Abstract
After spinal cord injury (SCI), the neurogenic bladder is observed to develop asynchronous bladder and external urethral sphincter (EUS) contractions in a condition known as detrusor-sphincter dyssnergia (DSD). Activation of the EUS spinal controlling center located at the upper lumbar spinal cord may contribute to reduce EUS dyssynergic contractions and decrease urethral resistance during voiding. However, this mechanism has not been well studied. This study aimed at evaluating the effects of epidural stimulation (EpS) over the spinal EUS controlling center (L3) in combination with a serotonergic receptor agonist on EUS relaxation in naive rats and chronic (6–8 wk) T8 SCI rats. Cystometrogram and EUS electromyography (EMG) were obtained before and after the intravenous administration of 5HT-1A receptor agonist and antagonist. The latency, duration, frequency, amplitude, and area under curve of EpS-evoked EUS EMG responses were analyzed. EpS on L3 evoked an inhibition of EUS tonic contraction and an excitation of EUS intermittent bursting/relaxation correlating with urine expulsion in intact rats. Combined with a 5HT-1A receptor agonist, EpS on L3 evoked a similar effect in chronic T8 SCI rats to reduce urethral contraction (resistance). This study examined the effect of facilitating the EUS spinal controlling center to switch between urine storage and voiding phases by using EpS and a serotonergic receptor agonist. This novel approach of applying EpS on the EUS controlling center modulates EUS contraction and relaxation as well as reduces urethral resistance during voiding in chronic SCI rats with DSD.
Keywords: electromyography, epidural stimulation, serotonergic receptors, urethral resistance
spinal cord injury (SCI) has a profound effect on the health of a patient's life. Researchers have noted that patients with SCI placed bowel/bladder, sexual function, and mobility as top priorities when assessing quality of life (25). Disorganized voiding reflexes, or detrusor-sphincter dyssynergia (DSD), develop in the majority of SCI patients. During voiding, the external urethral sphincter (EUS) dyssynergically contracts instead of relaxing completely, causing urine flow to be interrupted and the bladder pressure to rise (3). Commonly, DSD leads to urinary tract infections and long-term severe renal damages (30). The urgency and timeliness to better understand and modulate the changes occurring in the spinal circuits controlling lower urinary tract (LUT) function after SCI are evident.
The urinary bladder and EUS in rats receive their efferent innervation from parasympathetic neurons and EUS motoneurons located at the caudal lumbosacral levels (L6-S1) of the spinal cord. It is generally believed that these neurons are controlled by input from the pontine micturition center that is primarily responsible for coordinating the activity of the urinary bladder and urethral outlet during micturition (11). However, studies by Chang et al. (5) suggested that the EUS bursting pattern generator is located in the L3-4 spinal segments in female rats and is also important for generating the alternating relaxations and contractions of the EUS, an essential feature for efficient voiding in rodents. Thus the voiding phase in rodents is controlled by the upper lumbar levels (L3-4) instead of the motoneurons of the L6-S1 spinal segments in rats. A recent study reported that epidural stimulation (EpS) between L3 and L6 improves bladder function after severe SCI when combined with long-term locomotor training and serotonergic receptor agonists (15). The combinatorial treatment decreased nonvoiding contractions, leak point pressure, and urethral outlet resistance. Therefore, the present study, using a rat SCI model, was designed to evaluate the modulation of EUS tonic/bursting activity by using EpS over the upper lumbar spinal cord and to understand the switch between storage and voiding phases in the chronically injured spinal cord.
Furthermore, serotonergic 5HT-1A mechanisms play an important role on the modulation of LUT function, especially on EUS relaxation in rats, in part by prolonging the silent period within the bursting activity during voiding (6, 10, 12–13). While EpS elicits the inhibition of EUS tonic activity and further evokes bursting responses, a concurrent administration of a 5HT-1A receptor agonist may provide a synergistic effect. The present study was carried out as a combination of EpS on L3 spinal cord segment and serotonergic administration to investigate the mechanisms of EUS relaxation during voiding in rats with DSD.
METHODS
All animal use and procedures were performed in accordance with the guidelines established by the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. A total of 21 animals were used in the present study and included control (n = 9) and SCI (n = 12) groups.
Spinal cord transection.
Twelve adult female rats (Sprague-Dawley, weighing 210–250 g) underwent a complete spinal cord transection at spinal level T8 under gas anesthesia (isoflurane, 2–5%). The spinal cord transection was verified by visual inspection under the microscope. Cut ends were exposed and separated by Gelfoam (5). An analgesic (buprenorphine; 0.05 mg/kg) was given subcutaneously every 12 h for 48 h to relieve any postoperative pain. An antibiotic (Baytril; 5 mg/kg, sc) was given daily for 7 days to prevent urinary infections. Postoperatively, the bladder was manually expressed twice a day for 3 wk and then once a day until the end point (6–8 wk). At the end point, the animals underwent surgical preparation for EpS and urodynamic recordings.
Preparation for EpS and urodynamic recordings.
