Abstract
Aging and obesity both have a significant impact on central blood pressure (BP) regulation, and previous studies indicated that changes in central redox signaling with age may affect high-fat (HF) diet-induced cardiovascular responses. Therefore, we investigated the effects of 60% HF feeding on BP regulation in young adult (5 mo) and old (26 mo) Fischer-344 × Brown-Norway rats. Radiotelemetric transmitters were implanted to measure BP, heart rate (HR), locomotor activity, and spontaneous baroreflex sensitivity. Expression and activity of NADPH oxidase and ANG II type 1 receptor were assessed in the hypothalamus and in the nucleus tractus solitarii. Old animals gained more weight on HF diet compared with young, whereas central NADPH oxidase expression and activity elevated similarly in the two age groups. After an initial hypotensive and tachycardic response during the first week of HF feeding, BP in young animals increased and became significantly elevated after 6 wk of HF feeding. In contrast, BP in old animals remained depressed. Nighttime HR and locomotor activity decreased in both young and old rats fed with HF diet, but these changes were more significant in young rats. As a result, amplitudes of circadian variation of BP, HR, and activity that were originally higher in young rats declined significantly and became similar in the two age groups. In conclusion, our experiments led to the surprising finding that HF diet has a more serious impact on cardiovascular regulation in young animals compared with old.
Keywords: aging, obesity, NADPH oxidase, circadian variation, baroreflex sensitivity
elevated sympathetic nervous system (SNS) activity plays a central role in age- and obesity-related hypertension (2, 15, 16, 35, 40). Superoxide anion has been implicated in the mediation of sympathoexcitatory stimuli both in the hypothalamus and brain stem (11, 17, 27, 44), and we have previously demonstrated that high-fat (HF) diet-induced obesity increased the expression and activity of NADPH oxidase, a major source of superoxide anion, in the hypothalamus, while antioxidant defense mechanisms remain unchanged (10). On the other hand, although aging does not increase central NADPH oxidase expression, it reduces hypothalamic expression of antioxidant enzymes such as copper-zinc superoxide dismutase and catalase, resulting in increased oxidative stress (13). These findings suggest that sympathoexcitatory effects of aging and HF diet feeding could both be mediated by increased central oxidative stress, and their effects may be additive, since HF diet increases the production of superoxide, while age downregulates those enzymes that scavenge oxidative free radicals. Thus age could possibly magnify the adverse effects of obesity on central blood pressure (BP) regulation. On the other hand, previous studies in young and old rats (13) demonstrated that central ANG II-mediated and stress-induced pressor responses were diminished with age, even though these responses are also mediated, at least in part, by superoxide anion (11, 27), indicating that age has a complex effect on central BP regulatory mechanisms, and reduced central antioxidant capacity does not necessarily result in augmented superoxide-mediated sympathoexcitation and BP elevation.
Therefore, in this study, we set out to investigate the effects of HF diet-induced obesity on BP regulation in young adult and old Fischer 344 × Brown Norway (F344 × BN) rats fed with standard rat chow or a 60% HF diet. We have analyzed changes in circadian patterns of BP, heart rate (HR), and locomotor activity as well as spontaneous baroreflex sensitivity (sBRS) using radiotelemetry and assessed changes in the expression and activity of NADPH oxidase and ANG II type 1 (AT1) receptor in the hypothalamus and in the nucleus tractus solitarii (NTS).
MATERIALS AND METHODS
Experimental Animals
Five- and 26-mo-old male F344 × BN rats were obtained from Harlan (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for 1 wk. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals, and all procedures were approved by the local Institutional Animal Care and Use Committee. Rats were housed individually with a 12:12-h light-dark cycle (0600 to 1800 h).
Experimental Design
Young (n = 10) and old (n = 10) rats were equipped with radiotelemetric transmitters (model PA-C40; Data Sciences International, St. Paul, MN) for the measurement of BP and HR under isoflurane anesthesia with the cannulation of the abdominal aorta. After 4 wk of recovery, BP and HR were recorded for 3 wk in young and old animals fed with standard rat chow (15% fat; 3.3 kcal/g; diet-2018; Harlan Teklad, Madison, WI). Next, animals were switched to a HF diet (60% fat; 5.2 kcal/g, D12492; Research Diets, New Brunswick, NJ) and were fed with this diet for 15 wk. BP, HR, and locomotor activity were recorded on selected days for a minimum of 24 h in undisturbed rats. During the initial control measurements, telemetry data were collected 2–3 days/wk. During the first week of HF feeding, data were collected continuously for 7 days to analyze acute changes in BP and HR in response to HF diet. Next, data collection became less frequent to conserve battery power of the transmitters. The telemetry system also allowed us to assess spontaneous locomotor activity of the rats. Activity was calculated by the data acquisition system from variation in signal strength received from the transmitter and was expressed in arbitrary units (AU).
Additional groups of young (n = 8) and old (n = 8) rats were fed with standard rat chow along the whole duration of the study. These animals were used as controls for metabolic assessment, mRNA and protein expression, and NADPH activity assays.
Spontaneous Baroreflex Sensitivity
sBRS was assessed as described previously (29, 42, 43). In short, on selected days, telemetric BP data were recorded for 5 min continuously with a sampling rate of 500 Hz. Pulse intervals (PI) and averaged mean arterial BP (MAP) values for every pulse were calculated using software developed in our laboratory. Respiration-induced fluctuations were filtered out by calculating moving averages of MAP and PI over 10 cardiac cycles. Spontaneously occurring ramps of increasing MAP of four beats or more were used to calculate baroreceptor reflex gain. For each pair of MAP and PI ramps, measurements were made at delays of three, four, and five beats. This is based on the delay time from a change in BP to a reflex response in PI in the rat as previously described (29). sBRS gain was calculated from the average of the slopes of these three PI-BP plots. For each animal, the above-described analysis was conducted on five separate 5-min segments recorded between 1000 and 1200 h. Final sBRS values for each animal on a given day were attained by averaging results from the five segments.
