Abstract
Background and Aims:
Pulmonary aspiration of gastric contents is a serious complication of anaesthesia. The aim of this study was to determine, with the help of ultrasound, the gastric volume and content in fasted patients presenting for elective surgeries and correlate the results with fasting times and co-morbidities of the patients.
Methods:
The study was conducted in 100 adult patients presenting for elective surgery. A preoperative bedside gastric ultrasound scan was done in supine and right lateral position. Gastric contents were noted, and gastric volume was calculated at the level of the gastric antrum. Gastric volume was estimated by measuring antral cross-sectional area (CSA) and using a mathematical model. Gastric volume in the right lateral decubitus (RLD) position was taken as the final reading. Analysis of variance and Student's t-test were done for statistical significance and P < 0.05 was considered statistically significant.
Results:
Six out of 100 patients had solid gastric contents and 16 had >1.5 ml/kg clear liquids, although they had been fasting between 10 and 15 hours. Patients suffering from diabetes and chronic kidney disease had statistically significant increase in CSA in both supine and RLD. We also found increase in estimated gastric volume as the BMI of the patients increased.
Conclusion:
Our study showed that fasting for more than 6–10 hours does not guarantee an empty stomach. Those with co-morbidities like diabetes mellitus, obesity and chronic kidney disease (CKD) appear more prone to have unsafe gastric contents.
Key words: Gastric antrum, gastric content, gastric ultrasound, gastric volume, point-of-care, ultrasound
INTRODUCTION
Perioperative aspiration of gastric contents is a serious complication of anaesthesia and is associated with high morbidity and mortality.[1,2] It can result in severe pneumonia requiring mechanical ventilator support in up to one-third of patients with a mortality of 5%, representing up to 9% of all anaesthesia-related deaths.[3,4] The severity of the resulting respiratory compromise is thought to be related to both the volume and nature of the aspirate, with particulate matter carrying the highest risk.[5] Preoperative fasting guidelines help limit the risk in elective patients with minimal co-morbidities. However, fasting intervals are not applicable or reliable in urgent or emergency surgeries or for patients with certain medical conditions.
In anaesthesiology and acute care medicine, there is a growing interest in bedside evaluation of gastric ‘fullness’ to assess pulmonary aspiration risk. With the advent of portable ultrasound machines, performing point-of-care ultrasound has become relatively easy and feasible. Gastric ultrasound examination is finding a place as a point-of-care tool for aspiration risk assessment. It can identify the nature of the gastric content, i.e., empty, clear fluid and solid and when clear fluid is present, its volume can be quantified.[6]
The aim of the study was to examine the gastric contents and volume in fasting patients prior to elective surgery by using bedside ultrasound. The primary objective was to see if there is any correlation between fasting times and gastric content and estimated gastric volume in patients presenting for elective surgery. The secondary objective was to see if the gastric contents and gastric volume were any different in patients with conditions which supposedly predispose them to delayed gastric emptying like diabetes, chronic kidney disease and obesity compared to those without such conditions.
METHODS
This prospective observational correlation study was approved by the Institutional Ethics Committee. Ultrasound abdomen is not usually a premise of anaesthesiologists and to chart this new territory, we did practice gastric content and volume identification on normal volunteers with varying fasting times. We also took the help of a radiologist on performing the ultrasound abdomen and confirming our findings for the first 20 cases until we were deemed proficient to continue on our own. For the subsequent cases, we sent the ultrasonography images to a radiologist for reconfirmation of our findings.
The study was conducted between 1 June 2016 and 1 March 2017 in 100 adult (both male and female) patients aged between 18 and 80 years of American Society of Anesthesiology (ASA) physical status 1–3, presenting for elective surgeries who had fasted for more than 6 h. Pregnant women, morbidly obese and patients unable to turn and lie in lateral position were excluded from the study. Patients satisfying the inclusion and exclusion criteria were identified in the ward. They were given information about the gastric ultrasound examination study. A written informed consent was obtained. A brief history was taken from each patient regarding co-morbidities like diabetes mellitus (DM), chronic kidney disease and medication history. Patient's age, height, body weight, ASA grading, preoperative VAS score and number of hours of fasting were also noted. None of the patients were given any premedication.