Both control (n = 9) and SCI (n = 12) groups were given urethane (1.2 g/kg sc) 1 h before the surgical preparation. Each animal was placed on a water-circulating heating pad. An established method was used to place the EpS wires (16). In brief, a laminectomy was performed at a thoracic level of T12-L2. For EpS over L3 (L3-EpS), the spinal segments of L2-3 were exposed for placements of epidural stimulating electrodes. Two Teflon-coated stainless steel wires (AS632; Cooner Wire) were placed over the midline at dorsal surface of the spinal cord. The stimulation electrodes were made by removing a small portion (∼1-mm notch) of the Teflon coating to expose the stainless steel wire on the surface facing the spinal cord and then suturing them to the dura of L2 and L3 as the anode and cathode between 2 and 4 mm, respectively, to provide the bipolar/biphasic stimulation by an isolated unit and an electrical stimulator (SIU5 and S88; GRASS Technologies). The dura remained intact. In some animals, the EpS was tested over L6 (L6-EpS). The EpS wires were placed over the midline at dorsal surface of the spinal segments and sutured to the dura of L6-S1 as the anode and cathode, respectively, to provide the bipolar/biphasic stimulation. A bladder catheter and EUS EMG wires were placed using established methods (5, 6). Briefly, a midline lower abdominal incision was made to expose the bladder. A flared tip of a PE-50 catheter (Instech Laboratories) was inserted into the top of bladder dome and secured by a surgical thread. The bladder catheter was then connected to an infusion pump (KD Scientific) and a pressure sensor (Biopac Systems) via a three-way stopcock. Two 50-μm PFA-insulated platinum-iridium wire electrodes (A-M Systems) were inserted into EUS bilaterally using a 27-gauge needle. Wire electrodes were then connected to the amplifier (Biopac Systems). The infusion rate of saline was set at 0.12 ml/min. EpS was applied at 1- to 1.5-fold of the threshold intensity (1–5 V) at 10–40 Hz and a 0.1- to 0.3-ms pulse width for 50–70 s. The threshold intensity was set at the appearance of EUS bursting responses because EUS bursting has been an important index for voiding in rodents. The stimulation intensity was adjusted to avoid hindlimb movements and reduce the interference from the muscle twitching. The elicited EUS responses were collected before and after drug administration. 8-Hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT, a 5HT-1A receptor agonist; 0.5 mg/kg; Sigma-Aldrich, St Louis, MO) was administrated intravenously (iv). N-{2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl}-N-(2-pyridinyl) cyclohexanecarboxamide trihydrochloride (WAY100635, a 5HT-1A receptor antagonist; 0.3 mg/kg; Sigma-Aldrich) was given iv to evaluate the effect of 8-OH-DPAT on EUS activity. Both of 8-OH-DPAT and WAY100635 were dissolved in 0.9% of normal saline at the final dose of 1 mg/ml, aliquot in 1-m tube, and then stored in the −20°C freezer. These tubes were used within 2 wk since they were made.
Outcome measurements.
During continuous bladder infusion, three voiding contractions were evaluated 60 min after infusion. Outcome measures from cystometrogram and EUS electromyography (EMG) activity included the maximum intravesical pressure, resting pressure, and contraction duration as well as the maximum amplitude and area under curve (AUC). The latency of the first void (LFV) was measured as the latency from the start of empty bladder filling to the onset of the first void. The bladder capacity was calculated as the volume (ml) of [LFV (min) × infusion rate (0.12 ml/min)]. The latency to EUS bursting, duration of inhibited EUS tonic activity, duration of evoked bursting activity, and maximum amplitude, AUC, and firing frequency of evoked EUS bursting were measured for three responses and then averaged in each animal before and after drug administration.
Statistical analysis.
Data without drugs were analyzed by t-test to examine the differences between two groups using Prism 4.0 (GraphPad Software). Repeated t-test was applied to compare the drug effect of different doses. Quantitative data are expressed as means ± SE. We regarded P < 0.05 to indicate a statistically significant difference between groups.
RESULTS
Identification of EpS-evoked EUS reflexes.
In control rats (n = 9) EUS EMG recordings demonstrated a low amplitude tonic activity during bladder filling. This tonic activity increased gradually as the infused volume approached the voiding threshold. During bladder contractions, EUS EMG patterns markedly changed, consisting of increased tonic and bursting activity (Fig. 1). The bladder was partially distended to examine the increase of EUS tonic activity before onset of EpS. After EUS activity was measured, EpS parameters were optimized to prevent unwanted hind limb movement by utilizing lower intensity (voltages). A range of frequencies (10–40 Hz) and pulse widths (0.1–0.3 ms) was examined (Fig. 2). The optimal parameters of EpS were determined as 40 Hz and 0.2-ms pulse width because such EpS evoked not only inhibition of EUS tonic activity but also EUS bursting. Each stimulation episode occurred for 50–70 s with a resting time of 4–5 min. EUS EMG responses to EpS were not observed if the waiting time was <4 min under these stimulating parameters. L6-EpS evoked continuous EUS tonic contraction (Fig. 3A) until EpS stopped. L3-EpS elicited several EUS responses including an initial increase of EUS activity, followed by inhibition of EUS activity and onset of evoked EUS bursting (Fig. 3B) in control rats.