Tissue Harvesting and Preparation
Animals were overanesthetized with pentobarbital (120 mg/kg ip), and blood was collected through cardiac puncture. Next, the circulatory system was perfused with 60 ml of cold saline, and the brain was removed. First, the hypothalamus was isolated, then a 2-mm-thick brain section was cut 12 mm posterior to the bregma using a rat brain slicer matrix, and bilateral micropunches of 1 mm in diameter (Stoelting, Wood Dale, IL) containing the NTS were removed. Hypothalamus and NTS samples were immediately frozen in liquid nitrogen.
NADPH Oxidase Activity
NADPH oxidase activity was measured with a lucigenin-enhanced chemiluminescence assay using hypothalamus homogenates. Tissue samples were incubated in artificial cerebrospinal fluid at 37°C in a luminometer (BMG Fluostar Optima). Scintillation counts were obtained for 30 min in the presence of NADH (100 μM) and lucigenin (5 μM), and background-corrected values were normalized to protein content for each sample.
Reverse Transcriptase-Polymerase Chain Reaction and Western Blot
AT1, NADPH oxidase (NOX)-2, p47, and p67 mRNA expression was analyzed using relative quantitative RT-PCR and a QuantumRNA 18S Internal Standards kit (Ambion, Austin, TX). PCR was performed by multiplexing primers for AT1 (sense: 5′-CAGCTTGGTGGTGATTGTC; antisense: 5′-GCCATCGGTATTCCATAGC), NOX-2 (sense: 5′-CCTATGACTTGGAAATGGAT; antisense: 5′-CAGAGCCAGTAGAAGTAGAT), p47 (sense: 5′-CCACACCTCTTGAACTTCTTC; antisense: 5′-CTCTAGTCAGCGATGGC), p67(sense: 5′-AGACACCTTGAACTACCATCC; antisense: 5′-CTGCTCTTCTGCTTTCTTCC), and 18S primers. Protein levels of NADPH oxidase subunits NOX-2, p47phox, and p67phox were determined using Western blot, as previously described (12).
Metabolic Effects of HF Feeding
Blood samples were taken via cardiac puncture from nonfasted animals at the time of death. Serum insulin and leptin levels were measured using enzyme-linked immunosorbent assay and RIA, respectively (Millipore, Billerica, MA). Glucose levels were assessed using a kit from Biovision (Mountain View, CA), whereas total cholesterol and triglyceride levels were measured by assays from Wako Diagnostics (Richmond, VA).
Statistical Analysis
Data were analyzed by one-way ANOVA or repeated-measures ANOVA. When the main effect was significant, a post hoc test was applied to determine individual differences between means. Relationships between BP and HR as well as locomotor activity and HR were analyzed using the Pearson Product Moment Correlation test and linear regression analysis. A value of P < 0.05 was considered significant.
RESULTS
Body weight and daily caloric intake of old chow-fed animals were significantly higher compared with young at the beginning of the experiment, and there were no significant differences between groups of similar ages (Fig. 1). When animals were switched to HF diet, caloric intake increased significantly in both young and old animals (peak increases were ∼70% and ∼90%, respectively); however, young animals compensated for the higher-calorie content of the food by decreasing their daily food intake after ∼1.5 wk, and caloric intake in young HF rats returned back to normal. In contrast, caloric intake increased by a greater extent in old HF rats and remained elevated for a longer period of time (4 wk). Therefore, although both young HF and old HF rats became significantly heavier than their chow-fed counterparts, old HF animals gained more weight and at a higher rate compared with young.
Fig. 1.
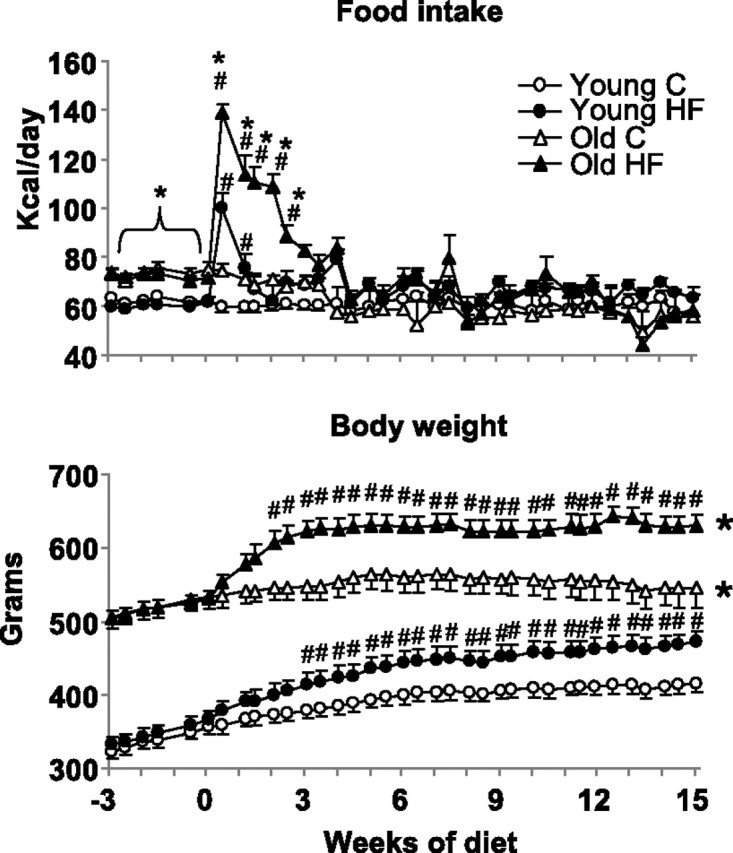
Changes in caloric food intake (top) and body weight (bottom) of young and old animals fed with standard rat chow (C) or a 60% high-fat (HF) diet. Initially, all groups were fed with standard rat chow (between week −3 and 0). Next, from week 0, rats in HF groups were fed with a 60% HF diet. *Caloric food intake was significantly higher (P < 0.05) in old vs. young HF groups from the beginning of the study until day 17 of diet treatment. Body weight was significantly higher (P < 0.05) in old vs. young rats regardless of diet for the whole duration of the study. #P < 0.05 HF vs. age-matched controls.
