Abstract
Introduction:
The ultimate goal for CML management is risk stratification of the patients to design the appropriate treatment approach. The Sokal, Euro and EUTOS risk scores were established to prognosticate the patients on therapy.
Aim:
To perform a comparative assessment of the Sokal, Euro and EUTOS prognostic score in Indian CML-CP patients on imatinib.
Methods:
This is a retrospective study performed in 260 Ph+ CML-CP patients who were administered oral imatinib (400 mg/day).
Results:
166/260 were males and 94/260 were females (M: F::1.6:1) with median age 35 years (range 20-70). 92 (35.38%), 125 (48.07%) and 43 (16.5%) patients were divided into low, intermediate and high risk Sokal score respectively. 102 (39.23%), 106 (40.76%) and 52 (20%) patients were discriminated into low, intermediate and high risk Euro score respectively. 210 (80.7%) and 50 (19.2%) patients were divided into low and high risk EUTOS score. Cumulative incidence of MMR for low, intermediate and high-risk Sokal score was 87%, 76% and 84% respectively (P = 0.016). Incidence of MMR in low, intermediate and high-risk Euro score was 93%, 85% and 68% respectively (P = 0.001). Incidence of MMR was 80 % and 81% for low and high risk EUTOS score (P = 0.764). Both EFS and OS are significantly correlated with Sokal score (P = 0.004, P = 0.007) and Euro score (P = 0.009, P = 0.001) but not with EUTOS score (P = 0.581, P = 0.927).
Conclusion:
The present study highlights the significant prognostic role of Sokal and Euro score in predicting the treatment outcome of the CML- CP patients on imatinib.
Keywords: Chronic myeloid leukemia-chronic phase, Euro, European Treatment and Outcome Study, imatinib, prognostication, Sokal
Introduction
Chronic myeloid leukemia (CML) is a clonal malignant disorder of pluripotent hematopoietic stem cells characterized by insidious onset of symptoms, progressive splenomegaly, marrow hypercellularity, anemia, and leukocytosis with myeloid cells in all stages of maturation. If untreated, this disease follows a typical course, from chronic phase (CP) toward an ill-defined accelerated phase (AP) to the final blast crisis (BC) within 3–5 years.[1,2] Majority of patients are diagnosed in CML-CP and median survival of these patients is 5–6 years in the absence of the treatment.[3] If the CML patients are left untreated, their progression from the accelerated phase to blast crisis typically occurs within 2 to 15 months. Furthermore in these patients, median survival of the patients in blast crisis is 3-6 months in absence of treatment.[4]
Over the years, many prognostic scoring models have been developed for risk stratification of CML. The three principal risk scores such as Sokal, Euro, and European Treatment and Outcome Study (EUTOS) were established in different eras of CML therapy with implications for prognosis and disease outcome.[5,6,7,8] Sokal score was developed in the era of chemotherapy, while Euro score was proposed in the time of interferon-alpha administration and EUTOS score was projected in Tyrosine Kinase Inhibitors (TKIs) era. Sokal and Euro score discriminated the patients into high, intermediate, and low-risk groups, but EUTOS score differentiated the patients into high- and low-risk groups.[5,6,7]
Worldwide, several studies from different regions have compared the clinical significance of these prognostic scoring systems and found mixed opinions regarding their utility.[9,10,11,12,13,14,15,16] Few studies observed that EUTOS score is better than Sokal and Euro score in predicting the prognosis of CML patients; however, Sokal and Euro scores were found better than EUTOS in other studies.[7,9,10,11,12,13,14,15] Sokal and Euro score were successfully used to differentiate all risk patients treated with imatinib according to 5-year overall survival (OS); however, EUTOS score has failed to show a significant association.[14] Initially, the EUTOS score was successful to predict the probability of complete cytogenetic response (CCyR) within 18 months of imatinib initiation and progression-free survival; however, Sokal and Euro scores failed to show significant efficacy.[7] EUTOS is the only CML prognostic score that has been developed in the TKIs era. Hence, its validation is important for the clinical management of CML. The usefulness of this score in predicting survival and outcome in CML-CP patients treated with TKIs was questioned, as mentioned in literature.[13,14,15]
To the best of our knowledge, there are very limited data from Asian population regarding the comparative analysis of prognostic scores in CML patients receiving front-line imatinib therapy.[14,15,16] In view of this, we assessed the most widely used prognostic risk models (Sokal score, Euro, and EUTOS score) and compared their efficacy as a predictive and prognostic tool in a cohort of 260 Indian CML-CP patients on imatinib.
