Abstract
Aim:
To evaluate the effect on the nonsteroidal anti-inflammatory drug indomethacin on Clostridium difficile infection (CDI) severity.
Materials & methods:
Indomethacin was administered in two different mouse models of antibiotic-associated CDI in two different facilities, using a low and high dose of indomethacin.
Results:
Indomethacin administration caused weight loss, increased the signs of severe infection and worsened histopathological damage, leading to 100% mortality during CDI. Indomethacin-treated, antibiotic-exposed mice infected with C. difficile had enhanced intestinal inflammation with increased expression of KC, IL-1β and IL-22 compared with infected mice unexposed to indomethacin.
Conclusion:
These results demonstrate a negative impact of nonsteroidal anti-inflammatory drugs on antibiotic-associated CDI in mice and suggest that targeting the synthesis or signaling of prostaglandins might be an approach to ameliorating the severity of CDI.
Keywords: : antibiotic-associated CDI, C. difficile infection severity, indomethacin, NSAID, prostaglandins
Clostridium difficile is a Gram-positive, anaerobic bacterium that is able to form spores. It is the leading pathogen responsible for antibiotic-associated diarrhea worldwide [1]. Symptoms associated with C. difficile infection (CDI) vary from asymptomatic colonization to severe diarrhea, pseudomembranous colitis, toxic megacolon, colonic perforation and even death [2]. During recent years, morbidity and mortality of CDI increased and emerged as a major health threat in developed countries, particularly for older adults [3]. Mortality rates from CDI usually approach 5% of total cases, but can reach 20% during outbreaks [4].
One way to reduce the burden of CDI is to identify modifiable risk factors for disease and recurrence. While antibiotic use is clearly the most important risk factor for CDI, we and others groups have identified several additional modifiable risks, including the use of gastric acid suppressive therapy [5], blood product transfusion [6], cigarette smoking [7] and certain antidepressant medications [8]. Epidemiological studies have noted an association between CDI risk and the use of nonsteroidal anti-inflammatory drugs (NSAIDs) [5,9–11] underscored by a recent meta-analysis [12]. NSAIDs inhibit prostaglandin (PG) synthesis by blocking the actions of cyclooxygenase enzymes [13]. NSAIDs are among the most commonly prescribed drugs in the USA, with more than 98 million prescriptions filled and an estimated 23 million Americans using over-the-counter NSAIDs in 2012 [14,15]. Relevant to CDI, older adults are major consumers of NSAIDs [15]. The plausibility of a link between NSAID use and CDI is bolstered by the association between NSAID use and flare-ups of inflammatory bowel disease [16] and the occasional occurrence of NSAID-induced colitis [17–20]. We recently reported a correlation between certain gut microbiome family members and the use of specific NSAIDs, such as ibuprofen, in adults [21,22] which might impact the susceptibility to CDI. Indomethacin is one of the most potent NSAIDs [13]; indeed, previous studies have reported that indomethacin induces gastrointestinal injuries associated with ulceration as the most severe manifestation of epithelial erosion (partial decrease in thickness) and a decrease in the mucus layer as a more mild pathology [23–25]. It has also been associated with the development of colitis in humans [26,27].
In this study, we addressed the impact of NSAID use on the severity of CDI in two different mouse models of antibiotic-associated CDI using both a low and high dose of indomethacin. These results suggest that inhibiting endogenous PG synthesis with NSAIDs, specifically with indomethacin, can worsen antibiotic-associated CDI.
Material & methods
Animals
All experiments were conducted with the approval of the Animal Care and Use Committees of the University of Michigan (MI, USA; protocol #3553), Vanderbilt University (TN, USA; protocol #M1600272-00) and Andrés Bello University (Santiago, Chile; protocol #019/2017). Six- to eight-week-old C57BL/6 (male or female) were purchased from Jackson Laboratories (ME, USA). Mice were cohoused for at least 2 weeks prior to each experiment. Mice were housed in individual cages with autoclaved water, food and bedding and all experiments and manipulations were performed in a biosafety level 2 laminar flow hood (NuAire, MN, USA).
Bacterial strains
C. difficile strain 630 (ATCC BAA-1382) a laboratory and genetically tractable strain, and strain R20291 (a NAP1/B1/027 isolate that has caused epidemics of CDI [28,29]) were used in this study. The culture and production of spores for R20291 and 630 strains followed our published protocol [30]. C. difficile strains were cultured under anaerobic conditions in an anaerobic chamber Bactron III-2 (Shel Lab, OR, USA) in 3.7% Brain Heart Infusion Broth (BD, NJ, USA) supplemented with 0.5% Yeast Extract (BD, NJ, USA); (BHIS) for 5 days at 37°C. Spores were harvested with sterile MilliQ water and purified in density gradient with 50% Nicodenz (Axis Shield, Oslo, Norway). Then, spores were counted in a Neubauer chamber and stored at 5 × 109 spores/ml at -80°C.