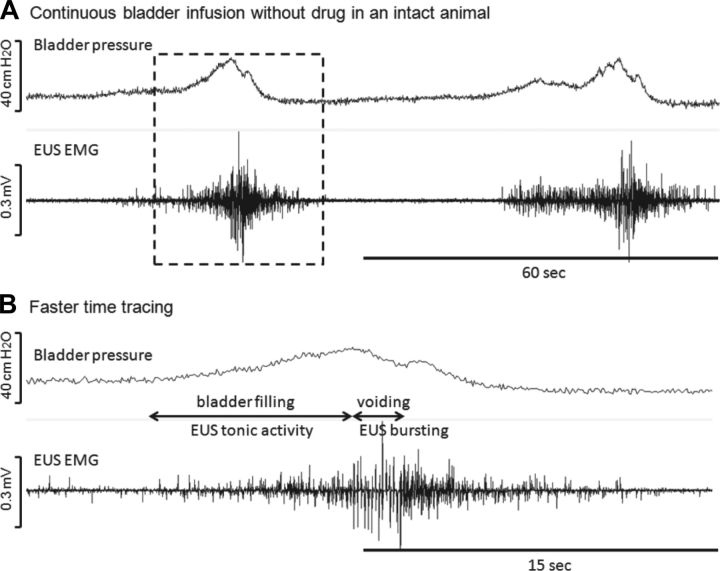
Fig. 1.
Continuous bladder infusion in an intact rat. A: regular voiding contractions responding to the saline infusion of the bladder are shown. B: faster time tracing shows external urethral sphincter (EUS) bursting during voiding. To summarize, the rat exhibits EUS tonic activity during storage phase and switches to bursting when voiding. The infusion rate was 0.12 ml/min.
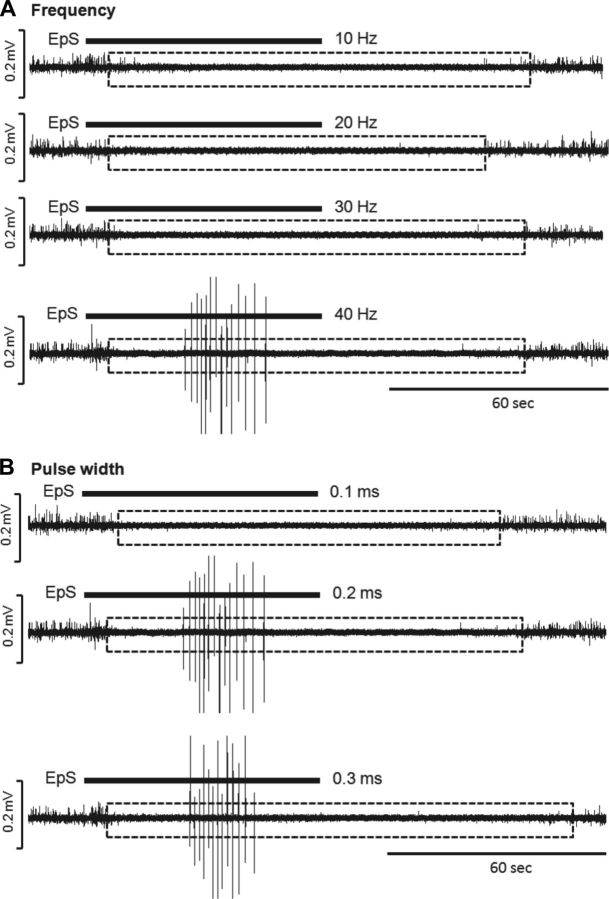
Fig. 2.
Representative examples acquired at different ranges of frequencies (A) at a 0.2-ms pulse width and pulse widths (B) at 40 Hz in a naive rat. The intensity was 5 V, 1.5-fold of threshold without lower extremities' movement. Note the bursting reflex was used as an indicator of voiding reflex because bursting only appeared during voiding in rodents. Therefore, the suggestive optimal stimulation was a 40-Hz, 0.2-ms pulse width at 1- to 2-fold over threshold intensity.
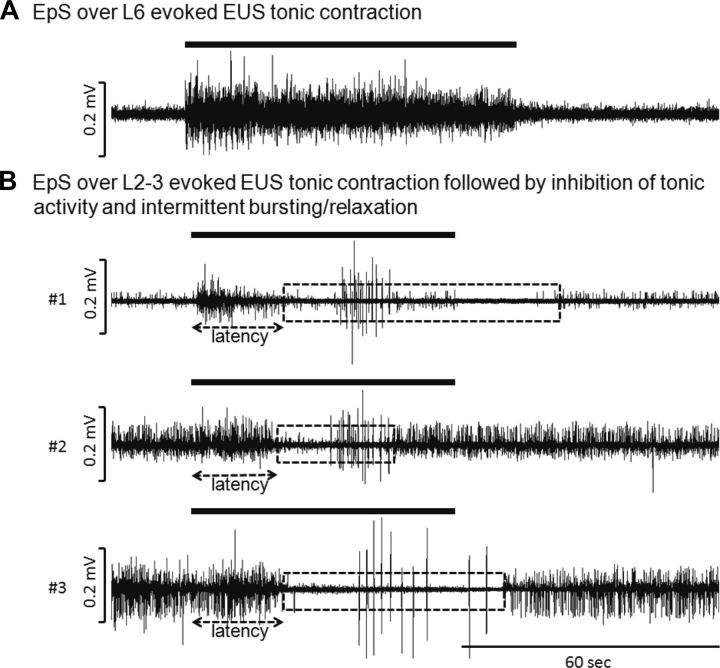
Fig. 3.
Representative examples of EUS bursting reflex elicited by epidural stimulation (EpS) on L6 (A) or L3 (B) spinal segment in 3 intact rats. Note EpS suppressed EUS tonic activity, and elicited EUS bursting reflex. In animals 1 and 3, the duration of suppressed tonic activity was longer and continued after EpS has been stopped. It may indicate that EpS was given close to EUS bursting pattern generator compared with the EpS location at animal 2. Dotted boxes indicate the duration of inhibited EUS tonic activity evoked by EpS. The solid bar above the tracings indicated the duration of EpS (4–6 V, 0.2-ms pulse width at 40 Hz).