Materials and Methods
This was a retrospective study performed in 260 Ph+ (Philadelphia positive) CML-CP patients attending the Hematology Outpatient Department of the AIIMS, New Delhi, from 2012 to 2016. CML-CP was diagnosed according to the WHO 2008 criteria and diagnosis was performed by reverse transcriptase-polymerase chain reaction (PCR) for the Ph chromosome. These patients consisted of 166 males and 94 females with a median age of 35 years. All these patients were started on oral imatinib 400 mg/day (Novartis, Bale, Switzerland). The study was approved by the Institutional Ethics Committee and written informed consent was obtained from all participants.
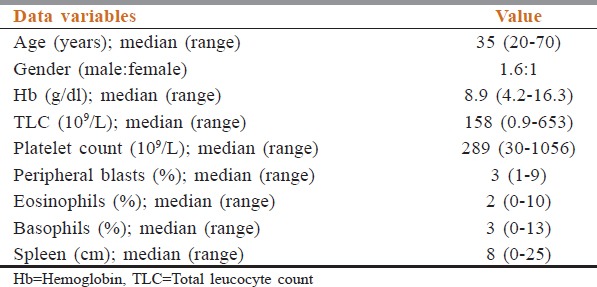
Baseline characteristics of the patients including age, gender, spleen size, hemoglobin (Hb), total leukocyte count (TLC), differential leukocyte count (percentage of myeloblasts, basophils, and eosinophils), and platelet count in peripheral blood were recorded. At the time of diagnosis, we calculated and categorized Sokal score, Euro score, and EUTOS risk score according to formulae given in Table 1.
Table 1.
Sokal score, Euro score, and European Treatment and Outcome Study score: Calculation and risk categorization
Responses were defined as previously described by Kantarjian et al.[17] Complete hematologic response was defined as TLC <10 × 109/L, platelet count <450 × 109/L, no immature cells (blasts, promyelocytes, or myelocytes) in the peripheral blood, and disappearance of all signs and symptoms related to leukemia, including palpable splenomegaly. Real-time quantitative PCR was performed on peripheral blood every 6–12 monthly to look for molecular response which was defined as a major molecular response (MMR) if the BCR-ABL/ABL ratio was <0.10% on the International Scale.[18]
All statistical analyses were performed using STATA 11 (College Station, TX, USA). Cumulative incidence of MMR, event-free survival (EFS), and OS for period of 4 years were calculated with Kaplan–Meier method.[19] Different risk curves were compared with log-rank test.[20] Survival curves were estimated according to the method of Kaplan and Meier, and statistical differences between curves were assessed by the log-rank test. EFS was defined as a loss of molecular and hematological response, progression to AP/blast phase on imatinib and death or last follow-up. OS was defined as the time period elapsed between initial diagnosis and death or last follow-up.[21]
Results
The baseline characteristics of 260 CML-CP patients are shown in Table 2. The median age at diagnosis was 35 years (range: 20–70 years) with (male:female: 1.6:1) and a median follow-up period of the patients was 38 months (range: 2–48 months). The median Hb was 8.9 g/dl (range: 4.2–16.3), median TLC 158 × 109/L (range: 0.9–653) with median blast percentage 3 (range: 1–9), and median basophil percentage 3 (range: 0–13). Splenomegaly was found in 72/260 (27.6%) patients, and the median spleen size below the left costal margin was 8 cm (range: 0–25 cm).