Mouse model of CDI
The research groups in Chile and in USA have different animal models established in their laboratories, therefore, different antibiotics and strains were employed to induce intestinal dysbiosis. We used a range of indomethacin doses in mice (2 and 10 mg/kg), within the low and high therapeutic range for mice [31]. In Chile, the effect of a low dose of indomethacin during CDI with an epidemically relevant strain was evaluated. Briefly, antibiotic mixture was administrated as previously described with modifications [32]. Briefly, a mix containing 40 mg/kg kanamycin (Sigma, MO, USA), 3.5 mg/kg gentamicin (Sigma), 4.2 mg/kg colistin (Sigma), 21.5 mg/kg metronidazole (Sigma) and 40mg/kg vancomycin (Laboratorio Chile, Santiago, Chile) was administrated via gavage for 3 days. Three days later (1 day prior to infection), an intraperitoneal injection of 30mg/kg clindamycin (Sigma) and a low dose (2 mg/kg) of indomethacin (Sigma) were administrated daily to all mice during 7 days by oral gavage at a dose of 2 mg/kg in 20% ethanol solution (Merck, Darmstadt, Germany) (Figure 1A). Mice not treated with indomethacin received an equal amount of ethanol. On day 0, mice were infected with 6 × 107 spores of C. difficile R20291 strain. In the USA, the effect of a higher dose of indomethacin (10 mg/kg) was evaluated. Briefly, mice were treated for 5 days with the antibiotic cefoperazone in the drinking water followed by 48 h of normal water as has been previously described [30,33]. On day 0, all mice received 10 mg/kg of indomethacin by oral gavage for 7 days, and infected with 1 × 104 spores of the C. difficile strain 630 (Figure 3A). Indomethacin or vehicle control (DMSO) (Sigma) was administered daily by gavage. In both countries, mice were daily monitored and weight loss and clinical score than were used to determine the end point of each animal. Sickness behaviors were monitored daily, and fecal samples, cecum content and colonic tissue collected.
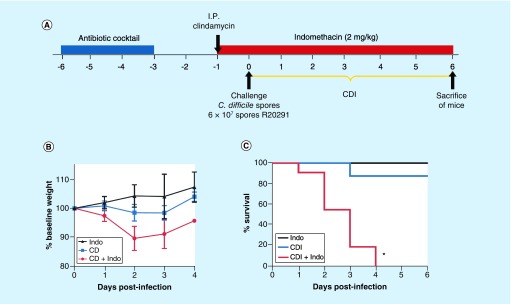
Figure 1. . Indomethacin increases the severity of Clostridium difficile infection R20291 strain in mice.
(A) Overview of the experimental design schematics C. difficile infection in a mouse model. Six to ten weeks old C57BL/6 mice (n = 3 Indo; n = 8 CDI; n = 11 CDI + Indo) were treated with an antibiotic cocktail for 3 days followed by 2 days of recovery and then an intraperitoneal dose of clindamycin. One day after antibiotics, mice were orally gavaged with 6 × 107 spores of C. difficile R20291 strain. 2 mg/kg of Indo were given daily by gavage, starting 1 day before the infection as indicated in red. (B) Weight was monitored daily and (C) survival. Error bars are standard error of the mean; *p < 0.001.
CDI: Clostridium difficile infection; Indo: Indomethacin.
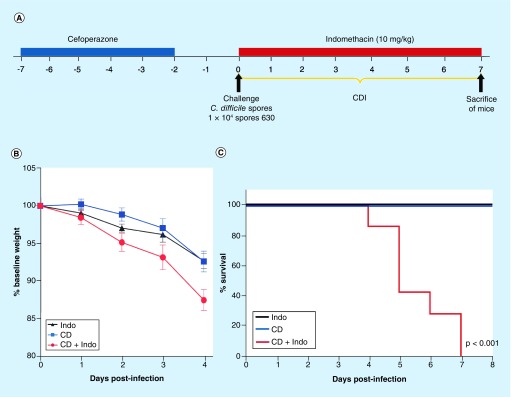
Figure 3. . Indomethacin worsens Clostridium difficile strain 630 in mice.
(A) Female 6–8-week-old C57BL/6 mice were treated with cefoperazone for 5 days followed by 2 days of recovery and then challenged by gavage with 1 × 104 spores of C. difficile 630 strain. Animals received 10 mg/kg of Indo) by gavage daily as indicated by red bar. (B) Weight loss and (C) mice were monitored for survival. **p < 0.01 by Log-rank (Mantel–Cox) test (n = 10 animals per group).
CDI: Clostridium difficile infection; Indo: Indomethacin.
