History and clinical signs
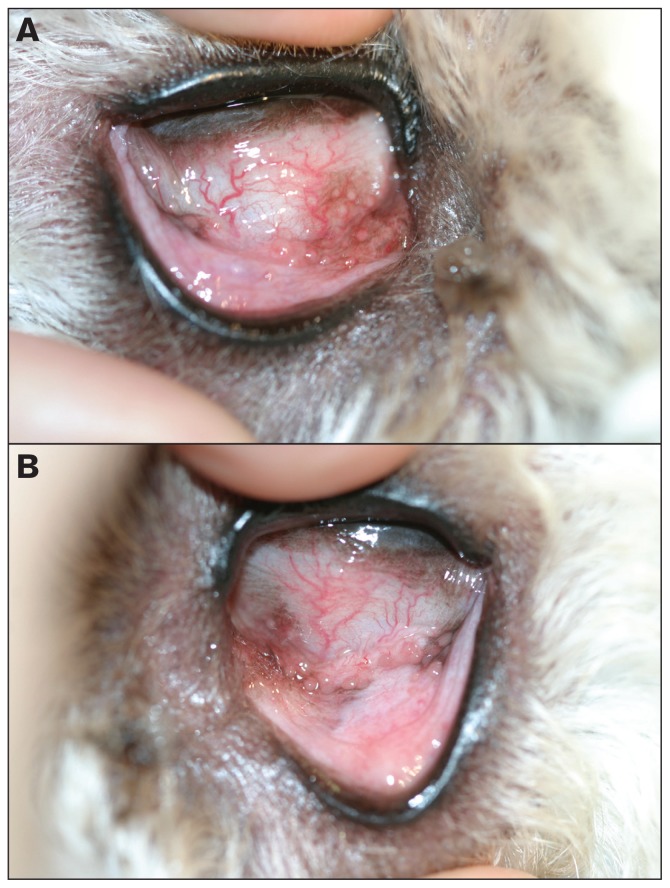
A 2-year-old spayed female golden doodle was examined by the ophthalmology service at the Western College of Veterinary Medicine. This dog was presented for red eyes and bilateral ocular discharge of a few weeks’ duration. The menace responses, and palpebral, oculocephalic, direct and consensual pupillary light reflexes were normal bilaterally. Schirmer tear test (Schirmer Tear Test Strips; Alcon Canada, Mississauga, Ontario) values were 26 and 20 mm/min in the right and left eyes, respectively. The intraocular pressures were estimated with a rebound tonometer (Tonovet; Tiolat, Helsinki, Finland) and were 14 and 12 mmHg in the right and left eyes, respectively. Fluorescein staining (Fluorets; Bausch & Lomb Canada, Markham, Ontario) of the corneas was negative bilaterally. On direct examination of both eyes there was mild, gray mucoid periocular discharge. Following application of 0.5% tropicamide (Mydriacyl; Alcon Canada, Mississauga, Ontario), examination of both eyes using a transilluminator (Welch Allyn Finoff Transilluminator; Welch Allyn, Mississauga, Ontario) and handheld biomicroscope (Kowa SL-15 Portable Slit Lamp; Kowa Co, Tokyo, Japan) revealed mild to moderate conjunctival hyperemia and multiple hyperplastic conjunctival follicles particularly in the ventromedial fornix. Indirect ophthalmoscopic (Heine Omega 200; Heine Instruments Canada, Kitchener, Ontario) examination was completed and did not reveal abnormalities in either eye. Photographs of the right and left conjunctival fornices at presentation are provided for your assessment (Figure 1).
Figure 1.
Clinical photographs of the right (A) and left (B) conjunctival fornices at presentation.
What are your clinical diagnoses, differential diagnoses, therapeutic plan, and prognosis?
Discussion
The clinical diagnosis is bilateral follicular conjunctivitis. Follicular conjunctivitis occurs secondary to chronic antigenic stimulation. Numerous characteristic-looking semitransparent conjunctival follicles are found which histopathologically represent aggregates of hyperplastic lymphocytes and plasma cells. A true hypersensitivity or allergic reaction is excluded based on the absence of mast cells and eosinophils. Conjunctival hyperemia and mucoid ocular discharge may also be part of the clinical presentation. The condition is most commonly diagnosed in younger dogs aged 2 y or less, and most often in dolichocephalic breeds that have deep conjunctival pockets owing to their deeper orbits (1).
