Abstract
Patients with liver cirrhosis are susceptible to infections due to various mechanisms, including abnormalities of humoral and cell-mediated immunity and occurrence of bacterial translocation from the intestine. Bacterial infections are common and represent a reason for progression to liver failure and increased mortality. Fungal infections, mainly caused by Candida spp., are often associated to delayed diagnosis and high mortality rates. High level of suspicion along with prompt diagnosis and treatment of infections are warranted. Bacterial and fungal infections negatively affect the outcomes of liver transplant candidates and recipients, causing disease progression among patients on the waiting list and increasing mortality, especially in the early post-transplant period. Abdominal, biliary tract, and bloodstream infections caused by Gram-negative bacteria [e.g., Enterobacteriaceae and Pseudomonas aeruginosa (P. aeruginosa)] and Staphylococcus spp. are commonly encountered in liver transplant recipients. Due to frequent exposure to broad-spectrum antibiotics, invasive procedures, and prolonged hospitalizations, these patients are especially at risk of developing infections caused by multidrug resistant bacteria. The increase in antimicrobial resistance hampers the choice of an adequate empiric therapy and warrants the knowledge of the local microbial epidemiology and the implementation of infection control measures. The main characteristics and the management of bacterial and fungal infections in patients with liver cirrhosis and liver transplant recipients are presented.
Keywords: Liver cirrhosis, Liver transplant recipients, Bacterial infections, Fungal infections, Multidrug resistant organisms, Management
Core tip: Infections are frequent in patients with liver cirrhosis, liver transplant candidates, and liver transplant recipients and are associated with increased morbidity and mortality. Knowledge of the risk factors, etiology, and type of infections is paramount for the management of severe bacterial and fungal infections in these patient populations. Increasing rates of infections due to multidrug-resistant pathogens have been reported worldwide and particularly affect liver transplant recipients. The type of bacterial and fungal infections along with their risk factors, management, and future research in patients with liver cirrhosis and liver transplant recipients are presented in the review.
INTRODUCTION
Liver cirrhosis (LC) represents a dynamic clinical entity characterized by various stages of progression[1]. From a clinical perspective, LC includes compensated and decompensated stages of disease characterized by different features, prognoses, and predictors of death. Specifically, decompensated cirrhosis is associated with portal hypertension or liver insufficiency and their related complications, including recurrent variceal hemorrhage, refractory ascites, hyponatremia, and/or hepatorenal syndrome. All these clinically evident complications can be further aggravated by the occurrence of infections[1].
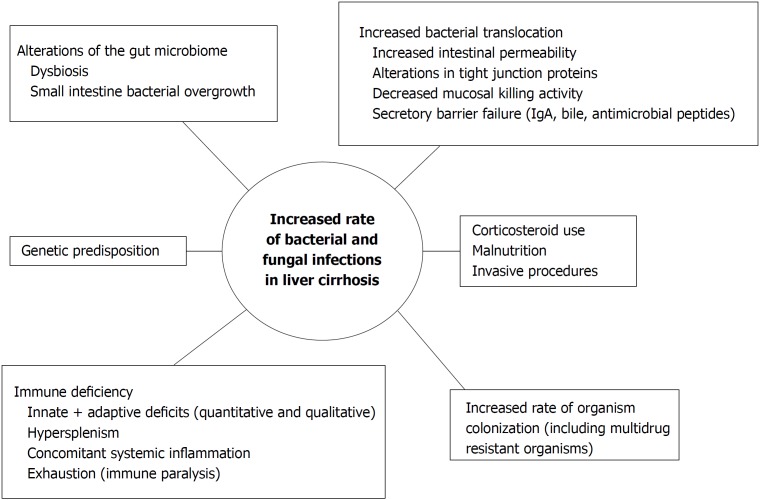
Various mechanisms predispose patients with LC to infections. Impairment of immune function has been well documented and is characterized by multiple immune deficiencies, involving not only local liver damage but also deficiencies in systemic innate and acquired immunity[2]. Increased gastrointestinal (GI) permeability and pathological bacterial translocation are considered key factors leading to increased infection susceptibility in LC (Figure 1)[3,4]. Although a clear correlation between bacterial translocation and spontaneous bacterial peritonitis (SBP, one of the most frequent infections in cirrhosis) has not been universally reported[5,6], various factors support this relationship. A GI pathogen such as Escherichia coli (E. coli), for example, represents a commonly isolated pathogen in cirrhosis and also a major cause of SBP (Table 1)[7,8]. Furthermore, a higher number of pathogenic bacteria, especially Enterobacteriaceae, have been found in the mucosal microbiota composition of the sigmoid in cirrhotic patients compared to healthy controls[9]. Host-related, hospital-related, and drug-related factors also contribute to increased susceptibility to infections in this population (Figure 1). The presence of concomitant comorbidities associated with liver disease, including obesity, alcohol consumption, malnutrition, viral hepatitis and/or HIV infection predisposes to bacterial and fungal infections[10]. Frequent and prolonged hospitalizations along with the use of invasive devices (e.g., urinary and central venous catheters, CVC) pose patients at risk of nosocomial infections such as pneumonia, CVC-related bacteremia, and urinary tract infections. Furthermore, the use of immunosuppressive agents remains frequent in this population[11].
Figure 1.
Factors leading to increased susceptibility to infections in patients with liver cirrhosis.
Table 1.
Main studies reporting the characteristics and mortality rates associated with infections in patients with liver cirrhosis
| Year | Prevalence of infections (%); patient n | Main infection sites (%) | Most common pathogens (%) | Mortality rates (%) | Ref. |
| 2018 | NR; n = 312 | Only BSI included; primary (32), SBP (16), UTI (11) | GNB (53) | 25 | [28] |
| 2017 | 61; n = 852 | Only BSI included; primary (60), abdominal (33), UTI (7), pneumonia (6) | GNB (60) | 23 | [22] |
| 2015 | 38; n = 401 | Pneumonia (22), UTI (21), SBP (19) | E. coli (72) | 31 | [23] |
| 2012 | 51; n = 207 | UTI (52), SBP (23), BSI (21) | GPB (56) | 24 | [19] |
| 2010 | 33; n = 150 | UTI (37), pneumonia (22), BSI (13) | GNB (62) | 37 | [18] |
| 2007 | 45; n = 233 | UTI (43), pneumonia (25), SBP (16) | GNB (65) | 18 | [20] |
| 2003 | 25; n = 135 | UTI (31), SBP (26), pneumonia (25) | NR | 9 | [25] |
| 2002 | 22; n = 70 | BSI (16), CVC-BSI (9), liver abscess (3) | GPB (67) | 29 | [24] |
| 2002 | 32; n = 507 | SBP (24), UTI (19), pneumonia (14), CVC-BSI (8) | GPB (47) | 22 | [17] |
| 2001 | 34; n = 361 | UTI (41), SBP (23), BSI (21), pneumonia (17) | E. coli (25) | 15 | [8] |
| 1994 | 39; n = 132 | SBP (44), UTI (26), pneumonia (16) | GNB (65), E. coli (62) | 29 | [27] |
| 1993 | 47; n = 170 | SBP (31), UTI (25), pneumonia (21) | GNB (72) | 17 | [26] |
NR: Not reported; BSI: Bloodstream infections; SBP: Spontaneous bacterial peritonitis; UTI: Urinary tract infections; CVC: Central venous catheter; GNB: Gram-negative bacteria; GPB: Gram-positive bacteria.
Infections remain one of the principal causes of morbidity and mortality also among liver transplant recipients (LTR)[12]. Bacterial and fungal infections following LT are frequent, occurring in more than 50% of patients mainly due to the type of surgical procedures that, compared to other solid organ transplants, are more complex and may presents complications such as abdominal abscess, bile leaks, and hepatic artery stenosis[13]. Bacterial infections account for up to 70% of all infections in LTR, followed by fungal and viral infections[14]. The interplay among key factors such as patients’ net state of immunosuppression, environmental exposure to specific organisms (e.g., nosocomial pathogens), and development of surgical complications affects the timing of specific post-LT infections[14]. The organism’s virulence, along with intensity and timing of the exposure, can also impact infections’ severity and outcome. Factors known to increase the risk of infections after LT include a Model for End-Stage Liver Disease (MELD) score greater than 30, reoperation (including retransplantation), renal replacement therapy, prolonged intensive care unit (ICU) stay, and older age[15].
An appropriate management of infections in patients with cirrhosis and following liver transplantation implies the knowledge of predisposing risk factors for infections in order to identify high-risk patients, the prompt use of correct diagnostic tools to recognize atypical disease presentations, and early adequate antimicrobial treatment and source control.
The possible etiologies of infections among patients with LC and LTR are diverse and may range from common bacterial and viral pathogens to opportunistic pathogens that are clinically relevant only for immunocompromised patients. In this review, the most common challenges and main principles for the management of bacterial and fungal infections are discussed. The review focuses mainly on nosocomial infections, including those caused by multidrug resistant organisms (MDRO), while other opportunistic infections are not presented in details.
BACTERIAL INFECTIONS IN PATIENTS WITH LIVER CIRRHOSIS
Epidemiology
Bacterial infections are common in patients with LC and can occur at various stages of liver disease, representing the primary cause of admission to emergency departments in this patient population[16]. Among patients admitted to emergency departments, increasing rates of SBP and hepatorenal syndrome have been documented[16]. Patients with LC present a high prevalence of bacterial infections, with 10% reporting more than one episode of infection within the same hospitalization[17,18]. Second infections were reported as independent predictors of mortality in hospitalized patients with LC and appeared preventable in the majority of cases[19].
According to reports from different countries, the overall prevalence of bacterial infections in hospitalized patients with LC varies from 22% to 51% (Table 1)[17-28]. Multiple factors have been associated with the occurrence of infections, including increased MELD scores, alcoholic liver disease, protein malnutrition, and GI bleeding[7,8,19,21,29]. Infections often represent the trigger for clinical deterioration or progression to liver decompensation[18,20]. For example, bacterial infections remain a leading cause of acute on chronic liver failure[19]. In a study including 50 cirrhotic patients, the presence of an infectious episode worsened liver function in 62% of cases[18]. Patients with infections were more likely to develop ascites, hepatic encephalopathy, hyponatremia, hepatorenal syndrome, or septic shock compared to noninfected ones. Furthermore, SBP can lead to severe renal failure, which is also associated with poor clinical outcomes[20,30]. In a report encompassing 104 cirrhotic patients with bacterial infections, 34% presented infection-induced renal failure associated with GI infections and SBP. Multivariate analysis confirmed that lack of infection resolution was an independent factor for renal failure (P = 0.03)[20].
On the other hand, clinical complications of LC can represent a risk factor for development of infections. Examples are GI bleeding, associated with bacterial infections in up to 45% of patients[7] and SBP, usually reported in patients with ascites, showing prevalence rates that vary between 15% and 25%[8]. Besides SBP, other frequent infections in cirrhotic patients include urinary tract infections, pneumonia, and skin and soft tissue infections (SSTI) (Table 1)[18,20].
Few reports have also documented high rates of bacterial meningitis among patients with cirrhosis compared to those without liver disease, including pneumococcal infections[31]. Increased creatinine serum levels were associated with mortality in cirrhotic patients with meningitis[32]. The overall incidence of bacteremia, urinary tract infections, pneumonia, meningitis, tuberculosis, and liver abscess appeared increased more than tenfold in LC, and mortality rates of each episode were 3 to 10 times higher than in non-cirrhotic patients[33]. A 10-fold higher risk of bloodstream infections (BSI) in LC compared to the general population was identified in a Danish study showing 47%, 45%, and 8% of Gram-negative, Gram-positive, and polymicrobial BSI, respectively, with overall 30-d case-fatality rate of 0.53[34]. BSI in cirrhotic patients can lead to complications such as deep-seated metastatic infections, including endotipsitis (in patients with transjugular intrahepatic portosystemic shunt) and infective endocarditis[35]. Streptococcus bovis endocarditis, in particular, has been associated with advanced liver disease[36]. Types, etiology, and mortality of bacterial infections in LC are reported in Table 1.
