Abstract
The etiology of digestive pathologies such as irritable bowel syndrome (IBS), inflammatory bowel diseases (IBD) and cancer is not yet fully understood. In recent years, several studies have evidenced circadian variations in mechanisms involved in digestive health. In situations of disturbed circadian rhythms (chronodisruption) where the central clock and the peripheral clocks receive incoherent signals, the synchronicity is lost producing implications for health. This lack of coordination could alter the tissue function and cause long term damage to the organs. Life habits such as sleep, physical exercise, social interaction, and feeding times are determinants for stability and integrity of circadian rhythms. In recent years, experimental and clinical studies have consistently evidenced that the alteration of circadian rhythms is associated with the development of digestive pathologies mainly linked to dismotility or changes in microbiota composition. Likewise, it seems reasonable to deep into the importance of chronodisruption as a factor that may participate in the development of pathologies such as IBS, IBD and digestive cancers. Moreover, life habits respecting circadian rhythms should be promoted for the prevention of these diseases. Further studies will allow us a better understanding of the mechanisms acting at molecular level, and the development of new therapeutic targets.
Keywords: Circadian rhythms, Gastrointestinal diseases, Irritable bowel syndrome, Inflammatory bowel disease, Digestive cancers
Core tip: Chronodisruption, or circadian rhythm disruption, has been associated with impairments in metabolic functions and organ physiology. In this review we focus on the link between circadian rhythms and digestive pathologies such as irritable bowel syndrome, inflammatory bowel diseases and cancers of the digestive tract. The purpose of this review is to highlight what is known about the negative impact of chronodisruption on pathogenesis of these digestive diseases and provide reasons for future research.
INTRODUCTION
Light and dark cycles of 24 h, which are due to rotation of the earth, serve as the dominant environmental factor affecting living organisms. Temporal organization within an organism is critical for maintenance of homeostasis as well as adaptation to changing external conditions. The term «circadian rhythms» describes endogenously generated rhythms that occur approximately every 24 h and play a fundamental role in the survival and evolution of life by ensuring that an organism’s internal physiology remains synchronized with the external environment.
In mammals, several mechanisms contribute to circadian variations. While the suprachiasmatic nucleus in the hypothalamus is the master circadian pacemaker, most tissues, including other brain regions and different organs, harbor self-sustained cellular circadian clocks. The suprachiasmatic nucleus is aligned with the light-dark cycle via the retina; this serves as the primary stimulus for melatonin production in the pineal gland[1] and coordinates all other oscillators throughout the body with external time by binding to melatonin receptors in the organs[2]. At the molecular level, circadian rhythms emerge from Circadian Locomotor Output Cycles Protein Kaput (Clock) genes and proteins, comprising a network of interconnected autoregulatory transcriptional-translational molecular clocks present in virtually every cell of the body. These “clock genes” form a regulation loop with a period lasting approximately twenty-four hours. By regulating the expression of other genes coding for proteins, enzymes and factors related to metabolic homeostasis, the clock genes cooperate to induce circadian variations all over the organism[3]. Circadian clocks are self-sustained and intrinsic, but their rhythm can be entrained by environmental signals, called “Zeitgeber” (timing cue), including light, temperature, and quality of lifestyle habits, such as sleep, physical exercise, social interaction, and very importantly, feeding times[3-6]. A lack of coordination of these elements leads to desynchronization of the circadian rhythms and impacts health.