The ultrasound examination was performed in the pre-surgical waiting area in the operation theatre complex prior to being wheeled into the operation theatre.
SonoSite M-Turbo® portable ultrasound machine was used to conduct the study. A curved array low frequency (2–5 MHz) probe was used in abdominal scan mode settings. The epigastrium was scanned in a sagittal plane sweeping the transducer from the left to right subcostal margins. Scanning was first done in the supine position followed by scanning in the right lateral position [Figure 1].
Figure 1.
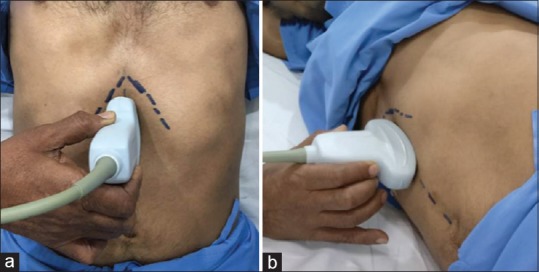
Scanning positions: (a) supine and (b) right lateral
The gastric antrum was identified just below the left lobe of liver and pancreas where the aorta/superior mesenteric artery act as important landmarks. A still image of the antrum was then taken between peristaltic contractions [Figure 2].
Figure 2.
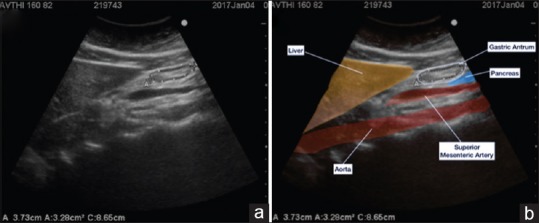
Still image of the antrum (empty with bulls eye appearance) (a) and coloured depiction of organs and cross sectional area calculation by using the free tracing tool (b)
According to the appearance on the ultrasound, the contents were identified as either empty, clear liquids, or solids [Figure 3]. Cross-sectional area (CSA) was measured using the free-hand tracing tool built into the SonoSite ultrasound machine. Gastric volume was calculated using the formula described by Perlas et al.[7]
Figure 3.
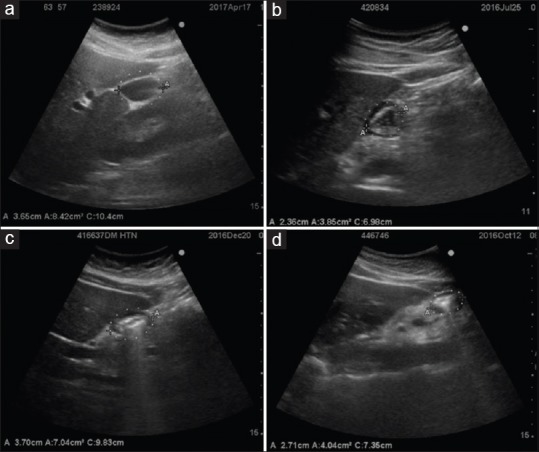
Appearance of gastric antrum with clear liquids (a), clear liquids with gas (starry sky appearance) (b), solids with blurring of posterior wall (c) and frank solids (d)
Volume (ml) = 27.0 + 14.6 × right-lateral CSA − 1.28 × age.[7]
For an outcome variable on correlation of gastric volume with CSA in right lateral decubitus position (RLD) with minimum correlation of 0.50, 90% statistical power and 5% level of significance, a sample size of 60 was calculated. However, we were able to study 100 patients in the study duration. Analysis of variance was used to find the significance of study parameters between three or more groups of patients; Student's t-test (two tailed, independent) was used to find the significance of study parameters on continuous scale between two groups (inter-group analysis) on metric parameters. Chi-square/Fisher exact test was used to find the significance of study parameters on categorical scale between two or more groups, non-parametric setting for qualitative data analysis. The statistical software, namely SPSS 18.0, and R environment ver. 3.2.2 were used for the analysis of the data, and Microsoft Word and Excel have been used to generate graphs, tables etc.
