Otitis externa is a common complaint in veterinary medicine. In general practice, the percentage of canine cases presenting for otitis externa ranges from 7.5% to 16.5% (1). Otitis externa is defined as inflammation of the external ear canal consisting of the pinna, and the vertical and horizontal ear canals up to the level of the tympanum. In many instances of otitis externa, secondary infection with bacteria or a fungus such as Malassezia pachydermatis, will be present. Pseudomonas aeruginosa, Staphylococcus pseudintermedius, Escherichia coli, and Klebsiella spp. are among the most common bacterial pathogens in cases of infectious otitis externa, with Pseudomonas aeruginosa being the most common Gram-negative isolate (1,2). Pseudomonas aeruginosa is a ubiquitous Gram-negative bacillus found in soil, water, and decaying organic matter. It is not a normal inhabitant of the canine ear and when it leads to infection, it can be challenging to manage (1,2). Selecting antibiotics for treatment can be problematic due to the bacterium’s resistance to many classes of antibiotics, and treatment is further complicated by the growing number of multidrug resistant strains (3,4).
Clinical presentation
Previous reports indicate that younger individuals are more prone to development of otitis externa and these same studies document no gender predilection (5). However, breed predispositions do exist and breeds such as cocker spaniels, Jura des Alpes, Brittany spaniels, golden retrievers, and West Highland white terriers are overrepresented (5). Some older studies also show that Labrador retrievers, miniature poodles, Afghans, Scottish terriers, fox terriers, Maltese, and German shepherds are also predisposed (1).
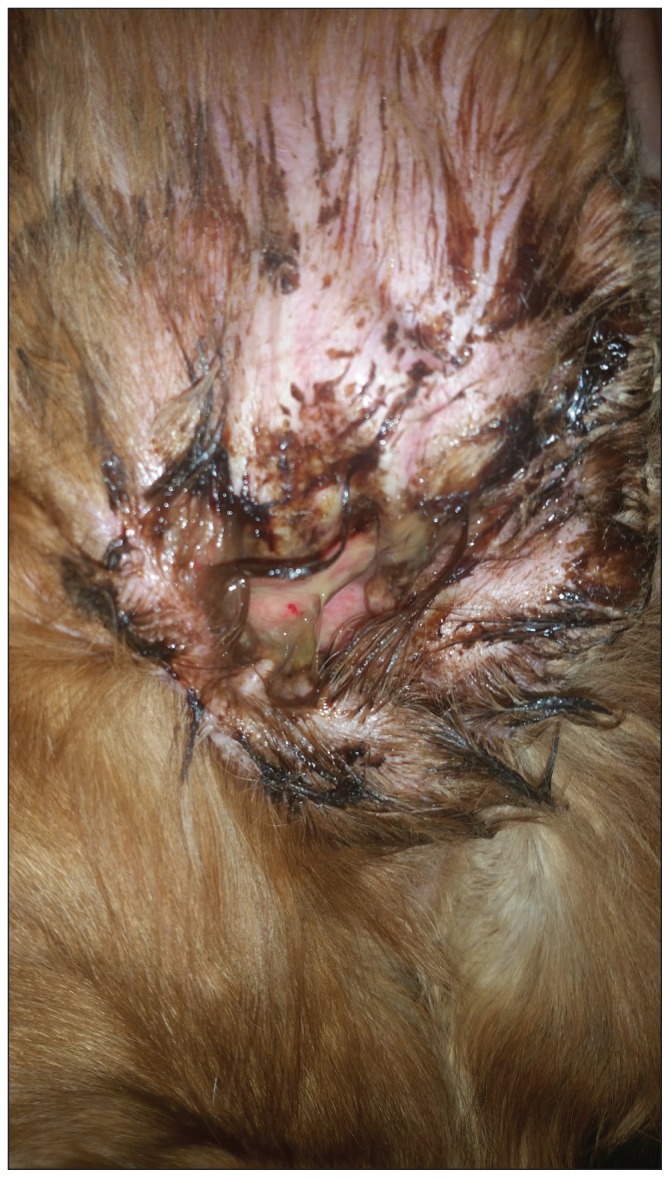
Dogs presenting with Pseudomonas otitis externa (Figure 1) may exhibit one or a combination of the following clinical signs: head shaking, aural pruritus, malodor from the ear, erythema, alopecia, signs of self trauma to the pinnae and pre-auricular region, shyness around the head, discharge from the ear canal, aural hematoma, and ulceration of the external ear canal (1,2). If the infection has extended deeper into the middle or inner ear, causing an otitis media or otitis interna, neurological signs such as head tilt, vestibular disease, hearing loss, and pain when opening the mouth or swallowing might also be present (2). Patients may display pain on palpation of the ear canal if the canal is thickened, firm, and less pliable following chronic disease. This leads to a more guarded prognosis as it is indicative of proliferative changes to the canal (1,2). The vertical and horizontal canals can also mineralize and will be hard upon palpation. In these cases surgery is often required (2). In any case of otitis externa, it is of utmost importance to perform a thorough dermatologic examination of the patient as approximately 76% of cases of chronic otitis externa will have other dermatologic lesions (1). The location and type of these lesions can help identify the primary factor in development of the chronic otitis externa.