Serum cholesterol levels measured at the end of the experiment in nonfasted animals were elevated significantly in old control rats compared with young (Table 1), but HF feeding had no effect on cholesterol levels in either age group. Triglyceride levels were also higher in old control rats compared with young control, and HF feeding increased triglyceride values both in young and old rats, but the percent increase was noticeably higher in young. Glucose and insulin levels were similar in young and old control rats and were elevated after HF feeding in both age groups. Leptin was elevated in old control compared with young control rats. HF feeding increased leptin levels in both young and old animals, and, although the diet treatment resulted in a significantly higher final serum leptin concentration in old HF rats, the percent increase in serum leptin was greater in the young HF group.
Table 1.
Metabolic effects of HF diet feeding
| Cholesterol, mg/dl | Triglycerides, mg/dl | Glucose, mg/dl | Insulin, ng/ml | Leptin, ng/ml | |
|---|---|---|---|---|---|
| Young control | 61.6 ± 3.7 | 71.0 ± 5.8 | 88.3 ± 4.2 | 1.92 ± 0.76 | 11.2 ± 1.5 |
| Young HF | 64.7 ± 2.8 | 156.9 ± 7.2## | 115.8 ± 8.8# | 6.28 ± 1.70# | 33.4 ± 2.0## |
| Old control | 125.3 ± 5.0** | 120.2 ± 9.4** | 80.1 ± 6.0 | 1.62 ± 0.44 | 24.8 ± 2.1** |
| Old HF | 128.3 ± 2.8** | 161.7 ± 7.9# | 110.8 ± 7.1## | 4.97 ± 0.66# | 42.2 ± 2.5*## |
Values are means ± SE. HF, high-fat diet fed.
P < 0.05 and
P < 0.01 with age.
P < 0.05 and
P < 0.01 with HF feeding.
Effect of HF Feeding on BP, HR, and Locomotor Activity
Baseline.
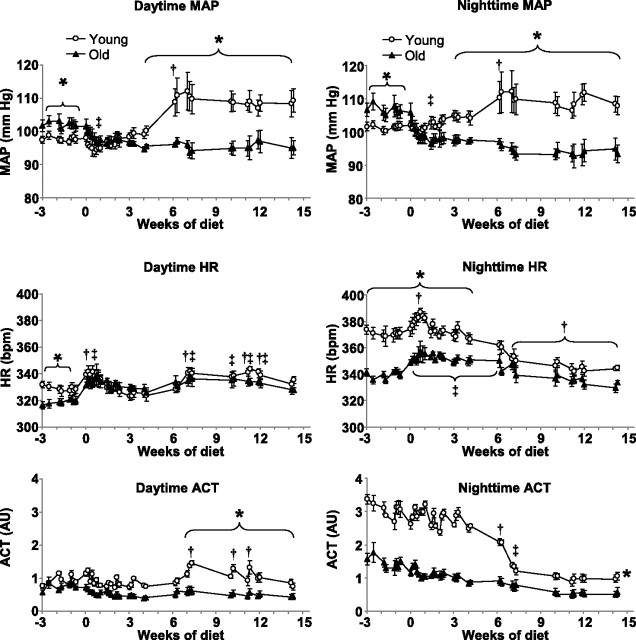
MAP was significantly elevated in the old chow-fed rats compared with young chow-fed, both day- and nighttime (by 4.0 ± 1.3 and 5.1 ± 1.7 mmHg, respectively, Fig. 2). Daytime HR was lower in old rats on most days, although not every day, while nighttime HR was elevated markedly (by 31 ± 4 beats/min) in young rats compared with old. Activity was similarly low in young and old rats during the day, but young animals were more than twofold more active during the night.
Fig. 2.
Mean arterial blood pressure (MAP), heart rate (HR), and locomotor activity (ACT) measured during daytime (between 0600 and 1800 h, left) and nighttime (between 1800 and 0600 h, right) in young and old animals. Rats were fed standard rat chow between week −3 and 0 and a 60% HF diet beginning from week 0. AU, arbitrary units. *P < 0.05 young vs. old. †P < 0.05 HF feeding vs. baseline in young rats, indicates the first data point when HF effect became significant, except for HR and daytime ACT figures, where it indicates individual data points when values were significantly different from baseline. ‡P < 0.05 HF feeding vs. baseline in old animals, indicates the first data point when HF effect became significant, except for HR figures, where it indicates individual data points when values were significantly different from baseline.
Acute response to HF diet.
Replacing the standard diet with 60% HF diet induced an initial decrease in MAP in both young and old rats, which lasted for ∼1 wk (Fig. 2). The hypotensive response to HF feeding was only significant in old rats [for example, nighttime MAP decreased by 2.6 ± 1.4 mmHg in young and by 7.6 ± 2.7 mmHg (P < 0.05) in old rats], and, as a result, the initial difference in MAP disappeared between young and old groups. In contrast with MAP, HR increased rapidly in both young and old rats in response to change in diet (nighttime HR increased by 20 ± 4 beats/min in young and by 17 ± 5 beats/min in old rats, P < 0.05 for both). Interestingly, this response was more rapid than the decrease in MAP, for example, daytime HR became elevated significantly on the first day of HF treatment both in young and old rats compared with their baseline HR values. Similarly, nighttime HR increased significantly on the first day in old rats but only became elevated in the young rats after 1 wk. Day- and nighttime activity levels were not affected significantly during the first few weeks of HF diet.
Long-term effects of HF feeding.
After the first week of HF feeding, MAP started to increase in young animals and became significantly elevated compared with baseline MAP after 6 wk of HF feeding (by 10.3 ± 5.1 mmHg, P < 0.05, Fig. 2). Next, MAP plateaued and remained elevated until the end of the experiment. In contrast, MAP in old animals remained significantly lower than baseline and also compared with MAP in young animals. Daytime HR values in both age groups declined back to baseline levels following the peak during the first wk of HF feeding but modestly elevated again after 7 wk of diet. However, the initial difference between young and old rats disappeared. Nighttime HR started to decline during the 2nd wk of HF feeding in young animals but remained steady in old rats. After 6 wk of HF feeding, HR decreased in old rats, too, but the decline in young rats was much more significant; therefore, the initial difference between young and old rats disappeared after 4 wk of HF feeding. For example, by the end of diet treatment, nighttime HR decreased by 32 ± 5 beats/min in young rats (P < 0.05) but decreased only by 7 ± 5 beats/min in old (not significant). Daytime activity increased in young rats after 6 wk of HF but remained unaffected in old animals. In contrast, nighttime activity decreased both in young and old rats following 6 wk of diet, but the decline was much more significant in young animals (−1.9 ± 0.13 AU, P < 0.05) compared with old (−0.8 ± 0.12 AU, P < 0.05), resulting in similar, although still statistically higher, activity levels.