In the digestive tract, a broad range of vital functions and mechanisms display circadian variations. In the gut, circadian clocks could regulate digestive physiology and maintain intestinal barrier function. For example, in the mouth, the volume of saliva produced is more important during the day than the night[7]. In association, mouth microbiota composition and diversity varies and is influenced by meal times[8]. Further, there is evidence of circadian rhythms in the peristalsis of the digestive tube. Thus, gastric emptying rates are longer in the evening than in the morning, nocturnal propagation velocities of the migrating motor complex are slower, and colonic motor activity is minimal during sleep[9]. The gastrointestinal tract is the most important source of the chemicals melatonin and serotonin outside the central nervous system. Both of these chemicals play an important role in gastrointestinal motility. Furthermore, melatonin plays a major role in the synchronization of central and peripheral oscillators allowing the adaptation of the internal milieu to external environment. Recently, the role of melatonin in the host-microbiota communication within the gut has been emphasized[10]. Both permeability of the digestive tract[11] and the secretion of mucus and digestive enzymes[12] are different at nighttime. Moreover, immune parameters ensuring digestive health follow a 24-h period. Recent studies highlight circadian regulation of innate and adaptive gut immunity[13]. These daily changes are reflected in the gut microbiota diversity and composition[14]. Gastrointestinal ecosystem has a diurnal variation according to the state of food/fasting and the time of day. But the microbiota also impacts the host circadian rhythms[15]. A change in the diet can rapidly shift the composition of the gut microbiome that in turn might be responsible for the reprogramming of circadian rhythmicity[16,17].
Circadian regulation also plays a large role in liver metabolism, as maintenance of plasma glucose, regulation of lipids, including triglycerides, cholesterol and free fatty acids follow circadian rhythms. Bile acids are also under circadian regulation to synchronize with periods of feeding and fasting[18].
It should be highlighted the specificity in the response of distinct peripheral clocks to food challenges[19]. Diet macronutrient variations (high-fat diet, ketogenic diet) trigger differential effects on liver and intestine clocks.
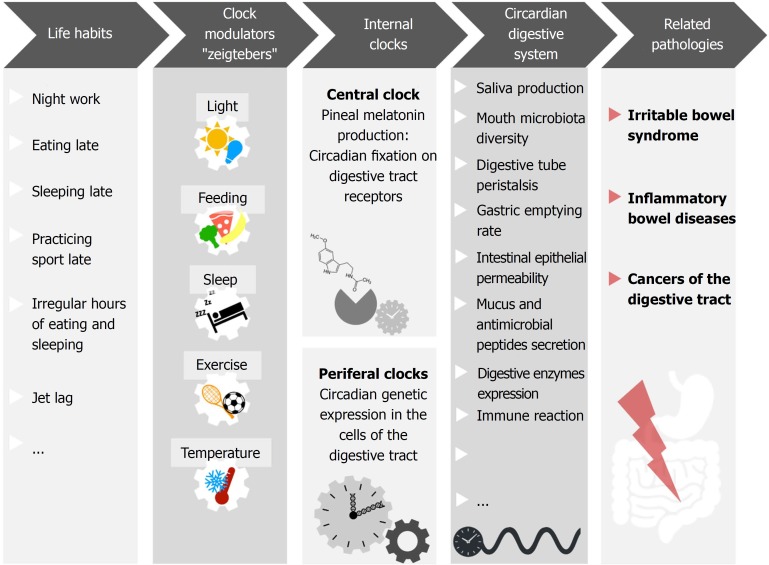
Altogether, these parameters contribute to digestive health, and their dysfunction is related to several pathologies. Given their association with circadian rhythms, it is logical to suspect that chronodisruption may play a part in gastrointestinal diseases pathogenesis (Figure 1).
Figure 1.
Numerous mechanisms involved in gastrointestinal homeostasis display daily rhythms. For example, saliva production, mouth microbiota diversity, digestive tube peristalsis, gastric emptying rate, intestinal epithelial permeability, mucus and antimicrobial peptides secretion, digestive enzymes expression, immune reaction and gut microbiota diversity. Night shift work, light at night, late evening food, late sleep or late physical exercise, as well as irregular meal schedules and jet lag, have been shown to affect the coherence/synchronization of internal circadian rhythms with the environment, affecting the homeostatic rhythms of digestive health parameters. This sequence favors the development of gastrointestinal pathologies such as irritable bowel syndrome, inflammatory bowel diseases, and cancers of the digestive tract.
In this editorial, we focus on the link between circadian rhythms and three types of pathologies: irritable bowel syndrome (IBS), inflammatory bowel disease (IBD) and cancer.