Diagnosis and treatment
Due to the increased risk of sepsis and multiorgan failure among cirrhotic patients, prompt identification of symptoms and signs of septic shock and assessment of organ function is paramount[37]. The diagnostic workup should also aim at identifying the source of infection by blood cultures, urine culture, chest X-ray, and lung or abdominal CT scan according to patients’ medical history and clinical presentation. Performance of paracentesis with neutrophil count and microbiological culture of ascitic fluid is recommended in all cirrhotic patients hospitalized with ascites to role out SBP[38,39]. Other microbiological tests include sputum and/or bronchoalveolar lavage cultures if pneumonia is suspected, stool cultures (including assays for Clostridium difficile diagnosis) in case of GI symptoms, and wound - or intra-abdominal - cultures, when indicated[37].
Data on antimicrobial treatment options in LC are limited, thus recommendations are often based on expert opinion or inferred from studies in non-cirrhotic populations[37]. A study analyzing bloodstream infections in patients with cirrhosis found that timely initiation of an appropriate antimicrobial therapy had a major impact on patients’ outcome[40]. Multidrug resistance is a major predictor of inappropriate therapy in LC. For these reasons, antimicrobial treatment should be promptly initiated and, when possible, adjusted according to the microbiological results. Empirical treatment should take into consideration the local epidemiology, including the rates of antimicrobial resistance, the site of infection, and patients’ clinical presentation (e.g., septic shock).
Patients with SBP usually have infections caused by enteric pathogens, such as Enterobacteriaceae and Enterococcus spp.; infections due to P. aeruginosa are also possible, especially among hospitalized patients[11]. Third generation cephalosporins (e.g., ceftriaxone, cefotaxime, or the antipseudomonal cephalosporin ceftazidime) or beta-lactam/beta-lactamase inhibitor combinations such as piperacillin/tazobactam (active also against Enterococcus spp.) are frequently used to treat SBP[11]. Although ciprofloxacin represents a potential option for SBP treatment, rates of resistances associated to quinolone use remains high at various centers and among patients receiving long-term norfloxacin prophylaxis[37]. Tigecycline presents good intra-abdominal penetration and is active against Enterococci [including Enterococcus faecium (E. faecium)], Staphylococcus aureus (S. aureus) and Enterobacteriaceae; nevertheless, its bacteriostatic activity and reduced serum concentrations limit tigecycline use in patients with sepsis. Other broad-spectrum antibiotics, such as carbapenems, should be reserved to the treatment of severe infections or in areas with a high prevalence of ESBL-producing strains[11,37]. Enterobacteriaceae and Enterococcus spp. are also common causes of urinary tract infections and can be treated with a third generation cephalosporin or beta-lactam/beta-lactamase inhibitor combinations[11]. In uncomplicated, non-bacteremic infections, oral options such as cotrimoxazole or nitrofurantoin can be used, according to the pathogen’s susceptibility. The use of quinolones, however, should be limited due to their high potential for antimicrobial resistance selection.
Nosocomial pneumonia represents a frequent life-threatening infection in LC. Antimicrobial options include a beta-lactam (e.g., ceftazidime, a beta-lactam/beta-lactamase inhibitor combination, or a carbapenem) with or without a quinolone such as ciprofloxacin[11]. If risk factors for MRSA are documented (e.g., MRSA colonization or previous infection), treatment with vancomycin or linezolid can be considered. SSTI, most frequently cellulitis, can be caused by both Gram-negative and Gram-positive pathogens in cirrhotic patients[41].
In severe nosocomial infections, the association of a broad-spectrum antibiotic active against Gram-negative bacilli (e.g., piperacillin/tazobactam or meropenem) with an anti-MRSA drug (e.g., vancomycin, daptomicin) is recommended[11].
Outcome
Although the diagnosis and treatment of infections have improved over the decades, their occurrence still significantly impact on the mortality of patients with liver cirrhosis[29]. In these patients, GI bacterial overgrowth and translocation favor the occurrence of various infections, while the increase of endotoxins levels and cytokines can induce systemic inflammatory responses leading to septic shock, multiorgan dysfunction, and death[29].
A systematic review including 178 studies showed better outcomes in noninfected compared to infected patients with cirrhosis (OR = 3.75, 95%CI: 2.12-4.23)[42]. Mortality of infected patients was 30% at 1 mo and increased to 63% after 12 mo following an infection. Infections and mortality appeared more frequent in patients with Child-Pugh C compared to A or B stage (P = 0.003 and P = 0.0002, respectively)[21]. A prospective study including 312 BSI (53% due to Gram-negative and 47% due to Gram-positive bacteria) showed 30-d mortality rates of 25%[40]. Risk factors associated with mortality included delayed (> 24 h) antibiotic treatment (P < 0.001), inadequate empirical therapy (P < 0.001), and increased Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score (P < 0.001). A study analyzing the characteristics of infections in cirrhotic compared to non-cirrhotic patients hospitalized in ICU identified higher prevalence of infections (59% vs 51%, P < 0.01), increased rates of abdominal infections, and higher number of Gram-positive infections (including methicillin-resistant Staphylococcus aureus, MRSA) in the LC group[43]. In a study showing mortality rates of 37% among LC patients with hospital-acquired infections, sepsis was an independent factor for hospital death (P = 0.005; 95%CI: 1.7-21.4)[18]. Another study identified higher in-hospital mortality in cirrhotic compared to non-cirrhotic patients (42% vs 24% respectively, P < 0.001) and among those with septic shock (30% vs 49%, P < 0.05)[43]. Based on these data, a novel prognostic stage in the course of cirrhosis was proposed, defining the group of “critically ill decompensated patients”, characterized by a risk of progression to death or liver transplant of 60%[42].
FUNGAL INFECTIONS IN LC
Fungal infections in patients with liver cirrhosis are mainly caused by Candida spp. and could represent, if not promptly recognized, a cause of treatment failure[44,45]. Studies on fungal infections in LC often refer only to Candida spp., are usually limited to retrospective cohorts, and can be biased by underreporting and under diagnosis. Nevertheless, data shown in the few available reports appear worrisome in terms of unfavorable outcomes.
Candidemia may arise both endogenously and exogenously in patients with LC, but nearly always occurs when prolonged antibiotic exposure is documented[40,44]. The use of antibiotics for prevention of SBP, in particular, is frequent and may favor an excessive growth of fungi in the intestinal flora, potentially causing fungal translocation in the peritoneal cavity and development of spontaneous fungal peritonitis (SFP)[46]. SFP is characterized by PMN counts of ≥ 250 cells/mm3 in the ascitic fluid along with positive fungal cultures (with or without concomitant SBP) and no apparent intraabdominal sources of infection, while fungal ascites is defined by lower PMN counts[47]. Culture positivity ranged from 0 to 11% in various studies[47]. SFP is mainly caused by C. albicans, is frequently nosocomial, and appears associated to higher mortality compared to PBS[47]. Advanced liver disease and GI bleeding have been advocated as risk factors for SFP, since higher GI permeability may be required to favor the translocation of large pathogens such as fungi[47]. SFP should also be considered in the diagnostic workup of hospitalized cirrhotic patients with impaired renal function, such as those with refractory ascites[45]. Other risk factors for SFP include prolonged hospitalizations and performance of invasive procedures[48]. Fungal infections characterized by high mortality have been described in LC patients hospitalized in ICU and in those with alcoholic hepatitis[44,45,49,50]. A retrospective analysis of 120 cirrhotic patients identified the presence of fungal colonization as an independent factor for mortality (P = 0.047)[49]. A study including 185 patients with culture-positive infections documented Candida spp. in 19 (10%) cases. Of these, 58% were SFP and 42% candidemia. Only 47% of fungal infections were diagnosed and treated with antifungal agents, while the remaining patients died. Mortality rates at one month were 58% and 29% in patients with fungal infections compared to those with bacterial infection, respectively (P = 0.001)[44]. Similarly, another study encompassing 126 cirrhotic patients with culture-positive ascites identified SFP in 14/126 (11%) patients. Only 43% of patients with cultures positive for fungi received antifungal treatment[45]. In a prospective multicenter study, Candida spp. represented 7% of all BSI and was associated with prolonged hospitalizations, prior surgery, CVC placement, neutropenia, and prior antimicrobial use[40]. Compared to other infections, Candida BSI had the strongest association with inappropriate empirical therapy[28]. Bassetti et al[51] previously analyzed 169 episodes of candidemia and 72 intra-abdominal candidiasis in cirrhotic patients, showing high rates of ICU admission (50%), non-albicans Candida infections (46%), and occurrence of septic shock (35%). Thirty-day mortality was 35.3% and was independently associated with candidemia (OR = 2.2, 95%CI: 1.2-4.5), septic shock (OR = 3.2, 95%CI: 1.7-6), and absence of adequate antifungal treatment (OR = 0.4, 95%CI: 0.3-0.9)[51].
These data emphasize the importance of performing fungal cultures and maintaining a high level of suspicion in patients with LC, especially those with impaired renal function and/or receiving antimicrobial treatment with limited clinical response, to ensure early treatment and ultimately reduce mortality (Table 2)[52-54].
Table 2.
Management of fungal infections in patients with liver cirrhosis
| Type | Characteristics | Management | Ref. |
| SFP, fungemia, disseminated fungal infection (mainly Candida spp.) | Delayed diagnosis and therapy. Lack of clinical signs and suspicion. Frequent concomitant SBP. High mortality. | Suspect if peritonitis is not improved after 48 h of empirical antibiotic treatment. Perform fungal cultures (ascites and blood). | [44,45,52,53] |
| Antifungal prophylaxis | Factors influencing mortality less known. Mortality higher than SBP due to delayed diagnosis. | Indicated for SBP (high risk, previous episode, GI bleeding). No clear indication for fungal infections. Consider in: ICU patients without improvement > 48 h, high prevalence (> 5%) regions, risk factors (corticosteroids, prolonged microbial use, CVC, TPN, high APACHE score, dialysis). | [48,54] |
| Antifungal treatment | Recommendations for fungal infections in LC. | Prompt initiation. Echinocandins as first-line treatment (e.g., fungemia, nosocomial SFP or critically ill with CA-SFP). Fluconazole indicated if less severe infections. De-escalation if patient is stable and sensitivity tests available. | [52-54] |
SFP: Spontaneous fungal peritonitis; SBP: Spontaneous bacterial peritonitis; GI: Gastrointestinal; CVC: Central venous catheter; TPN: Total parenteral nutrition; APACHE: Acute Physiology and Chronic Health Evaluation; LC: Liver cirrhosis; CA: Community-acquired.
Early administration of antifungal treatment has been associated with improved outcomes, especially in patients with severe infections[55,56].
Novel molecules (e.g., azoles such as isavuconazole) and new antifungal classes (e.g. echinocandines) have become available for the treatment of invasive fungal infections in the last decades. In patients with LC concerns in the efficacy and safety of antifungals appear linked to resistance to antifungals, patients’ reduced tolerance, and altered drug pharmacokinetics caused by advanced liver disease.
Although fluconazole is still widely used due to its favorable pharmacokinetics and tolerability, a shift to non-albicans strains showing lower fluconazole susceptibility has been reported[57]. Echinocandins are currently recommended as first line treatment in critically ill patients and in case of reduced susceptibility to fluconazole[58]. Despite evidence of resistance has emerged especially in C. glabrata, overall resistance rates to echinocandines remains low[59]. Compared to azoles such as voriconazole, echinocandins present reduced liver toxicity and better tolerability[60]. While dose adjustments are not recommended for any severity of liver disease for micafungin and andulafungin, reduction of caspofungin maintenance dose from 50 to 35 mg/d is suggested[61]. This dose reduction, however, may not be appropriate in critically ill patients who may have sub-therapeutic exposure and efficacy. In patients with liver cirrhosis receiving voriconazole, therapeutic drug monitoring is recommended due to the correlation between trough plasma concentration and occurrence of adverse effects[62].
MANAGEMENT OF INFECTIONS IN CIRRHOTIC PATIENTS
As a general rule, an infection should be suspected in all cirrhotic patients with unexpected clinical deterioration (e.g., new onset of porto-systemic encephalopathy, worsening of renal or liver function tests) due to the known impact of infections on liver disease progression[37].
A prompt diagnosis of infectious processes in patients with liver disease can be hampered by various factors that may act as confounders or mask bacterial and/or fungal infections, thus potentially delaying an effective treatment. Due to the immune impairment that accompanies LC, systemic responses and classical symptoms of infections may be reduced and difficult to diagnose. Furthermore, LC itself may be a cause of low-grade fever in up to 20% of patients[63]. Opportunistic infections can also occur and their recognition may be less immediate, or require longer times to obtain culture positivity. Targeted microbiological cultures (blood, urine and ascites cultures) before administration of antimicrobials are recommended, and the use of markers (e.g., galactomannan, beta-D-glucan) could be considered if fungal infections are suspected[64]. Similarly to other immunocompromised patients, high-resolution chest CT should be preferred to X-rays for pulmonary infections[37]. Besides prompt diagnosis, early appropriate antimicrobial and/or antifungal treatment remain key factors in the management of LC patients with severe infections.
