Abstract
The lung epithelial glycocalyx is a carbohydrate-enriched layer lining the pulmonary epithelial surface. Although epithelial glycocalyx visualization has been reported, its composition and function remain unknown. Using immunofluorescence and mass spectrometry, we identified heparan sulfate (HS) and chondroitin sulfate within the lung epithelial glycocalyx. In vivo selective enzymatic degradation of epithelial HS, but not chondroitin sulfate, increased lung permeability. Using mass spectrometry and gel electrophoresis approaches to determine the fate of epithelial HS during lung injury, we detected shedding of 20 saccharide-long or greater HS into BAL fluid in intratracheal LPS-treated mice. Furthermore, airspace HS in clinical samples from patients with acute respiratory distress syndrome correlated with indices of alveolar permeability, reflecting the clinical relevance of these findings. The length of HS shed during intratracheal LPS-induced injury (≥20 saccharides) suggests cleavage of the proteoglycan anchoring HS to the epithelial surface, rather than cleavage of HS itself. We used pharmacologic and transgenic animal approaches to determine that matrix metalloproteinases partially mediate HS shedding during intratracheal LPS-induced lung injury. Although there was a trend toward decreased alveolar permeability after treatment with the matrix metalloproteinase inhibitor, doxycycline, this did not reach statistical significance. These studies suggest that epithelial HS contributes to the lung epithelial barrier and its degradation is sufficient to increase lung permeability. The partial reduction of HS shedding achieved with doxycycline is not sufficient to rescue epithelial barrier function during intratracheal LPS-induced lung injury; however, whether complete attenuation of HS shedding is sufficient to rescue epithelial barrier function remains unknown.
Keywords: heparan sulfate, epithelial glycocalyx, syndecan, acute respiratory distress syndrome
The glycocalyx, a carbohydrate-enriched layer lining the cell surface, has long been an understudied cellular structural element. Artifactual degradation of the glycocalyx during tissue fixation has made visualizing and studying cell glycocalyces difficult, especially in whole-tissue preparations (1). The use of periodic acid–Schiff and colloidal iron staining allowed for the first description of the glycocalyx by identifying negatively charged carbohydrates on the surface of numerous cell types (2). Since these initial studies, electron microscopy and immunohistochemistry have been increasingly used to visualize glycocalyces on numerous cell types in several different organs, including on the pulmonary endothelial and epithelial surfaces (3, 4). The pulmonary endothelial glycocalyx is known to contribute to critical vascular functions, such as endothelial barrier integrity, mechanotransduction of shear stress, regulation of vascular tone, and inhibition of leukocyte adhesion (5–9). In contrast, the structure and function of the pulmonary epithelial glycocalyx, and its fate during lung injury, remain nearly entirely unknown.
Heparan sulfate (HS) is a glycosaminoglycan (GAG) that critically contributes to the structure and function of the glycocalyx in numerous cell types (10, 11). HS is anchored to the cell surface through covalent bonds to transmembrane proteoglycans; together, these protein–HS complexes are termed “HS proteoglycans” (HSPGs). HS is synthesized in the Golgi apparatus through copolymerization of glucosamine and glucuronic acid disaccharide units. The resulting linear copolymer may be further modified by epimerization of glucuronic acid to iduronic acid, as well as sulfation at specific sites within the repeating dissacharide unit: at the amino (N-), 6-O, or 3-O position of glucosamine and/or the 2-O position of iduronic acid (see Figure E2A in the data supplement) (12). Sequences of sulfation of HS at various sites impart HS with a landscape of negative charges that allows HS to bind proteins with specificity, thereby regulating their function through electrostatic interactions. Although others have demonstrated expression of proteoglycans in the lung epithelium and have studied the effect of HS-modifying enzymes on lung epithelial cells, the structure and function of epithelial cell surface HS in the healthy adult lung is unknown (13–16).
During tissue inflammation and injury, the structure and function of glycocalyx HS can be disrupted (8, 17, 18). After nonpulmonary sepsis-induced (i.e., endothelial-targeted) lung injury, endothelial glycocalyx HS is degraded by heparanase (a HS-specific mammalian endoglycosidase), releasing small (≤8 saccharide-long) HS fragments into the circulation (19). This septic HS degradation disrupts the critical vascular functions of the endothelial glycocalyx, resulting in lung edema and neutrophil adhesion—two pathogenic hallmarks of sepsis-induced acute lung injury and the acute respiratory distress syndrome (ARDS) (8). In contrast, epithelial insults do not appear to primarily target the endothelial glycocalyx, as human studies demonstrate a relative paucity of circulating HS and HSPGs after “direct” (i.e., epithelial-targeted) pulmonary injury (19, 20). Instead, these direct pulmonary insults may degrade epithelial HS. Bleomycin-induced lung injury induces matrix metalloproteinase (MMP)-7–mediated shedding of syndecan-1 (an epithelial HSPG) into the alveolar space, and in vitro models of alveolar epithelial injury are characterized by shedding of syndecans 1 and 4 via activation of disintegrin and metalloproteinase (ADAM)-17 (13, 14). Reactive oxygen species (ROS) may additionally serve as HSPG “sheddases” during acute lung injury, as proteoglycan and HS fragmentation can be prevented by extracellular superoxide dismutase (EC-SOD) (21). Despite this previous literature, the specific effect of HS shedding on lung injury and epithelial permeability in vivo has not been studied. As such, we aimed to identify the presence and function of HS within the healthy lung epithelial glycocalyx, and we hypothesized that intact epithelial HS is critical for alveolar barrier function maintenance, and that shedding of HS from the lung epithelium contributes to the increased lung permeability during intratracheal injury.