IBS
IBS is a functional gastrointestinal disorder diagnosed clinically and affecting approximately 11% of the population. Patients suffer abdominal pain and altered bowel habits[20]. The causes and mechanistic basis of this disease are not fully understood. Currently, IBS is considered a multifactorial disease that implies visceral hypersensitivity, an alteration in the relationship between the enteric nervous system and the central nervous system, a modification of the gut microflora, increased intestinal permeability and probably minimal intestinal inflammation. Several studies suggest that circadian rhythms play a role in the development and severity of IBS.
Human studies have demonstrated that lifestyle factors, such as disturbed sleep and working the night shift, are associated with a higher prevalence of IBS[21-24]. Consistent with this observation, significantly altered melatonin excretion was detected in the urine of women with IBS[24]. According to the hypothesis that circadian rhythm disruption directly impacts motility, melatonin could be potentially useful in IBS, especially for pain symptoms and bowel motility in constipation-predominant IBS. Some evidence supports the hypothesis that melatonin is involved in the modulation of pain and has analgesic effects; patients displayed an improvement in symptoms with melatonin supplementation[25,26]. However, the high rate of placebo effect observed in patients with IBS means that therapeutic uses of melatonin for pain need further investigation. In another study, IBS patients experienced a decrease in abdominal pain duration and distension intensity, along with an increase in rectal distension pain threshold, with probiotic supplementation (VSL3#). Such improvement occurred in parallel with modulation of melatonin profiles, with patients showing increased morning melatonin levels with VSL#3 treatment. It is possible that probiotic treatment regulates melatonin, leading to an improvement in symptoms[27].
Sleep impairment is not the only risk factor for impaired circadian rhythms. Alimentation schedules participate actively in circadian variations by mobilizing the mechanisms involved in digestion at different times of the day. Many hormones oscillate in a daily fashion in the anticipation of feeding, including ghrelin, leptin, corticosterone, insulin, glucagon, and glucagon-like peptide-1, suggesting the circadian rhythm plays an important role in the regulation of metabolic processing of food. IBS has been associated with habits such as snacking between main meals[28] and eating irregularly[29]. Beyond IBS, disturbed sleep is associated with fewer meals per day and more frequent snacking. Future studies should investigate the benefits of eating and sleeping at fixed hours on the symptoms of IBS.
IBD
IBD comprises both ulcerative colitis (UC), which is characterized by continuous damage located in the colon, and Crohn’s disease (CD), which is characterized by discontinuous alterations that can be located anywhere in the digestive tract, from the mouth to the anus. IBD is typically diagnosed at a young age (20-30 years old), has a relapsing and remitting disease course, and has no known cure. This combination of factors leads to a significant healthcare burden.
Genetics, immune responses and environment have been considered as the major etiologic factors of IBD. Available evidence suggests that both deregulated innate and adaptive immune pathways contribute to the aberrant intestinal inflammatory response in patients with IBD. There are multiple aspects of immune function that are under circadian control, such as host-pathogen interactions, trafficking of leukocytes, and the activation of innate and adaptive immunity[30]. Among the environmental risk factors, dietary elements have received considerable attention, particularly with the spread of the “Western” diet, which is high in fat and protein but low in fruit and vegetables. The hypothesis that dietary factors influence gut inflammation may be explained through several biological mechanisms, including antigen presentation, change in prostaglandin balance, and alteration of the microflora. Emerging evidence suggests that sleep also plays an important role, as circadian rhythms and melatonin could act as regulators of inflammation in the gastrointestinal tract. With prolonged sleep loss, there are elevations in monocytes and natural killer cells which form the source for the secretion of inflammatory cytokines[31]. Thus, disturbed sleep and chronic inflammation in IBD could form a self-perpetuating feedback loop, with the chronic inflammation of IBD worsening sleep and decreased sleep exacerbating the production of inflammatory cytokines and the inflammatory milieu. In mice, it has been demonstrated that functional circadian rhythms are necessary to maintain the enteric epithelial barrier. Impairment of circadian rhythms by genetic mutation of the clock genes that control circadian rhythms, including the 3 Period genes (Per1–3), or desynchronization of environmental signals result in more severe colitis, epithelial homeostasis alteration, increase of necrosis and diminution of secretory cells[32]. Recent studies have found an alteration in the expression of circadian genes in patients with IBD. Almost all circadian genes were reduced in both intestinal biopsies and peripheral blood mononuclear cells and showed a negative correlation with activity score. Furthermore, greater sensitivity to inflammatory damage and exacerbation of colitis were seen in mice who went through a phase shift in the light-dark cycle, resulting in increased secretion of pro-inflammatory cytokines and activation of inflammatory-related signaling pathways[33].