Infections caused by multidrug resistant organisms (MDRO) may represent a cause of treatment failure favoring poor outcomes. Prevalence of MDRO in LC patients reflects the global resistance burden of different countries, thus knowledge of the local patterns of susceptibility is paramount to optimize empirical and targeted therapy in severe infections. Two studies in Italy and Greece identified prevalence rates of MDR infections of 27% and 19%, respectively, mainly caused by ESBL-producing E. coli and carbapenem-resistant K. pneumoniae[65,66]. MDRO accounted for nearly one-third of BSI in cirrhotic patients in a European multicenter study that identified inadequate empirical therapy as an independent cause of 30-d mortality[40]. Infections were associated with previous antimicrobial exposure and invasive procedures. Most common MDRO were ESBL-producing Enterobacteriaceae (14%), while the highest mortality rates (> 40%) were associated with carbapenem-resistant Enterobacteriaceae, Candida spp., and E. faecium.
INFECTIONS IN PATIENTS ON THE TRANSPLANT WAITING LIST
Patients on the waiting list are frail and often require multiple hospitalizations, which in turn can favor infections and deteriorate liver function or lead to multiorgan failure. Occurrence of severe infections put patients at risk of dropout from transplant waiting lists, potentially reducing the possibility to undergo LT and causing a destructive impact on the natural progression of cirrhosis. A prospective study evaluating 136 LT candidates developing bacterial infections showed that the majority were delisted or died (42%), while 35% underwent LT[67]. Similarly, occurrence of SBP was documented as a cause of death of removal from the waiting list in 38% of patients with advanced cirrosis[68]. Although higher post-transplant mortality has not been clearly correlated with occurrence of pre-transplant infections, other factors such as increased MELD score, prolonged post-LT intubation and hospitalization were documented among infected LT candidate compared with noninfected ones[69]. Various studies investigating the outcome of LTR after recovery from an infection prior to LT showed an increase length of hospital stay, higher rates of postoperative infections and increased isolation of MDRO compared to patients without infection, although similar survival rates were reported[70]. Careful management of these patients, especially in case of repeated hospitalization, is warranted.
INFECTIONS IN LIVER TRANSPLANT RECIPIENTS
Bacterial infections: Timing
Bacterial infections, especially those caused by nosocomial pathogens, are more common during the early post-transplant period (0-1 mo). Surgical complications can lead to wound infections, peritonitis, hepatic artery thrombosis, and biliary tract ischemia that can cause biloma or strictures, increasing the risk of recurrent cholangitis[12,71,72]. Other factors contributing to bacterial infections in the early postoperative period include mechanical ventilation, prolonged ICU stay, alteration of the mucocutaneous barrier, vascular and urinary catheterization, and profound immunosuppression[73]. A retrospective study including 463 LTR over a 3-year period identified at least one infection in 41% of cases, with biliary tract infections and infections due to staphylococci representing the most common types[72].
Complications occurring during transplantation that imply a more complex and prolonged surgical procedure, such as development of ischemia-reperfusion injury and high amount of blood transfused intraoperatively, may favor surgical site infections[74,75]. A prospective study including LTR with BSI identified CVC-BSI (31%), pneumonia (24%), and abdominal and/or biliary infections (14%) as most common sources of bacteremia. Diabetes mellitus (P = 0.03) and serum albumin level less than 3.0 mg/dL (P = 0.02) were predictors of bacteremia. Mortality at 14 d was higher in patients with BSI compared with nonbacteremic infections (28% vs 4%, P = 0.03)[75]. Risk factors for mortality among patients with BSI after LT include ICU stay, abnormal laboratory findings (e.g., greater serum bilirubin level and prothrombin time) and lack of febrile response[75].
Infections in the donors, if controlled, are not considered a contraindication for transplant. However, since they may represent a source of post-transplant bacterial infections, an accurate screening of donors is recommended (Table 3)[76,77]. Opportunistic infections (e.g., herpesvirus infections, nocaridosis, tuberculosis, etc.) are considered more common between 1 and 6 mo post-transplant, although pneumonia and intra-abdominal infections can still occur during this period. Risk factors that may favor bacterial infections during the intermediate post-transplantation period include over-immunosuppression, allograft rejection, biliary tract complications, and re-transplantation[73]. In a study analyzing early (< 6 mo) vs late post-transplant infections (> 6 mo), the incidence decreased from 11.5 episodes/1000 transplant-days in the first month to 1.9 and 0.3 between 1 mo and 6 mo and after 6 mo, respectively[78]. Gram-positive and Gram-negative bacteria-related infections were equally distributed (14.8% of all infections). A specific risk factor for late infections was the performance of a biliary derivation to jejune, favoring cholangitis and secondary peritonitis in LTR. Risk for late bacterial infections varies according to the recipient’s graft and immune status, with high-risk patients characterized by recurrent rejection and allograft dysfunction requiring intense immunosuppression[73]. Community-acquired infections, however, remain common following LT even among low-risk patients. A high level of suspicion for late bacterial infections should be maintained due to potentially atypical or less expected infection presentations.
Table 3.
Management of infections in liver transplant recipients
| Population/ infection | Risk factor and type of infection | Management | Ref. |
| Liver transplant candidates/all infections | Donor-derived. Active/latent infections. Vaccine-preventable infection. | Donor screening. Careful patient history and physical examination. Identification of infections requiring therapy. Immunization. | [160-165] |
| Liver transplant recipients/bacterial | Nosocomial infections (ICU, invasive devices). Recurrent infections (anatomical defects). Immunosuppression. | Peri-transplant antibiotic prophylaxis (< 48 h). Prompt diagnostic workup (uncommon presentations, opportunisms). Source control when needed. | [76,83,160] |
| Liver transplant candidates and recipients/MDRO | Colonization (MRSA, VRE, CRE) linked to increased risk of infections. Risk of transmission between patients and across wards. | Surveillance cultures (CRE, VRE, MRSA) and decolonization (MRSA). Infection control (hand hygiene, isolation, contact precautions). | [102,112,164] |
LT: Liver transplantation; ICU: Intensive care unit; MDRO: Multidrug-resistant organisms; MRSA: Methicillin-resistant Staphylococcus aureus; VRE: Vancomycin-resistant enterococci; CRE: Carbapenem-resistant Enterobacteriaceae.
Management of infections in LTR includes prompt initiation of antimicrobial treatment and adequate source control (e.g., CVC removal, surgical debridement).
Type of post-transplant bacterial infections
Bloodstream infections: BSI represent an important cause of mortality in LTR[79]. BSI mainly occur during the first post-operative month and appear to be predictors of long-term survival in transplant recipients. A study encompassing 704 LTR at a single center over a 10-year period showed an incidence of BSI of 37% with an overall mortality of 16%[79]. The majority of BSI (39%) occurred within 10 d after LT. Most frequently isolated pathogens were Enterobacteriaceae (41%), S. aureus (19.8%), Enterococci (13.1%), P. aeruginosa (8.8%), and yeasts (7.1%). A similar study including only Gram-negative bloodstream infections identified an incidence of 210/1000 person-years within the first month following transplantation. Compared to kidney transplant recipients, LTR were more likely to develop early infections and had higher BSI-associated mortality[80]. Potential sources of BSI include intra-abdominal infections (IAI), CVC-BSI, pneumonia, and, less frequently, urinary tract infections. Need for re-operation, prolonged use of indwelling vascular catheters, and acute graft rejection represent predisposing factors for BSI[79]. Gram-negative bacilli such as E. coli, K. pneumoniae, and P. aeruginosa are often the most commonly isolated pathogens, although enterococci, viridans streptococci, and polymicrobial infections are frequently reported among LTR[79-81]. Blood cultures from CVC and peripheral vein represent the gold standard for the diagnosis of BSI and CVC-BSI. If pneumonia or urinary tract infections are suspected, additional cultures (e.g., sputum, bronchoalveolar lavage, or urine cultures) and imaging (e.g., chest CT scan or kidney imaging) should be performed. Management of persistent BSI also warrants the investigation of deep-seated infections (e.g., endocarditis, intra-abdominal abscesses, etc.) and, when possible, prompt source control measures such as removal of vascular catheters and drainage of collections[37].
Surgical site infections: Surgical site infections (SSI) can occur in up to 10% of patients undergoing LT. SSI are more frequently associated to the early post-transplant period and are mainly caused by Enterococcus spp., E. coli, and S. aureus[82,83]. Although they carry a relatively low mortality risk, SSI are associated with increased morbidity and length of hospital stay. In patients with suspected SSI, obtaining purulent discharge cultures and appropriate imaging (e.g., ultrasounds or CT scan) of a collection is important to achieve a timely diagnosis. Management of SSI is usually based on a combined approach, including surgical debridement and targeted antimicrobial therapy. A prospective study including 107 (9%) patients developing SSI identified as independent risk factors choledochojejunal or hepaticojejunal reconstruction, previous liver or kidney transplant, and transfusion of more than 4 red blood cell units[83].
Intra-abdominal infections: IAI represent common infections, accounting for up to 50% of early bacterial infections following LT, and include intraabdominal abscesses, peritonitis, and cholangitis[84-86]. IAI can be polymicrobial and are mainly caused by Enterococci, staphylococci, Pseudomonas spp., Enterobacteriaceae, and anaerobes[84]. Risk factors for IAI are often related to complications during transplantation and their severity is increased by hepatic artery thrombosis and arterial stenosis[84,87]. Compared with SSI, IAI can have a major impact on patients’ outcome. A study encompassing 57 LTR with biloma showed higher rates of mortality, graft loss and need for re-transplantation compared to patients without IAI[84,87]. Predictors of mortality were renal insufficiency (P = 0.02) and infections due to Candida spp. or Gram-negative bacteria.
Adequate imaging, such as ultrasounds, CT scan, or MRI, along with prompt source control are often essential to assure an appropriate management of IAI. Surgical approaches include percutaneous drainage of infected foci and control of peritoneal contamination by diversion or resection (e.g., biliary strictures or stones). Patients with diffuse peritonitis from a perforated viscus should undergo prompt emergency surgery. Intraoperative samples and cultures from recently (< 48 h) inserted drains or ascitic fluid collected in blood culture vials should always be performed to achieve a microbiological diagnosis.
Difficult-to-treat bacteria and MDRO
Although any bacteria can potentially be isolated after LT, infections are mainly caused by Enterobacteriaceae, P. aeruginosa, enterococci (including E. faecium), viridans streptococci, and S. aureus[88-90]. Even if an increase in Gram-negative pathogens responsible for infections in LTR has been documented, Gram-positive bacteria remain the most frequent agents of CVC-BSI[91].
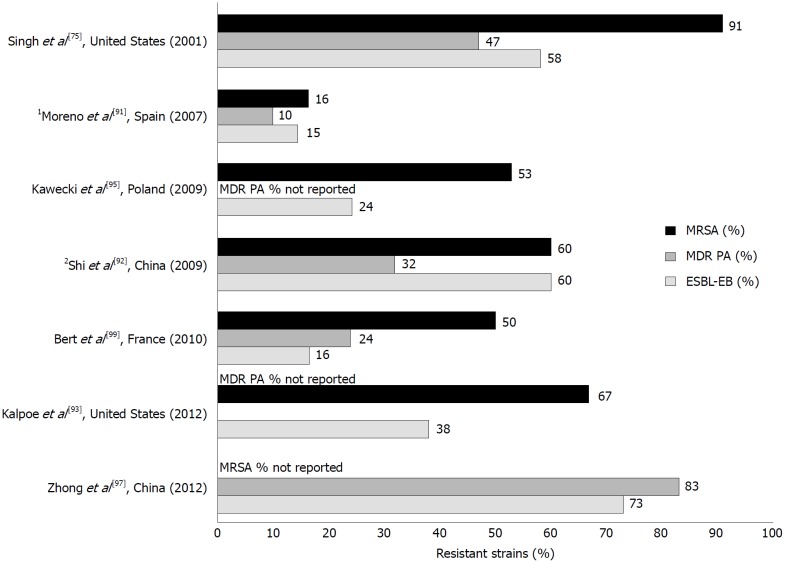
Similar to liver cirrhosis, an increasing number of antimicrobial resistant bacteria has been documented among LTR, with prevalence rates varying significantly according to the geographic areas and among different centers[92]. Most case series reporting rates of MDR Gram-negative in solid organ transplant recipients, however, were from endemic areas, resulting in relatively high percentages ranging from 18% to 50%[93,94]. Very few reports specifically documented the rates of resistance among the most frequently isolated pathogens in LTR (Figure 2)[79,91-93,95-98], and larger prospective studies are necessary to understand the global impact of these infections. Table 4 summarizes the current antimicrobial options to treat MDRO.