Methods
Materials
Materials used are listed in the supplementary Methods.
Animals
The animals used are listed in the supplementary Methods.
Alveolar Epithelial HS and Chondroitin Sulfate Degradation
To confirm the presence, and determine the sulfation pattern and effect of epithelial HS and chondroitin sulfate (CS) degradation, we anesthetized mice with inhaled isoflurane and, using a mouse laryngoscope, intratracheally instilled 15 U active or heat-inactivated (HI) heparinase I/III in 40 μl to degrade epithelial HS, or 2 U active or HI chondroitinase ABC in 40 μl to degrade epithelial CS. For experiments in which mice were treated with heparinase I/III–generated HS fragments, 8.75 μg full-length HS (Celsus Laboratories), was treated with 4.375 U active or HI heparinase I/III for 3 hours at 37°C. The heparinase–HS mixture was then heat inactivated at 100°C for 15 minutes and intratracheally instilled. Animals were killed 12, 24, and/or 72 hours after instillation and samples collected as detailed in the supplementary Methods.
Intratracheal LPS-induced Lung Injury
To induce intratracheal LPS-mediated lung injury, animals were intratracheally instilled, as described previously here, with 3 mg/kg LPS from Escherichia coli O55:B5 or PBS (30 μl) and killed 12 hours, 2 days, 4 days, and/or 6 days after instillation. For experiments containing TNF-α protease inhibitor-2 (TAPI-2), mice were treated concurrently with intratracheal LPS and 1 mg/kg TAPI-2, a dose sufficient to inhibit ADAM-17 (22), and killed 2 days after instillation. For experiments containing doxycycline, mice were treated with 70 mg/kg oral doxycycline hyclate by gavage (a dose sufficient to inhibit broad-spectrum MMP activity [23]) every 24 hours beginning 3 days before intratracheal LPS until being killed 2 days after intratracheal LPS instillation. For experiments using EC-SOD overexpressing and MMP-9 knockout (MMP9ko) mice, animals were killed 2 days after intratracheal LPS instillation. After death, samples were collected as detailed in the supplementary Methods.
Heat and Moisture Exchanger Collection
The Vanderbilt University Medical Center Institutional Review Board approved this minimal-risk study with a waiver of informed consent, as heat and moisture exchanger (HME) filters are part of routine clinical care, and used filters are typically discarded. Subjects were eligible if they were invasively mechanically ventilated and had bilateral radiographic infiltrates consistent ARDS (24). HME filters were collected and processed as described in the supplementary Methods.
Isolation, Quantification, and Size Determination of HME Fluid, BAL Fluid, and Plasma HS and CS
HS and CS were isolated from plasma, BAL fluid, and HME fluid by GAG disaccharide digestion (25). HS and CS disaccharide analyses were then performed using liquid chromatography–tandem mass spectrometry (LC-MS/MS) multiple reaction monitoring (MRM), as detailed in the supplementary Methods. BAL sulfated GAG size was determined by PAGE and Alcian blue/silver staining, as detailed in the supplementary Methods.
Lung/Alveolar Permeability, Edema, and Inflammation Quantification
BAL cell counts, total BAL protein, BAL albumin, and lung wet/dry ratio were quantified as detailed in the supplementary Methods.
Molecular Biology Analyses
Immunohistochemistry, Western blotting, gelatin zymography, RNA isolation, cDNA synthesis, and qRT-PCR were performed as detailed in the supplementary Methods.
Statistical Analyses
Statistical analyses were performed as detailed in the supplementary Methods.
Results
Epithelial Glycocalyx HS Contributes to Epithelial Barrier Function
To confirm the existence of HS within the pulmonary epithelial glycocalyx, we treated mice with intratracheal heparinase I/III, a combination of recombinant bacterial enzymes that specifically degrade HS, to remove any epithelial HS in vivo (Figure 1A). We then stained inflated, frozen, unfixed mouse lung tissue for HS. Mice treated with enzymatically inactive (HI) heparinase I/III demonstrated diffuse expression of alveolar epithelial HS, as visualized by HS (HS 10E4 antibody) colocalization with the peripheral airway/alveolar epithelial marker, Lycopersicon esculentum agglutinin (LEA) lectin (Figure 1B). In contrast, mice treated with enzymatically active heparinase I/III demonstrated a patchy loss of epithelial HS expression, consistent with the patchy distribution of intratracheal instillation, confirming antibody specificity and epithelial HS loss. HS degradation by intratracheal heparinase I/III instillation was constrained to the epithelium, as LC-MS/MS demonstrated HS shedding into the alveolar airspace (Figure 1C), but not the circulation (Figure 1D). There was minimal airway HS expression by HS 10E4 immunofluorescence (Figure E1), suggesting that HS fragments released into the BAL after heparinase I/III largely reflect alveolar epithelial HS. Any contribution of airway mucus HS (i.e., not epitheilial anchored) is likely reflected in the BAL HS of mice treated with HI heparinase I/III. Taken together, these data suggest that the increase in BAL HS measured after intratracheal heparinase I/III instillation largely reflects fragmentation of alveolar epithelial cell surface HS. However, we cannot exclude the epithelial basement membrane/extracellular matrix as an additional source of BAL HS after heparinase I/III. Furthermore, we cannot exclude the contribution of airway epithelial HS that may not be detected by the 10E4 antibody (26).
Figure 1.
