 in C57BL/6 mice.
Figure 2.
Germ-free (GF) mice have reduced responses to O3 compared with specific-pathogen-free (SPF) mice. Shown are (A) airway responsiveness, (B) BAL neutrophils, (C) BAL LTB4, and (D) BAL protein in O3-exposed GF mice and age- and sex-matched SPF mice treated with water. Also shown are (E) airway responsiveness and (F) BAL neutrophils in O3-exposed GF mice treated with water versus the antibiotic cocktail (AMNV). Results are mean ± SE of data from n = 8 per group. *P < 0.05 compared with GF.
Ampicillin, Metronidazole, and Vancomycin, but Not Neomycin, Attenuate O3-induced AHR
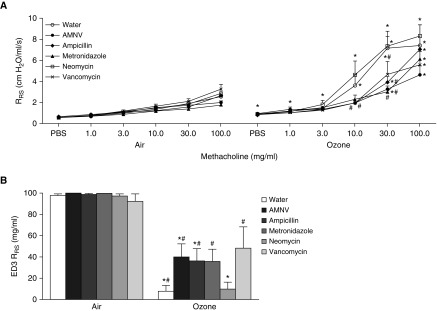
As described above, after O3 exposure, AMNV-treated SPF mice had reduced airway responsiveness compared with water-treated mice. To narrow down possible microbial taxa involved in modulating airway responsiveness, we measured airway responsiveness in SPF mice treated for 2 weeks either with the AMNV cocktail or with each antibiotic in the cocktail given separately. None of the antibiotics alone or in combination had any effect on the body weight (data not shown) or airway responsiveness of air-exposed mice (Figure 3A, left panel). Compared with water treatment, AMNV treatment reduced O3-induced AHR (Figure 3A, right panel), as described above (Figure 1). Treatment with ampicillin alone, metronidazole alone, or vancomycin alone also resulted in reduced O3-induced AHR, whereas mice treated with neomycin alone responded similarly to the water-treated control group (Figure 3A, right panel). Similar results were obtained when we computed the ED3 RRS, the dose of methacholine required to cause a 3 cm H2O/ml/s change in RRS (Figure 3B). These findings eliminate microbial clades that are affected by neomycin as candidate modulators of nonallergic AHR after O3 exposure.
Figure 3.
Treatment with ampicillin, metronidazole, and vancomycin, but not neomycin, attenuates O3-induced AHR. Mice were treated with water, an antibiotic cocktail (AMNV), or individual antibiotics (ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L, and vancomycin 0.5 g/L). Shown are (A) RRS and (B) effective dose 3 (ED3), the dose of methacholine required to cause an increase in RRS of 3 cm H2O/ml/s. ED3 was assigned a value of 100 if RRS did not increase by 3 cm H2O/ml/s by the last dose of methacholine. Results are mean ± SE of data from n = 6–8 per group. *P < 0.05 compared with air (see expanded scale in Figure E1 to determine which groups were different at the low doses); #P < 0.05 compared with water.
Bacterial Genera Depleted in Mice with Attenuated O3-induced AHR
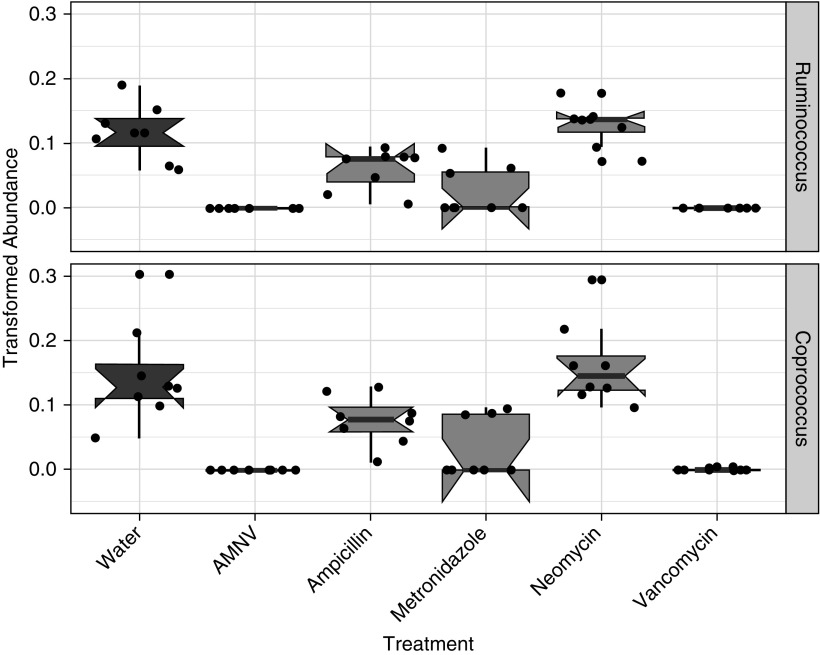