Table 2.
Baseline characteristics of the 260 chronic myeloid leukemia-chronic phase patients
The distribution of the patients according to various risk groups is shown in Table 3. Using Sokal score, 92 (35.38%), 125 (48.07%), and 43 (16.5%) patients were divided into low-, intermediate-, and high-risk score, respectively. As per the Euro score, 102 (39.23%), 106 (40.76%), and 52 (20%) patients were discriminated into low-, intermediate-, and high-risk score, respectively. According to the EUTOS score, 210 (80.7%) patients were categorized as low risk, while 50 (19.2%) patients had a high-risk score.
Table 3.
The distribution of the patients and incidence of major molecular response according to risk scores (Sokal, Euro, and European Treatment and Outcome Study)
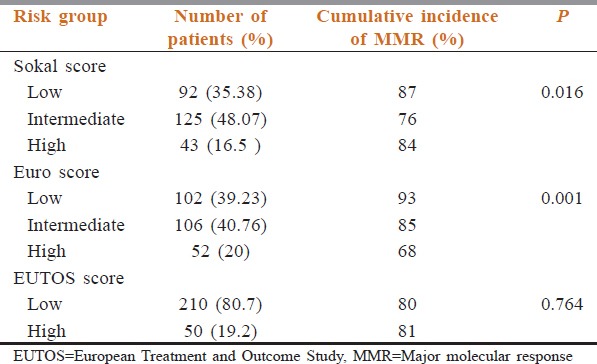
Cumulative incidence of MMR according to all the three risk scores is shown in Table 3. According to Sokal score, the cumulative incidence of MMR was 87%, 76%, and 84% for low-, intermediate-, and high-risk score, respectively (P = 0.016). As per Euro score, cumulative incidence of MMR was 93%, 85%, and 68% for low-, intermediate-, and high-risk group, respectively (P = 0.001). Cumulative incidence of MMR for low- and high-risk group as per EUTOS score was 80% and 81% (P = 0.764).
Kaplan–Meier analysis for EFS is shown in Figure 1. Estimated 4-year EFS for low-, intermediate-, and high-risk Sokal scores was 96%, 90%, and 61%, respectively (P = 0.004). EFS for Euro score was 97%, 89%, and 72%, respectively (P = 0.009). Estimated EFS for low and high EUTOS score was 87% and 84%, respectively (P = 0.581). Estimated 4-year OS analysis for the three risk scores is shown in Figure 2. OS was 97%, 84%, and 70% (P = 0.007) for low-, intermediate-, and high-risk Sokal group, respectively. OS was 83%, 87%, and 67% (P = 0.001) for low-, intermediate-, and high-risk Euro group, respectively. OS for low- and high-risk EUTOS scores was not significantly different, being 81% and 92%, respectively (P = 0.927).
Figure 1.
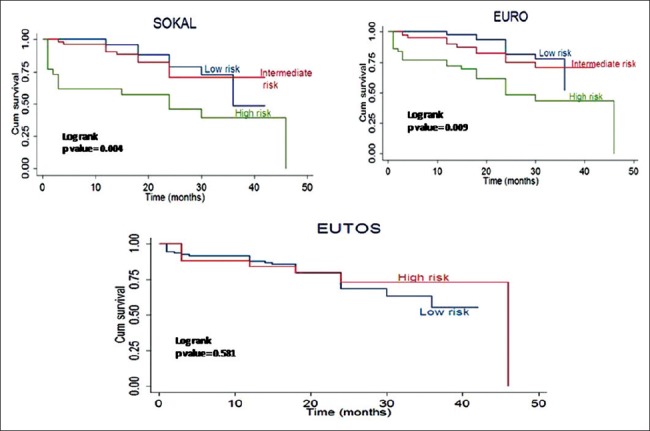
Event-free survival analysis of chronic myeloid leukemia-chronic phase patients as per risk scores by Kaplan–Meier method; Sokal, Euro, and European Treatment and Outcome Study
Figure 2.