In recent years, several studies have linked IBD to intestinal dysbiosis. Likewise, there is evidence of the induction of a modification of the microbiota produced for chronodisruption. Thus, the alteration of the microbiota can be one of the mechanisms by which circadian rhythms disruption favors IBD development[34].
Several human studies support the hypothesis that IBD is associated with circadian rhythms. In a prospective study of women who were enrolled in the Nurses’ Health Study I and II, the association between sleep duration an incidence of CD and UC was examined. The authors found that both short sleep duration (less than 6 h) or long sleep duration (more than 9 h) were associated with an increased risk of UC but not with CD[35].
At the genetic level, the influence of the rs2797685 variant of the clock gene Per3 on susceptibility and behavior of IBD has been suggested. Allele and genotype frequencies of rs2797685 were significantly increased in both CD and UC patients. Moreover, the rs2797685 variant of the Per3 gene is associated with both early onset and more aggressive forms of CD, highlighted by increased use of immunosuppressants and more frequent stricturing and fistulizing disease requiring surgery[36].
Several studies have assessed genes associated with circadian rhythms in IBD patients. By genome-wide cDNA microarray analysis, the transcriptome of endoscopic mucosal biopsies of patients with IBD) were analyzed, focusing on the expression of circadian genes in CD and UC. Damaged tissue and adjacent healthy tissue were compared. This study revealed an alteration in the expression of 50 genes associated with circadian rhythms in IBD damaged tissues when compared to adjacent tissues. Some of these genetic alterations were different between UC and CD patients. In CD specimens, the core clock genes ARNTL2 and RORA were up-regulated, while CSNK2B, NPAS2, Per1 and Per3 were down-regulated. Conversely, in UC patients, ARNTL2, CRY1, CSNK1E, RORA and TIPIN were up-regulated, while NR1D2 and Per3 were down-regulated[37]. Consistent with these findings, another study demonstrated a decrease in the expression of clock genes in the biopsies of damaged tissue in IBD patients compared to healthy controls. Interestingly, this decrease was more important in UC patients than in CD patients. The reduced clock genes expression was not only evidenced in intestinal biopsies but also in circulating mononucleated cells[33].
Taken together, these results suggest that genes associated with circadian rhythms are implicated in the physiopathology of IBD.
DIGESTIVE CANCERS
In recent decades, the number of digestive cancers has increased worldwide. Although digestive cancer is a multifactorial disease, the marked geographic variation, time trends, and migratory effects on cancer incidence suggest that environmental or lifestyle factors are major contributors to the etiology of this disease. Recently, the alteration of circadian rhythms has emerged as a suspected factor in this increase. Indeed, several studies have associated night shift work and induced chronodisruption with colorectal[38] and stomach cancer[39]. However, other studies failed to demonstrate this association[40]. Another study demonstrated a link between circadian variations, measured with wrist temperature variation, and colorectal cancer survival[41]. Thus, circadian rhythms are associated to prognostic factors.
The alteration of the microbiota that occurs with circadian rhythms disruption has been hypothesized as one of the causes of colorectal cancer. In mice, abnormal microbial community structure was found to be associated with inflammation and tumorigenesis in the colon[42].
