Abstract
AIM
To evaluate waiting list (WL) registration and liver transplantation (LT) rates in patients with hepatitis C virus (HCV)-related cirrhosis since the introduction of direct-acting antivirals (DAAs).
METHODS
All adult patients with cirrhosis listed for LT at Padua University Hospital between 2006-2017 were retrospectively collected using a prospectively-updated database; patients with HCV-related cirrhosis were divided by indication for LT [dec-HCV vs HCV/ hepatocellular carcinoma (HCC)] and into two interval times (2006-2013 and 2014-2017) according to the introduction of DAAs. For each patient, indications to LT, severity of liver dysfunction and the outcome in the WL were assessed and compared between the two different time periods. For patients receiving DAA-based regimens, the achievement of viral eradication and the outcome were also evaluated.
RESULTS
One thousand one hundred and ninty-four [male (M)/female (F): 925/269] patients were included. Considering the whole cohort, HCV-related cirrhosis was the main etiology at the time of WL registration (490/1194 patients, 41%). HCV-related cirrhosis significantly decreased as indication to WL registration after DAA introduction (from 43.3% in 2006-2013 to 37.2% in 2014-2017, P = 0.05), especially amongst dec-HCV (from 24.2% in 2006-2013 to 15.9% in 2014-2017, P = 0.007). Even HCV remained the most common indication to LT over time (289/666, 43.4%), there was a trend towards a decrease after DAAs introduction (from 46.3% in 2006-2013 to 39% in 2014-2017, P = 0.06). HCV patients (M/F: 43/11, mean age: 57.7 ± 8 years) who achieved viral eradication in the WL had better transplant-free survival (log-rank test P = 0.02) and delisting rate (P = 0.002) than untreated HCV patients.
CONCLUSION
Introduction of DAAs significantly reduced WL registrations for HCV related cirrhosis, especially in the setting of decompensated cirrhosis.
Keywords: Liver transplantation, Hepatitis C, Cirrhosis, Sustained virological response
Core tip: All-oral direct-acting antivirals significantly modify the natural history of hepatitis C virus (HCV) infection. According to our study, liver transplantation for HCV decompensated cirrhosis will decrease in the next future.
INTRODUCTION
Hepatitis C virus (HCV)-related liver disease has been the most common indication for liver transplantation (LT) in Western Europe for the last 20 years[1]. In Italy, due to the high prevalence of HCV in the general population[2,3], HCV-related decompensated cirrhosis and hepatocellular carcinoma (HCC) have been the main indications for LT to date[4]. The scenario of HCV treatment has evolved rapidly since 2011, due to the approval of oral direct-acting antiviral agents (DAAs). The first oral interferon-free drug available worldwide, sofosbuvir, was registered in Italy in 2014[5]. Since then, several highly-effective and well-tolerated DAA regimens have been approved, with real-life data confirming the high sustained virological response (SVR) rates seen in the registration trials, even in the setting of end-stage liver disease[6-8]. Improvement in liver function, as measured by Child-Turcotte-Pugh (CTP) and MELD scores, are reported in nearly two in three decompensated cirrhotic patients treated with DAAs[9,10]. These safe and effective new antiviral therapies will probably lead to a reduction of waiting list (WL) registrations due to HCV[11,12]. However, the role of DAAs in the Italian scenario has not been explored yet.
Therefore, the aims of our study were to evaluate the changing rates of waiting list (WL) registrations and LT for patients with HCV-related cirrhosis, with or without HCC, after introduction of DAAs.
MATERIALS AND METHODS
All patients registered on the WL for LT at Padua University Hospital between January 2006 and December 2017 were assessed. After WL registration, patients’ data were collected prospectively in an electronic database.
Patients with acute liver failure, with indications for LT other than cirrhosis, re-LT, or patients younger than 18 years old were excluded from the study. Patients were divided into two main groups according to indication to WL registration: patients with HCC and compensated liver disease (e.g. MELD < 15; HCC-group), and patients with decompensated disease, independently from presence of HCC (e.g. MELD ≥ 15 or complications of portal hypertension; dec-group). Patients with a combined diagnosis of HCV and other cause of liver disease (e.g., alcohol, autoimmune liver disease) were classified as HCV. Alcohol-related or HBV-related (or HBV/HDV) liver disease were diagnosed according to current guidelines[13], and they were assessed in separate groups. Similarly, all patients with autoimmune, cholestatic liver diseases or less common indications to LT were grouped together. Patients listed for cryptogenic cirrhosis who had a body mass index (BMI) > 30 were classified as metabolic liver disease[14,15], and grouped together with patients having a known diagnosis of non-alcoholic steatohepatitis (NASH). Patients with diagnosis of HCC were received WL prioritization according to previously published policy[16], updated in 2016[16,17]. Patients with HCV-related liver disease were treated from 2014 with DAAs, according to the recommendations of the national regulatory agency[5]. Each patient was strictly followed-up during and after treatment with DAA; only patients with durable clinical improvements after SVR achievement were delisted. Patients were also divided into two different time intervals: pre-DAA period (2006-2013) and post-DAA period (2014-2017).