Figure 2.
Studies reporting the percentage of infections caused by methicillin-resistant Staphylococcus aureus, MDR Pseudomonas aeruginosa, and extended-spectrum beta-lactamase-producing Enterobacteriaceae following liver transplantation[79,91-93,95-97]. 1Data reported for all solid organ transplants; 2MRSA data obtained from reference 98[98]. MRSA: Methicillin-resistant Staphylococcus aureus; MDR-PA: Multidrug-resistant Pseudomonas aeruginosa; ESBL-EB: Extended-spectrum-beta-lactamase Enterobacteriaceae.
Table 4.
Treatment options for multidrug resistant organisms in liver transplant recipients
| Pathogens | Recommendation | Antimicrobial regimens | Ref. |
| MDR Gram-positives | |||
| MRSA | Nasal decolonization with mupirocin. Daptomycin highly bactericidal in BSI; non effective in pulmonary infections. Linezolid and tigecycline bacteriostatic. | Vancomycin1/linezolid OR Daptomycin OR Tigecycline OR Novel anti-MRSA cephalosporins (ceftaroline, ceftobiprole)2. | [107-111] |
| VRE | Daptomycin highly bactericidal in BSI; non effective in pulmonary infections. Linezolid and tigecycline bacteriostatic. | Linezolid OR Daptomycin OR Tigecycline. | [113,121,122] |
| MDR Gram-negatives | |||
| ESBL-producing Enterobacteriaceae | Conflicting data on carbapenem superiority vs BLBLI. Meropenem recommended for high inoculum infections and unstable patients. | Carbapenems OR Piperacillin/tazobactam. | [175-177] |
| Carbapenem-resistant Enterobacteriaceae | Test antimicrobial susceptibility (also on colonizing strains). Some evidence of better outcomes with combination therapy vs monotherapy. New molecules promising but scarce data in LT. | Ceftazidime/avibactam, OR Combination regimen (at least two active drugs) including colistin/polymixin B, tigecycline, aminoglycosides1 (gentamycin, amikacin), IV fosfomycin, high-dose prolonged infusion carbapenems. For uncomplicated UTI, consider monotherapy (aminoglycosides, fosfomycin). | [127,137,138,175,178] |
| MDR P. aeruginosa | Test antimicrobial susceptibility. New molecules promising but scarce data in LT. | Combination regimen (at least two active drugs) including colistin, an anti-pseudomonal beta-lactam (if susceptible), aminoglycosides1, fosfomycin OR Ceftolozane/tazobactam, ceftazidime/avibactam | [175,179,180] |
Therapeutic drug monitoring recommended;
Approved for skin and soft tissue infections and community-acquired pneumonia (ceftaroline), community-acquired and hospital-acquired pneumonia excluding ventilator-associated pneumonia (ceftobiprole). MDR: Multidrug resistant; BLBLI: Beta-lactam/beta-lactamase inhibitor combination; BSI: Bloodstream infections; LT: Liver transplantation; MDRO: Multidrug-resistant organisms; MRSA: Methicillin-resistant Staphylococcus aureus; VRE: Vancomycin-resistant enterococci; CRE: Carbapenem-resistant Enterobacteriaceae.
Methicillin-resistant S. aureus: S. aureus is an important cause of BSI, pneumonia, wound infections and IAI in LTR, especially within the first 3 post-transplant months[88]. Isolation of methicillin-resistant S. aureus (MRSA) in LTR varies across centers and may cause up to 50% of BSI, with important implications for empirical therapy that may result inadequate[99]. MRSA isolation has been linked to several risk factors, including recent surgery (< 2 wk), cytomegalovirus primary infection, extended ICU stay, concomitant major post-transplant infections, peritonitis, and increased prothrombin time[99-101]. S. aureus carriers who are transplant candidates have a higher risk (24% to 87%) of post-LT infections and may benefit from decolonization prior to transplantation[102-104]. Pre-transplant identification of colonized patients and subsequent eradication of MRSA may be a valuable strategy for limiting S. aureus infections. Decolonization, however, is not permanent; hence it is difficult to determine the optimal timing to decolonize a patient. MRSA colonization is also possible following LT, according to local MRSA prevalence rates, infection control policies, and recipients’ general state of illness[105]. Infection control strategies aiming to reduce the transmission of MRSA through multifaceted interventions such as active surveillance, contact isolation, hand hygiene, environmental cleaning, decolonization of carriers, and antimicrobial stewardship are mandatory (Table 3)[106]. Each transplant program, however, should consider the local epidemiology as a key parameter to implement the infection control practices.
Although vancomycin remains the mainstay for treatment of MRSA, various limitations have been associated with its use, including lower efficacy for strains with MIC > 1.0 mg/L and MSSA-mediated infections, reduced tissue penetration, and increased renal toxicity compared to other available options[88,107,108]. Valid alternatives to vancomycin include linezolid, especially in the treatment of MRSA-related pneumonia, and daptomycin (Table 4)[108-111]. Furthermore, novel anti-MRSA options have recently become available for the treatment of MRSA, although most of them have only been approved for SSTI and real-world data, especially in the field of organ transplantation, are still limited[109].
Vancomycin-resistant Enterococci: Enterococcal infections are usually associated to CVC-BSI, catheter-associated urinary tract infections, and SSI[112]. Vancomycin resistance among Enterococci (VRE), especially E. faecium, currently represents a concern in various transplant centers[113]. VRE-colonized transplant recipients act as reservoirs for VRE transmission and carry an increased risk of infection, ICU stay, and death[114,115]. GI colonization with VRE among LTR is reported between 3% and 55%[116,117], while reported rates of VRE infections among colonized LTR range between 12% and 32%[114,116-118]. A study including LTR who developed bilomas in the early post-transplant period showed that the most common responsible pathogens were Enterococci (37%); of these, 50% were VRE[87]. Common risk factors for VRE infections include antimicrobial use, biliary leaks and strictures, and surgical re-exploration or percutaneous drainage[114-118]. Contact isolation in patients with VRE colonization and infection is recommended (Table 3). Daptomycin and linezolid are commonly used for VRE infections in solid organ transplant recipients, although reduced susceptibility to these antimicrobials has already been reported even in patients without previous exposure to these molecules[119-122].
MDR Gram-negative bacteria: MDR among Gram-negative bacteria is particularly relevant in LTR due to the documented shift from Gram-positive to Gram-negative bacteria infections in the last decade[71,81]. E. coli, K. pneumoniae, and P. aeruginosa currently represent commonly isolated bacteria in BSI after LT[81,92]. Rates of MDRO causing infections in LTR have exponentially increased worldwide, reaching up to 50% in some centers[92,96]. Various risk factors have been associated with antimicrobial resistance, including reoperation, graft rejection, and abdominal infections[96]. Unfortunately, MDRO infections are recognized to cause increased mortality compared to non-resistant infections[79,92,96].
P. aeruginosa is an early nosocomial pathogen and represents a major cause of infection in LTR, accounting for about 6.5% of all BSI[81]. BSI caused by MDR P. aeruginosa compared to susceptible strains appeared significantly more frequent in transplant recipients compared to non-transplanted patients. P. aeruginosa infections caused by MDR strains reached 43% in the United States and up to 52% in China[92,123]. MDR P. aeruginosa causing nosocomial pneumonia in LTR has been reported between 50% and 65%[124].
Rates of extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in LTR vary between 6% to 13% according to the area, reaching up to 40% in endemic countries[125,126]. Risk factors for ESBL-associated infections include pre-transplant colonization, advanced liver disease, and reoperation. In a study encompassing 317 LTR, independent factors associated with preoperative fecal carriage of ESBL-producing Enterobacteriaceae included exposure to a beta-lactam agent in the month preceding transplantation (P < 0.001) and a history of SBP (P = 0.02)[125,126]. The occurrence of ESBL-associated infections has increased the use of carbapenems, that usually represent the last resort for antimicrobial therapy in immunocompromised patients. Carbapenem-resistance has subsequently developed, further complicating the management of LTR since the optimal treatment for carbapenem-resistance Enterobacteriaceae is not yet established[127]. Carbapenem-resistant K. pneumoniae (CRKP), in particular, has emerged as a major threat for immunocompromised and hospitalized patients worldwide and is associated with significant mortality[128,129]. A retrospective study evaluating 14 CRKP infection episodes after LT showed early onset of infection (median time from LT of 12 d) and mortality rates of 71%. Survival rates were significantly lower for patients with CRKP infections compared to those without (29% vs 86%, P < 0.001), and represented an independent risk factor for mortality along with MELD scores ≥ 30[93]. Isolation of CRKP in LTR represents a public health threat in endemic countries[94,130]. In one Unites States transplant center, CRKP accounted for 23% of all bacterial infections in LTR[93,82]. Risk for acquisitions of CRKP in the general population that are also commonly encountered in LTR include exposure to broad-spectrum antibiotics, need for invasive devices, and ICU stay[131]. Only recently, some studies have analyzed the presence of specific factors that can be predictive of CRKP infections in LTR. CRKP pre- and post-transplant colonization appeared as an important factor associated with CRKP infections. In a cohort of 41 CRKP rectal carriers (11 at LT and 30 post-LT), 20 patients developed CRKP infections[132]. Compared with 2% of non-colonized patients, rates of infections were 18% and 47% among carriers before and after LT, respectively. Besides carrier status, renal replacement therapy, mechanical ventilation for > 48 h, and HCV recurrence appeared correlated with CRKP infections[132]. Another recent study involving 54 patients with CRKP infections identified as independent risk factors for post-transplant infections the presence of CRKP colonization, reoperation, combined transplantation, MELD > 32, and dialysis[133].
There are currently no guidelines specifically addressing the management of MDRO infections in LTR. Recent recommendations from the Spanish group for the study of infection in transplant recipients (GESITRA) identified as a key point the characterization of the isolate’s phenotypic and genotypic resistance profile in order to select a targeted therapy that can be adjusted according to susceptibility results[134]. A specific surgical prophylaxis regimen is currently not recommended for patients colonized with carbapenem-resistant strains. Carrier status in LTR recipients, however, should be timely detected, and empirical therapy in case of infection should include active antibiotics based on available microbiological results. Even if donor and/or recipient colonization are associated with an increased risk of infection, carrier status currently do not represent a contraindication to transplantation, but warrants contact isolation precautions and strict hand hygiene compliance. Due to the high-mortality of these infections and while awaiting real-world data on new antibiotic options, preventive strategies and antimicrobial stewardship programs remain key steps to curtail the impact of carbapenem-resistant infections in this cohort.
Novel molecules targeting MDR Gram-negative are currently available, although data on their use in immunocompromised patients remain scarce[135]. Among new antimicrobial options, ceftazidime-avibactam has demonstrated promising activity against CRKP in preliminary studies[136-138], and meropenem-vaborbactam has recently been approved for the treatment of complicated urinary tract infections caused by carbapenem-resistant Enterobacteriaceae. Both compounds, however, are not active against strains harboring metallo-beta-lactamases (MBLs) that are common in certain geographic areas[139]. Results from observational studies including old antibiotics have shown better outcomes for combination therapy (Table 4), but these results remain conflicting and were not confirmed by all studies[140-142]. New compounds targeting carbapenem-resistant strains, including MBLs (e.g., cefiderocol, aztreonam-avibactam) are currently under investigation[143]. Regarding Pseudomonas spp., the novel beta-lactam/beta-lactamase inhibitor ceftolozane-tazobactam has shown good activity against MDR strains, including carbapenem-resistant isolates, with the exception of MBL producers[144].