Correlation between MAP, HR, and locomotor activity responses.
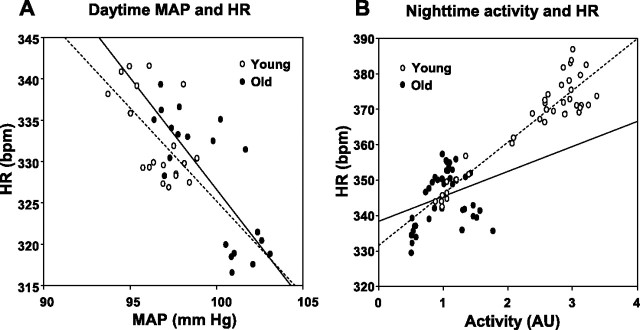
Analysis of MAP and HR responses during the acute phase of HF feeding (first 2 wk) indicated a significant correlation between MAP and HR in both age groups and both day- and nighttime. For example, for daytime MAP and HR, the correlation coefficient (r) was −0.61 (P < 0.01) in young and −0.78 (P < 0.01) in old animals (Fig. 3A). However, when analyzed over the whole duration of the experiment, correlation between daytime MAP and HR was positive in young rats (r = 0.359, P = 0.023) but negative in old animals (r = −0.694, P < 0.01). In contrast, correlation between nighttime MAP and HR was negative in young (r = −0.844, P < 0.01) while not significant in old rats. In addition, there was a strong correlation between nighttime locomotor activity and HR in young rats (r = 0.934, P < 0.01), whereas the activity-HR correlation was not significant in old rats (r = 0.299, P = 0.061, Fig. 3B).
Fig. 3.
A: relationship between daytime MAP and HR during the first 2 wk of HF feeding in young and old rats. Linear regression analysis indicated a slope of −2.25 in young rats (r2 = 0.372, P < 0.01, broken line) and a slope of −2.7 in old animals (r2 = 0.612, P < 0.01, solid line). B: relationship between nighttime locomotor activity and HR for the whole duration of the study in young and old rats. Linear regression analysis in young rats indicated a slope of 14.6 (r2 = 0.872, P < 0.01, broken line). In contrast, correlation between locomotor activity and HR in old rats (solid line) was not significant.
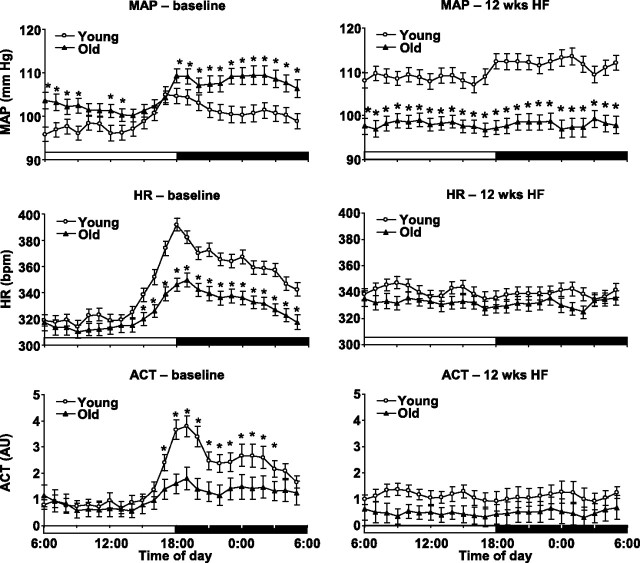
Circadian analysis of MAP, HR, and locomotor activity.
Age and HF feeding both had a significant impact on the circadian variation of MAP, HR, and activity. Variation in these parameters was significantly higher in young rats during the control phase of the study (Fig. 4, left). However, after 12 wk of HF diet, the amplitude of circadian variation declined markedly, and, while young animals still demonstrated a circadian MAP pattern, day-night difference in MAP completely diminished in old rats. In addition, circadian variation of HR and activity completely disappeared both in young and old animals (Fig. 4, right).
Fig. 4.
Circadian variation of MAP, HR, and ACT in young and old animals fed with standard rat chow (left) and after 12 wk of HF diet feeding (right). *P < 0.05 young vs. old.
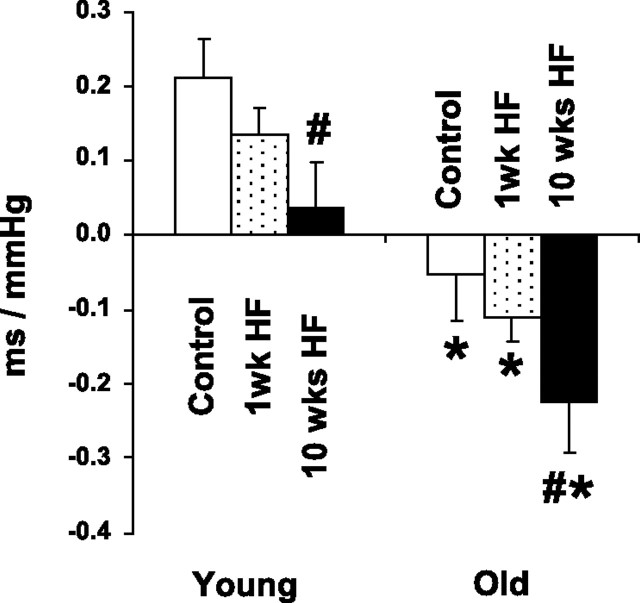
Spontaneous Baroreflex Sensitivity
In young chow-fed animals, sBRS was 0.211 ± 0.052 ms/mmHg, whereas in old chow-fed rats, sBRS analysis indicated a lack of baroreflex in the range of spontaneously occurring BP fluctuations (Fig. 5). After 1 wk of HF feeding, sBRS tended to be lower in both young and old animals, but these changes were not significant. However, after 10 wk of HF diet, sBRS in both young and old animals decreased significantly (0.036 ± 0.089 and −0.223 ± 0.108 ms/mmHg), resulting in a loss of baroreflex in the range of spontaneously occurring BP fluctuations in young rats and in a negative sBRS value for old animals.