Outcome data were collected at the latest available follow-up for each patient. Informed consent was obtained, and the study was approved by the local Ethical Committee (Comitato Etico per la Sperimentazione Clinica della Provincia di Padova, n. AOP/1405).
Statistical analysis
Categorical and continuous variables were calculated as frequencies and means ± SD, respectively, and were compared using the Fisher’s test or Student’s t-test, as appropriate. P values < 0.05 were considered statistically significant. Survival analyses were performed using Kaplan-Meier curves (log-rank test). Analyses were performed using SPSS software version 18 (Chicago, IL, United States).
RESULTS
A total of 1469 patients were listed for LT at Padua University Hospital from January 2006 to December 2017. Two hundred seventy-five patients (18.7%) were excluded from the study for the following reasons: 100 (6.8%) patients were younger than 18 years old at WL registration, 87 (5.9%) patients had undergone previous LT, and 88 (6%) patients did not have cirrhosis as indication for LT, leaving 1194 (81.3%) patients for the current analysis.
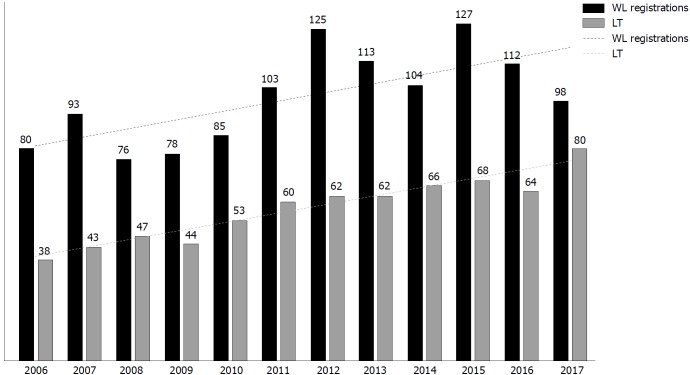
Considering this cohort, overall WL registration rates increased from 94/year in 2006-2013 to 110/year in 2014-2017, with a concomitant rise in the number of LT performed overtime, from 51/year in 2006-2013 to 69.5/year in 2014-2017 (Figure 1).
Figure 1.
Overall trends in waiting list registrations and liver transplantations at Padua University Hospital in the study period. WL: Waiting list; LT: Liver transplantation.
Trajectories of WL registrations for HCV-related disease before and after DAA introduction
Overall, HCV related liver disease (490/1194, 41%), with or without HCC, was the main indication for WL registration over time, followed by alcohol and HBV (24% and 15.5%, respectively). At WL registration, there were no differences between HCV and non-HCV patients in terms of age, gender, BMI, however HCV patients had higher prevalence of HCC (61.8% vs 42%; P = 0.001), and lower MELD score (15 ± 6 vs 16.7 ± 6.8, P = 0.01) compared with non-HCV patients (Table 1).
Table 1.
Characteristics at waiting list registration between hepatitis C virus and non- hepatitis C virus patients
| HCV n = 490 (%) | Non-HCV n = 704 (%) | P value | |
| Age (yr) | 56 ± 7.8 | 55 ± 10 | ns |
| Gender (male) | 393 (80) | 532 (75.5) | ns |
| BMI | 25 ± 4 | 25 ± 4 | ns |
| Blood group | ns | ||
| 0 | 206 (42) | 311 (44.2) | |
| A | 199 (40.6) | 282 (40) | |
| AB | 19 (3.8) | 30 (4.3) | |
| B | 66 (13.4) | 81 (11.5) | |
| HCC (yes) | 303 (61.8) | 295 (42) | 0.001 |
| Child-Pugh classes | 0.04 | ||
| A | 137 (28) | 163 (23) | |
| B | 185 (38) | 252 (36) | |
| C | 168 (34) | 289 (41) | |
| MELD at WL registration | 15 ± 6 | 16.7 ± 6.8 | 0.01 |
HCV: Hepatitis C virus; BMI: Body mass index; HCC: Hepatocellular carcinoma; MELD: Model of End-Stage Liver Disease; WL: Waiting list.