Fungal infections in liver transplant patients
Although better outcomes have been reported after the introduction of novel antifungals, invasive fungal infections remain an important cause of mortality in LTR, with reported rates between 25% and 81%[145,146]. Fungal infections are frequent in absence of antifungal prophylaxis and can occur in up to 42% of LTR[147,148]. Factors having an impact on the distribution and frequency of fungal infections include changes in surgical techniques, patient and donor organ characteristics, local fungal ecology and resistance, and the use of antifungal prophylaxis. The most common cause of invasive fungal infections in LTR is Candida spp. Candida infections, including candidemia, abdominal infections, and biliary infections, are mostly nosocomial and occur early after LT[149]. A retrospective study in LTR identified an overall incidence of fungal infections of 12%, with non-albicans Candida accounting for 55% of the infections; of these, half were caused by fluconazole-resistant C. parapsilosis[150]. One-year patient survival rates were significantly reduced among patients with fungal infections compared to those without (41% and 80%, respectively). Multivariate analysis showed that pre-transplant fungal colonization was associated with subsequent infections. Various other risk factors have been reported among patients developing fungal infections after LT, including high blood product volumes during surgery, early surgical re-exploration, choledochojejunostomy, retransplantation, fulminant hepatic failure, and severe renal impairment[145,147,148,151-153].
There is currently no consensus on pre-LT fungal prophylaxis regarding clinical indication, best regimens, and duration. Outcome benefits correlated with the use of antifungal prophylaxis among solid organ transplant recipients appear conflicting, and universal prophylaxis is currently not recommended[154-157]. Pre-transplant risk assessment, however, appears useful to identify patients who are at high-risk for the development of fungal infections[57,158].
Similar to liver cirrhosis, echinocandins and azoles are the most commonly used antifungals for the treatment of Candida spp. infections in LTR. Compared to the azoles, very few drug interactions have been reported with echinocandins. Only caspofungin use has been associated with relevant changes in the Cmax of tacrolimus (up to 20% reduction) and cyclosporine (up to 35% increase in plasma concentration of caspofungin)[159].
Lipid formulations of amphotericin B provide wide-spectrum options for patients that may be at risk of non-Candida infections. Voriconazole (and most recently, isavuconazole) remain the drug of choice for invasive aspergillosis, although limitations in its use in LTR and patients with compromised liver function are represented by significant drug interactions with immunosuppressants and occurrence of liver toxicity[159].
Management of infections in LTR
Bacterial and fungal infections in LTR are often associated to surgical complications, involving pathogens that are typically encountered in nosocomial infections and, more recently, MDRO. Clinical presentations of common bacterial infections may be atypical, and clinical and/or radiological findings may not be evident due to an impairment of the inflammatory responses caused by immunosuppressive therapies. Graft rejection can also be confused with infections. Early diagnoses can therefore be challenging and often require invasive diagnostic procedures that remain key to identify the correct cause of infection and promote a potentially successful therapy. The choice of an appropriate antimicrobial regimen for LTR can be particularly challenging due to the need of an urgent empiric therapy in severe infections, the increased rates of antimicrobial resistance, and the risk of drug toxicity and drug-drug interactions. Knowledge of the local epidemiology is particularly important since the empiric antimicrobial treatment should take into account the coverage of resistant pathogens that colonize or have been previously isolated in LTR, especially in areas that are endemic for MDRO or during outbreaks. Table 3 summarizes the main principles for the management of infections in LTR[160-164].
CONCLUSION
The impact of bacterial and fungal infections on the outcome of patients with liver cirrhosis, liver transplant candidates, and liver transplant recipients remain dramatic despite the advances in antimicrobial therapy and surgical techniques. The mechanisms associated with the increased risk of infections in LC are complex and include genetic predisposition, fecal dysbiosis, disruption of the intestinal barrier causing intestinal hyperpermeability, and multiple immunological deficits (Figure 1). In LTR, immunosuppression and risk factors associated with surgery and prolonged hospital stay represent the main causes for bacterial and fungal infections.
Research into the mechanisms favoring infections in cirrhosis is key to find potential areas of intervention. New strategies to modulate the gut-liver interaction are urgently needed since factors such as systemic inflammation and endotoxemia, that may cause life-threatening complications in LC (e.g., SBP and hepatic encephalopathy), are related to the gut environment[165,166]. Studies on the preservation of microbiome composition and function appeared promising in hematological patients undergoing hematopoietic stem cell transplantation[167]. Preliminary studies on gut microbiota-based therapeutics (e.g., probiotics, prebiotics, rifaximin, etc.) in cirrhosis are ongoing[166], but the impact of targeted intervention on gut dysbiosis, bacterial function, or metabolic state is still unclear.
Studies on cirrhosis-associated immune dysfunction appear also important to understand the patterns of pro-inflammatory or immunodeficient phenotypes that can lead to infection susceptibility and/or organ failure in different phases of liver disease, in order to identify potential markers or specific targets of disease progression[168,169].
Bacterial and fungal infections in LT candidates and recipients are often peculiar and may be characterized by confounding factors that favor delayed diagnosis and poor outcomes. For this reason, research dedicated to the development of rapid and accurate diagnostic tools is urgently needed. This is particularly relevant, considering the exponential increase in MDRO, in order to promote the correct use of antibiotics based on the results of microbiological cultures. Specifically, novel instruments of rapid diagnostics (e.g., matrix assisted laser desorption/ionization time-of-flight mass spectrometry, multiplex polymerase chain reaction platforms, etc.) may allow for prompt identification of pathogens, giving direction to clinicians dealing with severe infections in terms of broadening, discontinuation, and de-escalation of empiric regimens[170,171].
Furthermore, despite the availability of novel antibiotics in recent years, there is currently no consensus on optimal antimicrobial regimens that can safely and effectively treat infections caused by MDR Gram-negative bacteria in immunocompromised hosts. Results from pathogen-directed clinical trials employing novel antibiotics with broad activity are largely awaited. Limitations in the use of new molecules, however, include the scarce clinical experience from real-world studies, the lack of knowledge of their pharmacokinetics principles in immunocompromised patients, and an increased risk of toxicity, especially for combination therapies[172].
Finally, the optimization of current strategies directed towards the prevention and treatment of infections in patients with liver disease and LTR remain a key point for their successful management. Examples are represented by the implementation of dedicated antimicrobial stewardship programs and specific bundled interventions based on transplant centers’ local needs and epidemiology[171,172]. Studies evaluating antimicrobial stewardship programs in transplant recipients, for example, are limited but have shown promising results[173,174]. All these efforts, however, cannot be successful without the constant involvement of multidisciplinary teams, including transplant surgeons, hepatologists, specialists in infectious diseases and infection control, microbiologists, and pharmacologists[175-180].
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No conflict of interest exists.
Peer-review started: July 16, 2018
First decision: August 27, 2018
Article in press: October 5, 2018
P- Reviewer: Jha AK, Kim DJ, Zhu X S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
References
- 1.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–1449. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 4.Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457–1469. doi: 10.1111/liv.12271. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin J, Singla V, Arora I, Sood S, Joshi YK. Intestinal permeability and complications in liver cirrhosis: A prospective cohort study. Hepatol Res. 2013;43:200–207. doi: 10.1111/j.1872-034X.2012.01054.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim BI, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim HS, Kim DJ. Increased intestinal permeability as a predictor of bacterial infections in patients with decompensated liver cirrhosis and hemorrhage. J Gastroenterol Hepatol. 2011;26:550–557. doi: 10.1111/j.1440-1746.2010.06490.x. [DOI] [PubMed] [Google Scholar]
- 7.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 8.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunchorntavakul C, Chavalitdhamrong D. Bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. World J Hepatol. 2012;4:158–168. doi: 10.4254/wjh.v4.i5.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol. 2016;8:307–321. doi: 10.4254/wjh.v8.i6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez Mdel P, Martin P, Simkins J. Infectious Complications After Liver Transplantation. Gastroenterol Hepatol (NY) 2015;11:741–753. [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 2011;17 Suppl 3:S34–S37. doi: 10.1002/lt.22378. [DOI] [PubMed] [Google Scholar]
- 15.Sun HY, Cacciarelli TV, Singh N. Identifying a targeted population at high risk for infections after liver transplantation in the MELD era. Clin Transplant. 2011;25:420–425. doi: 10.1111/j.1399-0012.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- 16.Pant C, Olyaee M, Gilroy R, Pandya PK, Olson JC, Oropeza-Vail M, Rai T, Deshpande A. Emergency department visits related to cirrhosis: a retrospective study of the nationwide emergency department sample 2006 to 2011. Medicine (Baltimore) 2015;94:e308. doi: 10.1097/MD.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 18.Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS; NACSELD. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, Salinas F, Donà S, Fagiuoli S, Sticca A, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–229. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 21.Rosa H, Silvério AO, Perini RF, Arruda CB. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290–1293. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y, Tu B, Xu Z, Zhang X, Bi J, Zhao M, Chen W, Shi L, Zhao P, Bao C, et al. Bacterial distributions and prognosis of bloodstream infections in patients with liver cirrhosis. Sci Rep. 2017;7:11482. doi: 10.1038/s41598-017-11587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preveden T. Bacterial infections in patients with liver cirrhosis. Med Pregl. 2015;68:187–191. doi: 10.2298/mpns1506187p. [DOI] [PubMed] [Google Scholar]
- 24.Yoneyama K, Miyagishi K, Kiuchi Y, Shibata M, Mitamura K. Risk factors for infections in cirrhotic patients with and without hepatocellular carcinoma. J Gastroenterol. 2002;37:1028–1034. doi: 10.1007/s005350200173. [DOI] [PubMed] [Google Scholar]
- 25.de Mattos AA, Coral GP, Menti E, Valiatti F, Kramer C. [Bacterial infection in cirrhotic patient] Arq Gastroenterol. 2003;40:11–15. doi: 10.1590/s0004-28032003000100003. [DOI] [PubMed] [Google Scholar]
- 26.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 27.Toledo C, Flores C, Sáenz M, Jiménez P, Tejero A, Ibarra H, León J, Arce M. [Bacterial infections in hepatic cirrhosis] Rev Med Chil. 1994;122:788–794. [PubMed] [Google Scholar]