RESULTS
The results presented are from the study conducted in 100 adult patients who had fasted for more than 6 hours.
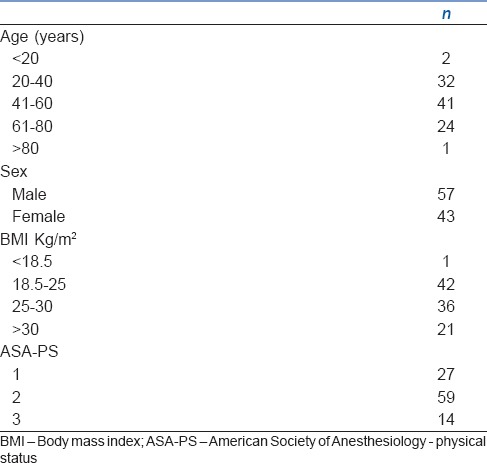
Demographic characteristics like age, sex and BMI along with the ASA grading of the patients are shown in Table 1.
Table 1.
Demographic characteristics
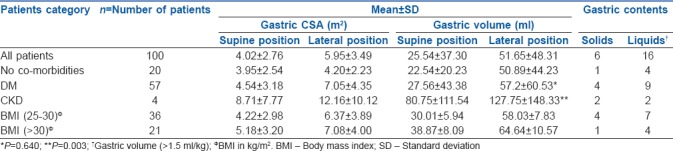
In the supine position when gastric content was assessed, 15% of patients had an empty antrum, 82% of patients had clear liquids and 3% of patients had solid contents. In the right lateral position, 9% of patients had an empty antrum, 85% of patients had clear liquids and 6% of patients had solid contents.
Of the six patients who had solids, one had no co-morbidities, two were diabetics, two had both DM and CKD and one was obese with a BMI of 35.
Gastric volume assessment in the supine position showed that 70% of patients had a gastric volume of less than 40 ml, 22% of patients had gastric volumes between 40 and 80 ml and 8% of patients had gastric volumes of more than 80 ml. In the right lateral position, 40% of patients had gastric volume less than 40 ml, 42% of patients had gastric volumes between 40 and 80 ml and 18% of patients had gastric volumes of more than 80 ml.
Sixteen patients had significant amount of clear liquids, that is more than 1.5 ml/kg. (The critical volume threshold of gastric fluid that by itself increases aspiration risk is considered to be above 1.5 ml/kg.)[8] Eleven of these patients were diabetics, two patients had CKD, one was obese and two had no apparent co-morbidities.
There was a significant increase in the CSA in both supine and right lateral positions in patients suffering from DM, as compared to non-DM patients though there was no significant difference between the fasting times (P-value in the supine position was 0.012 and in the right lateral position was 0.007). In the supine position, there was no significant difference in the volumes but the variation in volumes in supine position was more in DM patients as compared to non-DM patients. In the right lateral position, the volume was more than in the supine position but not statistically significant.
There was a significant difference in antral CSA (P = 0.001) as well as calculated gastric volume in both supine and right lateral positions (P = 0.003) in patients suffering from CKD as compared to patients with no co-morbidities though there was no significant difference between the fasting hours.
In our study, 21 patients were obese (BMI >30 kg/m2). We found that as the BMI increased from 25 to 35, there was a steady rise in the CSA in both supine and right lateral position. The increase of CSA in the supine position was statistically significant with the P value being 0.026. Similarly, when we compared the volumes, there was a steady rise in the volumes in both the positions, though the difference was not statistically significant.
The change in gastric volume from supine to RLD position was statistically significant in each subgroup as well as when they were analysed together. The P value being 0.001 in patients with DM, 0.088 in patients with chronic kidney disease, 0.001 in patients with increased BMI and 0.001 when all the patients were analysed together. Table 2 depicts the comparison of gastric volumes and gastric contents in patients with and without significant co-morbidities.