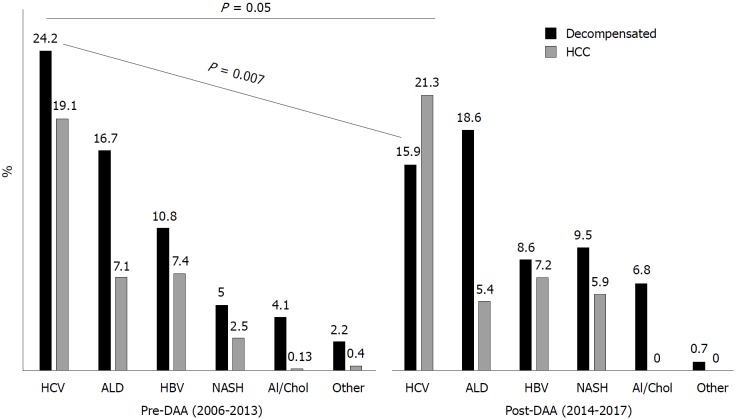
When WL registration rates were compared in the whole cohort between pre-DAA and post-DAA periods, HCV significantly decreased as indication to LT (43.3% in 2006-2013 vs 37.2% in 2014-2017, P = 0.05). Notably, there was a significant drop in the WL registration rates for dec-HCV (from 24.2% in 2006-2013 to 15.9% in 2014-2017, P = 0.007), whereas HCV/HCC remained a stable indication (from 19% in 2006-2013 to 21% in 2014-2017, P = 0.4, Figure 2).
Figure 2.
Trends in waiting list registration before and after direct-acting antiviral introduction. ALD: Alcoholic liver disease; DAA: Direct-acting antivirals; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HCC: Hepatocellular carcinoma; NASH: Non-alcoholic steatohepatitis.
Dec-HCV patients had similar characteristics al WL registration between two different interval time periods, whereas HCV/HCC patients in the last period were older (57 ± 7 years vs 59 ± 6 years, P = 0.03) and had lower CTP score (P = 0.01). The number of patients registered in the WL after achievement of SVR was higher in the post-DAA period, both amongst dec-HCV (7.3% in 2006-2013 vs 24.2% in 2014-2017, P = 0.004) and HCV/HCC (from 7% in 2006-2013 to 25.5% in 2014-2017, P = 0.001). Similarly, there was a significant increase in treatments while on the WL after DAA introduction, both amongst dec-HCV (from 4.9% in 2006-2013 to 20% in 2014-2017, P = 0.009) and HCV/HCC patients (from 4% in 2006-2013 to 26.5% in 2014-2017, P = 0.001) (Table 2).
Table 2.
Characteristics of hepatitis C virus patients at waiting list registration before and after direct-acting antiviral introduction, according to indication to waiting list registration (decompensated liver disease or hepatocellular carcinoma)
|
dec-HCV |
HCV/HCC |
|||||
| Pre-DAA n = 181 (%) | Post-DAA n = 70 (%) | P value | Pre-DAA n = 144 (%) | Post-DAA n = 94 (%) | P value | |
| Gender (male) | 139 (77) | 53 (75.7) | ns | 118 (82) | 82 (87) | ns |
| Age (yr) | 55 ± 8 | 55 ± 9 | ns | 57 ± 7 | 59 ± 6 | 0.03 |
| BMI | 25 ± 3 | 25 ± 4 | ns | 24.7 ± 3 | 25 ± 3.6 | ns |
| Refractory ascites (yes) | 74 (40) | 32 (45.7) | ns | - | - | |
| Blood group | ns | ns | ||||
| 0 | 86 (47.8) | 32 (45.7) | 62 (43) | 35 (39) | ||
| A | 70 (38.4) | 25 (35.7) | 52 (36) | 42 (44) | ||
| AB | 4 (2.2) | 4 (5.7) | 6 (4) | 5 (5) | ||
| B | 21 (11.5) | 9 (12.8) | 24 (17) | 12 (12) | ||
| HCC (yes) | 41 (22.5) | 24 (34.2) | ns | - | - | |
| Comorbidities | ns | ns | ||||
| None | 134 (74.1) | 44 (62.8) | 104 (72.2) | 76 (80) | ||
| HBV | 7 (3.8) | 2 (2.8) | 8 (5.5) | 3 (3.2) | ||
| Alcohol | 34 (18.6) | 19 (27.1) | 26 (18) | 10 (10.6) | ||
| Metabolic | 6 (3.2) | 5 (7.3) | 6 (4.3) | 5 (5.3) | ||
| Child-Pugh classes | ns | 0.01 | ||||
| A | 6 (3.3) | 3 (4.2) | 64 (44.5) | 64 (68) | ||
| B | 67 (37) | 25 (35.7) | 67 (46.5) | 26 (27) | ||