Fig. 5.
Spontaneous baroreflex sensitivity assessed in young and old rats fed with standard rat chow (control) and after HF diet feeding for 1 and 10 wk. *P < 0.05 young vs. old. #P < 0.05 HF vs. control.
Hypothalamic and NTS Effects of Age and HF Feeding
The catalytic NOX-2 subunit of NADPH oxidase remained unaffected by age in the hypothalamus, and HF diet increased its mRNA (Fig. 6A) and protein levels only in young rats. Compared with young controls, NOX-2 mRNA and protein were 27 ± 7 and 31 ± 9% higher in young HF rats, respectively (P < 0.05 for both). Hypothalamic mRNA levels of the p47 regulatory subunit decreased with age (by 26 ± 3%, Fig. 6B) but was elevated by HF feeding in both age groups (by 33 ± 6% in young and by 56 ± 10% in old HF rats compared with their age-matched controls; P < 0.05 for both). However, age failed to have a significant effect on hypothalamic p47 protein levels (p47 protein was 10 ± 6% higher in old compared with young controls), and HF diet-induced increases in p47 protein expression were only significant in young animals (19 ± 4% increase, P < 0.05), whereas the change was not significant in old rats (13 ± 9%). Another regulatory subunit of NADPH oxidase, p67, remained unaffected by age; mRNA (Fig. 6C) and protein levels were 11 ± 11 and 0.5 ± 5% higher in old controls compared with young. However, HF feeding elevated levels of p67 mRNA (by 21 ± 4% in young and by 30 ± 6% in old rats, P < 0.05 for both) and protein (by 26 ± 8% in young and by 27 ± 11% in old rats, P < 0.05 for both). NADPH oxidase activity was 29 ± 6% (P < 0.05) higher in chow-fed old animals compared with young controls and was augmented by HF feeding in both age groups (by 34 ± 6% in young and by 32 ± 9% in old rats, P < 0.05 for both, Fig. 5D).
Fig. 6.
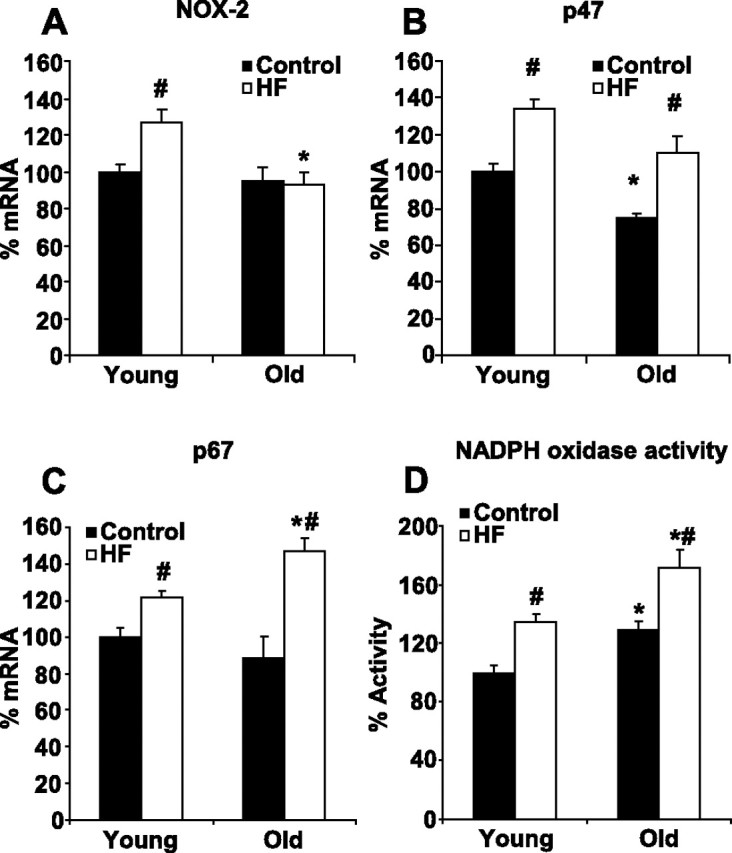
mRNA levels of NADPH oxidase NOX-2 (A), p47 (B), and p67 (C) subunits and NADPH oxidase activity (D) in the hypothalamus of young and old rats fed with standard chow or HF diet. *P < 0.05 young vs. old. #P < 0.05 HF vs. control.
In the NTS, NOX-2 mRNA was unaffected by both age and HF diet. However, p47 and p67 mRNA expression increased significantly with age (by 35 ± 8 and 37 ± 9%, P < 0.05 for both), and, while HF had no effect on p47 levels in the NTS, p67 mRNA elevated by 26 ± 8% in young and by 16 ± 5% in old rats (P < 0.05 for both). Age had no effect on hypothalamic AT1 mRNA levels, whereas AT1 mRNA in the NTS tended to be lower in old animals vs. young (−16 ± 6%, P = 0.052). However, HF feeding upregulated AT1 mRNA both in the hypothalamus (by 49 ± 11% in young and by 27 ± 5% in old, respectively; P < 0.05 for both) and in the NTS (by 20 ± 12% in young and by 24 ± 8% in old, respectively; P < 0.05 for both).
DISCUSSION
The major finding of this study is that, despite the greater increase in body weight in old rats, HF feeding has a significantly more adverse effect on cardiovascular regulation and locomotor activity in young animals.