Clinical score
A clinical score based on signs of illness was determined as previously described with modifications [34]. Briefly was categorized using the standard scoring system for CDI in mouse model as follows: Bodyweight, 0- is maintained or increased; 1- is less than 10%; 2- ≤20% or 3- less than 20%. Appearance, 0- normal; 1- lack of grooming apparent; 2- unkempt coat, eyes and nose may have discharges; 3- coat very unkempt, external orifices ungroomed, abnormal posture. Unprovoked behavior, 0- normal; 1- minor changes; 2- abnormal behavior, less mobile and less alert than normal; 3- unsolicited vocalization, extreme self-mutilation, expiratory grunts, very restless or does nor move at all. Behavior responses to external stimuli, 0- behavioral responses normal for the expected conditions, 1- shows some minor depression or minor exaggeration of responses, 2- shows moderate sign of abnormal responses, there may be a change of behavior, 3- animal reacts violently to stimuli, or muscular responses may be very weak as in precomatose state. Cutoff criteria for reaching end point were weight loss more than 20% or a clinical score equal or higher than 5.
Quantification of spores from feces
Fecal samples were hydrated with 500 μl in sterile MilliQ water for 16 h at 4°C, and then added 500 μl of absolute ethanol (Merck, Darmstadt, Germany), which were incubated for 30 min at room temperature. Samples were serially diluted and plated onto selective medium supplemented with 0.1% w/v taurocholate (Sigma), 16 μg/ml cefoxitin (Sigma), 250 μg/ml L-cycloserine and 0.6% w/v fructose taurocholate, cefoxitin, cycloserine, fructose and agar (TCCFA plates; Sigma). The plates were incubated anaerobically at 37°C for 48 h, colonies counted and results expressed as the Log10 (CFU/gram of feces).
Cytotoxicity assay
Cytotoxicity assay was performed as previously described [35]. Briefly, cytotoxicity was determined by the rounding of Vero cells in monolayer culture after exposure to supernatant of mouse cecal contents and to purified C. difficile toxins as control. Plates were incubated for 24 h at 37°C and cells were analyzed by inverted microscopy at 100× magnification.
Length of tissues
The colon and cecum were removed, linearized and the length was measured with a ruler.
Histopathology
The presence of submucosal edema, neutrophilic inflammation and epithelial damage in the colonic portion or cecum portion of the GI tract were scored in a blinded fashion by a tissue pathologist according to our previous methods [30,33].
Tissue inflammatory mediator expression
The induction of inflammatory mediators (cytokines) and prostaglandin E2 (PGE2) in cecal tissues were measured directly by ELISA per our previously reported methods [30].
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (CA, USA). Data are presented as means ± SEM of values determined from the indicated number of samples. Repeated measures analysis of variance (ANOVA) were used in body weights over time followed by Tukey multiple comparison post test or by using Mann–Whitney unpaired t-test as appropriate. Kruskal–Wallis (nonparametric) ANOVA was used for ordinal (ranked categorical) variables in histology and clinical scores. Kaplan–Meier plots were used to analyze survival in the challenged mice by Mantel–Cox Log-rank test. A p-value ≤0.05 was considered significant a priori.
Results
Indomethacin increases the mortality of CDI with strain R20291
We first evaluated whether oral administration of a low dose (2 mg/kg) of indomethacin 1 day prior to CDI and for a total of 7 days increased the severity of infection of C. difficile caused by strain R20291 (Figure 1A), a strain that is known to induce mild signs of illness in mice with spontaneous recovery of infected animals [36,37]. These experiments were conducted in Chile. A slight but not significant decrease in body weight loss was observed between indomethacin-exposed (uninfected) and indomethacin-unexposed C. difficile-infected mice (Figure 1B). By contrast, oral administration of indomethacin prior and during CDI caused a decrease in body weight as early as day 1 postinfection (Figure 1B). All indomethacin-treated (but uninfected) mice survived during the course of the experiment, while only one CDI-treated mouse succumbed (e.g., 88% survival of infected mice that were not treated with indomethacin) during the course of the experiment, however, no significant difference was observed between indomethacin treated, uninfected mice and antibiotic-exposed mice infected with C. difficile but not exposed to indomethacin (Log-rank p = 0.5404; Figure 1C). One hundred percent of the mice treated with indomethacin prior to and during CDI succumbed between days 1 and 4 after infection (Log-rank test, p < 0.001; Figure 1C), indicating that indomethacin exacerbated the severity of CDI in our mouse model.