It has been shown that in the colon cellular proliferation follows a circadian rhythm[43]. At some points of the cell cycle, the DNA is less protected and therefore more susceptible to damage. Thus, circadian variation of cell proliferation timing in the colon could limit DNA exposure to potential mutagen agents during digestion, serving as a protective factor. Furthermore, the low expression levels of the mitotic and anti-apoptotic gene Birc5/survivin significantly and specifically increased the sensitivity of colon epithelial cells to cyclin-dependent kinase inhibitors. This dynamic establishes a link between cell cycle, circadian rhythms and cellular sensitivity to cyclin-dependent kinase inhibitors, making this gene a potential target in anti-cancer treatment[43].
In patients affected by colorectal cancer, cancerous cells show a differential expression of clock genes such as Clock and Per when compared to healthy cells in the same patient[44-46], suggesting that clock genes may be interesting biomarkers in colorectal cancer[46]. Other clock genes, namely, Clock and Bmal1, have been shown to interfere with the cell cycle. They are over-expressed in human colorectal cancer cells and can suppress cell growth. Moreover, clock genes may suppress CyclinD1 expression, inhibiting the cell cycle between phases G1 and S[47]. Clock genes have also been shown to modulate the expression of the rat sarcoma viral oncogene (RAS)[48]. Alteration in clock genes expression could play a role in these respective pathways during the development of cancers and affect resistance to treatment.
It has been observed that tumor markers in the blood of colon cancer patients display different circadian variations, according to the stage of cancer (from I to IV)[49]. The expression of clock genes have been analyzed in metastases cells of colon cancer stage IV, and are disrupted in comparison to the expression of the same genes in healthy cells[50]. Following these findings, alterations were found in clock gene expression in the organs (kidney and liver) where colorectal cancer metastases had developed[51]. The possibility that tumors may modify peripheral clocks and induce variations from ordinary circadian rhythms in nearby cells suggests putative systemic participation in the chronodisruption. This phenomenon may explain the development of fatigue and weakness characteristically felt by cancer patients.
All of these genetic mechanisms are currently being investigated to determine the best way to treat cancers by taking circadian variations into consideration. Interesting associations between clock genes polymorphisms and colorectal cancer severity have been found[52]; in gastric cancer, survival prognosis is associated with the version of the allele of the clock gene[53]. Finally, several authors suggest using the analysis of clock genes polymorphisms to predict the response to chemotherapy[54].
CONCLUSION
In conclusion, the parameters implicated in digestive health display circadian variations. In recent decades, there has been an increase in lifestyle habits that interfere with circadian rhythms and often induce chronodisruption. When these internal clocks receive incoherent signals, they lose synchronization, and the mechanisms that they control are affected. This phenomenon likely plays an important role in the development of pathologies such as IBS, IBD and cancers. Strong evidence of the connection between circadian rhythm impairment and digestive pathologies have been published; however, additional studies are necessary to understand the molecular mechanisms involved.
The insights from the studies of circadian oscillations of innate and adaptive gut immunity joint to the host-microbial interactions may incorporate the chronopharmacology to increase the effectiveness of the agents used to modulate the immune response, i.e., to indicate the time-of-day-specific for the administration of antimicrobial and antiinflammatory therapy. Likewise, the potential benefits of melatonin as a co-adjuvant treatment in gastrointestinal diseases, especially IBS and IBD should be explored. These considerations open novel perspectives in preventive and therapeutic applications of chronobiology.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Peer-review started: July 27, 2018
First decision: August 27, 2018
Article in press: October 5, 2018
P- Reviewer: Camara-Lemarroy CR, Servillo G S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
Contributor Information
Pilar Codoñer-Franch, Department of Pediatrics, Obstetrics and Ginecology, University of Valencia, Valencia 46010, Spain; Department of Pediatrics, Dr. Peset University Hospital, Valencia 46017, Spain. pilar.codoner@uv.es.