Figure 1.
Otitis externa caused by Pseudomonas aeruginosa.
(Photograph provided by Dr. Anthony Yu).
Pathogenesis
Otitis externa develops due to primary, predisposing, and perpetuating factors. Primary factors are those that directly induce inflammation within the external ear canal, such as hypersensitivity disorders. In one study, the most common primary factors leading to otitis externa were: allergies, masses, endocrine disease, and autoimmune disease (6). Predisposing factors such as moisture act to increase the risk of development of otitis externa. Perpetuating factors such as otitis media occur following the development of otitis externa (1,2,6,7). Infectious agents have historically been categorized as perpetuating factors in otitis externa but are more recently being re-classified as secondary causes of otitis externa. Secondary causes will only lead to pathology in an abnormal ear or in combination with predisposing factors (1). In a study by Paterson et al (6), secondary infections with Pseudomonas were found to develop more quickly if there was a mass or autoimmune disease compared with allergies and endocrinopathies.
One virulence factor contributing to the ability of Pseudomonas to cause chronic otitis is its ability to form biofilms. Biofilms increase antimicrobial resistance by protecting the bacteria from the immune system and by preventing penetration of antimicrobials. Pseudomonas aeruginosa isolates from cases of canine otitis externa have been documented to form biofilms in about 40% of cases, and biofilm formation increases the minimum inhibitory concentration of antimicrobials needed to treat the infection (8).
Diagnosis
As for any dermatologic disease, obtaining a thorough history is the first step in diagnosis. An accurate history can not only help determine if an otitis externa may be present, but can also help identify the primary factor in development of otitis.
Otoscopic examination will help the clinician identify if the tympanum is intact, the type of lesions and discharge within the external ear canal, and whether the canal is stenotic. Otoscopic examination can also help rule out the presence of a foreign body. Both ears should be examined starting with the unaffected ear if the otitis externa is unilateral; this allows for comparison between the 2 ears. Separate cones should be used for each ear or the cone should be disinfected between use in the ears to prevent transmission of infection. Sedation or anesthesia might be required in a patient which is in pain to allow for a thorough otoscopic examination. Alternatively, if the patient is in pain, or the canal is stenotic, 7 to 14 days of anti-inflammatory therapy with an oral glucocorticoid such as prednisone or dexamethasone can precede the otoscopic examination. This will decrease inflammation and open up the ear canal to allow better visualization.
Cytology is the most valuable test to help confirm the presence of infectious otitis externa. Cotton-tipped swabs should be inserted into the ear to the level of the vertical and horizontal canal, or where a lesion is noted. The sample taken can then be smeared onto a glass slide, heat-fixed, stained with Diff-Quik stain (Baxter Canada, Alliston, Ontario), and viewed under the microscope. The presence of rod-shaped bacteria should increase the suspicion of Pseudomonas as the infectious agent responsible for the otitis externa. White blood cells may also be noted in the sample. The presence of these cells, especially if phagocytosis of bacteria is noted, is indicative of a response from the individual’s immune system to the infection (1). As Pseudomonas and other rod-shaped bacteria are not commensals of the canine external ear canal, any time rods are noted on cytology, this indicates an infection is present and treatment is warranted.