- 28.Bartoletti M, Giannella M, Caraceni P, Domenicali M, Ambretti S, Tedeschi S, Verucchi G, Badia L, Lewis RE, Bernardi M, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. 2014;61:51–58. doi: 10.1016/j.jhep.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Perdomo Coral G, Alves de Mattos A. Renal impairment after spontaneous bacterial peritonitis: incidence and prognosis. Can J Gastroenterol. 2003;17:187–190. doi: 10.1155/2003/370257. [DOI] [PubMed] [Google Scholar]
- 31.Mølle I, Thulstrup AM, Svendsen N, Schønheyder HC, Sørensen HT. Risk and case fatality rate of meningitis in patients with liver cirrhosis. Scand J Infect Dis. 2000;32:407–410. doi: 10.1080/003655400750044999. [DOI] [PubMed] [Google Scholar]
- 32.Barahona-Garrido J, Hernández-Calleros J, Téllez-Avila FI, Chávez-Tapia NC, Remes-Troche JM, Torre A. Bacterial meningitis in cirrhotic patients: case series and description of the prognostic role of acute renal failure. J Clin Gastroenterol. 2010;44:e218–e223. doi: 10.1097/MCG.0b013e3181d88d53. [DOI] [PubMed] [Google Scholar]
- 33.Vilstrup H. Cirrhosis and bacterial infections. Rom J Gastroenterol. 2003;12:297–302. [PubMed] [Google Scholar]
- 34.Thulstrup AM, Sørensen HT, Schønheyder HC, Møller JK, Tage-Jensen U. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31:1357–1361. doi: 10.1086/317494. [DOI] [PubMed] [Google Scholar]
- 35.Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence. 2016;7:309–319. doi: 10.1080/21505594.2016.1141162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripodi MF, Adinolfi LE, Ragone E, Durante Mangoni E, Fortunato R, Iarussi D, Ruggiero G, Utili R. Streptococcus bovis endocarditis and its association with chronic liver disease: an underestimated risk factor. Clin Infect Dis. 2004;38:1394–1400. doi: 10.1086/392503. [DOI] [PubMed] [Google Scholar]
- 37.Fagiuoli S, Colli A, Bruno R, Burra P, Craxì A, Gaeta GB, Grossi P, Mondelli MU, Puoti M, Sagnelli E, et al. Management of infections in cirrhotic patients: report of a consensus conference. Dig Liver Dis. 2014;46:204–212. doi: 10.1016/j.dld.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351–1355. doi: 10.1016/0016-5085(88)90372-1. [DOI] [PubMed] [Google Scholar]
- 39.Mendler MH, Agarwal A, Trimzi M, Madrigal E, Tsushima M, Joo E, Santiago M, Flores E, David G, Workman A, et al. A new highly sensitive point of care screen for spontaneous bacterial peritonitis using the leukocyte esterase method. J Hepatol. 2010;53:477–483. doi: 10.1016/j.jhep.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Bartoletti M, Giannella M, Lewis R, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, et al. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin Microbiol Infect. 2018;24:546.e1–546.e8. doi: 10.1016/j.cmi.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Hamza RE, Villyoth MP, Peter G, Joseph D, Govindaraju C, Tank DC, Sreesh S, Narayanan P, Vinayakumar KR. Risk factors of cellulitis in cirrhosis and antibiotic prophylaxis in preventing recurrence. Ann Gastroenterol. 2014;27:374–379. [PMC free article] [PubMed] [Google Scholar]
- 42.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1-1256.e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Gustot T, Felleiter P, Pickkers P, Sakr Y, Rello J, Velissaris D, Pierrakos C, Taccone FS, Sevcik P, Moreno C, et al. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int. 2014;34:1496–1503. doi: 10.1111/liv.12520. [DOI] [PubMed] [Google Scholar]
- 44.Alexopoulou A, Vasilieva L, Agiasotelli D, Dourakis SP. Fungal infections in patients with cirrhosis. J Hepatol. 2015;63:1043–1045. doi: 10.1016/j.jhep.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 45.Bucsics T, Schwabl P, Mandorfer M, Peck-Radosavljevic M. Prognosis of cirrhotic patients with fungiascites and spontaneous fungal peritonitis (SFP) J Hepatol. 2016;64:1452–1454. doi: 10.1016/j.jhep.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 46.Fiore M, Leone S. Spontaneous fungal peritonitis: Epidemiology, current evidence and future prospective. World J Gastroenterol. 2016;22:7742–7747. doi: 10.3748/wjg.v22.i34.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang SY, Yu SJ, Lee JH, Kim JS, Yoon JW, Kim YJ, Yoon JH, Kim EC, Lee HS. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014;33:259–264. doi: 10.1007/s10096-013-1953-2. [DOI] [PubMed] [Google Scholar]
- 48.Shizuma T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: A literature review. World J Hepatol. 2018;10:254–266. doi: 10.4254/wjh.v10.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lahmer T, Messer M, Mayr U, Saugel B, Noe S, Schultheiss C, Thies P, Spinner C, Nennstiel S, Schwerdtfeger C, et al. Fungal “colonisation” is associated with increased mortality in medical intensive care unit patients with liver cirrhosis. Mycopathologia. 2015;179:63–71. doi: 10.1007/s11046-014-9825-6. [DOI] [PubMed] [Google Scholar]
- 50.Lahmer T, Messer M, Schwerdtfeger C, Rasch S, Lee M, Saugel B, Schmid RM, Huber W. Invasive mycosis in medical intensive care unit patients with severe alcoholic hepatitis. Mycopathologia. 2014;177:193–197. doi: 10.1007/s11046-014-9740-x. [DOI] [PubMed] [Google Scholar]
- 51.Bassetti M, Peghin M, Carnelutti A, Righi E, Merelli M, Ansaldi F, Trucchi C, Alicino C, Sartor A, Toniutto P, et al. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: a multicenter study. Intensive Care Med. 2017;43:509–518. doi: 10.1007/s00134-017-4717-0. [DOI] [PubMed] [Google Scholar]
- 52.Gravito-Soares M, Gravito-Soares E, Lopes S, Ribeiro G, Figueiredo P. Spontaneous fungal peritonitis: a rare but severe complication of liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1010–1016. doi: 10.1097/MEG.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 53.Fiore M, Maraolo AE, Leone S, Gentile I, Cuomo A, Schiavone V, Bimonte S, Pace MC, Cascella M. Spontaneous peritonitis in critically ill cirrhotic patients: a diagnostic algorithm for clinicians and future perspectives. Ther Clin Risk Manag. 2017;13:1409–1414. doi: 10.2147/TCRM.S144262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nadim MK, Durand F, Kellum JA, Levitsky J, O’Leary JG, Karvellas CJ, Bajaj JS, Davenport A, Jalan R, Angeli P, et al. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016;64:717–735. doi: 10.1016/j.jhep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 57.Grossi PA, Gasperina DD, Barchiesi F, Biancofiore G, Carafiello G, De Gasperi A, Sganga G, Menichetti F, Montagna MT, Pea F, et al. Italian guidelines for diagnosis, prevention, and treatment of invasive fungal infections in solid organ transplant recipients. Transplant Proc. 2011;43:2463–2471. doi: 10.1016/j.transproceed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 58.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 59.Shields RK, Nguyen MH, Clancy CJ. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis. 2015;28:514–522. doi: 10.1097/QCO.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeoh SF, Lee TJ, Chew KL, Lin S, Yeo D, Setia S. Echinocandins for management of invasive candidiasis in patients with liver disease and liver transplantation. Infect Drug Resist. 2018;11:805–819. doi: 10.2147/IDR.S165676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mistry GC, Migoya E, Deutsch PJ, Winchell G, Hesney M, Li S, Bi S, Dilzer S, Lasseter KC, Stone JA. Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations. J Clin Pharmacol. 2007;47:951–961. doi: 10.1177/0091270007303764. [DOI] [PubMed] [Google Scholar]
- 62.Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, Hui J, Zhai S. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71:1772–1785. doi: 10.1093/jac/dkw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, Vida A, Kappelmayer J, Lakatos PL, Antal-Szalmas P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012;32:603–611. doi: 10.1111/j.1478-3231.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 64.Bassetti M, Marchetti M, Chakrabarti A, Colizza S, Garnacho-Montero J, Kett DH, Munoz P, Cristini F, Andoniadou A, Viale P, et al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive Care Med. 2013;39:2092–2106. doi: 10.1007/s00134-013-3109-3. [DOI] [PubMed] [Google Scholar]
- 65.Salerno F, Borzio M, Pedicino C, Simonetti R, Rossini A, Boccia S, Cacciola I, Burroughs AK, Manini MA, La Mura V, et al. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37:71–79. doi: 10.1111/liv.13195. [DOI] [PubMed] [Google Scholar]
- 66.Alexopoulou A, Papadopoulos N, Eliopoulos DG, Alexaki A, Tsiriga A, Toutouza M, Pectasides D. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33:975–981. doi: 10.1111/liv.12152. [DOI] [PubMed] [Google Scholar]
- 67.Reddy KR, O’Leary JG, Kamath PS, Fallon MB, Biggins SW, Wong F, Patton HM, Garcia-Tsao G, Subramanian RM, Thacker LR, et al. High risk of delisting or death in liver transplant candidates following infections: Results from the North American Consortium for the Study of End-Stage Liver Disease. Liver Transpl. 2015;21:881–888. doi: 10.1002/lt.24139. [DOI] [PubMed] [Google Scholar]
- 68.Mounzer R, Malik SM, Nasr J, Madani B, Devera ME, Ahmad J. Spontaneous bacterial peritonitis before liver transplantation does not affect patient survival. Clin Gastroenterol Hepatol. 2010;8:623–628.e1. doi: 10.1016/j.cgh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Sun HY, Cacciarelli TV, Singh N. Impact of pretransplant infections on clinical outcomes of liver transplant recipients. Liver Transpl. 2010;16:222–228. doi: 10.1002/lt.21982. [DOI] [PubMed] [Google Scholar]
- 70.Lin KH, Liu JW, Chen CL, Wang SH, Lin CC, Liu YW, Yong CC, Lin TL, Li WF, Hu TH, et al. Impacts of pretransplant infections on clinical outcomes of patients with acute-on-chronic liver failure who received living-donor liver transplantation. PLoS One. 2013;8:e72893. doi: 10.1371/journal.pone.0072893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Delden C. Bacterial biliary tract infections in liver transplant recipients. Curr Opin Organ Transplant. 2014;19:223–228. doi: 10.1097/MOT.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 72.Antunes M, Teixeira A, Fortuna P, Moya B, Martins A, Bagulho L, Pereira JP, Bento L, Perdigoto R, Barroso E, et al. Infections After Liver Transplantation: A Retrospective, Single-center Study. Transplant Proc. 2015;47:1019–1024. doi: 10.1016/j.transproceed.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Romero FA, Razonable RR. Infections in liver transplant recipients. World J Hepatol. 2011;3:83–92. doi: 10.4254/wjh.v3.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 75.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transpl. 2000;6:54–61. doi: 10.1002/lt.500060112. [DOI] [PubMed] [Google Scholar]
- 76.Fishman JA, Issa NC. Infection in organ transplantation: risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24:273–283. doi: 10.1016/j.idc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Delmonico FL. Cadaver donor screening for infectious agents in solid organ transplantation. Clin Infect Dis. 2000;31:781–786. doi: 10.1086/314000. [DOI] [PubMed] [Google Scholar]
- 78.San Juan R, Aguado JM, Lumbreras C, Díaz-Pedroche C, López-Medrano F, Lizasoain M, Gavalda J, Montejo M, Moreno A, Gurguí M, et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant. 2007;7:964–971. doi: 10.1111/j.1600-6143.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 79.Bert F, Larroque B, Paugam-Burtz C, Janny S, Durand F, Dondero F, Valla DC, Belghiti J, Moreau R, Nicolas-Chanoine MH. Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transpl. 2010;16:393–401. doi: 10.1002/lt.21991. [DOI] [PubMed] [Google Scholar]
- 80.Al-Hasan MN, Razonable RR, Eckel-Passow JE, Baddour LM. Incidence rate and outcome of Gram-negative bloodstream infection in solid organ transplant recipients. Am J Transplant. 2009;9:835–843. doi: 10.1111/j.1600-6143.2009.02559.x. [DOI] [PubMed] [Google Scholar]
- 81.Singh N, Wagener MM, Obman A, Cacciarelli TV, de Vera ME, Gayowski T. Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver Transpl. 2004;10:844–849. doi: 10.1002/lt.20214. [DOI] [PubMed] [Google Scholar]
- 82.Kawecki D, Chmura A, Pacholczyk M, Lagiewska B, Adadynski L, Wasiak D, Malkowski P, Sawicka-Grzelak A, Rokosz A, Szymanowska A, et al. Surgical site infections in liver recipients in the early posttransplantation period: etiological agents and susceptibility profiles. Transplant Proc. 2007;39:2800–2806. doi: 10.1016/j.transproceed.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 83.Asensio A, Ramos A, Cuervas-Mons V, Cordero E, Sánchez-Turrión V, Blanes M, Cervera C, Gavalda J, Aguado JM, Torre-Cisneros J; Red de Estudio de la Infección en el Trasplante - Grupo de Estudio de la Infección en el Trasplante. Effect of antibiotic prophylaxis on the risk of surgical site infection in orthotopic liver transplant. Liver Transpl. 2008;14:799–805. doi: 10.1002/lt.21435. [DOI] [PubMed] [Google Scholar]