Epithelial heparan sulfate (HS) degradation increases lung permeability but not inflammation. (A) Mice were instilled with 15 U intratracheal (IT) active or heat-inactivated (HI) heparinase I/III (Hep I/III or HI Hep I/III). (B) At 12 hours after instillation frozen lungs were sectioned and stained for HS (HS 10E4, green), and the peripheral airway/alveolar epithelium (Lycopersicon esculentum agglutinin [LEA] lectin, red). Scale bar: 100 μm. At 12, 24, and 72 hours after instillation, BAL fluid and plasma were obtained and (C and D) BAL and plasma HS concentration, (E) BAL protein, and (F) BAL albumin were measured. At 24 hours after instillation (G), lung wet/dry weight ratio was measured. At 12, 24, and 72 hours after instillation (H), BAL neutrophils were measured. (B) n = 3; (C–H) n = 4–7. *P < 0.05.
Mass spectrometry revealed that HS released from the lung epithelium after heparinase I/III was heavily N- and 2-O sulfated (NS2S), with 55% of HS disaccharides being jointly sulfated at these two sites, indicating that the alveolar epithelial glycocalyx HS is enriched with sulfation at the N- and 2-O positions (Figure E2B).
We measured BAL protein, BAL albumin, and lung wet/dry ratio, and performed BAL neutrophil cell counts in mice treated with intratracheal active or HI heparinase I/III to determine if epithelial HS contributes to the maintenance of alveolar epithelial barrier function or if epithelial HS degradation increases lung inflammation. Intratracheal active heparinase I/III treatment increased BAL total protein and albumin 12, 24, and 72 hours after instillation in comparison to HI heparinase I/III treatment (Figures 1E and 1F), indicating that epithelial HS contributes to the lung epithelial barrier to protein. In contrast, there was no change in lung wet/dry ratio in mice treated with active heparinase I/III in comparison to heat-inactived heparinase I/III, suggesting that epithelial HS degradation does not contribute to the lung epithelial barrier to fluid (Figure 1G). We detected no increase in BAL neutrophils after heparinase I/III treatment (Figure 1H), indicating that the increase in protein permeability after heparinase I/III treatment was not due to alveolar inflammation.
To determine if other negatively charged GAGs also contribute to the epithelial barrier, we treated mice with intratracheal chondroitinase ABC, a mixture of recombinant bacterial CS lyases, to remove epithelial CS in vivo (Figure E3A). Although treatment with active chondroitinases removed alveolar epithelial surface CS (Figure E3B), loss of CS did not result in an increase of BAL protein or BAL neutrophils (Figures E3C and E3D), suggesting that not all sulfated GAGs contribute to the lung epithelial barrier.
Heparinase I/III–generated HS Fragments Do Not Increase Lung Epithelial Permeability
Although others have shown that HS fragments can act as damage-associated molecular patterns (DAMPs) and induce inflammation through Toll-like receptor 4 signaling, we detected no increase in BAL neutrophilia after heparinase I/III treatment (Figure 1H) (27, 28). To confirm that heparinase I/III–generated HS fragments do not increase lung epithelial protein permeability by functioning as DAMPs, we treated mice with full-length HS pretreated ex vivo with either heparinase I/III (at 37°C for 3 h) to generate HS fragments, or HI heparinase I/III as control. After ex vivo HS fragmentation, the heparinase–HS mixture was then HI before intratracheal instillation (Figure 2A). As HS is resistant to heat inactivation, this approach ceases heparinase I/III activity without interfering with any DAMP-like effect of HS (29). We observed no increase in BAL protein or neutrophils after intratracheal instillation of heparinase I/III–treated HS in comparison to HI heparinase I/III–treated HS (Figures 2B and 2C). Furthermore, we detected no increase in mRNA expression of proinflammatory cytokines TNF-α, IL-6, or IL-1β in whole lung tissue from mice treated with intratracheal heparinase I/III–treated HS in comparison to HI heparinase I/III–treated HS (Figure 2D). Although there was a trend toward increased TNF-α expression in whole lung from mice treated with intratracheal heparinase I/III–treated HS (in comparison to HI heparinase I/III–treated HS), the overall absence of BAL neutrophilia in mice treated with heparinase I/III (Figure 1H) or heparinase-I/III–treated HS (Figure 2C) is supporting evidence that intratracheal heparinase I/III does not significantly increase alveolar inflammation. Taken together, these results indicate that HS on the epithelial cell surface contributes to the lung epithelial barrier to protein, and that the HS fragments generated after heparinase I/III treatment are not responsible for the increase in lung permeability observed after intratracheal heparinase I/III instillation.
Figure 2.
Exogenous Hep I/III–generated HS fragments do not increase lung permeability or inflammation. (A) Exogenous HS (8.75 μg) was pretreated with 4.375 U of active or HI Hep I/III. The heparinase–HS mixture was then heat inactivated and intratracheally instilled. At 24 hours after intratracheal instillation of active or HI Hep I/III–treated HS, (B) BAL protein, (C) BAL neutrophils, and (D) whole lung TNF-α, IL-6, and IL-1β mRNA expression was measured. (B–D) n = 4–5.