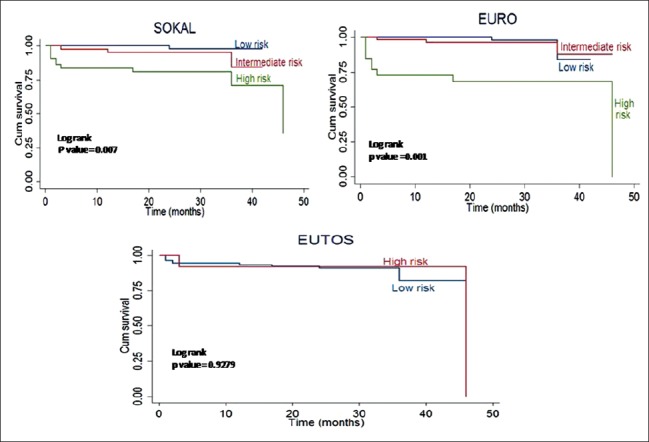
Overall survival analysis of chronic myeloid leukemia-chronic phase patients as per risk scores by Kaplan–Meier method; Sokal, Euro, and European Treatment and Outcome Study
Discussion
Over the years, various attempts have been made for the better prognostication of CML-CP patients at the time of diagnosis. Various baseline factors such as clonal chromosome abnormalities in Ph+ cells, specific multidrug resistance polymorphisms, and the detailed molecular analysis of the genome have been reported to influence the response to imatinib and OS in CML patients.[22,23,24] However, due to requirement of technical expertise and cumbersome time taking techniques for the assessment of above-mentioned factors, these cannot be routinely investigated in daily clinical practice. Hence, on the basis of easily available clinical and hematological parameters, the various scoring models have been designed for prognostic evaluation at diagnosis. In the past three decades, risk stratification for CML patients primarily relied on Sokal and Euro scores which were developed in the year 1984 and 1988, respectively.[25,26,27] Then Europeans designed EUTOS score during the TKI era in year 2011, which was based on more than 2000 CML-CP patients treated with imatinib.[7]
Studies validating the role of these scores in prognostication of CML patients on imatinib have shown conflicting results. The reasons for the mixed observations with these scores are: first, the heterogeneity in the study population and second, the type or dose of the treatment in the form of TKIs as per various studies. Therefore, the aim of our study was to analyze the predictive values of all the three prognostic scoring systems in CML-CP patients on 400 mg/day imatinib. We found that both the Sokal and Euro scores were clinically effective prognostic indicators but not the EUTOS score. In contrast to the Sokal and Euro scores, which predicted the significant cumulative incidence of MMR, EFS, and OS, the EUTOS score was not able to predict the same.
Our patients had a median age of 35 years at presentation, which is in contrast to studies in Western population in which median age is between 40 and 50 years.[28] This signifies the important finding of this study that the presentation of CML occurs in comparatively younger Indian population.[29] Thus, it is really very important to assess the prognostic capability of these scores in predicting clinical outcome in this predominantly young adult population.