- 84.Safdar N, Said A, Lucey MR, Knechtle SJ, D’Alessandro A, Musat A, Pirsch J, McDermott J, Kalayoglu M, Maki DG. Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin Infect Dis. 2004;39:517–525. doi: 10.1086/422644. [DOI] [PubMed] [Google Scholar]
- 85.Akin K, Ozturk A, Guvenc Z, Isiklar I, Haberal M. Localized fluid collections after liver transplantation. Transplant Proc. 2006;38:627–630. doi: 10.1016/j.transproceed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Kim YJ, Kim SI, Wie SH, Kim YR, Hur JA, Choi JY, Yoon SK, Moon IS, Kim DG, Lee MD, et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl Infect Dis. 2008;10:316–324. doi: 10.1111/j.1399-3062.2008.00315.x. [DOI] [PubMed] [Google Scholar]
- 87.Said A, Safdar N, Lucey MR, Knechtle SJ, D’Alessandro A, Musat A, Pirsch J, Kalayoglu M, Maki DG. Infected bilomas in liver transplant recipients, incidence, risk factors and implications for prevention. Am J Transplant. 2004;4:574–582. doi: 10.1111/j.1600-6143.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 88.Garzoni C, AST Infectious Diseases Community of Practice. Multiply resistant gram-positive bacteria methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus (MRSA, VISA, VRSA) in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S41–S49. doi: 10.1111/j.1600-6143.2009.02892.x. [DOI] [PubMed] [Google Scholar]
- 89.Muñoz P, AST Infectious Diseases Community of Practice. Multiply resistant gram-positive bacteria: vancomycin-resistant enterococcus in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S50–S56. doi: 10.1111/j.1600-6143.2009.02893.x. [DOI] [PubMed] [Google Scholar]
- 90.van Delden C, Blumberg EA; AST Infectious Diseases Community of Practice. Multidrug resistant gram-negative bacteria in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S27–S34. doi: 10.1111/j.1600-6143.2009.02890.x. [DOI] [PubMed] [Google Scholar]
- 91.Moreno A, Cervera C, Gavaldá J, Rovira M, de la Cámara R, Jarque I, Montejo M, de la Torre-Cisneros J, Miguel Cisneros J, Fortún J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant. 2007;7:2579–2586. doi: 10.1111/j.1600-6143.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 92.Shi SH, Kong HS, Xu J, Zhang WJ, Jia CK, Wang WL, Shen Y, Zhang M, Zheng SS. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis. 2009;11:405–412. doi: 10.1111/j.1399-3062.2009.00421.x. [DOI] [PubMed] [Google Scholar]
- 93.Kalpoe JS, Sonnenberg E, Factor SH, del Rio Martin J, Schiano T, Patel G, Huprikar S. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2012;18:468–474. doi: 10.1002/lt.23374. [DOI] [PubMed] [Google Scholar]
- 94.Bergamasco MD, Barroso Barbosa M, de Oliveira Garcia D, Cipullo R, Moreira JC, Baia C, Barbosa V, Abboud CS. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl Infect Dis. 2012;14:198–205. doi: 10.1111/j.1399-3062.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 95.Kawecki D, Chmura A, Pacholczyk M, Lagiewska B, Adadynski L, Wasiak D, Czerwinski J, Malkowski P, Sawicka-Grzelak A, Kot K, et al. Bacterial infections in the early period after liver transplantation: etiological agents and their susceptibility. Med Sci Monit. 2009;15:CR628–CR637. [PubMed] [Google Scholar]
- 96.Singh N, Gayowski T, Rihs JD, Wagener MM, Marino IR. Evolving trends in multiple-antibiotic-resistant bacteria in liver transplant recipients: a longitudinal study of antimicrobial susceptibility patterns. Liver Transpl. 2001;7:22–26. doi: 10.1053/jlts.2001.20769. [DOI] [PubMed] [Google Scholar]
- 97.Zhong L, Men TY, Li H, Peng ZH, Gu Y, Ding X, Xing TH, Fan JW. Multidrug-resistant gram-negative bacterial infections after liver transplantation - spectrum and risk factors. J Infect. 2012;64:299–310. doi: 10.1016/j.jinf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Shi SH, Kong HS, Jia CK, Xu J, Zhang WJ, Wang WL, Shen Y, Zhang M, Zheng SS. Coagulase-negative staphylococcus and enterococcus as predominant pathogens in liver transplant recipients with Gram-positive coccal bacteremia. Chin Med J (Engl) 2010;123:1983–1988. [PubMed] [Google Scholar]
- 99.Bert F, Bellier C, Lassel L, Lefranc V, Durand F, Belghiti J, Mentré F, Fantin B. Risk factors for Staphylococcus aureus infection in liver transplant recipients. Liver Transpl. 2005;11:1093–1099. doi: 10.1002/lt.20491. [DOI] [PubMed] [Google Scholar]
- 100.Singh N, Paterson DL, Chang FY, Gayowski T, Squier C, Wagener MM, Marino IR. Methicillin-resistant Staphylococcus aureus: the other emerging resistant gram-positive coccus among liver transplant recipients. Clin Infect Dis. 2000;30:322–327. doi: 10.1086/313658. [DOI] [PubMed] [Google Scholar]
- 101.Florescu DF, McCartney AM, Qiu F, Langnas AN, Botha J, Mercer DF, Grant W, Kalil AC. Staphylococcus aureus infections after liver transplantation. Infection. 2012;40:263–269. doi: 10.1007/s15010-011-0224-3. [DOI] [PubMed] [Google Scholar]
- 102.Bert F, Galdbart JO, Zarrouk V, Le Mée J, Durand F, Mentré F, Belghiti J, Lambert-Zechovsky N, Fantin B. Association between nasal carriage of Staphylococcus aureus and infection in liver transplant recipients. Clin Infect Dis. 2000;31:1295–1299. doi: 10.1086/317469. [DOI] [PubMed] [Google Scholar]
- 103.Desai D, Desai N, Nightingale P, Elliott T, Neuberger J. Carriage of methicillin-resistant Staphylococcus aureus is associated with an increased risk of infection after liver transplantation. Liver Transpl. 2003;9:754–759. doi: 10.1053/jlts.2003.50142. [DOI] [PubMed] [Google Scholar]
- 104.Russell DL, Flood A, Zaroda TE, Acosta C, Riley MM, Busuttil RW, Pegues DA. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant. 2008;8:1737–1743. doi: 10.1111/j.1600-6143.2008.02304.x. [DOI] [PubMed] [Google Scholar]
- 105.Santoro-Lopes G, de Gouvêa EF, Monteiro RC, Branco RC, Rocco JR, Halpern M, Ferreira AL, de Araújo EG, Basto ST, Silveira VG, et al. Colonization with methicillin-resistant Staphylococcus aureus after liver transplantation. Liver Transpl. 2005;11:203–209. doi: 10.1002/lt.20338. [DOI] [PubMed] [Google Scholar]
- 106.Singh N, Squier C, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Impact of an aggressive infection control strategy on endemic Staphylococcus aureus infection in liver transplant recipients. Infect Control Hosp Epidemiol. 2006;27:122–126. doi: 10.1086/500651. [DOI] [PubMed] [Google Scholar]
- 107.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 108.Bassetti M, Righi E. Safety profiles of old and new antimicrobials for the treatment of MRSA infections. Expert Opin Drug Saf. 2016;15:467–481. doi: 10.1517/14740338.2016.1142528. [DOI] [PubMed] [Google Scholar]
- 109.Bassetti M, Righi E, Carnelutti A. New therapeutic options for skin and soft tissue infections. Curr Opin Infect Dis. 2016;29:99–108. doi: 10.1097/QCO.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 110.Len O, Montejo M, Cervera C, Fariñas MC, Sabé N, Ramos A, Cordero E, Torre-Cisneros J, Martín-Dávila P, Azanza JR, Pahissa A, Gavaldà J; DAPTOSOT (Daptomycin in Solid Organ Transplantation) Study Group. Daptomycin is safe and effective for the treatment of gram-positive cocci infections in solid organ transplantation. Transpl Infect Dis. 2014;16:532–538. doi: 10.1111/tid.12232. [DOI] [PubMed] [Google Scholar]
- 111.Radunz S, Juntermanns B, Kaiser GM, Treckmann J, Mathe Z, Paul A, Saner FH. Efficacy and safety of linezolid in liver transplant patients. Transpl Infect Dis. 2011;13:353–358. doi: 10.1111/j.1399-3062.2011.00617.x. [DOI] [PubMed] [Google Scholar]
- 112.Bucheli E, Kralidis G, Boggian K, Cusini A, Garzoni C, Manuel O, Meylan PR, Mueller NJ, Khanna N, van Delden C, et al. Impact of enterococcal colonization and infection in solid organ transplantation recipients from the Swiss transplant cohort study. Transpl Infect Dis. 2014;16:26–36. doi: 10.1111/tid.12168. [DOI] [PubMed] [Google Scholar]
- 113.Patel G, Snydman DR; AST Infectious Diseases Community of Practice. Vancomycin-resistant Enterococcus infections in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:59–67. doi: 10.1111/ajt.12099. [DOI] [PubMed] [Google Scholar]
- 114.McNeil SA, Malani PN, Chenoweth CE, Fontana RJ, Magee JC, Punch JD, Mackin ML, Kauffman CA. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin Infect Dis. 2006;42:195–203. doi: 10.1086/498903. [DOI] [PubMed] [Google Scholar]
- 115.Gearhart M, Martin J, Rudich S, Thomas M, Wetzel D, Solomkin J, Hanaway MJ, Aranda-Michel J, Weber F, Trumball L, et al. Consequences of vancomycin-resistant Enterococcus in liver transplant recipients: a matched control study. Clin Transplant. 2005;19:711–716. doi: 10.1111/j.1399-0012.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 116.Dominguez EA, Davis JC, Langnas AN, Winfield B, Cavalieri SJ, Rupp ME. An outbreak of vancomycin-resistant Enterococcus faecium in liver transplant recipients. Liver Transpl Surg. 1997;3:586–590. [PubMed] [Google Scholar]
- 117.Bakir M, Bova JL, Newell KA, Millis JM, Buell JF, Arnow PM. Epidemiology and clinical consequences of vancomycin-resistant enterococci in liver transplant patients. Transplantation. 2001;72:1032–1037. doi: 10.1097/00007890-200109270-00009. [DOI] [PubMed] [Google Scholar]
- 118.Hagen EA, Lautenbach E, Olthoff K, Blumberg EA. Low prevalence of colonization with vancomycin-resistant Enterococcus in patients awaiting liver transplantation. Am J Transplant. 2003;3:902–905. doi: 10.1034/j.1600-6143.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 119.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niebel M, Perera MT, Shah T, Marudanayagam R, Martin K, Oppenheim BA, David MD. Emergence of linezolid resistance in hepatobiliary infections caused by Enterococcus faecium. Liver Transpl. 2016;22:201–208. doi: 10.1002/lt.24328. [DOI] [PubMed] [Google Scholar]
- 121.King ST, Usery JB, Holloway K, Koeth L, Cleveland KO, Gelfand MS. Successful therapy of treatment-emergent, non-clonal daptomycin-non-susceptible Enterococcus faecium infections. J Antimicrob Chemother. 2011;66:2673–2675. doi: 10.1093/jac/dkr343. [DOI] [PubMed] [Google Scholar]
- 122.Linden PK, Pasculle AW, Manez R, Kramer DJ, Fung JJ, Pinna AD, Kusne S. Differences in outcomes for patients with bacteremia due to vancomycin-resistant Enterococcus faecium or vancomycin-susceptible E. faecium. Clin Infect Dis. 1996;22:663–670. doi: 10.1093/clinids/22.4.663. [DOI] [PubMed] [Google Scholar]
- 123.Johnson LE, D’Agata EM, Paterson DL, Clarke L, Qureshi ZA, Potoski BA, Peleg AY. Pseudomonas aeruginosa bacteremia over a 10-year period: multidrug resistance and outcomes in transplant recipients. Transpl Infect Dis. 2009;11:227–234. doi: 10.1111/j.1399-3062.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 124.Shi SH, Kong HS, Jia CK, Zhang WJ, Xu J, Wang WL, Shen Y, Zhang M, Zheng SS. Risk factors for pneumonia caused by multidrug-resistant Gram-negative bacilli among liver recipients. Clin Transplant. 2010;24:758–765. doi: 10.1111/j.1399-0012.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- 125.Bert F, Larroque B, Paugam-Burtz C, Dondero F, Durand F, Marcon E, Belghiti J, Moreau R, Nicolas-Chanoine MH. Pretransplant fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae and infection after liver transplant, France. Emerg Infect Dis. 2012;18:908–916. doi: 10.3201/eid1806.110139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bert F, Larroque B, Dondero F, Durand F, Paugam-Burtz C, Belghiti J, Moreau R, Nicolas-Chanoine MH. Risk factors associated with preoperative fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in liver transplant recipients. Transpl Infect Dis. 2014;16:84–89. doi: 10.1111/tid.12169. [DOI] [PubMed] [Google Scholar]
- 127.Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G; Critically Ill Patients Study Group of the European Society of Clinical Microbiology and Infectious Disease (ESCMID); Hellenic Society of Chemotherapy (HSC) and Società Italiana di Terapia Antinfettiva (SITA) Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24:133–144. doi: 10.1016/j.cmi.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 128.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen M, Eschenauer GA, Bryan M, O’Neil K, Furuya EY, Della-Latta P, Kubin CJ. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 2010;67:180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 130.Pereira MR, Scully BF, Pouch SM, Uhlemann AC, Goudie S, Emond JE, Verna EC. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl. 2015;21:1511–1519. doi: 10.1002/lt.24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Swaminathan M, Sharma S, Poliansky Blash S, Patel G, Banach DB, Phillips M, LaBombardi V, Anderson KF, Kitchel B, Srinivasan A, et al. Prevalence and risk factors for acquisition of carbapenem-resistant Enterobacteriaceae in the setting of endemicity. Infect Control Hosp Epidemiol. 2013;34:809–817. doi: 10.1086/671270. [DOI] [PubMed] [Google Scholar]