When the bladder was empty with modest EUS tonic activity, L3-EpS-evoked EUS bursting was shown but not the inhibition of EUS tonic activity. When the bladder was filled (62 ± 6% of the bladder capacity based on LFV of each animal, n = 9), both EUS bursting and inhibition of EUS tonic activity were evoked by L3-EpS. L3-EpS-evoked EUS bursting was compared when the bladder was empty or distended in control rats. There were no significant EUS bursting changes of latency, duration, frequency, AUC, and maximum amplitude (Table 1). These evoked EUS responses were eliminated after an acute bilateral pudendal nerve transection or T8 spinal transection (Fig. 4). A bilateral pelvic nerve transection did not affect these L3-EpS-evoked EUS responses.
Table 1.
Measurements of L3-EpS-evoked EUS bursting responses when the bladder was empty or distended in the control rats
| Latency, s | Duration, s | Frequency, Hz | Amplitude, mV | AUC, mV/s | |
|---|---|---|---|---|---|
| Empty bladder (n = 9) | 29.63 ± 3.54 | 18.15 ± 1.75 | 1.14 ± 0.15 | 0.19 ± 0.04 | 0.34 ± 0.15 |
| Distended bladder (n = 9) | 30.91 ± 4.00 | 15.97 ± 2.99 | 1.11 ± 0.29 | 0.17 ± 0.14 | 0.28 ± 0.14 |
EpS, epidural stimulation; EUS, external urethral sphincter; AUC, area under curve.
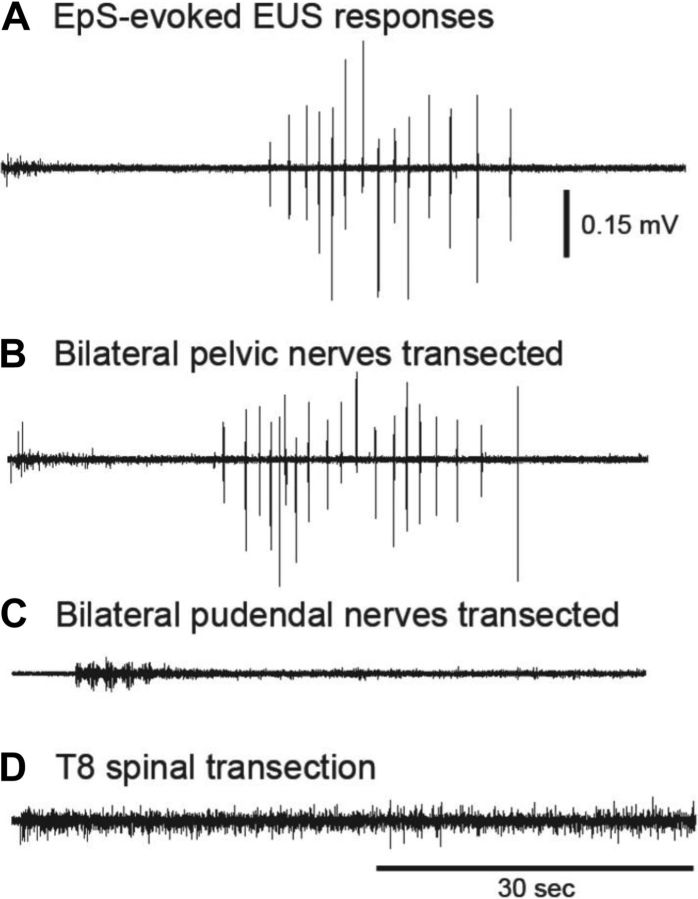
Fig. 4.
Verification of the pathway of evoked EUS responses in intact rats (A) when EpS was applied over L3. The animal received bilateral pelvic nerve transection (B), and it did not affect evoked EUS responses. Then, the same animal underwent bilateral pudendal nerve transection (C). The evoked EUS responses were eliminated. In other words, the evoked EUS responses are suggested to be conducted by pudendal nerve. In another rat, an acute T8 transection (D) alone showed that the evoked EUS responses were eliminated when the pathway to the pontine micturition center was blocked acutely.
After administration of 8-OH-DPAT, a 5HT-1A agonist, the maximum amplitude and AUC of evoked EUS bursting were significantly increased in control rats (Table 2). WAY100635, a 5HT-1A antagonist, reversed the effect of 8-OH-DPAT and decreased the frequency and AUC of EUS bursting. The inhibition of EUS activity was significantly prolonged by WAY100635 compared with the condition without drug (Table 2).
Table 2.
Statistical analysis of evoked EUS EMG responses in naive animals before and after drugs
| Naive Animals | Without Drug | 8-OH-DPAT | 8-OH-DPAT + WAY100635 |
|---|---|---|---|
| n | 9 | 9 | 7 |
| Inhibition of EUS activity, s | 39 ± 6 | 33 ± 4 | 61 ± 16† |
| Latency to the beginning of inhibition, s | 29 ± 4 | 22 ± 3 | 23 ± 3 |
| Duration of EUS bursting, s | 18 ± 2 | 21 ± 3 | 19 ± 3 |
| Frequency of EUS bursting, Hz | 1.7 ± 0.3 | 1.8 ± 0.3 | 0.3 ± 0.1† |
| Amplitude of EUS bursting, mV | 0.15 ± 0.03 | 0.23 ± 0.06* | 0.11 ± 0.03 |
| AUC of EUS bursting, mV/s | 0.33 ± 0.08 | 0.67 ± 0.04* | 0.16 ± 0.06† |
Inhibition of EUS EMG activity includes the duration of EUS bursting. WAY100635 (0.3 mg/kg iv) was given after 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT; 0.5 mg/kg iv).