An aerobic bacterial culture and susceptibility is required if systemic antibiotics are to be prescribed for a case of otitis externa. A culture should never be performed without concurrent cytology verifying bacteria are present within the ear canal. Most cases of otitis externa can be adequately treated using topical antimicrobials and selection of these can be based on cytology findings. Culture and susceptibility tests show the serum level of a drug required to kill the organism. Concentrations of topically administered antimicrobials often reach levels that exceed those achieved in the circulation (9). Previous studies have shown that clinical response to topically applied antibiotics does not correlate with antimicrobial susceptibility results (10). If the canal is not ulcerated or eroded, systemic antibiotics are unlikely to reach therapeutic concentrations in the ear canal (9). However, if ulceration is present along the external ear canals, as if often the case with Pseudomonas, systemic antimicrobials will penetrate the ear canal more readily. If systemic antibiotics are to be prescribed, this should be done following an aerobic bacterial culture and susceptibility. This culture will definitively identify the species of bacteria and will allow identification of the susceptibility patterns of that organism which can guide antibiotic selection. Pseudomonas are inherently resistant to many antimicrobials due to their low cell permeability, beta lactamases, and efflux pumps. They can develop resistance easily if treatment is ineffective, if treatment is too short, or if an inappropriate antibiotic is selected (6). Pseudomonas is often susceptible to oral fluoroquinolones, but if the bacterium becomes resistant to this family of antibiotics, other systemic treatment options are often expensive, have to be administered via injection, or are not licensed for the treatment of animals (7).
Treatment
Treatment of Pseudomonas otitis externa revolves around the following: identifying and correcting the primary cause for the otitis externa, removing any debris from the ear canal, treating concurrent infections, and controlling inflammation within the ear/reversing pathological changes in the ear (7,11).
To identify and treat the primary cause of the otitis externa, further questions concerning history may be asked. A thorough dermatological examination should also be undertaken to see whether there are other clinical signs suggestive of an underlying etiology.
Cleaning or flushing of the ear will help to remove any debris, purulent material, or secondary infection in the external ear canal. The type and amount of exudate present will determine which cleaner is most beneficial. If owners are unable to clean the patient’s ear at home, then a thorough flushing of the ear in hospital using sterile saline or an ear cleaner is warranted. This flushing should be performed under general anesthesia. Some topical antimicrobials, such as the aminoglycosides, are inactivated in purulent material; therefore, cleaning/flushing is imperative before their use to allow for better activity of the antimicrobial (7). In Canada, there are no licensed chlorhexidine-based ear cleaners so if the clinician wants to use an antimicrobial ear cleaner, options would include those with a low pH (low levels of acetic or boric acid) or those containing isopropyl alcohol (7). Alcohol-based cleaners can potentially lead to a pain response if ulceration is noted along the ear canal. In previous studies, acetic acid has been found to be most effective against Pseudomonas, especially when used as a 2% solution (12). TrizEDTA will damage bacterial cell walls by chelating minerals in the wall allowing better penetration of topical antimicrobials. The use of TrizEDTA will help increase antimicrobial efficacy which is especially important in cases of Pseudomonas otitis externa. Cleaning with TrizEDTA should be done approximately 15 to 30 minutes before instilling topical antimicrobials and does not appear to be ototoxic (1,7). TrizEDTA has been shown, in vitro, to reduce the minimum inhibitory concentration (MIC) of marbofloxacin and gentamicin for multidrug resistant P. aeruginosa (13). It has also been shown in vitro to reduce the MIC for biofilm imbedded P. aeruginosa for certain antimicrobials (14).
Cytology can aid in empirical selection of an antimicrobial to treat infectious otitis externa. Fluoroquinolones, gentamicin, and polymixin B are usually effective against Pseudomonas (6,9). Ototoxicity has been previously reported with gentamicin instilled topically into the ear canal with a ruptured tympanic membrane. However, one study showed no vestibulotoxic or ototoxic effects when gentamicin was instilled twice daily into ears with ruptured tympanic membranes for a 3-week period (15). This indicates that this ototoxicity might be overemphasized. Most often a first line antimicrobial such as Polymixin B should be selected for acute episodes of Pseudomonas otitis externa, based on cytology and otoscopic findings (7). When using topical medication, it is most important to make sure an adequate volume of the medication is being used. A volume can be recommended based on the dog’s size. For example, in small breed dogs 0.25 mL can be instilled, medium-sized dogs: 0.5 mL, large breed: 0.75 mL, and giant breeds a full 1 mL/ear. Topical medications are used once or twice daily depending on the medication selected (11).
If the otitis externa is chronic or recurrent, proliferative changes will likely be present. In these cases decreasing swelling, tissue proliferation, and inflammation is another goal of therapy. Glucocorticoids (especially dexamethasone), can help reverse the ototoxic effects of Pseudomonas infection (7). Oral dexamethasone dosed at 0.05 mg/kg body weight (BW) every 24 h or oral prednisone dosed at 0.5 to 1 mg/kg BW, q12 to 24h should be sufficient to control inflammation and this dose can be tapered over 2 to 3 weeks pending response and severity of disease (1,7). Cyclosporine can also aid in reducing inflammation and treating chronic otitis externa. Rechecks should occur every 2 to 4 weeks as medications are being tapered to assess response to the treatment protocol. With any oral anti-inflammatory drug, dosage should be decreased over time to the lowest effective maintenance dose that will treat the underlying pathology and the primary factor of the otitis externa. Topical therapy should still be recommended for chronic cases but use of a second line or third line antimicrobial will be warranted. Treatment duration in these cases may be up to 4 weeks.