Using the Sokal score, 92 (35.38%), 125 (48.07%), and 43 (16.5%) patients were divided into low-, intermediate-, and high-risk score, respectively. As per Euro score, 102 (39.23%), 106 (40.76%), and 52 (20%) patients were divided into low-, intermediate-, and high-risk score, respectively. Our findings are in line with previous studies from European population and Western population.[5,7,14,16] According to the EUTOS score, 210 patients (80.7%) and 50 patients (19.2%) were in low-risk and high-risk score respectively, which is similar to the study from European population but in contrast to study from Chinese population by Tao et al.[10,11,12,13]
According to Sokal score, cumulative incidence of MMR was 87%, 76%, and 84% for low-, intermediate-, and high-risk group, respectively (P = 0.016). As per Euro score, cumulative incidence of MMR was 93%, 85%, and 68% for low-, intermediate-, and high-risk group, respectively (P = 0.001). Both EFS and OS were significantly correlated with Sokal score (P = 0.004 and P = 0.007) and Euro score (P = 0.009 and P = 0.001). Our findings are also confirmed by Yamamoto et al., Marin et al., and Jabbour et al. in Japanese, German, and Western population, respectively. These studies indicated the significance of Sokal and Euro score in forecasting the outcome in CML patients and also questioned the validity of the EUTOS scoring system.[14,15,16] However, contradictory results were suggested by Hoffman et al. in German population.[12]
As per EUTOS score, cumulative incidence of MMR for low- and high-risk group was 80% and 81%, respectively (P = 0.764). No significant correlation was observed for EFS and OS with EUTOS score (P = 0.581 and P = 0.927). There are conflicting opinions, regarding the use of EUTOS system for the prognostic evaluation of CML.[9,10,11,12,13,14,15,16] In contrast to study by Hoffman et al.and Tao et al. in German population and Chinese population, respectively, who revealed the significant correlation of EUTOS score with treatment outcome,[10,12] numerous studies from Japanese, German, and Western population did not find any significant association of this score with prognostication of the patients. Our findings are in line with the above-mentioned studies by Jabbour et al., Marin et al., and Yamamoto et al.[13,14,15]
Conclusion
The present study highlighted the significant prognostic role of Sokal and Euro score in predicting the treatment outcome of the CML-CP patients. One of the possible reasons for parallel results in Sokal and euro scores may be due to the involvement of similar factors in their calculation such as age, platelet count, and blast cell count. The present study was not able to validate the effectiveness of the EUTOS score in predicting the clinical outcome. This difference in findings of the EUTOS score may be due to the following reasons; first, it is calculated by two parameters as used in calculating Sokal and Euro score, i.e., basophil count and splenomegaly, and second, there was the comparatively small proportion of the patients in the high-risk group. Hence, we recommend the routine application of Sokal and Euro score in prognostication of CML-CP patients on imatinib. Future projects involving the development of new prognostic models may open the window for prognostication of CML patients at presentation and will provide insight into selecting the most appropriate therapy for a better outcome in this modern era of TKIs therapy.
Financial support and sponsorship
The first author Sunita Chhikara acknowledges the Indian Council of Medical Research, New Delhi, for the research fellowship.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors wish to acknowledge Mr. Rajender Chhokar and Mr. Arun Dalsus for their technical support.
References
- 1.Trask PC, Mitra D, Iyer S, Candrilli SD, Kaye JA. Patterns and prognostic indicators of response to CML treatment in a multi-country medical record review study. Int J Hematol. 2012;95:535–44. doi: 10.1007/s12185-012-1043-8. [DOI] [PubMed] [Google Scholar]
- 2.Verbeek W, König H, Boehm J, Kohl D, Lange C, Heuer T, et al. Continuous complete hematological and cytogenetic remission with molecular minimal residual disease 9 years after discontinuation of interferon-alpha in a patient with Philadelphia chromosome-positive chronic myeloid leukemia. Acta Haematol. 2006;115:109–12. doi: 10.1159/000089476. [DOI] [PubMed] [Google Scholar]
- 3.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–72. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 4.Cwynarski K, Roberts IA, Iacobelli S, van Biezen A, Brand R, Devergie A, et al. Stem cell transplantation for chronic myeloid leukemia in children. Blood. 2003;102:1224–31. doi: 10.1182/blood-2002-12-3637. [DOI] [PubMed] [Google Scholar]
- 5.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99. [PubMed] [Google Scholar]