Analysis of the clinical score revealed increase of signs of illness due to administration of indomethacin (Figure 2A). No significant differences (Kruskal–Wallis test, day 0 p = 0.5; day 1 p = 0.5774) were observed on days 0 and 1 posttreatment. However, after day 2 postinfection, significant differences were noted (Kruskal–Wallis test, p = 0.0154); administration of indomethacin prior and during CDI led to a clinical score of 5.9 ± 1.47 that represented a significant increase compared with C. difficile infected mice not exposed to indomethacin (1.0 ± 0.42) and uninfected mice treated with the NSAID (0 ± 0). Similarly, on days 3 and 4 after infection, significant differences were observed (Kruskal–Wallis test, day 2 p = 0.0154; day 3 p = 0.014; day 4 p = 0.0152), as the administration of indomethacin prior and during CDI caused a significant increase in the clinical illness scores (Figure 2A). No detectable C. difficile spores were observed in uninfected, indomethacin-treated mice during the course of the experiment; by contrast, similar levels of C. difficile were observed during the course of the experiment in CDI mice and indomethacin–CDI-treated mice (Figure 2B). Similarly, cytotoxic titers of cecum contents were similar (Mann–Whitney unpaired, p = 0.81) between CDI and CDI/indomethacin-treated mice (Figure 2C). Analysis of the length of the GI tract revealed a decrease in the length of the colon (Mann–Whitney unpaired, p = 0.06) from 8.80 ± 0.39 in C. difficile-infected mice to 7.82 ± 0.22 in CDI/indomethacin-treated mice, and a significant decrease (Mann–Whitney unpaired, p = 0.007) in the cecum length from 2.60 ± 0.13 in C. difficile-infected mice to 2.04 ± 0.09 in CDI/indomethacin-treated mice (Figure 2D). No difference in cecum width was observed between treatments (Mann–Whitney unpaired, p = 0.606). Collectively, these results demonstrate that administration of a low dose indomethacin prior to and during mild CDI, did not affect C. difficile colonization and cytotoxicity, but exacerbated the signs of severe disease in mice.
Figure 2. . Indomethacin intensifies the signs of Clostridium difficile R202091 strain infection in mice.
C57BL/6 mice treated with an antibiotic cocktail were challenged with 6 × 107 spores of C. difficile R20291 strain, following treatment with (Indo) according to the experimental design in Figure 1 (n = 3 Indo; n = 8 CDI; n = 11 CDI + Indo). (A) Clinical score was monitored daily, (B) Fecal C. difficile spore shedding, (C) Cecum content cytotoxicity and (D) length of colon. Data obtained from two independent experiments. In (A), ‘0’ indicates no clinical score. Error bars are standard error of the mean. *p ≤ 0.05.
CDI: Clostridium difficile infection; Indo: Indomethacin.
Effect of cefoperazone-induced dysbiosis & indomethacin on mice infected with C. difficile strain 630
To validate these findings in a different CDI model, we tested the impact of a high dose of indomethacin (10 mg/kg) exposure on broad spectrum cefoperazone-treated mice infected with the well-characterized C. difficile strain 630 (Figure 3A). This strain normally causes a very mild, self-resolving colitis in cefoperazone-exposed mice [33]. These experiments were conducted in the USA. C. difficile-infected mice had a similar decrease in body weight as indomethacin-treated mice (Figure 3B). However, administration of 10 mg/kg of indomethacin to CDI-treated mice caused a significant decrease in body weight after 4 days of infection compared with infected mice not exposed to indomethacin (Figure 3B). No mortality was observed in uninfected mice treated with indomethacin or in mice infected with C. difficile strain 630 but not treated with the NSAID (Figure 3C); however, administration of indomethacin to C. difficile-inoculated mice during the initiation of infection caused 100% mortality by day 7 after infection (Log-rank Mantel–Cox test, p < 0.01; Figure 3C). These results demonstrate that administration of indomethacin- to cefoperazone-treated mice infected with C. difficile leads to increased mortality.
Histopathological analysis of cecal tissue revealed that histological score (9.2 ± 1.24) of mice treated with indomethacin during CDI was significantly higher than infected mice not exposed to the drug (6.6 ± 0.81; Mann–Whitney unpaired t-test p = 0.032) and uninfected mice treated with indomethacin (1.5 ± 2.9; Mann–Whitney unpaired t-test p = 0.016; Figure 4A). No differences in C. difficile colonization between mice treated with indomethacin during CDI and C. difficile-infected mice (two-tailed Student t-test, p = 0.91) were evidenced in cecum content (Figure 4B). We evaluate the extent to which administration of indomethacin decreased the levels of PGE2 in cecal and colonic tissues. As expected, administration of indomethacin significantly reduced PGE2 levels in these tissue (Figure 4C), suggesting that PGE2 might be implicated in reduced mortality and histopathological damage produced during CDI.
Figure 4. . Effect of indomethacin on bacterial burden, tissue prostaglandin E2 and histological inflammation levels in mice infected with C. difficile strain 630.
C57BL/6 mice treated with an antibiotic cocktail were challenged with C. difficile 630 strain, following treatment with (Indo) according to the experimental design in Figure 3. (A) Mice were harvested by day 4 after infection and cecum histological scores, (B) C. difficile colonization level in cecal content (C) and PGE2 were measured. The data are presented as means ± SEM. **p < 0.01, compared with uninfected group by Student t-test, two-tailed; n = 5/group.