| C | 108 (59.6) | 42 (60) | 13 (9) | 4 (4) | ||
| MELD score | 18.7 ± 6 | 19.3 ± 6 | ns | 11 ± 3.5 | 11 ± 3.4 | ns |
| HCV Genotype1 | ns | ns | ||||
| 1a | 18 (15.1) | 14 (23) | 11 (10.2) | 16 (18.8) | ||
| 1b | 68 (57.1) | 34 (55.7) | 67 (62) | 39 (45.8) | ||
| 2 | 12 (10) | 3 (4.9) | 10 (9.3) | 4 (4.7) | ||
| 3 | 16 (13.4) | 6 (9.8) | 15 (13.9) | 22 (25.8) | ||
| 4 | 5 (3.3) | 4 (6.5) | 5 (4.6) | 4 (4.7) | ||
| SVR achievement | ||||||
| None | 159 (87.8) | 39 (55.7) | 128 (89) | 45 (47.8) | ||
| Before WL registration | 13 (7.1) | 17 (24.2) | 0.004 | 10 (7) | 24 (25.5) | 0.001 |
| IFN based | 13 (7.1) | 2 (2.8) | 10 (7) | 6 (6.3) | ||
| During WL registration | 9 (4.9) | 14 (20) | 0.009 | 6 (4) | 25 (26.5) | 0.001 |
HCV genotype was available in 373 patients at the time of WL registration. HCV: Hepatitis C virus; HCC: Hepatocellular carcinoma; DAA: Direct-acting antivirals; BMI: Body mass index; HBV: Hepatitis B virus; MELD: Model of End-Stage Liver Disease; SVR: Sustained virological response; WL: Waiting list.
Trajectories of LT for HCV-related disease before and after DAA introduction
Overall, HCV related disease remained the first indication to LT over time (43.4%), followed by alcohol and HBV (22.6% and 16%, respectively). At the time of LT, HCV patients were older than non-HCV patients (57 ± 7.5 vs 55 ± 10, P = 0.02), and had lower CTP score (P = 0.03) and MELD score (17 ± 8 vs 18 ± 8, P = 0.01) (Table 3).
Table 3.
Characteristics at the time of liver transplantation between hepatitis C virus and non-hepatitis C virus patients n (%)
| HCV n = 289 | Non-HCV n = 377 | P value | |
| Age (yr) | 57 ± 7.5 | 55 ± 10 | 0.02 |
| Gender (male) | 240 (83) | 288 (76) | 0.04 |
| Blood group | ns | ||
| 0 | 118 (40.8) | 167 (44.2) | |
| A | 124 (43) | 147 (39) | |
| AB | 12 (4.1) | 19 (5) | |
| B | 35 (12.1) | 44 (11.8) | |
| Child-Pugh classes | |||
| A | 82 (25) | 84 (22.2) | |
| B | 99 (36.7) | 116 (30.7) | 0.03 |
| C | 108 (38.2) | 177 (47) | |
| MELD score | 17 ± 8 | 19 ± 8 | 0.01 |
| Waiting time (mo) | 9.6 ± 12 | 10 ± 14 | ns |
HCV: Hepatitis C virus; MELD: Model of End-Stage Liver Disease.
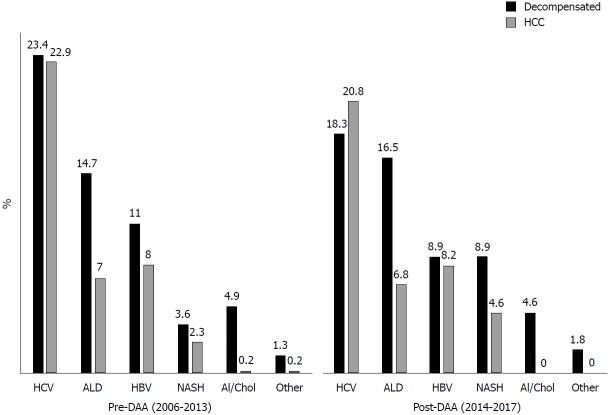
Overall, there a was a trend towards a decrease in LTs for HCV related disease after DAA introduction (from 46.3% in 2006-2013 to 39% of overall LT in 2014-2017, P = 0.06). When HCV patients were stratified according to the main indication to transplantation, LT rates for decompensated disease (from 23.4% in 2006-2013 to 18.3% in 2014-2017, P = 0.1) and for HCC (from 22.9% in 2006-2013 to 20.8% in 2014-2017, P = 0.5) did not significantly change (Figure 3).
Figure 3.
Trends in liver transplantation before and after direct-acting antiviral introduction. ALD: Alcoholic liver disease; DAA: Direct-acting antivirals; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HCC: Hepatocellular carcinoma; NASH: Non-alcoholic steatohepatitis.