The canine ocular surface has evolved an intricate mucosal immune system in order to respond to environmental and microbial affronts, while simultaneously tolerating self-antigens and commensal microflora. Similar to other mucosal sites in the body, the conjunctiva has conjunctival associated lymphoid tissue (CALT). In canine conjunctival lymphoid follicles, antigens are taken up by follicle-associated epithelium via M-cells in order to incite the production and activity of effector cells (lymphocytes and plasma cells) (1,2). These conjunctival lymphoid aggregates can be seen in non-disease states and are identified most commonly on the bulbar surface of the third eyelid in close association with the gland of the third eyelid. In follicular conjunctivitis, lymphocyte and plasma cell populations differentiate and proliferate such that these follicles appear larger and in greater number, and can also be found anywhere on the conjunctival surface.
Unlike cats and horses, dogs are a species that have few types of primary conjunctivitis. In dogs, the term conjunctivitis is most often a misnomer as most cases of “red eyes” are in fact conjunctival injection or hyperemia occurring secondary to more common diseases affecting other ocular tissues (i.e., keratitis, uveitis, orbital disease, glaucoma, scleritis). For example, conjunctivitis associated with quantitative and qualitative tear film deficiencies, and lymphoplasmacytic infiltration of the third eyelid in dogs with pannus, are not primary conjunctivitis disorders but rather reflect the secondary effects of a primary ocular disease process. For this reason, it is important for practitioners to complete a comprehensive ophthalmic examination to exclude more likely ocular diseases, before diagnosing primary conjunctivitis in dogs. Differentials for primary conjunctivitis in dogs are few and include infectious conjunctivitis (viral such as canine herpes virus-1 and distemper virus, parasitic such as Thelazia), contact hypersensitivity conjunctivitis, and ligneous conjunctivitis of the Doberman pinscher (3–6).
Therapy for follicular conjunctivitis is symptomatic and includes regular irrigation of the conjunctival pockets using an eye wash or saline solution and/or short-term topical ophthalmic corticosteroid application q6h. The dog in this case received twice daily (q12h) irrigation of the conjunctival pockets of both eyes and topical dexamethasone 0.1% (Maxidex ophthalmic suspension; Alcon, Mississauga, Ontario) in both eyes starting at q6h, tapering down the frequency over 6 wk. Clinical signs resolved completely and have not recurred. Occasionally, gentle debridement of the conjunctival follicles with a dry cotton tip applicator can aid in improved penetration of topical medication and full resolution for less responsive cases.
The prognosis following these simple therapies is excellent. Most dogs will not have recurrence of clinical signs, especially as they age. However, some dogs may require long-term therapy via regular lavage of their conjunctival fornices to reduce antigen exposure or through the intermittent use of topical corticosteroid (1,6).
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Gelatt KN, Gilger BC, Kern TJ. Veterinary Ophthalmology. 2-volume set. Ames, Iowa: Wiley; 2013. [Google Scholar]
- 2.Giuliano EA, Moore CP, Phillips TE. Morphological evidence of M cells in healthy canine conjunctiva-associated lymphoid tissue. Graefes Arch Clin Exp Ophthalmol. 2002;240:220–226. doi: 10.1007/s00417-002-0429-3. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey DT, Ketring KL, Glaze MB, Knight B, Render JA. Ligneous conjunctivitis in four Doberman pinschers. J Am Anim Hosp Assoc. 1996;32:439–447. doi: 10.5326/15473317-32-5-439. [DOI] [PubMed] [Google Scholar]
- 4.Gervais KJ, Pirie CG, Ledbetter EC, Pizzerani S. Acute primary canine herpesvirus-1 dendritic ulcerative keratitis in an adult dog. Vet Ophthalmol. 2012;15:133–138. doi: 10.1111/j.1463-5224.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 5.Lia RP, Traversa D, Agostini A, Otranto D. Field efficacy of moxidectin 1 per cent against Thelazia callipaeda in naturally infected dogs. Vet Rec. 2004;154:143–145. doi: 10.1136/vr.154.5.143. [DOI] [PubMed] [Google Scholar]
- 6.Glaze MB. Ocular allergy. Semin Vet Med Surg (Small Anim) 1991;6:296–302. [PubMed] [Google Scholar]