Age-Related Changes in the Metabolic Effects of HF Feeding
significantly with advancing age. Body weight and adiposity increase steadily with age, whereas sensitivity to leptin is decreased in the hypothalamus, resulting in increased susceptibility to HF-induced obesity (33). Our results confirm these previous findings, since switching diet from standard rat chow to 60% HF diet resulted in an increased caloric intake that lasted almost four times longer in old rats compared with young. The inefficient control of food intake in response to higher caloric content also meant that old rats gained more weight at a faster rate during HF treatment. Serum leptin levels rose parallel with increases in obesity, and, although it reached significantly higher levels in old rats, the percent increase from control leptin level was larger in young animals following HF feeding. Leptin has been shown to affect central BP regulatory mechanisms increasing SNS activity and BP (5, 8), and leptin-induced sympathoexcitation has been implicated in the pathomechanism of obesity-related hypertension (1). However, even though exogenously infused leptin increases SNS activity and BP, we demonstrated in a previous study that HF diet-induced elevation of BP cannot be reversed with central infusion of a leptin antagonist (38). Results from our current study further prove that serum leptin level alone cannot be responsible for elevated BP, since BP in old animals failed to increase in response to HF diet despite the extremely high serum leptin levels.
The F344 × BN rat was chosen for our studies because these animals demonstrate a gradual increase in body weight (23), similar to what occurs in humans (34). The F344 × BN rat has a steady weight gain paralleled by an increase in adiposity from 3 to 25 mo of age, followed by a decline at 30 mo. In humans, there is a gradual increase in obesity in both men and women from age 25 to around 65, after which body weight declines. The gradual increase in body weight with age in the F344 × BN rats is very different from the rapid increase in body weight observed in other rat strains, for example, in the Sprague Dawley rats, and the similarities between our rats and what occurs in humans suggest that the F344 × BN rat is a reasonable model for human aging and obesity. In addition, analysis of HF diet-induced changes could prove to be impossible in rat strains where aged rats demonstrate significant obesity even on chow diet. However, because of the distinctive characteristics of the F344 × BN rats, including moderate weight gain, but also lower incidences of many age-related diseases and significantly longer life span (24), the cardiovascular effects of HF feeding observed during our studies could be specific to this rat strain. Nevertheless, the metabolic changes in young F344 × BN rats were similar to those reported by previous studies conducted in Sprague Dawley rats of similar age following HF/high-energy diet feeding, including elevated glucose and insulin levels and reduced insulin resistance as well as dyslipidemia (4, 19, 32, 37).
Acute Cardiovascular Effects of HF Feeding
One objective of our experiments was to evaluate acute BP and HR responses at the onset of HF diet feeding. Therefore, the current experiments were designed so that BP and HR were recorded continuously during the first week of HF feeding. The observed immediate increase in HR corresponds with previous studies suggesting a sympathoexcitatory effect of increased caloric intake; however, the decline in BP during the first week of diet was unexpected. Because HR increased during this time, we have to assume that there was a decrease in peripheral vascular resistance or a decrease in cardiac muscle contractility. Conversely, a significant negative correlation between BP and HR values during this phase of the experiment could indicate that the increase in HR was mediated by baroreflex in response to a decrease in BP. However, correlation coefficients were relatively low, and, interestingly, changes in BP were delayed in relation to the increases in HR. Therefore, it is doubtful that the tachycardic response was mediated by baroreflex. Further studies are needed to reveal the mechanisms underlying this acute hypotensive response and to determine whether these responses are specific to the F344 × BN strain or the 60% HF diet.
Long-Term Cardiovascular Effects of HF Feeding
It has been shown by others (6) and us (10) that HF diet feeding for an extended period results in a modest elevation of BP and a disruption of circadian patterns in young Sprague Dawley rats. In this study, we demonstrated that young F344 × BN rats respond to HF diet similarly, and BP became significantly elevated after 6–7 wk of diet. Surprisingly, BP of old animals failed to increase in response to HF diet during this time. In fact, BP declined further, resulting in significantly lower BP values in old HF rats compared with young HF. On the other hand, HF feeding had similar effects on the circadian pattern of BP, reducing the amplitude of fluctuation in both young and old rats, but it resulted in a complete loss of circadian variation in old rats because of the originally lower BP variation at this age.
The effect of HF feeding on HR and locomotor activity was also more severe in young animals compared with old. HF diet reduced circadian variation of HR and activity in both young and old rats, but the decline in nighttime HR and activity was much more significant in young rats, and HF feeding completely obliterated the age-related difference and the circadian patterns of HR and activity. Nightime HR and activity values had a strong correlation in young animals, implying that diminished circadian rhythm of HR may be a consequence of reduced locomotor activity. In addition, reduction of nighttime activity in young animals was coupled with an increased activity during the resting daytime period, indicating that sleep patterns may have also been disrupted in young rats by HF feeding. These severe effects of HF diet on circadian patterns are of utmost significance, since disturbed circadian pattern has been shown to be a risk factor for cardiovascular diseases (3, 36, 41). Although the underlying mechanisms are unclear, developing leptin resistance may have played a key role in HF diet-induced inactivity, since inhibition of hypothalamic leptin signaling has been shown to reduce voluntary wheel running in rats (25). In addition, the percent increase in serum leptin, which is an indicator of leptin resistance, was considerably higher in young vs. old rats, which correlates with the greater decline in locomotor activity. On the other hand, because old animals were already leptin resistant even on control diet, changes in leptin sensitivity and locomotor activity during HF feeding were not as significant as in young rats.
In addition to the above-described effects, HF feeding also lowered sBRS values in both age groups. This meant a loss of baroreflex in the range of spontaneously occurring BP fluctuations in young rats and in a negative sBRS value for old animals. The significance of zero or negative sBRS values is not clear, but unpublished data from our laboratory indicate that baroreceptor reflex, assessed with intravenously infused hypotensive and hypertensive drugs over a much wider pressure range, is maintained in old animals, although with a reduced sensitivity, even when the sBRS value is close to zero. This could mean that fine tuning of HR to small spontaneous fluctuations of BP is lost (in old control and young HF rats) or even reversed (old HF rats), whereas larger changes in BP, such as those induced by intravenous infusion of vasoactive drugs, are still compensated with adjustments of HR, although with a lower sensitivity. Such loss of the fine tuning ability might be responsible for the increased BP variation (standard deviation and variation coefficient) observed in elderly patients (20), since significantly larger changes in BP are needed to trigger a compensatory response. Such an increase in BP variation not only makes assessment of antihypertensive treatments more difficult but it may also contribute to the development of cardiovascular diseases (22).