Others have reported that oral treatment with vancomycin alone does not alter the lung microbiome even though it does affect the gut microbiome (30), likely because vancomycin does not cross the gut epithelium after oral administration (31). Because vancomycin alone was able to attenuate O3-induced AHR (Figure 3), we focused our attention on the gut microbiome. To determine the effect of antimicrobials on the murine gut microbiome, we conducted microbial community 16S rRNA gene amplicon surveys from mouse stool collected with or without antimicrobial treatment (see supplemental Methods). Statistical association testing of taxonomic profiles and treatment variables demonstrated that both the cocktail and individual antibiotic treatments resulted in significant perturbations in gut microbial ecology (Figure E5). Consistent with other reports (32), several members of the Firmicutes, Bacteroidetes, Actinobacteria, Deferribacteres, and Tenericutes phyla were depleted by the antibiotic treatments, with concurrent enrichment for γ-Proteobacteria clades (Figure E5B). The phylum-level relative abundances of mice given water and neomycin were quite similar (Figure E5B), but significant effects of the neomycin treatment were observed at the genera level (Figure E6), including Anaeroplasma spp and Ruminococcus gnavus, which indicated the overall antibiotic efficacy of the neomycin dose administered. Because our data showed that all individual antibiotic treatments except for neomycin attenuated O3-induced AHR (Figure 3), we investigated taxa that were significantly affected in the AMNV, ampicillin-alone, metronidazole-alone, and vancomycin-alone treatment groups, but not in the neomycin-alone treatment group. Relative to water-treated mice, Ruminococcus and Coprococcus genera of the Clostridiales order were reduced in AMNV-, ampicillin-, metronidazole-, and vancomycin-treated mice, but not in neomycin-treated mice (Figure 4), suggesting that functions of these two genera may be involved in the development of O3-induced AHR.
Figure 4.
Ruminococcus and Coprococcus genera decreased with antibiotic treatments that attenuated O3-induced AHR. 16S rRNA gene sequencing analysis indicated that the relative abundances of (A) Ruminococcus and (B) Coprococcus genera were significantly decreased in AMNV-, ampicillin-, metronidazole-, and vancomycin-treated animals but not in neomycin-treated mice. Relative abundance was assessed using MaAsLin (50) with a q value (false discovery rate corrected using the Benjamini-Hochberg correction method) < 0.25 considered significant. Each dot indicates one mouse, with n = 8 per treatment group.
Role of SCFAs
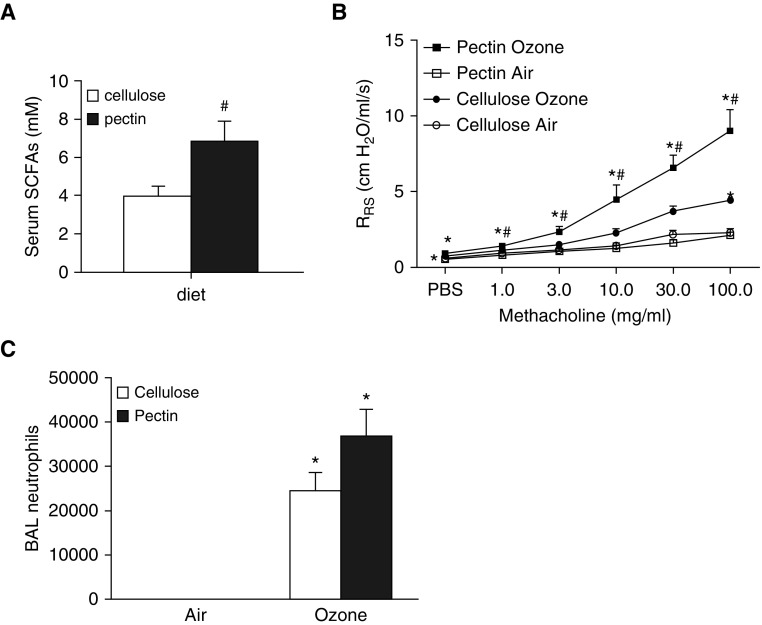
Ruminococcus and Coprococcus genera are notable fermenters of nondigestible carbohydrates, a process that yields SCFA end products, primarily acetate, propionate, and butyrate (33). SCFAs have previously been implicated in allergen-induced AHR (16). Hence, we compared serum SCFAs in water-, AMNV-, and neomycin-treated mice. As described above, AMNV treatment reduced O3-induced AHR, whereas neomycin treatment did not. Consistent with these results, serum propionate was significantly reduced in AMNV-treated mice (16.6 ± 4.3 μM; P < 0.01), but not neomycin-treated mice (43.8 ± 1.8 μM), compared with mice treated with control drinking water (39.6 ± 5.3 μM) and exposed to air. A similar trend was observed for serum acetate, but not butyrate (data not shown), consistent with