PGE2: Prostaglandin E2; SEM: Standard error of the mean.
Administration of indomethacin-enhanced intestinal inflammation during CDI
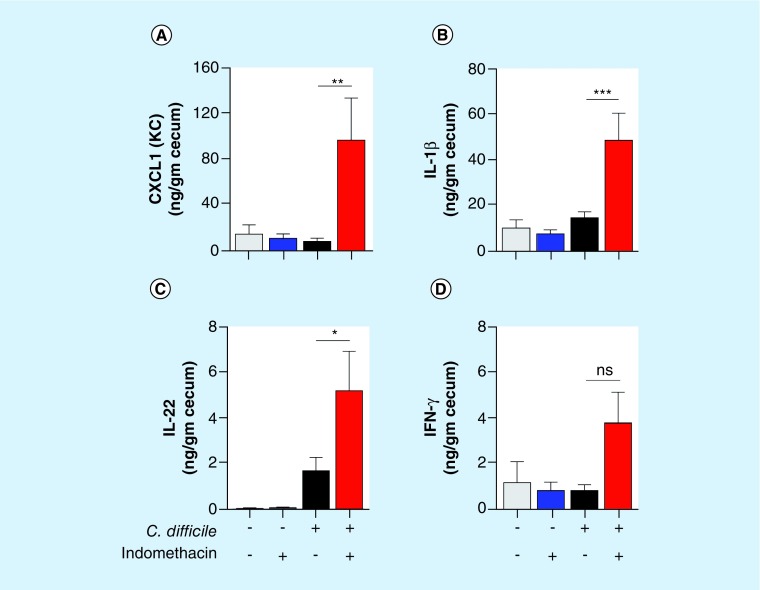
To evaluate the extent to which the indomethacin-mediated inhibition of PGs affected intestinal inflammation, we determine the levels of several cytokines in cecum harvested 4 days postinfection. Notably, administration of indomethacin in C. difficile-treated mice led to significant increases in the tissue levels of the chemokine CXCL1 (KC; Figure 5A), IL-1β (Figure 5B) and of IL-22 (Figure 5C). Indomethacin administration in C. difficile-treated mice also caused an increase in IFN-γ that was not significant by ANOVA (Figure 5D). Collectively, these results suggest that indomethacin-mediated inhibition of PGs exacerbates the manifestations the disease during CDI, leading to increased intestinal damage and mortality.
Figure 5. . Daily indomethacin results in enhanced intestinal inflammation.
C57BL/6 mice were treated with cefoperazone for 5 days following 2 days of recovery in regular drinking water and then challenged with 1 × 104 spores of C. difficile 630 strain. Animals received 10 mg/kg of indomethacin (Indo) by gavage every day starting 1 h before infection. Mice were euthanized and cecum harvested after 4 days postinfection for cytokine measurement (n = 10–13 mice/group). The data are presented as means ± SEM. ***p < 0.001, **p < 0.01, *p < 0.05 by ANOVA followed by Tukey post test.
ns: Not significant; SEM: Standard error of the mean.
Discussion
These studies demonstrate that exposure to the NSAID indomethacin, a nonselective inhibitor of both cyclooxygenase COX-1 and COX-2 isoforms, significantly worsened the severity of CDI in antibiotic-exposed mice. Important experimental support of epidemiological data linking NSAID use, specifically indomethacin, to an increased risk for CDI [5,9–11] is also provided. This work also suggests that there is a causal relationship between indomethacin use and an increased risk for (or severity of) CDI. The biological plausibility of a link between NSAID use and CDI is bolstered by the association between NSAID use and flare-ups of inflammatory bowel disease [16] and the occurrence of an NSAID-induced colitis [17–20,38,39].
A notable observation of this work was that previously animal model of CDI infected with R20291 [36], reported to cause moderate and self-limiting CDI, had an increased mortality upon administration of low indomethacin. Previous observations evidenced that the administration of indomethacin in patients shows increased intestinal permeability [40], colonic ulceration and bleeding [41], multiple perforations [42] and hemorrhage [43]. Indomethacin also affects the structural composition of the intestinal microbiota [44]. Although it is evident that indomethacin-induced intestinal alterations might impact the severity of CDI, the precise mechanisms underlying the increase in severity remain unclear and matter of further studies.