Our initial hypothesis that diminished central antioxidant capacity in old rats (13) would increase susceptibility to HF diet-induced central oxidative stress, sympathoexcitation, and BP elevation was not supported by our results. HF diet did increase AT1 receptor expression in both the hypothalamus and NTS in the two age groups, which corresponds with our previous findings (10). In addition, although there were age-related differences in the upregulation of NADPH oxidase subunits (for example, hypothalamic NOX-2 and p47 subunits only elevated in young rats in response to HF feeding), NADPH oxidase activity elevated similarly in the two age groups. However, the diet-induced increase in central superoxide level did not translate into higher BP values in old rats.
There are several possible explanations for this. First, as our previous studies have indicated, BP of old rats is less sensitive to centrally infused ANG II, and restraint stress-induced BP elevation is also diminished with age (13) despite the fact that both ANG II- and stress-induced hypertensive responses are mediated by superoxide anion (11, 26, 27). However, one important target of the ANG II-superoxide pathway is noradrenergic neurons in the hypothalamus and brain stem (7, 18, 46), and we have shown in previous studies that hypothalamic levels of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis, and dopamine β-hydroxylase are reduced markedly in old animals compared with young (9, 14). Although it is not yet clear whether this is due to a loss of noradrenergic cells in cardiovascular regulatory regions or to reduced catecholamine biosynthesis, it is possible that a decline in noradrenergic neurotransmission limits the ability of superoxide anion to increase SNS activity and BP. Another possibility is that, in old animals, the vasculature may not respond to increases in SNS outflow the same way as in young animals. Age has a significant impact on vascular function. Endothelium-dependent dilator responses are reduced significantly mainly because of chronic low-grade inflammation and oxidative stress (39). Therefore, elevated basal vascular tone in old animals may limit the ability of the SNS to induce further vasoconstriction. In addition, it has been shown that α1-adrenergic receptor-mediated vasoconstriction is also diminished with age in peripheral arteries (30, 45), possibly further reducing the sympathoexcitatory effect of HF feeding on peripheral vascular resistance and BP in old rats compared with young. To clarify the relative importance of central regulatory mechanisms and peripheral vascular function, further studies assessing sympathetic nerve activity parallel with changes in BP will be necessary.
In addition, a limitation of our study was that we did not directly measure markers of oxidative stress in hypothalamic and NTS tissue samples. Correlation analysis of these markers and BP and HR responses could reveal further details about the importance of central redox signaling in obesity- and age-related dysregulation of BP and the SNS.
In conclusion, our experiments led to the surprising finding that HF diet feeding has a more serious impact on cardiovascular regulation in young animals compared with old, and HF diet also has a striking effect on spontaneous locomotor activity of young animals. On the other hand, age-related changes in the central nervous system and/or in the peripheral vasculature make old animals resistant to the hypertensive effects of a HF/high-calorie diet. These findings are significant in view of the ever-increasing prevalence of childhood and adolescent obesity and their link to elevated risk of cardiovascular morbidity and mortality in adulthood (31). The importance of consuming a balanced diet and limiting daily caloric food intake is further emphasized by the finding that HF diet dramatically reduced locomotor activity and by the overwhelming evidence that sedentary lifestyle is linked with obesity and cardiovascular diseases (21, 28).
GRANTS
This study was supported by the Medical Research Service of the Department of Veterans Affairs and by an award from the American Heart Association. B. Basgut was supported by a TUBITAK scholarship.
DISCLOSURES
None.
REFERENCES
- 1. Aizawa-Abe M , Ogawa Y , Masuzaki H , Ebihara K , Satoh N , Iwai H , Matsuoka N , Hayashi T , Hosoda K , Inoue G , Yoshimasa Y , Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest : 1243–1252, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes MJ , Lapanowski K , Conley A , Rafols JA , Jen KL , Dunbar JC. High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Res Bull : 511–519, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Bianchi S , Bigazzi R , Baldari G , Sgherri G , Campese VM. Diurnal variations of blood pressure and microalbuminuria in essential hypertension. Am J Hypertens : 23–29, 1994. [DOI] [PubMed] [Google Scholar]
- 4. Carroll JF , Zenebe WJ , Strange TB. Cardiovascular function in a rat model of diet-induced obesity. Hypertension : 65–72, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Correia ML , Morgan DA , Sivitz WI , Mark AL , Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension : 936–942, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Dobrian AD , Davies MJ , Prewitt RL , Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension : 1009–1015, 2000. [DOI] [PubMed] [Google Scholar]
- 7. Dogan MD , Sumners C , Broxson CS , Clark N , Tumer N. Central angiotensin II increases biosynthesis of tyrosine hydroxylase in the rat adrenal medulla. Biochem Biophys Res Commun : 623–626, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Dunbar JC , Hu Y , Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes : 2040–2043, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Erdem SR , Broxson CS , Erdem A , Spar DS , Williams RT , Tumer N. The age-related discrepancy in the effect of neuropeptide Y on select catecholamine biosynthetic enzymes in the adrenal medulla and hypothalamus in rats. Neuropharmacology : 1280–1288, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Erdos B , Broxson CS , Cudykier I , Basgut B , Whidden M , Landa T , Scarpace PJ , Tumer N. Effect of high-fat diet feeding on hypothalamic redox signaling and central blood pressure regulation. Hypertens Res : 983–988, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Erdos B , Broxson CS , King MA , Scarpace PJ , Tumer N. Acute pressor effect of central angiotensin II is mediated by NAD(P)H-oxidase-dependent production of superoxide in the hypothalamic cardiovascular regulatory nuclei. J Hypertens : 109–116, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Erdos B , Broxson CS , Landa T , Scarpace PJ , Leeuwenburgh C , Zhang Y , Tumer N. Effects of life-long caloric restriction and voluntary exercise on age-related changes in levels of catecholamine biosynthetic enzymes and angiotensin II receptors in the rat adrenal medulla and hypothalamus. Exp Gerontol : 745–752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erdos B , Cudykier I , Woods M , Basgut B , Whidden M , Tawil R , Cardounel AJ , Tumer N. Hypertensive effects of central angiotensin II infusion and restraint stress are reduced with age. J Hypertens : 1298–1306, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Erdos B , Erdem SR , Erdem A , Broxson CS , Tumer N. Effect of age on angiotensin II-mediated downregulation of adrenomedullary catecholamine biosynthetic enzymes. Exp Gerontol : 806–809, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Esler M , Hastings J , Lambert G , Kaye D , Jennings G , Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol : R909–R916, 2002. [DOI] [PubMed] [Google Scholar]