reports of others indicating that butyrate produced by gut microbiota is largely cleared before reaching the systemic circulation by intestinal epithelial cells that use it for energy production (34). Other investigators have reported roles for SCFAs in microbiome-dependent effects on allergic airways disease, insulin sensitivity, and colitis (16–19). To evaluate the role of microbiota-derived SCFAs in pulmonary responses to O3, mice were placed on diets containing pectin (30% by weight). Pectin is a plant fiber that is readily fermented by gut bacteria, yielding SCFAs (16, 35). Control mice were placed on diets that were compositionally identical except that the plant fiber cellulose, which is not readily fermented by bacteria, was substituted for pectin. Indeed, compared with cellulose treatment, pectin treatment resulted in an approximately 75% increase in total serum SCFAs (Figure 5A), consistent with results of others (16). Body weight did not differ between cellulose- and pectin-treated mice (data not shown). There was no effect of diet on airway responsiveness in air-exposed mice (Figure 5B), but O3 caused a greater increase in airway responsiveness in pectin-treated mice than in cellulose-treated mice. In contrast, there was no significant difference in BAL neutrophils in pectin- versus cellulose-treated mice (Figure 5C).
Figure 5.
Effect of dietary fiber on O3-induced AHR. (A) Serum short-chain fatty acid (SCFA) levels (acetate plus propionate plus butyrate) in mice treated with diets in which 30% by weight of the food derived from either pectin or cellulose and exposed to air. (B) Airway responsiveness and (C) BAL neutrophils in cellulose- and pectin-treated mice exposed to room air or O3. Results are mean ± SE of data from n = 6–8 mice per group. *P < 0.05 compared with air; #P < 0.05 compared with cellulose.
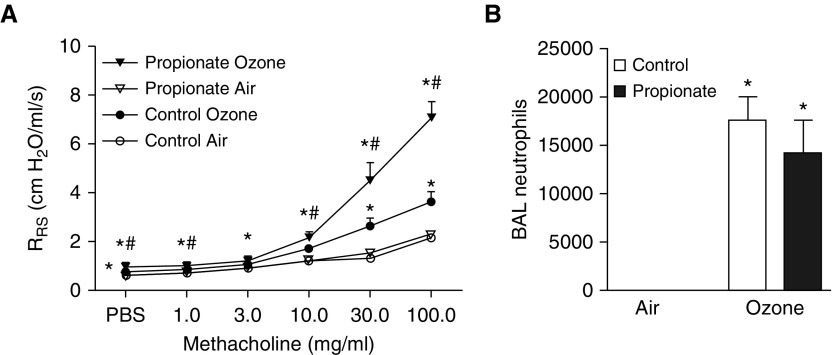
To determine whether exogenous administration of SCFAs has the capacity to impact responses to O3, we administered exogenous propionate to SPF mice for 3 days via their drinking water and then exposed them to O3. We chose to administer propionate because others have shown that it alters allergen-induced responses in the airways (16). Propionate treatment did not affect body weight (data not shown). Airway responsiveness was not affected by propionate treatment in air-exposed mice, but was significantly greater in propionate-treated versus saline-treated (control) mice exposed to O3 (Figure 6A). In contrast, BAL neutrophils were not affected by propionate treatment (Figure 6B), similar to results obtained with pectin treatment (Figure 5C). Together, our results suggest that microbiota-derived SCFAs are involved in the development of O3-induced AHR in mice.
Figure 6.
Effect of exogenous propionate on O3-induced AHR. (A) Airway responsiveness and (B) BAL neutrophils of saline-treated (control) versus propionate-treated mice exposed to room air or O3. Results are mean ± SE of data from n = 10–14 per group. *P < 0.05 compared with air; #P < 0.05 compared with control.