Marie Gombert, Department of Pediatrics, Obstetrics and Ginecology, University of Valencia, Valencia 46010, Spain; Department of Biotechnology, University of La Rochelle, La Rochelle 17000, France.
References
- 1.Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int J Mol Sci. 2014;15:16848–16884. doi: 10.3390/ijms150916848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter GD, Cade JE, Grant PJ, Hardie LJ. Nutrition and the circadian system. Br J Nutr. 2016;116:434–442. doi: 10.1017/S0007114516002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, Cuenca-García M, Plada M, Diethelm K, Kafatos A, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond) 2011;35:1308–1317. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Sastre P, Gómez-Abellán P, Martinez-Nicolas A, Ordovás JM, Madrid JA, Garaulet M. Evening physical activity alters wrist temperature circadian rhythmicity. Chronobiol Int. 2014;31:276–282. doi: 10.3109/07420528.2013.833215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter R, Hardeland R, Rol MA, Madrid JA. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014;15:23448–23500. doi: 10.3390/ijms151223448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawes C. Circadian rhythms in human salivary flow rate and composition. J Physiol. 1972;220:529–545. doi: 10.1113/jphysiol.1972.sp009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collado MC, Engen PA, Bandín C, Cabrera-Rubio R, Voigt RM, Green SJ, Naqib A, Keshavarzian A, Scheer FAJL, Garaulet M. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J. 2018;32:2060–2072. doi: 10.1096/fj.201700697RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogerwerf WA. Role of clock genes in gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 2010;299:G549–G555. doi: 10.1152/ajpgi.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulose JK, Wright JM, Patel AG, Cassone VM. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS One. 2016;11:e0146643. doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A. Circadian rhythms, alcohol and gut interactions. Alcohol. 2015;49:389–398. doi: 10.1016/j.alcohol.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pácha J, Sumová A. Circadian regulation of epithelial functions in the intestine. Acta Physiol (Oxf) 2013;208:11–24. doi: 10.1111/apha.12090. [DOI] [PubMed] [Google Scholar]
- 13.Bando JK, Colonna M. Innate lymphoid cell function in the context of adaptive immunity. Nat Immunol. 2016;17:783–789. doi: 10.1038/ni.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A. Circadian Rhythm and the Gut Microbiome. Int Rev Neurobiol. 2016;131:193–205. doi: 10.1016/bs.irn.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA. 2015;112:10479–10484. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Tognini P, Liu Y, Eckel-Mahan KL, Baldi P, Sassone-Corsi P. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 2016;17:1292–1303. doi: 10.15252/embr.201642463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tognini P, Murakami M, Sassone-Corsi P. Interplay between Microbes and the Circadian Clock. Cold Spring Harb Perspect Biol. 2018:10. doi: 10.1101/cshperspect.a028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrell JM, Chiang JY. Circadian rhythms in liver metabolism and disease. Acta Pharm Sin B. 2015;5:113–122. doi: 10.1016/j.apsb.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tognini P, Murakami M, Liu Y, Eckel-Mahan KL, Newman JC, Verdin E, Baldi P, Sassone-Corsi P. Distinct Circadian Signatures in Liver and Gut Clocks Revealed by Ketogenic Diet. Cell Metab. 2017;26:523–538.e5. doi: 10.1016/j.cmet.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morito Y, Aimi M, Ishimura N, Shimura S, Mikami H, Okimoto E, Sato S, Ishihara S, Kushiyama Y, Katsube T, et al. Association between sleep disturbances and abdominal symptoms. Intern Med. 2014;53:2179–2183. doi: 10.2169/internalmedicine.53.2591. [DOI] [PubMed] [Google Scholar]