Strengths of our study include the robust and consistent, damaging impact of indomethacin on CDI in mice, even when strains of C. difficile varied, antibiotic pretreatment varied, and dosing regimens of indomethacin varied. Results were also qualitatively similar despite the fact that experiments were conducted by separate teams led by investigators in Chile (D Paredes-Sabja) and the USA (DM Aronoff). Some differences were noted between the two modes. One was that mouse weight was significantly affected in the model of infection with strain 630, but not with strain R20291. In addition, 100% of animals treated with indomethacin and with CDI died either by day 7 or day 4, respectively. These differences could be the reflect of the difference of the used models, C. difficile strain and doses of indomethacin. In addition to this, we note that NSAID use exacerbated inflammatory responses to CDI and future studies will be needed to identify the extent to which such changes drive the enhanced severity of disease. Limitations of our work include: the use of only one strain of mice (C57BL/6), the lack of analysis of the length of colon and cecum from mice treated with indomethacin alone and a lack of knowledge of the mechanism(s) whereby indomethacin exposure increases the susceptibility of antibiotic-exposed mice to CDI but, the mechanism might be related to direct epithelial cell or mucosal injury, induction of dysbiosis or vasoconstriction [45,46]. However, the mechanism remains to be elucidated and ongoing studies are being conducted to elucidate the molecular mechanism that involves NSAIDs and severity of CDI. We are presently focused on deciphering the answers to these unknowns. An additional limitation of our work is the use of indomethacin as a representative of NSAID class. Further experiments with more commonly used NSAIDs would allow generalization our findings.
The clinical importance in humans of this phenomena is that the use of NSAIDs might be one factor that leads CDI to be severe enough that a patient presents to the hospital for diagnosis and management. Many cases of CDI are mild or self-limited and therefore are not diagnosed because a patient does not present to a healthcare provider for testing or the healthcare provider does not think of the diagnosis [47,48]. If NSAID exposure increases the severity of cases that would otherwise be mild or self-limited this would increase the likelihood of CDI being diagnosed. NSAIDs in that case would increase the risk for a CDI diagnosis (by increasing the severity). In addition, older adults are the primary users of NSAIDs [15] and are at high risk for severe CDI [49], so the combination of NSAID exposure could be an important determinant of disease severity in older adults, which clinical studies will need to explore.
Conclusion
Our findings demonstrate a negative impact of NSAIDs on antibiotic-associated CDI in mice and suggest that targeting the synthesis or signaling of PGs might be an approach to ameliorating the severity of CDI. Further studies would be required the evaluate the impact of PGs in reducing the burden of CDI.
Future perspective
In this work, we evaluated the use of an NSAID, indomethacin in the increase of the severity to CDI, however, the use of all other NSAID remains to be elucidated. A limitation of this work is that we did not report whether NSAID use per se, in the absence of antibiotic use, could render an animal susceptible to CDI. Our unpublished data suggest this is not the case but further studies are needed. The molecular mechanism whereby NSAID use increases the severity of CDI also remains unclear.
Summary points.
Risk Factors for Clostridium difficile infection
Antibiotic use is the main risk factor for C. difficile infection (CDI), but other risk factors such as gastric acid suppressive therapy, blood product transfusion, cigarette smoking and antidepressant medication have been reported.
Association between the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and CDI have been noted, and NSAIDs are among the most highly prescribed drugs in the USA.
Indomethacin increase the severity of CDI
Administration of indomethacin to C. difficile-infected mice decreases in the body weight, mortality, histopathological score and inflammation during CDI.
Mechanism of action
Indomethacin inhibits prostaglanding production by cyclooxygenase-1 and -2 but the exact nature of the effect on CDI pathogenesis is unclear. The molecular mechanism remains to be elucidated.
Clinical importance if this work
The use of indomethacin (NSAID) might be one factor that increases the severity of CDI to be severe enough to make patients seek medical help.
Conclusion
The combination of Indomethacin (NSAID) and CDI could be an important determinant of the disease severity, but clinical studies will be needed to corroborate this results.