- 6.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. Anew prognostic score for survival of patients with chronic myeloid leukemia treated with interferon Alfa. Writing committee for the collaborative CML prognostic factors project group. J Natl Cancer Inst. 1998;90:850–8. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 7.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood. 2011;118:686–92. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Savani BN. Impact of risk score calculations in choosing front-line tyrosine kinase inhibitors for patients with newly diagnosed chronic myeloid leukemia in the chronic phase. Eur J Haematol. 2014;93:179–86. doi: 10.1111/ejh.12356. [DOI] [PubMed] [Google Scholar]
- 9.Yahng SA, Jang EJ, Choi SY, Lee SE, Kim SH, Kim DW, et al. Prognostic discrimination for early chronic phase chronic myeloid leukemia in imatinib era: Comparison of Sokal, Euro, and EUTOS scores in Korean population. Int J Hematol. 2014;100:132–40. doi: 10.1007/s12185-014-1600-4. [DOI] [PubMed] [Google Scholar]
- 10.Tao Z, Liu B, Zhao Y, Wang Y, Zhang R, Han M, et al. EUTOS score predicts survival and cytogenetic response in patients with chronic phase chronic myeloid leukemia treated with first-line imatinib. Leuk Res. 2014;38:1030–5. doi: 10.1016/j.leukres.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio M, Binotto G, Calistri E, Maino E, Tiribelli M. Gruppo Triveneto LMC. EUTOS score predicts early optimal response to imatinib according to the revised 2013 ELN recommendations. Ann Hematol. 2014;93:163–4. doi: 10.1007/s00277-013-1974-z. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann V, Baccarani M, Hasford J, Guilhot J, Saussele S, Rosti G, et al. The EUTOS CML score aims to support clinical decision-making. Blood. 2012;119:2966–7. doi: 10.1182/blood-2012-01-402511. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour E, Cortes J, Nazha A, O’Brien S, Quintas-Cardama A, Pierce S, et al. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors: A single institution experience. Blood. 2012;119:4524–6. doi: 10.1182/blood-2011-10-388967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto E, Fujisawa S, Hagihara M, Tanaka M, Fujimaki K, Kishimoto K, et al. European treatment and outcome study score does not predict imatinib treatment response and outcome in chronic myeloid leukemia patients. Cancer Sci. 2014;105:105–9. doi: 10.1111/cas.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin D, Ibrahim AR, Goldman JM. European treatment and outcome study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol. 2011;29:3944–5. doi: 10.1200/JCO.2011.37.6962. [DOI] [PubMed] [Google Scholar]
- 16.Kuntegowdanahalli LC, Kanakasetty GB, Thanky AH, Dasappa L, Jacob LA, Mallekavu SB, et al. Prognostic and predictive implications of Sokal, Euro and EUTOS scores in chronic myeloid leukaemia in the imatinib era-experience from a tertiary oncology centre in southern india. Ecancermedicalscience. 2016;10:679. doi: 10.3332/ecancer.2016.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 18.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan GL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 20.Peto R, Pike MC. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973;29:579–84. [PubMed] [Google Scholar]
- 21.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European leukemiaNet. J Clin Oncol. 2009;27:6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luatti S, Castagnetti F, Marzocchi G, Baldazzi C, Gugliotta G, Iacobucci I, et al. Additional chromosomal abnormalities in Philadelphia-positive clone: Adverse prognostic influence on frontline imatinib therapy: A GIMEMA working party on CML analysis. Blood. 2012;120:761–7. doi: 10.1182/blood-2011-10-384651. [DOI] [PubMed] [Google Scholar]
- 23.Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J, et al. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2008;112:2024–7. doi: 10.1182/blood-2008-03-147744. [DOI] [PubMed] [Google Scholar]
- 24.Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. Acommon BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–8. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 25.de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: Incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–63. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 26.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 27.Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 28.Tardieu S, Brun-Strang C, Berthaud P, Michallet M, Guilhot F, Rousselot P, et al. Management of chronic myeloid leukemia in France: A multicentered cross-sectional study on 538 patients. Pharmacoepidemiol Drug Saf. 2005;14:545–53. doi: 10.1002/pds.1046. [DOI] [PubMed] [Google Scholar]
- 29.Bansal S, Prabhash K, Parikh P. Chronic myeloid Leukemia data from India. Indian J Med Paediatr Oncol. 2013;34:154–8. doi: 10.4103/0971-5851.123711. [DOI] [PMC free article] [PubMed] [Google Scholar]