HS Is Released into the Airspace during Intratracheal LPS-induced Lung Injury in Mice and ARDS in Humans
Given the observed contributions of HS to the pulmonary epithelial barrier, we sought to determine if epithelial HS shedding occurs during acute lung injury, a disease state characterized by increased lung epithelial permeability. Although other groups have shown that syndecans are shed from the epithelial cell surface in response to inflammatory insults, HS shedding during direct lung injury has, to our knowledge, never been investigated (13, 14). We induced acute lung epithelial injury in mice via intratracheal instillation of 3 mg/kg LPS, a dose sufficient to induce alveolar hyperpermeability (as measured by BAL protein) and histologic injury (Figures 3A–3C). Using LC-MS/MS MRM, we observed that, during injury, there was increased alveolar, but not plasma, HS 12 hours, 2 days, and 4 days after intratracheal LPS administration, in comparison to PBS control (Figures 3D and 3E). The increase in alveolar HS after LPS instillation persisted after BAL high-speed centrifugation (data not shown), suggesting that the HS detected in the BAL fluid is shed and not anchored to dead and sloughed epithelial cells. In contrast to the NS2S-enriched HS detected in the BAL fluid after intratracheal heparinase I/III, the BAL HS was heavily unsulfated in mice treated with intratracheal LPS (92%, 84%, and 85% unsulfated [0S] 12 h, 2 d, and 4 d after LPS instillation; Figure E4). Consistent with our findings that epithelial HS functions to maintain epithelial barrier function, in animals treated with intratracheal LPS, alveolar HS shedding correlated with the degree of lung permeability as measured by BAL protein (Figure 3F). Together, these results indicate that HS shedding/degradation may contribute to the lung epithelial barrier dysfunction during intratracheal LPS-induced lung injury.
Figure 3.
Increased airspace HS is detected during intratracheal LPS-induced lung injury in mice and in patients with acute respiratory distress syndrome (ARDS). (A) Mice were intratracheally instilled with 3 mg/kg LPS or PBS as control. At 12 hours and 2, 4, and 6 days after intratracheal instillation, (B) BAL protein was measured. (C) Hematoxylin and eosin staining was performed on lung sections from Day 2 after intratracheal LPS and PBS instillation. At 12 hours and 2, 4, and 6 days after intratracheal instillation, (D) BAL and (E) plasma HS was measured. Scale bars: 100 μm. (F) At 12 hours and 2 and 4 days after intratracheal instillation, BAL protein–HS correlation was analyzed by linear regression. (G) Protein and HS were measured in heat and moisture exchanger (HME) fluid from patients with ARDS, and HME protein–HS correlation was analyzed by linear regression. (B, D, and E) n = 3–9; (C) n = 3; (F) n = 10; (G) n = 15. *P < 0.05.
We next sought to determine whether HS shedding/degradation similarly occurs in human patients with ARDS, and if the amount of airspace HS correlates with lung permeability. In patients with ARDS, alveolar edema fluid contents can be accurately measured in fluid collected from endotracheal tube HMEs, allowing for a minimally invasive approach to airspace sampling in critically ill patients (30). We collected HME fluid from 15 patients with ARDS and measured HME total protein and HS concentrations (Table E1). In accordance with our findings in acute lung injury in mice, HME HS concentration correlated with the total protein concentration in patients with ARDS (Figure 3G). In addition, we detected a modest trend toward an increase in HME HS in patients with ARDS with “direct lung injury” due to pneumonia/aspiration in comparison to patients with “indirect lung injury” due to sepsis (Figure E5A). To ensure that the three patients with sepsis and ARDS were not “uninjured” subjects skewing the correlation between HME HS and HME protein, we performed sensitivity analyses excluding these three patients, as well as three additional patients with similarly low HME HS and protein. After excluding these patients, the HME HS–protein correlation still remained significant (Figure E5B), strengthening our findings that alveolar HS shedding may contribute to the epithelial barrier dysfunction during lung injury. Furthermore, the HME HS in patients with ARDS was similarly heavily unsulfated (66% 0S), like the BAL HS detected during intratracheal LPS-induced lung injury (Figure E5C). Together, these observations support the clinical relevance of our animal findings.
HS Shed during Intratracheal LPS-induced Lung Injury Is Long and Is Accompanied by Shedding of Epithelial HSPGs SYNDECAN-1 and Syndecan-4
We sought to identify the sheddase(s) responsible for alveolar HS shedding and to determine if sheddase inhibition reduces lung permeability after intratracheal LPS. Several enzymes and reactive molecules have the ability to degrade HSPGs, either by fragmenting HS into small oligosaccharides or by releasing full-length HS through cleavage of the anchoring proteoglycan (Figure 4A) (17). Our group has previously shown that nonpulmonary sepsis induces heparanase-mediated degradation of endothelial HS into small (six- to eight-saccharide) circulating fragments (8, 19). In contrast, after intratracheal LPS instillation, we observed shedding of long HS polysaccharides (≥20 saccharides in length) into the airspace (Figure 4B), suggesting the presence of sheddases targeting the proteoglycan core protein. Accordingly, we observed an increase in BAL syndecan-1 (sevenfold) and syndecan-4 (20.4-fold) ectodomains from mice treated with LPS in comparison to PBS, further suggesting that release of alveolar HS into the BAL after LPS occurs via cleavage of the proteoglycan anchor (Figures 4C and 4D).
Figure 4.
Increased airspace HS after intratracheal LPS instillation is long, and is accompanied by increased airspace syndecan-1 and syndecan-4. (A) Heparanase, reactive oxygen species (ROS), matrix metalloproteinase (MMP)-2, -7, and -9, and activation of disintegrin and metalloproteinase (ADAM)-17 cleave HS proteoglycans at distinct sites. (B) PAGE and Alcian blue/silver staining was performed on BAL fluid 2 and 4 days after intratracheal LPS instillation. dp = degree of polymerization; GAG = glycosaminoglycan. (C and D) BAL syndecan-1 and syndecan-4 were measured via Western blot from mice 2 days after intratracheal LPS instillation. (B) n = pool of 6–7 individual samples; (C and D) n = 5. *P < 0.05.