Characteristics of dec-HCV patients at time of LT did not differ across the two periods, except for a higher prevalence of HCC at the time of transplant in the post-DAA period (20.8% in 2006-2013 vs 37.3% in 2014-2017, P = 0.04). Similarly, no significant differences were found on characteristics of HCV/HCC patients at time of LT between the two periods. Prevalence of patients transplanted after achievement of SVR was significantly higher in the post-DAA period, both amongst dec-HCV (from 8.8% in 2006-2013 to 29.4% in 2014-2017, P = 0.01) and HCV/HCC (from 11% in 2006-2013 to 41% in 2014-2017, P = 0.0001) (Table 4).
Table 4.
Characteristics of hepatitis C virus patients at liver transplantation before and after direct-acting antiviral introduction, according to indication to waiting list registration (decompensated liver disease or hepatocellular carcinoma) n (%)
|
dec-HCV |
HCV/HCC |
|||||
| Pre-DAA n = 91 | Post-DAA n = 51 | P value | Pre-DAA n = 89 | Post-DAA n = 58 | P value | |
| Gender (male) | 74 (81) | 40 (78.4) | ns | 77 (86.5) | 49 (84.5) | ns |
| Age (yr) | 54.8 ± 8 | 56 ± 8 | ns | 57 ± 7 | 58 ± 7 | ns |
| Refractory ascites (yes) | 37 (40.6) | 27 (53) | ns | - | - | |
| Blood group | ns | ns | ||||
| 0 | 41 (45) | 21 (41.7) | 37 (41.5) | 19 (32.7) | ||
| A | 35 (38) | 22 (43.3) | 39 (43.8) | 28 (54.9) | ||
| AB | 3 (3.3) | 3 (5.8) | 4 (4.5) | 2 (3.4) | ||
| B | 12 (13) | 5 (9.8) | 9 (10.2) | 9 (15.5) | ||
| HCC (yes) | 19 (20.8) | 19 (37.3) | 0.04 | - | - | |
| Child-Pugh classes | ns | 0.001 | ||||
| A | 3 (3.2) | 1 (1.9) | 38 (42.6) | 42 (72.4) | ||
| B | 27 (29.6) | 17 (33.3) | 42 (47.2) | 12 (20.6) | ||
| C | 61 (67.1) | 33 (64.7) | 9 (10) | 4 (6.8) | ||
| MELD at LT | 24 ± 6.5 | 23 ± 7.5 | ns | 11.7 ± 4 | 11.2 ± 3 | ns |
| SVR at time of LT (yes) | 8 (8.8) | 15 (29.4) | 0.002 | 10 (11) | 24 (41) | 0.0001 |
| SVR after IFN-based regimens | 7 (7.6) | 2 (3.9) | 9 (10.1) | 1 (1.7) | ||
| Waiting time (mo) | 8.3 ± 12 | 10.6 ± 12 | ns | 9 ± 7.7 | 10 ± 11 | ns |
HCV: Hepatitis C virus; HCC: Hepatocellular carcinoma; DAA: Direct-acting antivirals; BMI: Body mass index; MELD: Model of End-Stage Liver Disease; LT: Liver transplantation; SVR: Sustained virological response.
Outcome of HCV patients treated with DAA in the setting of LT
Since 2014, 88 out of 164 (53.5%) patients with HCV related cirrhosis were treated with DAA in the setting of LT (Table 5). Thirty-four (20%) were listed after achievement of SVR, whereas 54 (32.9%) were treated while on the WL. The SVR rate after first treatment with DAA was 88%. Ten patients relapsed after treatment with DAA and achieved SVR after a second treatment while they were on the WL. Sofosbuvir-based regimens were mostly used, whereas ribavirin was used in 43% of patients.
Table 5.
Characteristics of hepatitis C virus patients who achieved sustained virological response after treatment with direct-acting antivirals while in the waiting list n (%)
| SVR after DAA before the WL n = 34 | SVR after DAA in the WL n = 54 | |
| Age (yr) | 58 ± 8 | 57.7 ± 8 |
| Gender (male) | 25 (73.5) | 43 (79.6) |
| dec-HCV (yes) | 15 (44) | 44 (44.4) |
| MELD score before treament | - | 13 ± 5 |
| HCV genotype | ||
| 1a | 6 (17.6) | 6 (11) |
| 1b | 17 (50) | 34 (63) |
| 2 | 2 (5.8) | 2 (3.7) |
| 3 | 7 (20.5) | 7 (13) |
| 4 | 2 (5.8) | 5 (9.3) |
| Treatment regimens | ||
| Sofosbuvir | 4 (11.7) | 27 (50) |
| Sofosbuvir/Ledipasvir | 23 (67.6) | 18 (33.3) |
| Sofosbuvir/Daclatasvir | 3 (8.8) | 3 (5.5) |
| Sofosbuvir/Simeprevir | 1 (2.9) | 4 (7.4) |
| Other | 5 (14.7) | 2 (3.7) |
| Ribavirin use (yes) | 1 (2.9) | 37 (68.5) |
| MELD score after treament | 12.3 ± 4 | 13 ± 6 |
| Outcome | ||
| Waiting for LT | 16 (47) | 6 (11) |
| Delisted | 1 (2.9) | 10 (18.5) |
| LT | 10 (29.4) | 28 (51.8) |
| Drop out | 5 (14.7) | 2 (3.7) |
| Dead | 2 (5.8) | 8 (14.8) |
SVR: Sustained virological response; DAA: Direct-acting antivirals; HCV: Hepatitis C virus; MELD: Model of End-Stage Liver Disease; LT: Liver transplantation.