In cases of chronic otitis externa or where there is concern for otitis media, systemic antibiotics should be prescribed based on bacterial culture and susceptibility results. This systemic treatment should continue for a minimum of 4 weeks.
At the end of the prescribed treatment, cytology should be repeated to verify that there is a cytological cure of the secondary infection leading to otitis externa.
Footnotes
The Canadian Academy of Veterinary Dermatology (CAVD) is a not-for-profit organization that promotes veterinary dermatology in Canada and provides continuing education for veterinarians, animal health technicians/technologists, and veterinary students. The CAVD welcomes applications for membership (www.cavd.ca).
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Miller WH, Griffin CE, Campbell KL. Muller and Kirk’s Small Animal Dermatology. 7th ed. Toronto, Ontario: Elsevier; 2013. pp. 741–767. [Google Scholar]
- 2.Barnard N, Foster A. Pseudomonas otitis in dogs: A GP’s guide to treatment. In Practice. 2017;39:j892. doi: 10.1136/inp.j892. [DOI] [Google Scholar]
- 3.Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekic S, Matanovic K, Šeol B. Antimicrobial susceptibility of Pseudomonas aeruginosa isolates from dogs with otitis externa. Vet Rec. 2011;169:125. doi: 10.1136/vr.d2393. [DOI] [PubMed] [Google Scholar]
- 5.Saridomichelakis MN, Farmaki R, Leontides LS, Koutinas AF. Aetiology of canine otitis externa: A restrospective study of 100 cases. Vet Dermatol. 2007;18:341–347. doi: 10.1111/j.1365-3164.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 6.Paterson S, Matyskiewicz W. A study to evaluate the primary causes associated with pseudomonas otitis in 60 dogs. J Small Anim Pract. 2018;59:238–242. doi: 10.1111/jsap.12813. [DOI] [PubMed] [Google Scholar]
- 7.Nuttall T. Successful management of otitis externa. In Practice. 2016;38:17–21. [Google Scholar]
- 8.Pye C, Yu A, Weese JS. Evaluation of biofilm production by Pseudomonas aeruginosa from canine ears and the impact of biofilm on antimicrobial susceptibility in vitro. Vet Dermatol. 2013;24:446–449. doi: 10.1111/vde.12040. [DOI] [PubMed] [Google Scholar]
- 9.Morris DO. Medical therapy of otitis externa and otitis media. Vet Clin North Am Small Anim Pract. 2004;34:541–555. doi: 10.1016/j.cvsm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Robson D, Burton G, Bassett R. Correlation between topical antibiotic selection, in vitro bacterial antibiotic sensitivity and clinical response in 16 cases of canine otitis externa complicated by Pseudomonas aeruginosa. Proc North American Veterinary Dermatology Forum; Portland, Oregon. April 14–17, 2010. [Google Scholar]
- 11.Griffin C. Pseudomonas otitis. Proc World Small Animal Veterinary Association World Congress; San Diego, California. 2006. [Google Scholar]
- 12.Thorp MA, Kruger J, Oliver S, Nilssen EL, Prescott CA. The antibacterial activity of acetic acid and Burow’s solution as topical otological preparations. J Laryngol Otol. 1998;112:925–928. doi: 10.1017/s0022215100142100. [DOI] [PubMed] [Google Scholar]
- 13.Buckley LM, McEwan NA, Nuttall T. Tris–EDTA significantly enhances antibiotic efficacy against multidrug-resistant Pseudomonas aeruginosa in vitro. Vet Dermatol. 2013;24:519–e122. doi: 10.1111/vde.12071. [DOI] [PubMed] [Google Scholar]
- 14.Pye C, Singh A, Weese JS. Evaluation of the impact of tromethamine edetate disodium dihydrate on antimicrobial susceptibility of Pseudomonas aeruginosa in biofilm in vitro. Vet Dermatol. 2014;25:120–123. doi: 10.1111/vde.12115. [DOI] [PubMed] [Google Scholar]
- 15.Strain GM, Merchant SR, Neer TM, Tedford BL. Ototoxicity assessment of a gentamicin sulfate otic preparation in dogs. Am J Vet Res. 1995;56:532–538. [PubMed] [Google Scholar]