Discussion
Our data indicate that both GF mice and mice treated with certain types of antibiotics have reduced O3-induced AHR and inflammation. These data indicate that microbiota play a significant role in pulmonary responses to acute O3 exposure. Importantly, only the antibiotics that caused a significant reduction in two SCFA-producing genera were effective in reducing responses to O3. Taken in conjunction with our observations that 1) antibiotic treatments that attenuated O3-induced AHR also attenuated serum SCFA, 2) diets that increased serum SCFAs also increased O3-induced AHR, and 3) exogenous administration of the SCFA propionate augmented O3-induced AHR, our data suggest that the role of microbiota is mediated through their ability to produce SCFAs.
It is possible that the ability of antibiotics to attenuate responses to O3 was due to the antibiotics affecting bacteria in the lung rather than the gut. Such an explanation could account for the lack of efficacy of the neomycin treatment on O3-induced AHR, as after oral administration, both ampicillin and metronidazole are able to cross the gut epithelium, circulate systemically, and hence impact lung microbiota, whereas neomycin is not able to do so (31). However, vancomycin does not cross the gut epithelium after oral administration either (31), and others have reported no effect of oral vancomycin on lung bacterial community structure in mice even though vancomycin does substantially affect the gut microbiome (36), consistent with our observations. Nevertheless, vancomycin was as effective as ampicillin or metronidazole in reducing O3-induced AHR. Instead, the lack of a significant effect of neomycin versus the other antibiotics likely stems from differences in the gut bacteria targeted by these antibiotics.
Of the bacterial taxa affected by antibiotics, only two were significantly reduced by each of the antibiotic treatments that reduced O3-induced AHR (AMNV, ampicillin alone, metronidazole alone, and vancomycin alone) but not by treatment with neomycin alone. Bacteria in these two taxa are well-known plant degraders, fermenting complex carbohydrates into SCFAs such as acetate, propionate, and butyrate, including in human intestines (33, 37). Indeed, our data suggest that microbiota-derived SCFAs may contribute to the observed role of the microbiome in responses to O3, as both mice treated with a diet that promotes SCFA production by gut bacteria and mice treated with exogenous propionate had augmented AHR after O3 exposure.
Whereas SCFAs augmented O3-induced AHR, others have reported that increased circulating levels of SCFAs (secondary to a high-pectin diet) attenuate AHR induced by allergen (16, 18). The reason for these disparate effects of SCFAs likely lies in the different factors driving O3-induced versus allergen-induced AHR. Trompette and colleagues (16) showed that the ability of SCFAs to attenuate allergen-induced AHR was the result of reduced dendritic cell hematopoiesis leading to a diminished T-helper cell type 2 cell response. In contrast, dendritic cells do not appear to be involved in mediating the effects of O3 (38). Others have reported that the role of SCFAs in disease states differs depending on whether the innate or adaptive immune system is involved (39). For example, in models where the adaptive immune system is important, such as allergic airways disease (16) and experimental autoimmune encephalomyelitis (39), SCFA treatment is found to be beneficial. On the other hand, in models in which only the innate immune system is involved, such as the initial stages of antigen-induced arthritis and Parkinson’s disease, SCFAs exacerbate inflammation and symptom severity (39, 40). Hence, the pulmonary response to acute O3 exposure, which involves activation of the innate immune system (25), may be similarly negatively affected by SCFAs.
We do not know exactly how SCFAs affect O3-induced AHR. However, SCFAs can affect expression of certain cytokines, including some that have previously been implicated in O3-induced AHR and inflammation (30, 41). Nevertheless, when we assessed the BAL concentrations of IL-17A, osteopontin, IL-33, and GRP, each of which has been reported to play a role in O3-induced AHR in mice, cytokine levels did not differ between water-treated and antibiotic-treated mice, nor did we observe any change in BAL IL-17A, IL-33, or GRP in O3-exposed mice after propionate treatment (data not shown) even though AHR was affected. It is also possible that SCFAs could promote AHR by acting directly on airway smooth muscle cells, which express receptors for SCFAs (42). However, we found no change in serum propionate after administration of propionate via the drinking water (data not shown), indicating that the propionate we administered did not reach the lungs even though it was able to augment O3-induced AHR. These data are consistent with reports of others indicating that propionate administered via the gut is largely cleared by enterocytes and hepatocytes before reaching the systemic circulation (34, 43). These data suggest that intestinal epithelial cells and/or the liver are the likely sites of action of SCFAs in mediating the observed effects of propionate on the lung. Indeed, SCFAs cause the release of intestinal hormones such as GLP1 and PYY (44), and activate intestinal vagal afferents (45), either of which could affect the lungs.