MMPs Mediate Alveolar HS Shedding during Intratracheal LPS-induced Lung Injury
Although several enzymes and reactive molecules are known to cleave HSPG core proteins, ADAM-17, MMP-7, and ROS quenchable by EC-SOD have been shown to cleave syndecans from lung epithelial cells in vitro or in the airspace in vivo (13, 14, 21). To determine the mechanism of proteoglycan cleavage after intratracheal LPS, we accordingly employed in vivo pharmacologic and transgenic inhibitory approaches to target EC-SOD–quenchable ROS, ADAM-17, and MMPs. We observed no reduction in LPS-induced alveolar HS shedding (or BAL protein) in alveolar epithelial type II EC-SOD–overexpressing mice (Figures E6A and E6B) or in wild-type mice treated with the ADAM-17 inhibitor, TAPI-2 (at a dose demonstrated to prevent ADAM-17 activity [22]) (Figures E6C and E6D).
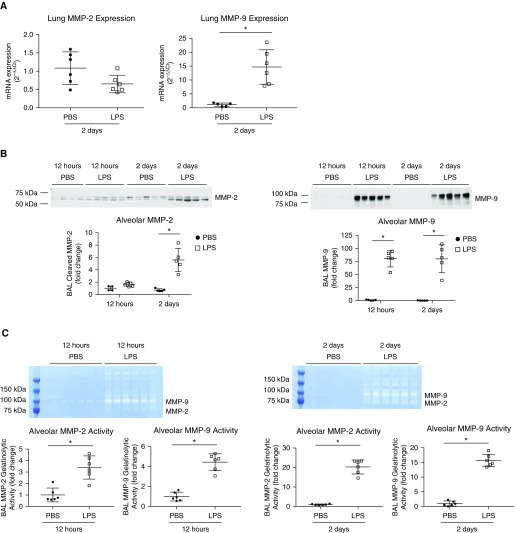
As MMP-2, -7, and -9 are also known to cleave HSPGs (17), we measured MMP-2, -7, and -9 mRNA expression in whole-lung tissue, BAL MMP-2, -7, and -9 protein, and BAL MMP-2 and -9 activity by gelatin zymography in mice treated with intratracheal LPS. We observed an increase in whole-lung MMP-9 mRNA expression in mice 2 days after intratracheal LPS instillation (Figure 5A), whereas there was no change in whole-lung MMP-2 mRNA, and undetectable or very low MMP-7 mRNA (in both groups; data not shown) in comparison to PBS. However, we detected an increase in both BAL MMP-2 and -9 protein (by Western blot) and activity (by zymography) in mice treated with intratracheal LPS (Figures 5B and 5C). Similar to whole-lung MMP-7 mRNA expression, BAL MMP-7 protein was undetectable in animals treated with intratracheal LPS or PBS (data not shown).
Figure 5.
Lung MMP-9 mRNA expression and BAL MMP-2 and -9 protein and activity are increased after intratracheal LPS instillation. (A) Whole-lung MMP-2 and -9 mRNA expression was measured in mice 2 days after intratracheal LPS or PBS instillation. (B) BAL MMP-2 and -9 protein and (C) gelatinolytic activity were measured by Western blot and zymography in mice 12 hours and 2 days after intratracheal LPS or PBS instillation. (A–C) n = 4–6. *P < 0.05.
To determine if MMPs, including MMP-2 and -9, mediate alveolar HS shedding, we treated mice daily with oral doxycycline, a broad-spectrum MMP inhibitor, from 3 days before intratracheal LPS instillation until the animals were harvested 2 days after LPS instillation (Figure 6A). In mice treated with intratracheal LPS and oral doxycycline, we observed a 42% decrease in both alveolar HS and syndecan-1, but no decrease in syndecan-4, in comparison to mice treated with intratracheal LPS and oral water as control (Figures 6B and 6D).
Figure 6.
Doxycycline partially inhibits the increase in airspace HS and syndecan-1, but not syndecan-4 or BAL protein, after intratracheal LPS instillation. (A) Mice were treated with 70 mg/kg oral doxycycline by gavage or water as control every 24 hours, starting 3 days before intratracheal instillation of LPS. Mice were harvested 2 days after intratracheal LPS instillation. BAL (B) HS, (C) syndecan-1, (D) syndecan-4, (E) protein, and (F) neutrophils were measured. (B) n = 9; (C and D) n = 6; (E) n = 9–10; (F) n = 10. *P < 0.05.
Given our initial findings that epithelial HS degradation increases lung permeability, we hypothesized that inhibition of HS shedding by doxycycline would partially restore alveolar barrier function and reduce BAL protein after intratracheal LPS instillation. In mice treated with doxycycline, we observed a nonsignificant trend toward protection against lung permeability (Figure 6E). In addition, in accordance with our findings that epithelial HS degradation does not increase lung inflammation, we observed no change in BAL neutrophils in mice treated with intratracheal LPS and oral doxycycline in comparison to mice treated with intratracheal LPS and oral water (Figure 6F). The limited effect of MMP inhibition on barrier protection, taken together with the data showing that intratracheal heparinase I/III increases permeability (Figure 1), indicates that HS shedding/degradation is sufficient to increase lung permeability, but is not necessary to induce the lung hyperpermeability observed during LPS-mediated intratracheal lung injury.