Considering only patients treated while in the WL, 28 (51%) patients underwent LT, whereas 10 (18%) were delisted due to improvement of liver disease, 8 (14.8%) died, and 2 (3.7%) dropped-out due to HCC progression. DAA-treated patients had a higher delisting rate due to improvement of liver disease than untreated HCV patients over time (18% vs 2.5%, P = 0.002), and a better transplant free survival (log-rank P = 0.02).
DISCUSSION
In recent decades, HCV-related cirrhosis has been considered the most prevalent indication for LT in the Western Countries, for both decompensated liver disease and HCC. The introduction of DAAs has significantly modified the natural history of HCV infection, and viral eradication (especially in patients with compensated cirrhosis) has been associated with an improvement of liver function. We consequently explored dynamics of long-term changes in WL registrations and LTs in Our Centre.
Spanning more than a decade, we found that HCV remained the main indication for WL registration not only before, but also after DAA introduction. This might be because the first-generation protease inhibitors were approved in Italy since January 2013[18], but their use was initially limited by side effects and low rates of SVR achievement (less than 50% in cirrhotic patients)[19]. Extended approval of DAA therapies was granted in Italy in 2014, producing a significant drop in the WL registrations for HCV related cirrhosis, moving from 43.3% in the pre-DAA period to 37.2% in 2014-2017. These results are consistent with data recently published by another tertiary center in Italy[20], where the proportion of dec-HCV patients decreased from 49% to 36% in the last years. Two possible explanations may be hypothesized; first, a significant change in HCV epidemiology in Italy in recent years, as shown by several studies[3,21,22], with the highest prevalence of HCV in patients over 70 years old (who are not eligible for LT anymore); second, the achievement of SVR after DAA therapies[23-25], leading to improvement in liver function and reduction of portal-hypertensive complications[26]. Thus, previously decompensated patients, potentially suitable for LT, would therefore not be placed on the WL.
On the contrary, in the setting of HCV related cirrhosis, proportion of WL registration for HCC remained stable after DAA introduction (from 19% in 2006-2013 to 21% in 2014-2017; P = ns). Notably, in the post-DAA period, HCC became the first indication to LT amongst HCV patients. This trend was confirmed also in patients with decompensated disease (e.g. MELD ≥ 15), in whom the prevalence of HCC at the time of transplant rose from 20.8% in 2006-2013 to 37.3% in 2014-2017. Development of HCC in patients previously treated with DAA is debated. Several studies[6,27-29] recently showed that HCC may still occur or recur in patients with SVR achievement after DAA therapy (probably due to changes in the immunological microenvironment after viral clearance), and these patients would probably still need for LT.
In our experience, nearly 18% of HCV patients were delisted after achieving SVR, in a significantly higher proportion than in the pre-DAA period. This reinforces the concept that SVR achievement could significantly improve liver function and increase delisting rate in the next future, even if we’ll expect that HCV patients will be cured before decompensation, without requiring WL registration. Furthermore, our data were consistent with those reported by Belli et al[30], who reported a delisting rate of 20% (though they only considered HCV-related decompensated cirrhosis patients without HCC).
Our study retrospectively collected all data regarding cirrhotic patients from WL registration to final outcome, using a prospectively updated electronic database. There were no significant changes in WL and LT policies during the study period, and the antiviral treatments were administered under the supervision of a tertiary center, in accordance with the changing criteria recommended by the Italian Regulatory Agency. This study has some limitations that need to be acknowledged, however. It was based on a single-center cohort and covered a lengthy period of time. These data, coming from a single-center cohort, might not be extended to the whole Italian scenario, due to its heterogenous epidemiology in terms of etiologies of liver disease. However, recently published data from Northern Italy seemed to be in accordance with our results[20]. Furthermore, criteria for NASH diagnosis significantly changed over, so we included patients with cryptogenic cirrhosis and a BMI > 30, as other Authors had done[15]. Lastly, we were assessing trends of the WL for LT, and some misdiagnosed comorbidities may have interfered with the natural history of several patients.