Footnotes
Financial & competing interests disclosure
This work was supported by Fondo Nacional de Ciencia y Tecnología de Chile (FONDECYT grant 1151025), from the and by a grant from Fondo de Fomento al Desarrollo Científico y Tecnológico (FONDEF) ID16|10038 to D Paredes-Sabja and by a doctoral fellowship to P Castro-Córdova (CONICYT 21161395). DM Aronoff and his team were funded by the NIH grants TR001723, AI121796 and the Vanderbilt Digestive Diseases Research Center NIH grant DK058404. BC Trindade was funded by a grant from the CAPES (grant number BEX 9179/11-9). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin. Infect. Dis. 2015;60(Suppl. 2):S66–S71. doi: 10.1093/cid/civ140. [DOI] [PubMed] [Google Scholar]
- 2.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J. Clostridium difficile-associated diarrhea and colitis. Infect. Control Hosp. Epidemiol. 1995;16(8):459–477. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–1041. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 6.Rogers MAM, Micic D, Blumberg N, Young VB, Aronoff DM. Storage duration of red blood cell transfusion and Clostridium difficile infection: a within person comparison. PLoS ONE. 2014;9(2):e89332. doi: 10.1371/journal.pone.0089332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers MAM, Greene MT, Saint S, et al. Higher rates of Clostridium difficile infection among smokers. PLoS ONE. 2012;7(7):e42091. doi: 10.1371/journal.pone.0042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers MAM, Greene MT, Young VB, et al. Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Med. 2013;11(1):121. doi: 10.1186/1741-7015-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suissa D, Delaney JAC, Dial S, Brassard P. Non-steroidal anti-inflammatory drugs and the risk of Clostridium difficile-associated disease. Br. J. Clin. Pharmacol. 2012;74(2):370–375. doi: 10.1111/j.1365-2125.2012.04191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnault H, Bourrier A, Lalande V, et al. Prevalence and risk factors of Clostridium difficile infection in patients hospitalized for flare of inflammatory bowel disease: a retrospective assessment. Dig. Liver Dis. 2014;46(12):1086–1092. doi: 10.1016/j.dld.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Søes LM, Holt HM, Böttiger B, et al. Risk factors for Clostridium difficile infection in the community: a case-control study in patients in general practice, Denmark, 2009–2011. Epidemiol. Infect. 2013;142(7):1437–1448. doi: 10.1017/S0950268813002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Permpalung N, Upala S, Sanguankeo A, Sornprom S. Association between NSAIDs and Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Can. J. Gastroenterol. Hepatol. 2016;2016:7431838. doi: 10.1155/2016/7431838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas S. The pharmacology of indomethacin. Headache. 2016;56(2):436–446. doi: 10.1111/head.12769. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol. Drug Saf. 2014;23(1):43–50. doi: 10.1002/pds.3463. [DOI] [PubMed] [Google Scholar]
- 15.Fowler TO, Durham CO, Planton J, Edlund BJ. Use of nonsteroidal anti-inflammatory drugs in the older adult. J. Am. Assoc. Nurse Pract. 2014;26(8):414–423. doi: 10.1002/2327-6924.12139. [DOI] [PubMed] [Google Scholar]
- 16.Kvasnovsky CL, Aujla U, Bjarnason I. Nonsteroidal anti-inflammatory drugs and exacerbations of inflammatory bowel disease. Scand. J. Gastroenterol. 2014;1:1–9. doi: 10.3109/00365521.2014.966753. [DOI] [PubMed] [Google Scholar]; • Provides clinical evidence of nonsteroidal anti-inflammatory drugs in exacerbation of inflammatory bowel disease.
- 17.Tonolini M. Acute nonsteroidal anti-inflammatory drug-induced colitis. J. Emerg. Trauma. Shock. 2013;6(4):301–303. doi: 10.4103/0974-2700.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riddell RH, Tanaka M, Mazzoleni G. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case-control study. Gut. 1992;33(5):683–686. doi: 10.1136/gut.33.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentric A, Pennec YL. Diclofenac-induced pseudomembranous colitis. Lancet. 1992;340(8811):126–127. doi: 10.1016/0140-6736(92)90459-g. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Gómez M, Suárez García E, Castro Fernández M. Pseudomembranous colitis induced by diclofenac. J. Clin. Gastroenterol. 1998;26(3):228. doi: 10.1097/00004836-199804000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Rogers MAM, Aronoff DM. The influence of nonsteroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2015;22(2):178.e1–178.e9. doi: 10.1016/j.cmi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abatan M, Lateef I, Taiwo V. Toxic effects of non-steroidal anti-inflammatory agents in rats. African J. Biomed. Res. 2009;9(3):219–223. [Google Scholar]
- 23.Dorshow RB, Hall-Moore C, Shaikh N, et al. Measurement of gut permeability using fluorescent tracer agent technology. Sci. Rep. 2017;7(1):10888. doi: 10.1038/s41598-017-09971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodie DA, Cook PG, Bauer BJ, Dagle GE. Indomethacin-induced intestinal lesions in the rat. Toxicol. Appl. Pharmacol. 1970;17(3):615–624. doi: 10.1016/0041-008x(70)90036-0. [DOI] [PubMed] [Google Scholar]; • First article to demonstrate that indomethacin induces lesions in the GI tract of animal model.
- 25.Stewart THM, Hetenyi C, Rowsell H, Orizaga M. Ulcerative enterocolitis in dogs induced by drugs. J. Pathol. 1980;131(4):363–378. doi: 10.1002/path.1711310408. [DOI] [PubMed] [Google Scholar]
- 26.Tas A, Celik H. Severe colitis due to indomethacin suppository. Rev. Esp. Enfermedades Dig. 2013;105(2):119–120. doi: 10.4321/s1130-01082013000200016. [DOI] [PubMed] [Google Scholar]
- 27.Coutrot S, Roland D, Barbier J, Van Der Marcq P, Alcalay M, Matuchansky C. Acute perforation of the colonic diverticula associated with short-term indomethacin. Lancet. 1978;312(8098):1055–1056. doi: 10.1016/s0140-6736(78)92385-1. [DOI] [PubMed] [Google Scholar]; • First article to report that short-term administration of indomethacin is associated with GI lesions in humans.