Table 2.
Comparison of gastric volumes and gastric contents in patients with and without significant co-morbidities
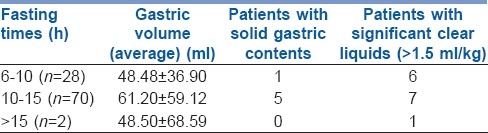
In our study, 28% patients had fasted between 6 and 10 h, 70% had fasted between 10 and 15 h and 2% patients had fasted for more than 15 h. Table 3 depicts the correlation of gastric volumes and gastric content with respect to fasting hours.
Table 3.
Correlation of gastric volumes and gastric content with respect to fasting hours
DISCUSSION
A full stomach prior to general anaesthesia has been widely recognised as a cause for concern ever since Mendelson, in 1946, described his dreaded ‘Mendelson's syndrome’ or acid aspiration syndrome.[9] This can lead to severe aspiration pneumonia with a mortality of up to 5%, representing up to 9% of all anaesthesia-related deaths.[3,4]
Patients presenting for urgent surgical procedures are usually not fasted and they may have significant gastric content despite long periods of fasting. Aspiration and the severity of the resulting respiratory compromise is thought to be related to both the volume and nature of the aspirate (content), with particulate matter carrying the highest risk.[10,11,12]
In the absence of data, it was considered safer to assume a ‘full stomach’ in emergency situations leading to either surgical cancellations, re-scheduling in elective cases or modification of interventions to prevent aspiration, such as a rapid sequence induction and tracheal intubation. In elective cases, preoperative fasting guidelines help limit the risk in patients with minimal co-morbidities. Most people believe that fasting a patient for more than 6 h places them in the ‘safe’ category for aspiration risk stratification.
Many techniques have been described to assess the contents of the stomach like paracetamol absorption, electrical impedance tomography, radio-labelled diet, polyethylene glycol dilution and gastric content aspiration.[13,14] These methods are not suitable in the perioperative period and none of these methods have proved foolproof or easy to use. However, with the advent of the newer portable ultrasound machines, one can accurately diagnose the presence of unsafe stomach contents non-invasively. It can help clinicians individualize aspiration risk at the bedside and guide anaesthetic management more appropriately.[13]
The present study was undertaken to see whether the assumption of 6 hrs providing adequate time for the stomach to empty was right both in patients who had no co-morbidities and in those with co-morbidities like DM, CKD and obesity. We used the ultrasound machine as the point-of-care tool as it was portable and easily available.
Initially, we encountered difficulties in identifying details of the stomach ultrasonographically. With practice, we found that the antrum is easy to identify once the left lobe of the liver, gall bladder and aortic/superior mesenteric artery pulsations are identified. Peristalsis in the antrum is confirmatory. An empty stomach may appear flattened, liquid is seen as hypoechoic, liquid and air as a ‘starry sky’ appearance and solids as frosted glass with blurring of the posterior wall [Figure 3].[15] Once learned, we found that the ultrasound is an easy non-invasive tool to use.
For proper risk stratification, scanning in both the supine and right lateral positions in all patients is very important.[16] The antrum may appear empty in the supine position but will appear fuller in the right lateral position. This apparent increase in gastric volume in the right lateral position is probably due to the gastric contents gravitating towards the gastric outlet.
When we compared the CSA and gastric volumes with respect to the fasting times, we found that patients who had fasted between 10 and 15 hrs had greater CSA and gastric volumes than patients who had fasted between 6 and 10 hrs. There is, therefore, no relationship between prolonged fasting and a safe gastric environment. Other authors have found that with prolonged fasting there is a decrease in the pH of gastric contents (pH <2.5) with increased gastric volumes due to increased secretion of gastric acid.[17,18,19] Six of our patients who had fasted for more than 6 hrs and eight who had fasted for more than 10 hrs had significant liquid (>1.5 ml/kg). Of the six patients in our study who had solid gastric contents, five had fasted for more than 10 hrs and one for 6–10 hrs. This would imply slow gastric emptying.