†P < 0.05, significant change compared with the recordings before drug administration.
Examinations of EpS-evoked EUS reflexes after spinal cord injury.
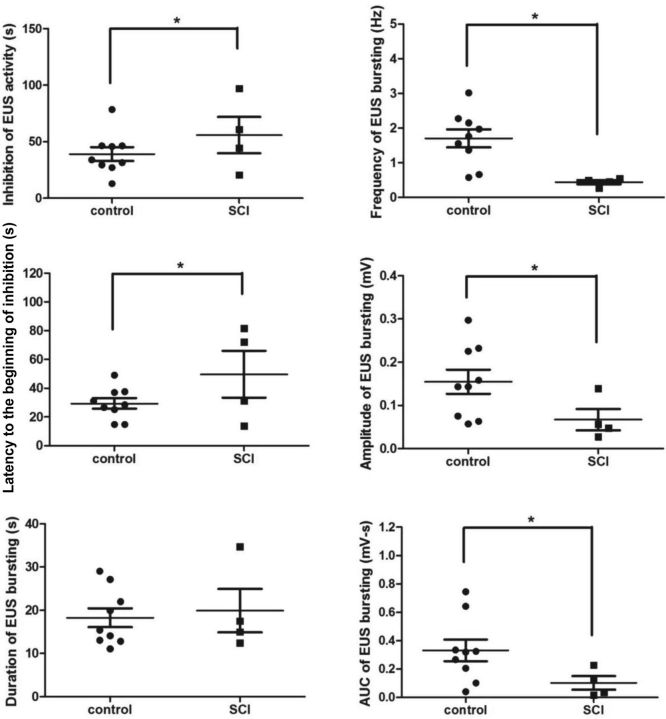
In rats with a chronic SCI (n = 12), only large amplitude EUS tonic activity (contraction) was produced during voiding (Fig. 5A). The bladder was partially distended by infusing saline, 60% of bladder capacity based on LFV of each animal (n = 12). L6-EpS evoked continuous EUS tonic contraction that persisted even after EpS was stopped (Fig. 5C). L3-EpS elicited inhibition of EUS activity (relaxation) in all SCI rats but only elicited EUS bursting in 4 out of 12 SCI rats. The latency of L3-EpS-evoked EUS responses was significantly delayed compared with controls (Fig. 6). The duration of EUS bursting did not significantly differ between SCI and control groups. The firing frequency, maximum amplitude, and AUC were significantly decreased in SCI rats (Fig. 6). SCI rats had prolonged inhibition of EUS tonic activity during L3-EpS compared with controls. L3-EpS-evoked EUS responses, including inhibition of tonic activity and intermittent relaxation/bursting, were present when the bladder was empty or distended.
Fig. 5.
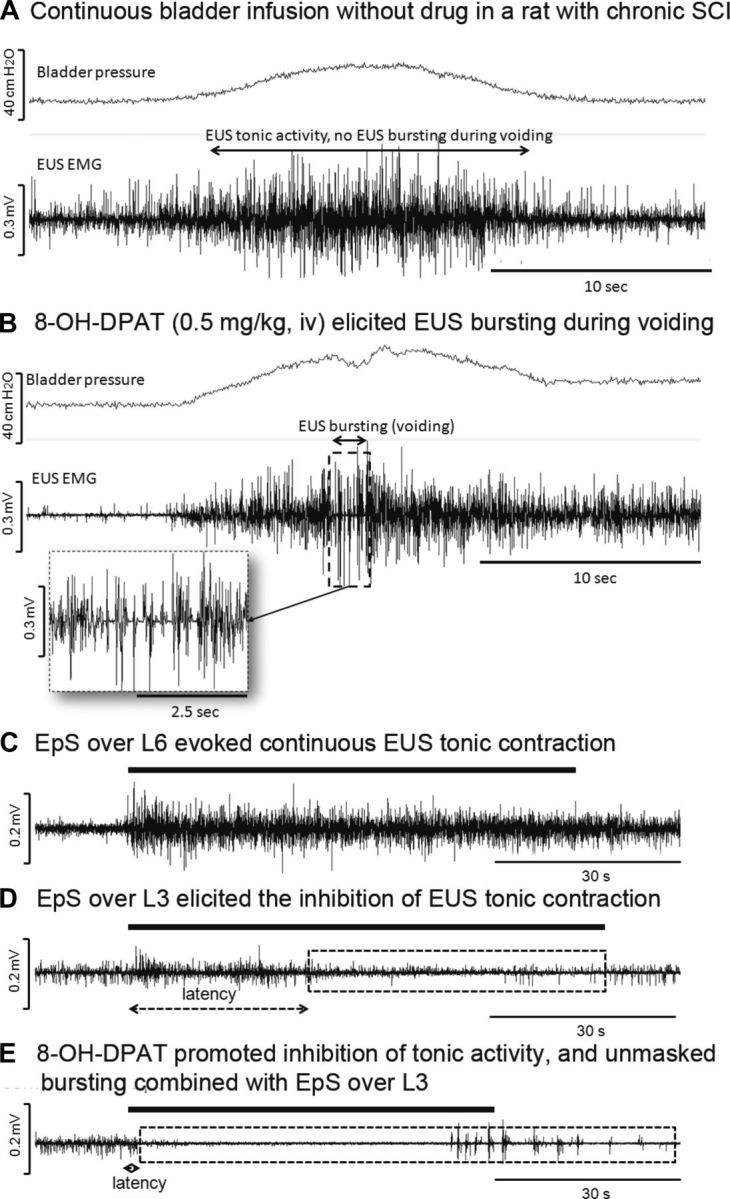
Representative examples of continuous bladder infusion in a rat with chronic spinal cord injury (SCI). The rat produced only EUS tonic activity during storage and voiding phases, representing detrusor sphincter dyssnergia (DSD) after SCI (A). No EUS relaxation was found during the high amplitude part of the tonic contractions during voiding. After administration of 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT; 0.5 mg/kg iv), the EUS bursting was unmasked during voiding (B). Increased EUS relaxation (silent periods between each individual burst) was also demonstrated after drug administration. The bladder infusion rate was 0.12 ml/min. When EpS was applied over L6, only continuous EUS tonic firing activity was seen (C). However, EpS over L3 evoked the inhibition of EUS tonic activity (storage) but no bursting (D). The latency was shortened and the duration of suppressed EUS tonic activity (storage) was prolonged by administration of 8-OH-DPAT (0.5 mg/kg iv; E).