Antibiotics and GF conditions attenuated not only O3-induced AHR but also O3-induced increases in BAL neutrophils. However, the mechanistic bases for the effects of the microbiome on AHR and neutrophils appear to differ. Whereas microbiota-dependent changes in SCFAs appear to account for the effects of the microbiome on O3-induced AHR, neither exogenous administration of SCFAs nor SCFA-producing diets had any significant effect on O3-induced changes in BAL neutrophils. Instead, microbiome-dependent effects on the production of LTB4, a chemotactic factor that is known to contribute to O3-induced neutrophil recruitment (46), may account for the effects of antibiotics and GF conditions on O3-induced neutrophil recruitment. BAL LTB4 was significantly reduced in both antibiotic-treated and GF mice. Others have reported that prebiotics that are capable of altering the microbiome also attenuate colonic LTB4 production in a rat colitis model (47). Although it is conceivable that microbiome-dependent effects on LTB4 also contributed to O3-induced AHR (48), we were unable to detect any effect of propionate administration on BAL LTB4 (data not shown) despite the marked effects of propionate on O3-induced AHR.
One technical issue requires consideration. We performed experiments in C57BL/6 mice from two different vendors: Taconic Farms and The Jackson Laboratory. Others have reported differences in the taxonomic composition of the gut microbiome in mice from these vendors (49), and our data (not shown) confirm such vendor differences. Given the observed role for the microbiome in pulmonary responses to O3, it may be surprising that mice from these two vendors had similar responses to O3 (compare water-treated mice in Figure 1 and SPF mice in Figure 2). In this respect, it is important to note that many different bacterial taxa can fill the same functional niche within the gut microbial community structure. Indeed, even though mice from the two vendors had different microbiomes, they had the same serum SCFA levels (data not shown), consistent with their similar responses to O3.
In summary, our study indicates a role for the gut microbiome in pulmonary responses to acute O3 exposure. Our findings also suggest that the ability of the microbiome to produce SCFAs likely contributes to this role. A better understanding of the relationship between the microbiome and asthma could lead to new diagnostic biomarkers for asthma and novel therapeutics, including probiotics and prebiotics.
Acknowledgments
Acknowledgment
The authors thank Dr. Lester Kobzik and Dr. Jeffrey Drazen for their insightful comments.
Footnotes
Supported by National Institutes of Health grants ES-013307, ES-024032, ES-022556, HL-007118, and ES-000002.
Author Contributions: Y.C., G.A.-A., H.T., D.I.K., T.A.B., J.D.B., J.A.M., C.H., and S.A.S. conceived and designed the experiments. Y.C., G.A.-A., H.T., D.I.K., and T.A.B. performed the experiments and analyzed the data. Y.C. wrote the paper. Y.C., G.A.-A., H.T., D.I.K., T.A.B., J.D.B., J.A.M., C.H., and S.A.S. reviewed, revised, and approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0404OC on March 12, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cernadas M. It takes a microbiome: commensals, immune regulation, and allergy. Am J Respir Crit Care Med. 2011;184:149–150. doi: 10.1164/rccm.201105-0828ED. [DOI] [PubMed] [Google Scholar]
- 2.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135:25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD.Asthma-associated differences in microbial composition of induced sputum J Allergy Clin Immunol 2013131346–352.e1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 6.Metsälä J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy. 2015;45:137–145. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- 7.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 8.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–38. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 10.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexis N, Urch B, Tarlo S, Corey P, Pengelly D, O’Byrne P, et al. Cyclooxygenase metabolites play a different role in ozone-induced pulmonary function decline in asthmatics compared to normals. Inhal Toxicol. 2000;12:1205–1224. doi: 10.1080/08958370050198548. [DOI] [PubMed] [Google Scholar]
- 12.Triche EW, Gent JF, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Low-level ozone exposure and respiratory symptoms in infants. Environ Health Perspect. 2006;114:911–916. doi: 10.1289/ehp.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauroux B, Sampil M, Quénel P, Lemoullec Y. Ozone: a trigger for hospital pediatric asthma emergency room visits. Pediatr Pulmonol. 2000;30:41–46. doi: 10.1002/1099-0496(200007)30:1<41::aid-ppul7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Schreinemachers D, et al. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991;4:72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Holtzman MJ, Cunningham JH, Sheller JR, Irsigler GB, Nadel JA, Boushey HA. Effect of ozone on bronchial reactivity in atopic and nonatopic subjects. Am Rev Respir Dis. 1979;120:1059–1067. doi: 10.1164/arrd.1979.120.5.1059. [DOI] [PubMed] [Google Scholar]