- 22.Kim HI, Jung SA, Choi JY, Kim SE, Jung HK, Shim KN, Yoo K. Impact of shiftwork on irritable bowel syndrome and functional dyspepsia. J Korean Med Sci. 2013;28:431–437. doi: 10.3346/jkms.2013.28.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells MM, Roth L, Chande N. Sleep disruption secondary to overnight call shifts is associated with irritable bowel syndrome in residents: a cross-sectional study. Am J Gastroenterol. 2012;107:1151–1156. doi: 10.1038/ajg.2011.486. [DOI] [PubMed] [Google Scholar]
- 24.Nojkov B, Rubenstein JH, Chey WD, Hoogerwerf WA. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. 2010;105:842–847. doi: 10.1038/ajg.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisniewska-Jarosinska M, Chojnacki J, Konturek S, Brzozowski T, Smigielski J, Chojnacki C. Evaluation of urinary 6-hydroxymelatonin sulphate excretion in women at different age with irritable bowel syndrome. J Physiol Pharmacol. 2010;61:295–300. [PubMed] [Google Scholar]
- 26.Danilov A, Kurganova J. Melatonin in Chronic Pain Syndromes. Pain Ther. 2016;5:1–17. doi: 10.1007/s40122-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186–194. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 28.Wasiluk D, Ostrowska L, Stefańska E, Janke A, Jurkowska G. Diet for women with irritable bowel syndrome - a preliminary study. Rocz Panstw Zakl Hig. 2017;68:151–160. [PubMed] [Google Scholar]
- 29.Guo YB, Zhuang KM, Kuang L, Zhan Q, Wang XF, Liu SD. Association between Diet and Lifestyle Habits and Irritable Bowel Syndrome: A Case-Control Study. Gut Liver. 2015;9:649–656. doi: 10.5009/gnl13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagel R, Bär F, Schröder T, Sünderhauf A, Künstner A, Ibrahim SM, Autenrieth SE, Kalies K, König P, Tsang AH, et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. 2017;31:4707–4719. doi: 10.1096/fj.201700141RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Yu R, Zhu L, Hou X, Zou K. Bidirectional Regulation of Circadian Disturbance and Inflammation in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1741–1751. doi: 10.1097/MIB.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 34.Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, Turek FW, Keshavarzian A. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9:e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, Richter JM, Schernhammer ES, Chan AT. Sleep duration affects risk for ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014;12:1879–1886. doi: 10.1016/j.cgh.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzoccoli G, Palmieri O, Corritore G, Latiano T, Bossa F, Scimeca D, Biscaglia G, Valvano MR, D’Incà R, Cucchiara S, et al. Association study of a polymorphism in clock gene PERIOD3 and risk of inflammatory bowel disease. Chronobiol Int. 2012;29:994–1003. doi: 10.3109/07420528.2012.705935. [DOI] [PubMed] [Google Scholar]
- 37.Palmieri O, Mazzoccoli G, Bossa F, Maglietta R, Palumbo O, Ancona N, Corritore G, Latiano T, Martino G, Rubino R, et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int. 2015;32:903–916. doi: 10.3109/07420528.2015.1050726. [DOI] [PubMed] [Google Scholar]
- 38.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 39.Gyarmati G, Turner MC, Castaño-Vinyals G, Espinosa A, Papantoniou K, Alguacil J, Costas L, Pérez-Gómez B, Martin Sanchez V, Ardanaz E, et al. Night shift work and stomach cancer risk in the MCC-Spain study. Occup Environ Med. 2016;73:520–527. doi: 10.1136/oemed-2016-103597. [DOI] [PubMed] [Google Scholar]