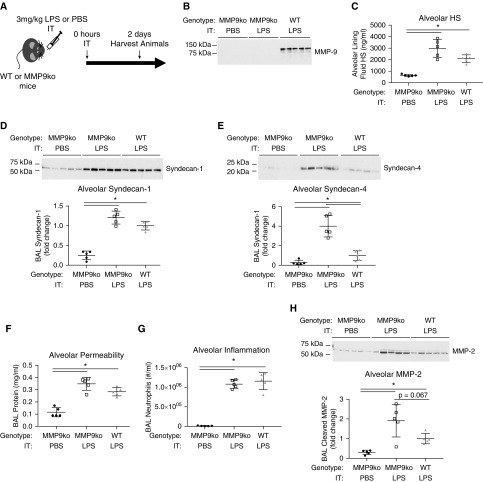
Considering that doxycycline partially attenuated alveolar HS and syndecan-1 shedding, and that MMP-9 protein and activity is abundantly increased in mice treated with intratracheal LPS, we sought to determine if MMP-9 mediates alveolar HS shedding during intratracheal LPS-induced lung injury by using MMP9ko mice (Figure 7A). After confirming a loss of BAL MMP-9 in MMP9ko mice (Figure 7B), we measured BAL HS, syndecan-1, and syndecan-4 in MMP9ko mice treated with intratracheal LPS in comparison to wild-type mice treated with intratracheal LPS and MMP9ko mice treated with intratracheal PBS as control. In contrast to our hypothesis, we detected a trend toward increased BAL HS and syndecan-1, and increased BAL syndecan-4 in MMP9ko mice treated with intratracheal LPS in comparison to wild-type mice treated with LPS (Figures 7C–7E). There was also a trend toward increased BAL protein, but no change in BAL neutrophils in LPS-treated MMP9ko mice in comparison to wild-type mice (Figures 7F and 7G). To investigate this paradoxical effect, we measured BAL MMP-2 protein to determine if there was a compensatory increase of MMP-2 in MMP9ko mice after LPS, as has been observed in other disease models (31, 32). Indeed, we observed a trend toward increased BAL MMP-2 protein in MMP9ko mice treated with intratracheal LPS in comparison to wild-type mice treated with intratracheal LPS (Figure 7H). These findings indicate that MMPs partially mediate alveolar HS shedding during intratracheal LPS-induced lung injury, and that inhibition of MMP-9 alone does not protect against alveolar HS shedding, potentially due to compensatory upregulation of other HSPG sheddases. Of note, MMP2ko mice have developmental defects in alveolization, preventing their use to specifically interrogate the role of MMP-2 in intratracheal LPS-induced HS shedding (33).
Figure 7.
MMP-9 knockout (MMP9ko) mice are not protected from alveolar HS, syndecan-1, or syndecan-4 shedding, or BAL protein or neutrophilia, and exhibit increased BAL MMP-2 protein after intratracheal LPS instillation. (A) MMP9ko and wild-type (WT) mice were treated with intratracheal LPS, and MMP9ko mice were treated with intratracheal PBS as control. (B) A loss of BAL MMP-9 was confirmed by Western blot in MMP9ko mice in comparison to wild-type mice. BAL (C) HS, (D) syndecan-1, (E) syndecan-4, (F) protein, (G) neutrophils, and (H) MMP-2 protein were measured. (B–H) n = 5. *P < 0.05.
Discussion
The epithelial glycocalyx remains a largely understudied component of the lung, despite its discovery nearly 50 years ago (3). Although the proteoglycans, syndecan-1 and syndecan-4, are known to be expressed in the lung epithelium, this report identifies the structure and function of HS on the surface of the healthy adult lung epithelium (13, 14). Using immunofluorescence and state-of-the-art LC-MS/MS MRM analyses (25), we not only demonstrated the presence of HS within the lung epithelium, but also that epithelial HS is enriched in disaccharides sulfated on the nitrogen of glucosamine and 2-position of iduronic acid (NS2S). These patterns of sulfation influence the biological function of HS, as the molecular distribution of negative charge imparted by sulfate groups allow HS to bind (with specificity) to positively charged residues of proteins and regulate their function (12, 34). Interestingly, NS2S HS is known to bind fibroblast growth factor-10, a master regulator of lung epithelial development and repair, and binding of fibroblast growth factor-10 to HS is required for its function in airway development (35–37). By determining the sulfation pattern of the lung epithelial HS, these findings may provide insight into additional lung epithelial HS binding partners and functions.
Our findings additionally demonstrate that lung epithelial HS contributes to the lung epithelial barrier to protein. These observations are consistent with findings published by Saumon and colleagues (38), demonstrating that instillation of airway protamine, a positively charged protein that can bind to and neutralize the negative charge of sulfated GAGs and other molecules, increases lung epithelial permeability and are similar to findings from the intestinal, bladder, and corneal epithelium (38–42). Although the mechanisms by which lung epithelial HS promotes barrier integrity remain unknown, findings from the lung endothelium and the corneal epithelium suggest that cell glycocalyx-HS regulates the expression and localization of tight junction, adherens junction, and gap junction proteins, structures known to be involved in epithelial and endothelial barrier function (39, 43). In addition, cell surface HS may serve as a nonsignaling, structural constituent of the epithelial barrier, similar to the “charged meshwork” of the endothelial glycocalyx that opposes transvascular protein flux (44). Future studies are needed to determine relative contributions of epithelial HS to barrier-regulating, outside-in cellular signaling processes, as well as to the physical barrier that opposes epithelial permeability.