In conclusion, our study showed the significant changes occurring in the LT scenario. Even if DAA therapies have significantly contributed to a decrease in the WL registration for dec-HCV, it will take more time for HCV to disappear as an indication for liver transplantation, especially in the setting of LT for HCC.
ARTICLE HIGHLIGHTS
Research background
Hepatitis C virus (HCV)-related cirrhosis has been the first indication for liver transplantation in Western Countries in the last decades. Introduction of all-oral direct-acting antivirals (DAAs) significantly modified the natural history of HCV related liver disease.
Research motivation
Our study aimed at evaluating the change in waiting list registrations and in liver transplantation for HCV related cirrhosis after DAAs introduction.
Research objectives
To evaluate the outcome of patients with HCV related cirrhosis, listed for liver transplantation at Padua University Hospital between 2006 and 2017. Patients were further divided according to two different time periods (2006-2013 vs 2014-2017) and according to indication to liver transplantation (decompensated disease vs hepatocellular carcinoma).
Research methods
The outcome of patients listed for liver transplantation (LT) for HCV related cirrhosis was retrospectively analysed using a prospectively updated database.
Research results
After DAAs introduction, HCV-related cirrhosis significantly decreased as indication to waiting list registration, especially among patients with decompensated disease. Considering liver transplantation, even HCV remained the most common indication to LT over time (289/666, 43.4%), there was a trend towards a decrease in the last time period (2013-2017). Furthermore, HCV patients who achieved viral eradication had better transplant-free survival than untreated HCV patients.
Research conclusions
The study demonstrated that HCV related cirrhosis might be a decreasing indication to liver transplantation, especially for decompensated liver disease. Viral eradication achieved with DAA-based regimens should reduce decompensation rates and need to LT. This study confirmed what already known in literature about the beneficial role provided by DAAs in patients with HCV related cirrhosis. Viral eradication obtained after DAAs- based regimens should reduce decompensation rates amongst patients with HCV related cirrhosis. Future studies are needed to confirm the changing scenario regarding indications to LT in Western countries.
Research perspectives
Viral eradication obtained after DAA therapy should reduce decompensation rates amongst patients with HCV related cirrhosis. To further investigate trends in waiting list registrations and liver transplantations for HCV related cirrhosis, especially in the setting of hepatocellular carcinoma. Larger, multicentre, prospective studies are the best methods for the future research.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was approved by all members of the Local Ethical Committee “Comitato Etico per la Sperimentazione Clinica della Provincia di Padova” (protocol n. 4466/AO/18; code AOP1405).
Informed consent statement: Informed consent protocol was approved by the Local Ethical Committee. Informed consent was collected and signed by all patients included in the study.
Conflict-of-interest statement: All the Authors declare no conflict of interest regarding this paper.
Data sharing statement: Individual de-identified participant data have been anonymously collected.
STROBE statement: The manuscript has been prepared according to the STROBE statement.
Peer-review started: June 7, 2018
First decision: July 11, 2018
Article in press: August 1, 2018
P- Reviewer: Kamimura K S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Alberto Ferrarese, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Giacomo Germani, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Martina Gambato, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Francesco Paolo Russo, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Marco Senzolo, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Alberto Zanetto, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Sarah Shalaby, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Umberto Cillo, Hepatobiliary Surgery and Liver Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Giacomo Zanus, Hepatobiliary Surgery and Liver Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Paolo Angeli, Internal Medicine, Department of Medicine, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy.
Patrizia Burra, Multivisceral Transplant Unit, Department of Surgery, Oncology and Gastroenterology, Padua University Hospital, via Giustiniani 2, Padua 35128, Italy. burra@unipd.it.
References
- 1.Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A; All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–688. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Scognamiglio P, Piselli P, Fusco M, Pisanti FA, Serraino D, Ippolito G, Girardi E; Collaborating Study Group. Declining unawareness of HCV-infection parallel to declining prevalence in Southern Italy. J Med Virol. 2017;89:1691–1692. doi: 10.1002/jmv.24840. [DOI] [PubMed] [Google Scholar]
- 3.Morisco F, Loperto I, Stroffolini T, Lombardo FL, Cossiga V, Guarino M, De Feo A, Caporaso N. Prevalence and risk factors of HCV infection in a metropolitan area in southern Italy: Tail of a cohort infected in past decades. J Med Virol. 2017;89:291–297. doi: 10.1002/jmv.24635. [DOI] [PubMed] [Google Scholar]
- 4.Angelico M, Cillo U, Fagiuoli S, Gasbarrini A, Gavrila C, Marianelli T, Costa AN, Nardi A, Strazzabosco M, Burra P, et al. Liver Match, a prospective observational cohort study on liver transplantation in Italy: study design and current practice of donor-recipient matching. Dig Liver Dis. 2011;43:155–164. doi: 10.1016/j.dld.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Agenzia Italiana del Farmaco. Available from: http://www.agenziafarmaco.gov.it.