Fig. 6.
Statistical analysis of EpS evoked EUS responses in control (n = 9) and SCI (n = 4) groups without drug administration. AUC, area under the curve. *P < 0.05, significant changes.
After administration of 8-OH-DPAT, EUS bursting during voiding was unmasked in the SCI group (Fig. 5B). All SCI rats showed EUS bursting evoked by L3-EpS. 8-OH-DPAT significantly shortened the latency of evoked EUS responses as well as enhanced the maximum amplitude, frequency, and AUC of evoked EUS bursting in all SCI rats (Fig. 5E and Table 3). WAY100635 reversed the effect of 8-OH-DPAT, and only 3 out of 12 SCI rats showed L3-EpS-evoked EUS responses. WAY100635 significantly decreased the duration of EUS bursting and the inhibition of EUS activity compared with the condition without drug (Table 3).
Table 3.
Statistical analysis of evoked EUS EMG responses in the animals with chronic SCI before and after drugs
| Chronic SCI | Without Drug | 8-OH-DPAT | 8-OH-DPAT + WAY100635 |
|---|---|---|---|
| n | 4 | 12 | 3 |
| Inhibition of EUS activity, s | 56 ± 16 | 52 ± 8 | 33 ± 14† |
| Latency to the beginning of inhibition, s | 50 ± 16 | 27 ± 4* | 45 ± 12 |
| Duration of EUS bursting, s | 20 ± 5 | 15 ± 2 | 9 ± 2† |
| Frequency of EUS bursting, Hz | 0.44 ± 0.06 | 0.60 ± 0.07* | 0.56 ± 0.05 |
| Amplitude of EUS bursting, mV | 0.07 ± 0.03 | 0.14 ± 0.03* | 0.04 ± 0.01 |
| AUC of EUS bursting, mV/s | 0.10 ± 0.05 | 0.21 ± 0.05* | 0.09 ± 0.03 |
SCI, spinal cord injury. Inhibition of EUS EMG activity includes the duration of EUS bursting. WAY100635 (0.3 mg/kg iv) was given after 8-OH-DPAT (0.5 mg/kg iv).
†P < 0.05, significant change compared with the recordings before drug administration.
DISCUSSION
The present study investigated the effects of spinal electrical stimulation over the dura matter at the dorsal surface of the L3 spinal cord segment in attempts to modulate EUS relaxation during voiding. L3-EpS evoked an inhibition of EUS tonic activity and activation of EUS bursting in control animals. L3-EpS inhibited EUS tonic activity in spinal cord transected rats, and L3-EpS in combination with a serotonergic receptor agonist (8-OH-DPAT) inhibited EUS tonic activity and unmasked EUS bursting. These results indicated that L3-EpS combined 8-OH-DPAT over the EUS spinal pattern generator promotes voiding and offers a potential new strategy for EUS relaxation during voiding to ameliorate DSD after SCI.
EpS of the L3 segment evokes spinal EUS reflexes in the present study and indicates possible linkage between the L3 and L6-S1 segments for LUT function, especially EUS activation. Previous histological and electrophysiological studies have shown possible connections between the L3-4 and L6-S1 spinal segments that might mediate the EUS bursting. In male rats, bursting activity was identified in the periurethral striated muscles (bulbocavernosus and ischiocavernous muscles) involved in ejaculation (20). The ejaculation reflex is mediated by a spinal pattern generator located in the L3-4 spinal segments (29). Because pseudorabies virus (PRV) tracing studies also revealed PRV-labeled neurons in this same region following injection of PRV into EUS of female rats (19), these L3-4 spinal neurons may also be responsible for the generation of EUS bursting activity during voiding (5). Other studies showed that electrical stimulation of the pudendal nerve, which contains afferents innervating the EUS, increased c-fos expression in the L3-4 spinal segments (31). In addition, electrical stimulation of the pelvic nerve, which contains afferents from the bladder and urethra, increased c-fos staining in neurons located between the L2 and S1 segments (2). These reports provide support for a circuitry connecting motor pathways in the L6-S1 spinal cord with a putative pattern generator in the L3-4 spinal segments. Our data suggest that, instead of direct stimulation of Onuf's nucleus at the lumbosacral levels (L6-S1), stimulation of the upper lumbar levels containing the EUS bursting pattern generator may provide an innovative approach to modulate LUT functions.