- 28.O'Connor JR, Johnson S, Gerding DN. Clostridium difficile Infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913–1924. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 29.He M, Miyajima F, Roberts P, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile . Nat. Genet. 2012;45(1):109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first report of the spread of C. difficile epidemic from healthcare sources.
- 30.Trindade BC, Theriot CM, Leslie JL, et al. Clostridium difficile-induced colitis in mice is independent of leukotrienes. Anaerobe. 2014;30:90–98. doi: 10.1016/j.anaerobe.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter T, Chau TT, Weichman BM. Effects of analgesics on bradykinin-induced writhing in mice presensitized with PGE2. Agents Actions. 1989;27(3–4):375–377. doi: 10.1007/BF01972826. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes. 2011;2(6):326–334. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton D, Griffiths P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985;116(16):431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 35.Plaza-Garrido A, Miranda-Cárdenas C, Castro-Córdova P, et al. Outcome of relapsing Clostridium difficile infections do not correlate with virulence-, spore- and vegetative cell-associated phenotypes. Anaerobe. 2015;36:30–38. doi: 10.1016/j.anaerobe.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Pizarro-Guajardo M, Díaz-González F, Álvarez-Lobos M, Paredes-Sabja D. Characterization of chicken IgY specific to Clostridium difficile R20291 spores and the effect of oral administration in mouse models of initiation and recurrent disease. Front. Cell. Infect. Microbiol. 2017;7:365. doi: 10.3389/fcimb.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zackular JP, Moore JL, Jordan AT, et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016;22(11):1330–1334. doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This work provides a breakthrough on factors that affect the microbiota and susceptibility to C. difficile infections.
- 38.Vikash, Dhameja N, Dixit VK. NSAID (Diclofenac) induced apoptotic colitis–a case report and review of literature. Indian J. Appl. Res. 2016;6(3):443–444. [Google Scholar]
- 39.Faucheron JL, Parc R. Non-steroidal anti-inflammatory drug-induced colitis. Int. J. Colorectal Dis. 1996;11(2):99–101. doi: 10.1007/BF00342469. [DOI] [PubMed] [Google Scholar]
- 40.Suenaert P, Bulteel V, Den Hond E, et al. In vivo influence of nicotine on human basal and NSAID-induced gut barrier function. Scand. J. Gastroenterol. 2003;38(4):399–408. doi: 10.1080/00365520310000834. [DOI] [PubMed] [Google Scholar]
- 41.Oren R, Ligumsky M. Indomethacin-induced colonic ulceration and bleeding. Ann. Pharmacother. 1994;28(7–8):883–885. doi: 10.1177/106002809402800713. [DOI] [PubMed] [Google Scholar]
- 42.Loh AHP, Ong LY, Liew WK, et al. Multiple indomethacin-induced colonic perforations in an adolescent. Singapore Med. J. 2011;52(4):e82–e84. [PubMed] [Google Scholar]; •• Study that investigates the impact of indomethacin in colonic perforations.
- 43.Langman MJ, Morgan L, Worrall A. Use of anti-inflammatory drugs by patients admitted with small or large bowel perforations and haemorrhage. Br. Med. J. (Clin. Res. Ed.) 1985;290(6465):347–349. doi: 10.1136/bmj.290.6465.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang X, Bittinger K, Xuanwen L, Abernethy DR, Bushman FD, Fitzgerald GA. Bidirectional interactions between indomethacin and the murine intestinal microbiota. Elife. 2015;4:e08973. doi: 10.7554/eLife.08973. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This work provides the first study of the impact of indomethacin and the intestinal microbiota.
- 45.Goldstein JL, Cryer B. Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc. Patient Saf. 2015;7:31–41. doi: 10.2147/DHPS.S71976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Syer SD, Wallace JL. Environmental and NSAID-enteropathy: dysbiosis as a common factor. Curr. Gastroenterol. Rep. 2014;16(3):377. doi: 10.1007/s11894-014-0377-1. [DOI] [PubMed] [Google Scholar]
- 47.Alcala L, Martin A, Marin M, et al. The undiagnosed cases of Clostridium difficile infection in a whole nation: where is the problem? Clin. Microbiol. Infect. 2012;18(7):E204–E213. doi: 10.1111/j.1469-0691.2012.03883.x. [DOI] [PubMed] [Google Scholar]
- 48.Goldenberg SD. Hidden burden of undiagnosed Clostridium difficile infection. Lancet Infect. Dis. 2014;14(12):1167–1168. doi: 10.1016/S1473-3099(14)71010-2. [DOI] [PubMed] [Google Scholar]
- 49.Shivashankar R, Khanna S, Kammer PP, et al. Clinical factors associated with development of severe-complicated Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2013;11(11):1466–1471. doi: 10.1016/j.cgh.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]