- 40.Devore EE, Massa J, Papantoniou K, Schernhammer ES, Wu K, Zhang X, Willett WC, Fuchs CS, Chan AT, Ogino S, et al. Rotating night shift work, sleep, and colorectal adenoma in women. Int J Colorectal Dis. 2017;32:1013–1018. doi: 10.1007/s00384-017-2758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lévi F, Dugué PA, Innominato P, Karaboué A, Dispersyn G, Parganiha A, Giacchetti S, Moreau T, Focan C, Waterhouse J, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int. 2014;31:891–900. doi: 10.3109/07420528.2014.924523. [DOI] [PubMed] [Google Scholar]
- 42.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD. The gut microbiome modulates colon tumorigenesis. MBio. 2013;4:e00692–e00613. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siffroi-Fernandez S, Dulong S, Li XM, Filipski E, Gréchez-Cassiau A, Peteri-Brünback B, Meijer L, Lévi F, Teboul M, Delaunay F. Functional genomics identify Birc5/survivin as a candidate gene involved in the chronotoxicity of cyclin-dependent kinase inhibitors. Cell Cycle. 2014;13:984–991. doi: 10.4161/cc.27868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Sun N, Lu C, Bei Y, Qian R, Hua L. Upregulation of circadian gene ‘hClock’ contribution to metastasis of colorectal cancer. Int J Oncol. 2017;50:2191–2199. doi: 10.3892/ijo.2017.3987. [DOI] [PubMed] [Google Scholar]
- 45.Momma T, Okayama H, Saitou M, Sugeno H, Yoshimoto N, Takebayashi Y, Ohki S, Takenoshita S. Expression of circadian clock genes in human colorectal adenoma and carcinoma. Oncol Lett. 2017;14:5319–5325. doi: 10.3892/ol.2017.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonnes S, Donatsky AM, Gögenur I. Expression of core clock genes in colorectal tumour cells compared with normal mucosa: a systematic review of clinical trials. Colorectal Dis. 2015;17:290–297. doi: 10.1111/codi.12847. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto W, Takenoshita S. OVEREXPRESSION OF BOTH CLOCK AND BMAL1 INHIBITS ENTRY TO S PHASE IN HUMAN COLON CANCER CELLS. Fukushima J Med Sci. 2015;61:111–124. doi: 10.5387/fms.2015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Athman R, Genov NN, Mazuch J, Zhang K, Yu Y, Fuhr L, Abreu M, Li Y, Wallach T, Kramer A, et al. The Ink4a/Arf locus operates as a regulator of the circadian clock modulating RAS activity. PLoS Biol. 2017;15:e2002940. doi: 10.1371/journal.pbio.2002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tóth K, Patai ÁV, Kalmár A, Barták BK, Nagy ZB, Galamb O, Wichmann B, Tulassay Z, Molnár B. Circadian Rhythm of Methylated Septin 9, Cell-Free DNA Amount and Tumor Markers in Colorectal Cancer Patients. Pathol Oncol Res. 2017;23:699–706. doi: 10.1007/s12253-016-0174-2. [DOI] [PubMed] [Google Scholar]
- 50.Huisman SA, Ahmadi AR, IJzermans JN, Verhoef C, van der Horst GT, de Bruin RW. Disruption of clock gene expression in human colorectal liver metastases. Tumour Biol. 2016;37:13973–13981. doi: 10.1007/s13277-016-5231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huisman SA, Oklejewicz M, Ahmadi AR, Tamanini F, Ijzermans JN, van der Horst GT, de Bruin RW. Colorectal liver metastases with a disrupted circadian rhythm phase shift the peripheral clock in liver and kidney. Int J Cancer. 2015;136:1024–1032. doi: 10.1002/ijc.29089. [DOI] [PubMed] [Google Scholar]
- 52.Qu F, Qiao Q, Wang N, Ji G, Zhao H, He L, Wang H, Bao G. Genetic polymorphisms in circadian negative feedback regulation genes predict overall survival and response to chemotherapy in gastric cancer patients. Sci Rep. 2016;6:22424. doi: 10.1038/srep22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajendran S, Benna C, Monticelli H, Spiro G, Menin C, Mocellin S. Germline variation of circadian pathway genes and prognosis of gastric cancer patients. Gut. 2018;67:779–780. doi: 10.1136/gutjnl-2017-314740. [DOI] [PubMed] [Google Scholar]
- 54.Lu H, Chu Q, Xie G, Han H, Chen Z, Xu B, Yue Z. Circadian gene expression predicts patient response to neoadjuvant chemoradiation therapy for rectal cancer. Int J Clin Exp Pathol. 2015;8:10985–10994. [PMC free article] [PubMed] [Google Scholar]