A variety of electrical stimulation treatments are currently under investigation or already clinically available for modulating voiding dysfunction. Functional electrical stimulation devices (23), peripheral nerve stimulation (26), and intraspinal stimulation (14, 22) methods are examples of electrical stimulation approaches currently in use and/or under investigation. These electrical stimulation methods (1, 14, 22, 24, 26) target the lumbosacral levels at L6-S2 to directly activate the preganglionic parasympathetic neurons and EUS motoneurons. While the majority of prior studies have focused on electrical stimulation at lumbosacral levels, the present study demonstrates a novel method of EpS over upper lumbar levels to modulate urethral sphincter activity (5).
Previous investigations have demonstrated early (ER) and late (LR) responses of pelvic-EUS reflexes elicited by pelvic nerve stimulation in spinally intact rats to represent EUS tonic and bursting activities, respectively (5). EpS-evoked EUS responses are similar to the ER and LR of pelvic-EUS reflexes. The ER and tonic activity were eliminated when L6-S1 segments were transected indicating EUS tonic activity is mediated by a spinal pathway. The present study confirmed that tonic activity was elicited when L6-EpS was applied in naive and spinal cord injured rats. In contrast to ER and EUS tonic activity mediated by L6-S1, the LR and EUS bursting are eliminated when L3-4 spinal segments are damaged indicating EUS bursting is mediated by a supinobulbospinal reflex pathway. L3-EpS evoked the EUS bursting. However, L3-EpS provides strong stimulation to elicit EUS bursting responses, regardless of whether the bladder is empty or distended. The LR of pelvic-EUS reflex requires bladder distension to be evoked by indirect stimulation of the L3-4 segments via pelvic nerve stimulation. Firing frequencies (1–2 Hz) of LR of pelvic-EUS reflexes and EpS-evoked EUS bursting indicate more EUS relaxation compared with the regular EUS bursting (6–8 Hz) when the bladder is infused by saline (4, 5, 9).
In the present study, when EpS was applied directly on L3, the spinal EUS bursting center, EUS tonic activity was first suppressed and then the bursting responses were evoked. When EpS was applied slightly closer to L2, EpS-evoked EUS responses showed shorter duration of suppressed tonic activity (Fig. 3B). However, when EpS was applied on L6, it only evoked continuous EUS tonic activity. These results indicated that the optimal stimulation site may be located closely to L3. The present results showed that EpS-evoked EUS responses are not affected by transecting the pelvic nerves, but they are eliminated when the bilateral pudendal nerves are cut. Therefore, all EpS-evoked EUS responses are conducted via the pudendal nerves, which innervate the EUS muscle (21). By acutely transecting the T8 spinal segment, all evoked responses were eliminated. In most chronic T8 SCI rats, L3-EpS evoked the inhibition of EUS tonic activity and EUS bursting when combined with 8-OH-DPAT.
Serotonergic 5HT-1A mechanisms have a long-standing history in promoting EUS activation in rodent models (5, 6, 8, 10, 12, 18, 28). 8-OH-DPAT, a 5HT-1A receptor agonist, produces a bladder contraction leading to micturition at subthreshold bladder pressures (18). In the ventral horn, 5HT-1A binding sites are very densely located in the spinal dorsolateral nucleus of the pudendal nerve (27). Studies also indicate that 5HT-1A receptors facilitate EUS bursting duration and relaxation in rats (6, 10), which is important to expulse the urine and empty the bladder. Here we showed that 8-OH-DPAT increased the amplitude and AUC of L3-EpS-evoked EUS bursting responses in intact rats. However, in the chronic SCI rats, L3-EpS only suppressed EUS tonic activity but did not elicit EUS bursting in 8 of 12 chronic SCI rats without 8-OH-DPAT. Additionally, 8-OH-DPAT further unmasked EUS bursting and shortened the latency of EUS bursting in all SCI rats. 8-OH-DPAT also increased the amplitude, AUC, and firing frequency of EUS bursting. The excitatory effect of 8-OH-DPAT on L3-EpS-evoked EUS responses is attributable to an action in the spinal cord because it occurred in chronic SCI rats. These results appear similarly to the ER of the pelvic-EUS reflex facilitated by 8-OH-DPAT in intact rats as well as in chronic T8 SCI rats and the LR of pelvic-EUS reflexes facilitated by 8-OH-DPAT in chronic T8 SCI rats (5).
To validate the effects of 8-OH-DPAT in EpS-induced EUS activity, a known 5HT-1A antagonist, WAY1000635, was used. In our study, after administration of 8-OH-DPAT, WAY100635 extensively prolonged the inhibition of EUS tonic activity in intact rats but reduced the inhibition of EUS tonic activity and EUS bursting responses in the SCI rats. Previous studies in neurologically intact rats revealed that WAY100635 alone not only reversed the excitatory effect of 8-OH-DPAT but also suppressed reflex bladder activity (5, 17, 28) and EUS bursting induced by bladder distension (5). This indicates that EUS bursting is facilitated by an endogenous serotonergic mechanism in anesthetized rats with an intact neuraxis. Therefore, serotonergic effects of prolonged EUS relaxation on EUS bursting center located in the L3 spinal segment can be expected from these two studies when indirect activation is provided by electrical stimulation of the pelvic afferents or directly on the L3 segment.