- 132.Giannella M, Bartoletti M, Morelli MC, Tedeschi S, Cristini F, Tumietto F, Pasqualini E, Danese I, Campoli C, Lauria ND, et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015;15:1708–1715. doi: 10.1111/ajt.13136. [DOI] [PubMed] [Google Scholar]
- 133.Freire MP, Oshiro IC, Pierrotti LC, Bonazzi PR, de Oliveira LM, Song AT, Camargo CH, van der Heijden IM, Rossi F, Costa SF, et al. Carbapenem-Resistant Enterobacteriaceae Acquired Before Liver Transplantation: Impact on Recipient Outcomes. Transplantation. 2017;101:811–820. doi: 10.1097/TP.0000000000001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aguado JM, Silva JT, Fernández-Ruiz M, Cordero E, Fortún J, Gudiol C, Martínez-Martínez L, Vidal E, Almenar L, Almirante B, Cantón R, Carratalá J, Caston JJ, Cercenado E, Cervera C, Cisneros JM, Crespo-Leiro MG, Cuervas-Mons V, Elizalde-Fernández J, Fariñas MC, Gavaldà J, Goyanes MJ, Gutiérrez-Gutiérrez B, Hernández D, Len O, López-Andujar R, López-Medrano F, Martín-Dávila P, Montejo M, Moreno A, Oliver A, Pascual A, Pérez-Nadales E, Román-Broto A, San-Juan R, Serón D, Solé-Jover A, Valerio M, Muñoz P, Torre-Cisneros J; Spanish Society of Transplantation (SET); Group for Study of Infection in Transplantation of the Spanish Society of Infectious Diseases and Clinical Microbiology (GESITRA-SEIMC); Spanish Network for Research in Infectious Diseases (REIPI) (RD16/0016) Management of multidrug resistant Gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant Rev (Orlando) 2018;32:36–57. doi: 10.1016/j.trre.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 135.Bassetti M, Righi E. New antibiotics and antimicrobial combination therapy for the treatment of gram-negative bacterial infections. Curr Opin Crit Care. 2015;21:402–411. doi: 10.1097/MCC.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 136.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, et al. Efficacy of Ceftazidime-avibactam Salvage Therapy in Patients with Infections Caused by KPC-producing Klebsiella pneumoniae. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy492. [DOI] [PubMed] [Google Scholar]
- 137.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob Agents Chemother. 2017:61. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis. 2018;66:163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cho JC, Zmarlicka MT, Shaeer KM, Pardo J. Meropenem/Vaborbactam, the First Carbapenem/β-Lactamase Inhibitor Combination. Ann Pharmacother. 2018;52:769–779. doi: 10.1177/1060028018763288. [DOI] [PubMed] [Google Scholar]
- 140.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 141.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 143.Bassetti M, Russo A, Carnelutti A, La Rosa A, Righi E. Antimicrobial resistance and treatment: an unmet clinical safety need. Expert Opin Drug Saf. 2018;17:669–680. doi: 10.1080/14740338.2018.1488962. [DOI] [PubMed] [Google Scholar]
- 144.Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, Doi Y, Hao B, Press EG, Cooper VS, et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin Infect Dis. 2017;65:110–120. doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Husain S, Tollemar J, Dominguez EA, Baumgarten K, Humar A, Paterson DL, Wagener MM, Kusne S, Singh N. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation. 2003;75:2023–2029. doi: 10.1097/01.TP.0000065178.93741.72. [DOI] [PubMed] [Google Scholar]
- 146.Rabkin JM, Oroloff SL, Corless CL, Benner KG, Flora KD, Rosen HR, Olyaei AJ. Association of fungal infection and increased mortality in liver transplant recipients. Am J Surg. 2000;179:426–430. doi: 10.1016/s0002-9610(00)00366-4. [DOI] [PubMed] [Google Scholar]
- 147.Collins LA, Samore MH, Roberts MS, Luzzati R, Jenkins RL, Lewis WD, Karchmer AW. Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J Infect Dis. 1994;170:644–652. [PubMed] [Google Scholar]
- 148.Briegel J, Forst H, Spill B, Haas A, Grabein B, Haller M, Kilger E, Jauch KW, Maag K, Ruckdeschel G. Risk factors for systemic fungal infections in liver transplant recipients. Eur J Clin Microbiol Infect Dis. 1995;14:375–382. doi: 10.1007/BF02114892. [DOI] [PubMed] [Google Scholar]
- 149.Bassetti M, Peghin M, Carnelutti A, Righi E, Merelli M, Ansaldi F, Trucchi C, Alicino C, Sartor A, Wauters J, et al. Invasive Candida Infections in Liver Transplant Recipients: Clinical Features and Risk Factors for Mortality. Transplant Direct. 2017;3:e156. doi: 10.1097/TXD.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raghuram A, Restrepo A, Safadjou S, Cooley J, Orloff M, Hardy D, Butler S, Koval CE. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007) Liver Transpl. 2012;18:1100–1109. doi: 10.1002/lt.23467. [DOI] [PubMed] [Google Scholar]
- 151.Nieto-Rodriguez JA, Kusne S, Mañez R, Irish W, Linden P, Magnone M, Wing EJ, Fung JJ, Starzl TE. Factors associated with the development of candidemia and candidemia-related death among liver transplant recipients. Ann Surg. 1996;223:70–76. doi: 10.1097/00000658-199601000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.George MJ, Snydman DR, Werner BG, Griffith J, Falagas ME, Dougherty NN, Rubin RH. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med. 1997;103:106–113. doi: 10.1016/s0002-9343(97)80021-6. [DOI] [PubMed] [Google Scholar]
- 153.Patel R, Portela D, Badley AD, Harmsen WS, Larson-Keller JJ, Ilstrup DM, Keating MR, Wiesner RH, Krom RA, Paya CV. Risk factors of invasive Candida and non-Candida fungal infections after liver transplantation. Transplantation. 1996;62:926–934. doi: 10.1097/00007890-199610150-00010. [DOI] [PubMed] [Google Scholar]
- 154.Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl. 2006;12:850–858. doi: 10.1002/lt.20690. [DOI] [PubMed] [Google Scholar]
- 155.Winston DJ, Pakrasi A, Busuttil RW. Prophylactic fluconazole in liver transplant recipients. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:729–737. doi: 10.7326/0003-4819-131-10-199911160-00003. [DOI] [PubMed] [Google Scholar]
- 156.Fortún J, Martín-Davila P, Moreno S, Barcena R, de Vicente E, Honrubia A, García M, Nuño J, Candela A, Uriarte M, et al. Prevention of invasive fungal infections in liver transplant recipients: the role of prophylaxis with lipid formulations of amphotericin B in high-risk patients. J Antimicrob Chemother. 2003;52:813–819. doi: 10.1093/jac/dkg450. [DOI] [PubMed] [Google Scholar]
- 157.Hadley S, Huckabee C, Pappas PG, Daly J, Rabkin J, Kauffman CA, Merion RM, Karchmer AW. Outcomes of antifungal prophylaxis in high-risk liver transplant recipients. Transpl Infect Dis. 2009;11:40–48. doi: 10.1111/j.1399-3062.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 158.Hashemizadeh Z, Badiee P, Malekhoseini SA, Raeisi Shahraki H, Geramizadeh B, Montaseri H. Observational Study of Associations between Voriconazole Therapeutic Drug Monitoring, Toxicity, and Outcome in Liver Transplant Patients. Antimicrob Agents Chemother. 2017:61. doi: 10.1128/AAC.01211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ullmann AJ. Review of the safety, tolerability, and drug interactions of the new antifungal agents caspofungin and voriconazole. Curr Med Res Opin. 2003;19:263–271. doi: 10.1185/030079903125001884. [DOI] [PubMed] [Google Scholar]
- 160.Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol. 2014;20:6211–6220. doi: 10.3748/wjg.v20.i20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.O'Leary JG, Reddy KR, Wong F, Kamath PS, Patton HM, Biggins SW, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:753–759.e1-2. doi: 10.1016/j.cgh.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fischer SA, Lu K; AST Infectious Diseases Community of Practice. Screening of donor and recipient in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:9–21. doi: 10.1111/ajt.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferrarese A, Zanetto A, Becchetti C, Sciarrone SS, Shalaby S, Germani G, Gambato M, Russo FP, Burra P, Senzolo M. Management of bacterial infection in the liver transplant candidate. World J Hepatol. 2018;10:222–230. doi: 10.4254/wjh.v10.i2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Santoro-Lopes G, de Gouvêa EF. Multidrug-resistant bacterial infections after liver transplantation: an ever-growing challenge. World J Gastroenterol. 2014;20:6201–6210. doi: 10.3748/wjg.v20.i20.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–G685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fukui H. Gut Microbiome-based Therapeutics in Liver Cirrhosis: Basic Consideration for the Next Step. J Clin Transl Hepatol. 2017;5:249–260. doi: 10.14218/JCTH.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Taur Y. Intestinal microbiome changes and stem cell transplantation: Lessons learned. Virulence. 2016;7:930–938. doi: 10.1080/21505594.2016.1250982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dirchwolf M, Podhorzer A, Marino M, Shulman C, Cartier M, Zunino M, Paz S, Muñoz A, Bocassi A, Gimenez J, et al. Immune dysfunction in cirrhosis: Distinct cytokines phenotypes according to cirrhosis severity. Cytokine. 2016;77:14–25. doi: 10.1016/j.cyto.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 169.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Egli A, Osthoff M, Goldenberger D, Halter J, Schaub S, Steiger J, Weisser M, Frei R. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) directly from positive blood culture flasks allows rapid identification of bloodstream infections in immunosuppressed hosts. Transpl Infect Dis. 2015;17:481–487. doi: 10.1111/tid.12373. [DOI] [PubMed] [Google Scholar]
- 171.Sato A, Kaido T, Iida T, Yagi S, Hata K, Okajima H, Takakura S, Ichiyama S, Uemoto S. Bundled strategies against infection after liver transplantation: Lessons from multidrug-resistant Pseudomonas aeruginosa. Liver Transpl. 2016;22:436–445. doi: 10.1002/lt.24407. [DOI] [PubMed] [Google Scholar]
- 172.Abbo LM, Ariza-Heredia EJ. Antimicrobial stewardship in immunocompromised hosts. Infect Dis Clin North Am. 2014;28:263–279. doi: 10.1016/j.idc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 173.Aitken SL, Palmer HR, Topal JE, Gabardi S, Tichy E. Call for antimicrobial stewardship in solid organ transplantation. Am J Transplant. 2013;13:2499. doi: 10.1111/ajt.12364. [DOI] [PubMed] [Google Scholar]
- 174.So M, Yang DY, Bell C, Humar A, Morris A, Husain S. Solid organ transplant patients: are there opportunities for antimicrobial stewardship? Clin Transplant. 2016;30:659–668. doi: 10.1111/ctr.12733. [DOI] [PubMed] [Google Scholar]
- 175.van Duin D, van Delden C; AST Infectious Diseases Community of Practice. Multidrug-resistant gram-negative bacteria infections in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:31–41. doi: 10.1111/ajt.12096. [DOI] [PubMed] [Google Scholar]
- 176.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 177.Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, et al. A Multinational, Preregistered Cohort Study of β-Lactam/β-Lactamase Inhibitor Combinations for Treatment of Bloodstream Infections Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2016;60:4159–4169. doi: 10.1128/AAC.00365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, Vazquez J, Zaitsev V, Bidair M, Chorvat E, et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. JAMA. 2018;319:788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huntington JA, Sakoulas G, Umeh O, Cloutier DJ, Steenbergen JN, Bliss C, Goldstein EJ. Efficacy of ceftolozane/tazobactam versus levofloxacin in the treatment of complicated urinary tract infections (cUTIs) caused by levofloxacin-resistant pathogens: results from the ASPECT-cUTI trial. J Antimicrob Chemother. 2016;71:2014–2021. doi: 10.1093/jac/dkw053. [DOI] [PubMed] [Google Scholar]
- 180.Walkty A, DeCorby M, Lagacé-Wiens PR, Karlowsky JA, Hoban DJ, Zhanel GG. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 study) Antimicrob Agents Chemother. 2011;55:2992–2994. doi: 10.1128/AAC.01696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]