During intratracheal LPS-induced lung injury and ARDS, a disease state characterized by increased lung permeability, we detected shedding of HS, accompanied by syndecan-1 and syndecan-4, into the airspace. These data complement the relative paucity of circulating HS observed in mice treated with intratracheal LPS (Figure 3E) or in patients with ARDS with pneumonia versus nonpulmonary sepsis (19, 20), indicating that during ARDS, HS shedding/degradation occurs in an injury-inducible, compartment-specific fashion. As such, HME/edema fluid and plasma HS may serve as biomarkers to quantify the extent of epithelial and endothelial injury, respectively, in patients with ARDS. Inexpensive and rapid identification of HS in the plasma and HME/edema fluid is theoretically possible using colorimetric dimethylmethylene blue detection; however, the sensitivity, specificity, and feasibility of using plasma and HME/edema fluid dimethylmethylene blue as a biomarker remains to be seen (45).
Furthermore, HS shedding into the airspace may prove more than just a biomarker during intratracheal LPS-induced lung injury and ARDS due to pneumonia/aspiration. Although we originally hypothesized that epithelial HS shedding may contribute to lung hyperpermeability during acute lung injury, epithelial HS shedding may alternatively be evolutionarily beneficial during lung injury and infection. Many viruses and bacteria use HS as a receptor to adhere to and infect host cells, including Staphylococcus aureus and Streptococcus pneumoniae, common causes of hospital- and community-acquired pneumonia (11, 46–48). In addition, soluble HS can act as a decoy receptor to bind and inhibit the adherence of S. aureus and S. pneumoniae to lung epithelial cells. Thus, epithelial HS shedding during infection may be beneficial to inhibit the propagation of infection by both depleting bacterial receptors on host cells and increasing soluble HS decoy receptors. This evolutionary importance would support the presence of redundant, insult-specific pulmonary epithelial sheddases. Indeed, administration of high-dose doxycycline (using doses previously demonstrated to inhibit lung MMP activity [23]) only partially reduced HS and syndecan-1 shedding and did not affect syndecan-4 shedding. Furthermore, MMP9ko mice were not protected from HS, syndecan-1, and syndecan-4 shedding (potentially due to a compensatory increase in MMP-2) during intratracheal LPS-induced lung injury. Together, these data suggest the presence of redundant sheddases responsible for HS release into the airspace in addition to MMPs.
In conclusion, we employed state-of-the-art glycomic approaches (including LC-MS/MS MRM analyses) to identify the presence and sulfation of HS within the lung epithelial glycocalyx. We identified a novel role for lung epithelial HS as a contributor to lung barrier function, showing that a loss of epithelial HS is sufficient to increase lung protein permeability. We determined that MMPs shed HS during intratracheal LPS-mediated lung injury and that HS shedding is sufficient, but not necessary, to induce lung permeability, as attenuation of HS shedding did not significantly reduce lung permeability after intratracheal LPS. These findings highlight novel functions of the lung epithelial HS that may serve as a foundation for future mechanistic investigations as well as human biomarker studies.
Acknowledgments
Acknowledgment
The authors acknowledge Peter Henson (National Jewish Health, Denver, CO) for his intellectual advice on the experiments performed and William Parks (Cedars Sinai, Los Angeles, CA) for technical advice on matrix metalloproteinase experiments.
Footnotes
This work was supported by National Institutes of Health grants HL125371 and HL132424.
Author Contributions: S.M.H., E.N.-G., R.L.Z., R.M.T., J.A.B., R.J.L., and E.P.S. contributed to the conception and design; S.M.H., X.L., X.H., J.B.M., K.O., S.A.M., Y.Y., Y.O., and F.Z. contributed to the analysis and interpretation of results; S.M.H., K.O., S.A.M., Y.Y., F.Z., E.N.-G., R.L.Z., J.A.B., R.J.L., and E.P.S. contributed to manuscript revision.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0428OC on March 27, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lai M, Lampert IA, Lewis PD. The influence of fixation on staining of glycosaminoglycans in glial cells. Histochemistry. 1975;41:275–279. doi: 10.1007/BF00497691. [DOI] [PubMed] [Google Scholar]
- 2.Rambourg A, Neutra M, Leblond CP. Presence of a “cell coat” rich in carbohydrate at the surface of cells in the rat. Anat Rec. 1966;154:41–71. doi: 10.1002/ar.1091540105. [DOI] [PubMed] [Google Scholar]
- 3.Brooks RE. Ruthenium red stainable surface layer on lung alveolar cells; electron microscopic interpretation. Stain Technol. 1969;44:173–177. doi: 10.3109/10520296909063346. [DOI] [PubMed] [Google Scholar]
- 4.Bignon J, Jaubert F, Jaurand MC. Plasma protein immunocytochemistry and polysaccharide cytochemistry at the surface of alveolar and endothelial cells in the rat lung. J Histochem Cytochem. 1976;24:1076–1084. doi: 10.1177/24.10.789758. [DOI] [PubMed] [Google Scholar]