- 6.Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WTH, MacDonald DC, Agarwal K, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Terrault NA, McCaughan GW, Curry MP, Gane E, Fagiuoli S, Fung JYY, Agarwal K, Lilly L, Strasser SI, Brown KA, et al. International Liver Transplantation Society Consensus Statement on Hepatitis C Management in Liver Transplant Candidates. Transplantation. 2017;101:945–955. doi: 10.1097/TP.0000000000001708. [DOI] [PubMed] [Google Scholar]
- 8.D’Ambrosio R, Degasperi E, Colombo M, Aghemo A. Direct-acting antivirals: the endgame for hepatitis C? Curr Opin Virol. 2017;24:31–37. doi: 10.1016/j.coviro.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Fernández Carrillo C, Lens S, Llop E, Pascasio JM, Crespo J, Arenas J, Fernández I, Baliellas C, Carrión JA, de la Mata M, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: Analysis of data from the Hepa-C registry. Hepatology. 2017;65:1810–1822. doi: 10.1002/hep.29097. [DOI] [PubMed] [Google Scholar]
- 10.Chhatwal J, Samur S, Bethea ED, Ayer T, Kanwal F, Hur C, Roberts MS, Terrault N, Chung RT. Transplanting hepatitis C virus-positive livers into hepatitis C virus-negative patients with preemptive antiviral treatment: A modeling study. Hepatology. 2018;67:2085–2095. doi: 10.1002/hep.29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. doi: 10.1002/hep.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 15.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Cillo U, Vitale A, Grigoletto F, Gringeri E, D’Amico F, Valmasoni M, Brolese A, Zanus G, Srsen N, Carraro A, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7:972–981. doi: 10.1111/j.1600-6143.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 17.Cillo U, Burra P, Mazzaferro V, Belli L, Pinna AD, Spada M, Nanni Costa A, Toniutto P; I-BELT (Italian Board of Experts in the Field of Liver Transplantation) A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model”. Am J Transplant. 2015;15:2552–2561. doi: 10.1111/ajt.13408. [DOI] [PubMed] [Google Scholar]
- 18.Ascione A. Boceprevir in chronic hepatitis C infection: a perspective review. Ther Adv Chronic Dis. 2012;3:113–121. doi: 10.1177/2040622312441496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viganò R, Mazzarelli C, Alberti AB, Perricone G. Change of liver transplantation list composition: Pre versus post direct-acting antivirals era. The Niguarda Hospital experience. Dig Liver Dis. 2017;49:317. doi: 10.1016/j.dld.2017.01.145. [DOI] [PubMed] [Google Scholar]
- 21.Stroffolini T, Sagnelli E, Gaeta GB, Sagnelli C, Andriulli A, Brancaccio G, Pirisi M, Colloredo G, Morisco F, Furlan C, et al. Characteristics of liver cirrhosis in Italy: Evidence for a decreasing role of HCV aetiology. Eur J Intern Med. 2017;38:68–72. doi: 10.1016/j.ejim.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Gardini I, Bartoli M, Conforti M, Mennini FS, Marcellusi A, Lanati E. HCV - Estimation of the number of diagnosed patients eligible to the new anti-HCV therapies in Italy. Eur Rev Med Pharmacol Sci. 2016;20:7–10. [PubMed] [Google Scholar]
- 23.Chhatwal J, Samur S, Kues B, Ayer T, Roberts MS, Kanwal F, Hur C, Donnell DM, Chung RT. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017;65:777–788. doi: 10.1002/hep.28926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curry MP, Charlton M. Sofosbuvir and Velpatasvir for Patients with HCV Infection. N Engl J Med. 2016;374:1688. doi: 10.1056/NEJMc1601160. [DOI] [PubMed] [Google Scholar]
- 25.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 26.Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, et al. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273–1283.e1. doi: 10.1053/j.gastro.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WT, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Reig M, Boix L, Mariño Z, Torres F, Forns X, Bruix J. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Semin Liver Dis. 2017;37:109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 30.Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, Morelli C, Donato F, Volpes R, Pageaux GP, Coilly A, Fagiuoli S, Amaddeo G, Perricone G, Vinaixa C, Berlakovich G, Facchetti R, Polak W, Muiesan P, Duvoux C; European Liver and Intestine Association (ELITA) Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524–531. doi: 10.1016/j.jhep.2016.05.010. [DOI] [PubMed] [Google Scholar]