- 16.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 17.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 18.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 20.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6:e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- 22.Barreno RX, Richards JB, Schneider DJ, Cromar KR, Nadas AJ, Hernandez CB, et al. Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to methacholine. Am J Physiol Lung Cell Mol Physiol. 2013;305:L118–L129. doi: 10.1152/ajplung.00080.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathews JA, Krishnamoorthy N, Kasahara DI, Hutchinson J, Cho Y, Brand JD, et al. Augmented responses to ozone in obese mice require IL-17A and gastrin-releasing peptide. Am J Respir Cell Mol Biol. 2018;58:341–351. doi: 10.1165/rcmb.2017-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, et al. Il-33 drives augmented responses to ozone in obese mice. Environ Health Perspect. 2017;125:246–253. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, Ge MQ, Kokalari B, Redai IG, Wang X, Kemeny DM, et al. Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2016;137:571–578. doi: 10.1016/j.jaci.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Cour CD, Norlén P, Håkanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept. 2007;143:118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Degen LP, Peng F, Collet A, Rossi L, Ketterer S, Serrano Y, et al. Blockade of GRP receptors inhibits gastric emptying and gallbladder contraction but accelerates small intestinal transit. Gastroenterology. 2001;120:361–368. doi: 10.1053/gast.2001.21174. [DOI] [PubMed] [Google Scholar]
- 29.Lach E, Haddad EB, Gies JP. Contractile effect of bombesin on guinea pig lung in vitro: involvement of gastrin-releasing peptide-preferring receptors. Am J Physiol. 1993;264:L80–L86. doi: 10.1152/ajplung.1993.264.1.L80. [DOI] [PubMed] [Google Scholar]
- 30.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biddle A, Stewart L, Blanchard J, Leschine S.Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities Diversity 20135627–640 [Google Scholar]
- 34.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen HL, Lin YM, Wang YC. Comparative effects of cellulose and soluble fibers (pectin, konjac glucomannan, inulin) on fecal water toxicity toward Caco-2 cells, fecal bacteria enzymes, bile acid, and short-chain fatty acids. J Agric Food Chem. 2010;58:10277–10281. doi: 10.1021/jf102127k. [DOI] [PubMed] [Google Scholar]
- 36.Barfod KK, Vrankx K, Mirsepasi-Lauridsen HC, Hansen JS, Hougaard KS, Larsen ST, et al. The murine lung microbiome changes during lung inflammation and intranasal vancomycin treatment. Open Microbiol J. 2015;9:167–179. doi: 10.2174/1874285801509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brand JD, Mathews JA, Kasahara DI, Wurmbrand AP, Shore SA. Regulation of IL-17A expression in mice following subacute ozone exposure. J Immunotoxicol. 2016;13:428–438. doi: 10.3109/1547691X.2015.1120829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2001;164:602–607. doi: 10.1164/ajrccm.164.4.2001016. [DOI] [PubMed] [Google Scholar]
- 42.Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, et al. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep. 2016;6:38231. doi: 10.1038/srep38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters SG, Pomare EW, Fisher CA. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut. 1992;33:1249–1252. doi: 10.1136/gut.33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 46.Hicks A, Goodnow R, Jr, Cavallo G, Tannu SA, Ventre JD, Lavelle D, et al. Effects of LTB4 receptor antagonism on pulmonary inflammation in rodents and non-human primates. Prostaglandins Other Lipid Mediat. 2010;92:33–43. doi: 10.1016/j.prostaglandins.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Lara-Villoslada F, de Haro O, Camuesco D, Comalada M, Velasco J, Zarzuelo A, et al. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. Eur J Nutr. 2006;45:418–425. doi: 10.1007/s00394-006-0610-2. [DOI] [PubMed] [Google Scholar]
- 48.Stevens WH, Vanderheyden C, Wattie J, Lane CG, Smith W, O’Byrne PM. Effect of a leukotriene B4 receptor antagonist SC-53228 on ozone-induced airway hyperresponsiveness and inflammation in dogs. Am J Respir Crit Care Med. 1995;152:1443–1448. doi: 10.1164/ajrccm.152.5.7582275. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov II, Frutos RdeL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]