- 5.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, et al. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285:L986–L995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 6.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, et al. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–H726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 10.Haeger SM, Yang Y, Schmidt EP. Heparan sulfate in the developing, healthy, and injured lung. Am J Respir Cell Mol Biol. 2016;55:5–11. doi: 10.1165/rcmb.2016-0043TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 12.Lindahl U, Kusche-Gullberg M, Kjellén L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 14.Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285:555–564. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Newman DR, Bonner JC, Sannes PL. Over-expression of human endosulfatase-1 exacerbates cadmium-induced injury to transformed human lung cells in vitro. Toxicol Appl Pharmacol. 2012;265:27–42. doi: 10.1016/j.taap.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Newman DR, Sannes PL. HSULF-1 inhibits ERK and AKT signaling and decreases cell viability in vitro in human lung epithelial cells. Respir Res. 2012;13:69. doi: 10.1186/1465-9921-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876–3889. doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- 18.Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1:e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289:8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy LS, Wickersham N, McNeil JB, Shaver CM, May AK, Bastarache JA, et al. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann Intensive Care. 2017;7:102. doi: 10.1186/s13613-017-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2008;10:261–268. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finigan JH, Faress JA, Wilkinson E, Mishra RS, Nethery DE, Wyler D, et al. Neuregulin-1–human epidermal receptor-2 signaling is a central regulator of pulmonary epithelial permeability and acute lung injury. J Biol Chem. 2011;286:10660–10670. doi: 10.1074/jbc.M110.208041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng HH, Narasaraju T, Phoon MC, Sim MK, Seet JE, Chow VT. Doxycycline treatment attenuates acute lung injury in mice infected with virulent influenza H3N2 virus: involvement of matrix metalloproteinases. Exp Mol Pathol. 2012;92:287–295. doi: 10.1016/j.yexmp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 25.Sun X, Li L, Overdier KH, Ammons LA, Douglas IS, Burlew CC, et al. Analysis of total human urinary glycosaminoglycan disaccharides by liquid chromatography–tandem mass spectrometry. Anal Chem. 2015;87:6220–6227. doi: 10.1021/acs.analchem.5b00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Born J, Salmivirta K, Henttinen T, Ostman N, Ishimaru T, Miyaura S, et al. Novel heparan sulfate structures revealed by monoclonal antibodies. J Biol Chem. 2005;280:20516–20523. doi: 10.1074/jbc.M502065200. [DOI] [PubMed] [Google Scholar]
- 27.Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, et al. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012;120:2899–2908. doi: 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodall KJ, Poon IK, Phipps S, Hulett MD. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One. 2014;9:e109596. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaudet JM, Weyers A, Solakyildirim K, Yang B, Takieddin M, Mousa S, et al. Impact of autoclave sterilization on the activity and structure of formulated heparin. J Pharm Sci. 2011;100:3396–3404. doi: 10.1002/jps.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil JB, Shaver CM, Kerchberger VE, Russell DW, Grove BS, Warren MA, et al. Novel method for noninvasive sampling of the distal airspace in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;197:1027–1035. doi: 10.1164/rccm.201707-1474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP–mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115:839–848. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Haeger SM, Suflita MA, Zhang F, Dailey KL, Colbert JF, et al. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol. 2017;56:727–737. doi: 10.1165/rcmb.2016-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, et al. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem. 2008;283:9308–9317. doi: 10.1074/jbc.M709995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volckaert T, De Langhe S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair. 2014;7:8. doi: 10.1186/1755-1536-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izvolsky KI, Zhong L, Wei L, Yu Q, Nugent MA, Cardoso WV. Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am J Physiol Lung Cell Mol Physiol. 2003;285:L838–L846. doi: 10.1152/ajplung.00081.2003. [DOI] [PubMed] [Google Scholar]
- 38.Saumon G, Soler P, Martet G. Effect of polycations on barrier and transport properties of alveolar epithelium in situ. Am J Physiol. 1995;269:L185–L194. doi: 10.1152/ajplung.1995.269.2.L185. [DOI] [PubMed] [Google Scholar]
- 39.Coulson-Thomas VJ, Chang SH, Yeh LK, Coulson-Thomas YM, Yamaguchi Y, Esko J, et al. Loss of corneal epithelial heparan sulfate leads to corneal degeneration and impaired wound healing. Invest Ophthalmol Vis Sci. 2015;56:3004–3014. doi: 10.1167/iovs.14-15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qing Q, Zhang S, Chen Y, Li R, Mao H, Chen Q. High glucose-induced intestinal epithelial barrier damage is aggravated by syndecan-1 destruction and heparanase overexpression. J Cell Mol Med. 2015;19:1366–1374. doi: 10.1111/jcmm.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bode L, Salvestrini C, Park PW, Li JP, Esko JD, Yamaguchi Y, et al. Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. J Clin Invest. 2008;118:229–238. doi: 10.1172/JCI32335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg Gynecol Obstet. 1990;171:493–496. [PubMed] [Google Scholar]
- 43.Mensah SA, Cheng MJ, Homayoni H, Plouffe BD, Coury AJ, Ebong EE. Regeneration of glycocalyx by heparan sulfate and sphingosine 1-phosphate restores inter-endothelial communication. PLoS One. 2017;12:e0186116. doi: 10.1371/journal.pone.0186116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108:384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19:216–228. doi: 10.1097/MCP.0b013e32835f27be. [DOI] [PubMed] [Google Scholar]
- 47.Ishiguro T, Takayanagi N, Yamaguchi S, Yamakawa H, Nakamoto K, Takaku Y, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med. 2013;52:317–324. doi: 10.2169/internalmedicine.52.8830. [DOI] [PubMed] [Google Scholar]
- 48.Rajas O, Quirós LM, Ortega M, Vazquez-Espinosa E, Merayo-Lloves J, Vazquez F, et al. Glycosaminoglycans are involved in bacterial adherence to lung cells. BMC Infect Dis. 2017;17:319. doi: 10.1186/s12879-017-2418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]