- 16. Esler MD , Turner AG , Kaye DM , Thompson JM , Kingwell BA , Morris M , Lambert GW , Jennings GL , Cox HS , Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol Regul Integr Comp Physiol : R278–R285, 1995. [DOI] [PubMed] [Google Scholar]
- 17. Gao L , Wang W , Li YL , Schultz HD , Liu D , Cornish KG , Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res : 937–944, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Gelband CH , Sumners C , Lu D , Raizada MK. Angiotensin receptors and norepinephrine neuromodulation: implications of functional coupling. Regul Pept : 141–147, 1998. [DOI] [PubMed] [Google Scholar]
- 19. Gollisch KS , Brandauer J , Jessen N , Toyoda T , Nayer A , Hirshman MF , Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab : E495–E504, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imai Y , Aihara A , Ohkubo T , Nagai K , Tsuji I , Minami N , Satoh H , Hisamichi S. Factors that affect blood pressure variability. A community-based study in Ohasama, Japan. Am J Hypertens : 1281–1289, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Janiszewski PM , Ross R. The utility of physical activity in the management of global cardiometabolic risk. Obesity (Silver Spring) , Suppl 3: S3–S14, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Kudo H , Kai H , Kajimoto H , Koga M , Takayama N , Mori T , Ikeda A , Yasuoka S , Anegawa T , Mifune H , Kato S , Hirooka Y , Imaizumi T. Exaggerated blood pressure variability superimposed on hypertension aggravates cardiac remodeling in rats via angiotensin II system-mediated chronic inflammation. Hypertension : 832–838, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Li H , Matheny M , Nicolson M , Tumer N , Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes : 2035–2039, 1997. [DOI] [PubMed] [Google Scholar]
- 24. Lipman RD , Chrisp CE , Hazzard DG , Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci : B54–59, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matheny M , Zhang Y , Shapiro A , Tumer N , Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol : R1254–R1261, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayorov DN , Head GA. AT1 receptors in the RVLM mediate pressor responses to emotional stress in rabbits. Hypertension : 1168–1173, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Mayorov DN , Head GA , De Matteo R. Tempol attenuates excitatory actions of angiotensin II in the rostral ventrolateral medulla during emotional stress. Hypertension : 101–106, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Mokdad AH , Marks JS , Stroup DF , Gerberding JL. Actual causes of death in the United States, 2000. J Am Med Assoc : 1238–1245, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Oosting J , Struijker-Boudier HA , Janssen BJ. Validation of a continuous baroreceptor reflex sensitivity index calculated from spontaneous fluctuations of blood pressure and pulse interval in rats. J Hypertens : 391–399, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Ramsey MW , Behnke BJ , Prisby RD , Delp MD. Effects of aging on adipose resistance artery vasoconstriction: possible implications for orthostatic blood pressure regulation. J Appl Physiol : 1636–1643, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Reilly JJ , Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) In press. [DOI] [PubMed] [Google Scholar]
- 32. Ricci MR , Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol : R610–R618, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Scarpace PJ , Matheny M , Moore RL , Tumer N. Impaired leptin responsiveness in aged rats. Diabetes : 431–435, 2000. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz RS. Obesity in the elderly. In: Handbook of Obesity, edited by , Bray GA , Bouchard C , James WPT. New York, NY: Dekker, 1998, p. 103–114. [Google Scholar]
- 35. Seals DR , Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol : H1895–H1905, 2004. [DOI] [PubMed] [Google Scholar]
- 36. Shimada K , Kawamoto A , Matsubayashi K , Nishinaga M , Kimura S , Ozawa T. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens : 875–878, 1992. [PubMed] [Google Scholar]
- 37. Silva AA , Kuo JJ , Tallam LS , Liu J , Hall JE. Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension : 259–264, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Tumer N , Erdos B , Matheny M , Cudykier I , Scarpace PJ. Leptin antagonist reverses hypertension caused by leptin overexpression, but fails to normalize obesity-related hypertension. J Hypertens : 2471–2478, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Ungvari Z , Kaley G , de Cabo R , Sonntag WE , Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci : 1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaz M , Jennings G , Turner A , Cox H , Lambert G , Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation : 3423–3429, 1997. [DOI] [PubMed] [Google Scholar]
- 41. Verdecchia P , Schillaci G , Guerrieri M , Gatteschi C , Benemio G , Boldrini F , Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation : 528–536, 1990. [DOI] [PubMed] [Google Scholar]
- 42. Waki H , Kasparov S , Katahira K , Shimizu T , Murphy D , Paton JF. Dynamic exercise attenuates spontaneous baroreceptor reflex sensitivity in conscious rats. Exp Physiol : 517–526, 2003. [DOI] [PubMed] [Google Scholar]
- 43. Waki H , Kasparov S , Wong LF , Murphy D , Shimizu T , Paton JF. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J Physiol : 233–242, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang G , Anrather J , Huang J , Speth RC , Pickel VM , Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci : 5516–5524, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wray DW , Nishiyama SK , Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol : H497–H504, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu K , Lu D , Rowland NE , Raizada MK. Angiotensin II regulation of tyrosine hydroxylase gene expression in the neuronal cultures of normotensive and spontaneously hypertensive rats. Endocrinology : 3566–3576, 1996. [DOI] [PubMed] [Google Scholar]