The present study examined the possibility of modulating the EUS spinal controlling center and investigated the mechanisms for switching between urine storage and voiding phases. The overall goal of this technique is to suppress the dyssynergic EUS contraction and then empty the bladder by the later EpS-evoked EUS bursting during voiding in chronic SCI rats with DSD. Although humans do not have EUS bursting during voiding, EpS on the EUS spinal controlling center may contribute to suppress dyssynergic EUS contraction while the bladder reaches voiding threshold in the SCI patients with DSD to expulse urine.
GRANTS
The present study is partially supported by the University of California through a continuation of the Roman Reed Spinal Cord Injury Research Fund of California and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.M.A. and H.H.C. conception and design of research; E.M.A., R.M.I., and H.H.C. performed experiments; E.M.A. and H.H.C. analyzed data; E.M.A. and H.H.C. drafted manuscript; E.M.A., R.M.I., L.A.H., and H.H.C. edited and revised manuscript; E.M.A., R.M.I., L.A.H., and H.H.C. approved final version of manuscript; L.A.H. and H.H.C. interpreted results of experiments; H.H.C. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Lisa Wulund for assisting with surgery and postoperative animal care.
REFERENCES
- 1.Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res : 227–239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birder LA, Roppolo JR, Iadarola MJ, de Groat WC. Electrical stimulation of visceral afferent pathways in the pelvic nerve increases c-fos in the rat lumbosacral spinal cord. Neurosci Lett : 193–196, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil : 272–280, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am J Physiol Regul Integr Comp Physiol : R224–R234, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections at different segmental levels indicate that different spinal circuits are involved in tonic and bursting external urethral sphincter activity in rats. Am J Physiol Renal Physiol : F1044–F1053, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang HH, Havton LA. Serotonergic 5-HT1A receptor agonist (8-OH-DPAT) ameliorates impaired micturition reflexes in a chronic ventral root avulsion model of incomplete cauda equina/conus medullaris injury. Exp Neurol : 210–217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen KJ, Chen LW, Liao JM, Chen CH, Ho YC, Ho YC, Cheng CL, Lin JJ, Huang PC, Lin TB. Effects of a calcineurin inhibitor, tacrolimus, on glutamate-dependent potentiation in pelvic-urethral reflex in anesthetized rats. Neuroscience : 69–76, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Chen SC, Cheng CL, Fan WJ, Chen JJ, Lai CH, Peng CW. Effect of a 5-HT1A receptor agonist (8-OH-DPAT) on external urethral sphincter activity in a rat model of pudendal nerve injury. Am J Physiol Regul Integr Comp Physiol : R225–R235, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol : 445–454, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cheng CL, de Groat WC. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. Am J Physiol Renal Physiol : F771–F778, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res : 59–84, 2005. [DOI] [PubMed] [Google Scholar]
- 12.de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology : 30–36, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol : R1699–R1706, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain Res : 19–30, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Horst M, Heutschi J, van den Brand R, Andersson KE, Gobet R, Sulser T, Courtine G, Eberli D. Multisystem neuroprosthetic training improves bladder function after severe spinal cord injury. J Urol : 747–753, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett : 339–344, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kakizaki H, Yoshiyama M, Koyanagi T, de Groat WC. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturitionreflex pathway in the rat. Am J Physiol Regul Integr Comp Physiol : R1407–R1413, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Lecci A, Giuliani S, Santicioli P, Maggi CA. Involvement of 5-hydroxytryptamine1A receptors in the modulation of micturition reflexes in the anesthetized rat. J Pharmacol Exp Ther : 181–189, 1992. [PubMed] [Google Scholar]
- 19.Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol : 584–602, 1997. [PubMed] [Google Scholar]
- 20.McKenna KE, Chung SK, McVary KT. A model for the study of sexual function in anesthetized male and female rats. Am J Physiol Regul Integr Comp Physiol : R1276–R1285, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Peng CW, Chen JJ, Cheng CL, Grill WM. Improved bladder emptying in urinary retention by electrical stimulation of pudendal afferents. J Neural Eng : 144–154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pikov V, Bullara L, McCreery DB. Intraspinal stimulation for bladder voiding in cats before and after chronic spinal cord injury. J Neural Eng : 356–368, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragnarsson KT. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord : 255–274, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Rijkhoff NJ, Wijkstra H, van Kerrebroeck PE, Debruyne FM. Urinary bladder control by electrical stimulation: review of electrical stimulation techniques in spinal cord injury. Neurourol Urodyn : 39–53, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Simpson LA, Eng JJ, Hsieh JT, Wolfe DL; Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma : 1548–1555, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai C, Booth AM, de Groat WC, Roppolo JR. Bladder and urethral sphincter responses evoked by microstimulation of S2 sacral spinal cord in spinal cord intact and chronic spinal cord injured cats. Exp Neurol : 171–183, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience : 235–252, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Testa R, Guarneri L, Poggesi E, Angelico P, Velasco C, Ibba M, Cilia A, Motta G, Riva C, Leonardi A. Effect of several 5-hydroxytryptamine(1A) receptor ligands on the micturition reflex in rats: comparison with WAY100635. J Pharmacol Exp Ther : 1258–1269, 1999. [PubMed] [Google Scholar]
- 29.Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science : 1566–1569, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. Urol Clin North Am : 459–473, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Wiedey J, Alexander MS, Marson L. Spinal neurons activated in response to pudendal or pelvic nerve stimulation in female rats. Brain Res : 106–114, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]