The American College of Gastroenterology states that patients suffering from diabetes, especially long standing diabetics, have gastroparesis.[20] In our study, there were 57 patients with DM and 43 non-diabetics. When we compared these two subgroups, both of whom had similar fasting times, the CSA in patients with diabetes was statistically higher (P = 0.012 and 0.007) compared to non-diabetics. However, the same did not translate when gastric volumes were estimated from the CSA. On further analysis, we realised that the gastric volume is calculated from a mathematical model which includes age as a parameter.[7] The average age of patients with DM was 58.8 years whereas that of non-diabetic patients was 38 years. This explains the disparity in gastric volumes and CSA [Table 2].
Patients suffering from chronic kidney disease show significant gastroparesis due to uraemia and they are expected to have increased gastric volumes despite fasting, making them prone to aspiration during sedation or general anaesthesia.[21] In our study, there were four patients suffering from chronic kidney disease all of whom had a statistically significant increase in the CSA and gastric volume in both supine and right lateral positions. However, as there were only four patients with CKD in our study, further evaluation with an appropriately powered study will need to be done before we can conclusively prove that patients with CKD have gastroparesis and make a recommendation for this subgroup.
Obese patients have increased gastric volumes despite fasting for more than 6 hrs.[22] In our study 25 patients were obese (BMI >30 kg/m2). We found that as the BMI increased from 25 to 35, there was a clinically significant rise in the gastric CSA in both supine and right lateral position [Table 2]. However, the mathematical model used does not include weight or BMI as a variable in the calculation of gastric volume.[7]
Gastric emptying is also affected by age, pain and preoperative medications.[22] We did a subgroup analysis to see if the patient's age, pain as assessed by VAS score and preoperative medication affected the gastric contents and volume. However, the numbers in each group were too small to have any clinical or statistical significance.
CONCLUSION
Our study showed that fasting for more than 6–10 hours does not guarantee an empty stomach irrespective of whether they have co-morbidities. Those with co-morbidities like DM, obesity and chronic kidney disease appear more prone to have unsafe gastric contents. The obese diabetic patients appear to be at greatest risk of having a full stomach.
It is therefore obvious that bedside ultrasound is an important tool in determining the status of stomach contents and could prove to be a tool used for stratification of aspiration risk. It would be useful in many clinical situations in which aspiration risk is unclear or undetermined and we hope it will eventually become a standard of care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank the staff of the Departments of Anaesthesia and Radiology for technical help and support.
REFERENCES
- 1.Neelakanta G, Chikyarappa A. A review of patients with pulmonary aspiration of gastric contents during anesthesia reported to the departmental quality assurance committee. J Clin Anesth. 2006;18:102–7. doi: 10.1016/j.jclinane.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Green SM, Krauss B. Pulmonary aspiration risk during emergency department procedural sedation – An examination of the role of fasting and sedation depth. Acad Emerg Med. 2002;9:35–42. doi: 10.1197/aemj.9.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Shime N, Ono A, Chihara E, Tanaka Y. Current status of pulmonary aspiration associated with general anesthesia: A Nationwide Survey in Japan. Masui. 2005;54:1177–85. [PubMed] [Google Scholar]
- 4.Lienhart A, Auroy Y, Péquignot F, Benhamou D, Warszawski J, Bovet M, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105:1087–97. doi: 10.1097/00000542-200612000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–71. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 6.Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–7. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 7.Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–63. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 8.Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;7:694–5. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 9.Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg. 2001;93:494–513. doi: 10.1097/00000539-200108000-00050. [DOI] [PubMed] [Google Scholar]
- 10.Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–9. doi: 10.1097/ALN.0b013e3181a97250. [DOI] [PubMed] [Google Scholar]
- 11.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Kozlow JH, Berenholtz SM, Garrett E, Dorman T, Pronovost PJ. Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999-2000. Crit Care Med. 2003;31:1930–7. doi: 10.1097/01.CCM.0000069738.73602.5F. [DOI] [PubMed] [Google Scholar]
- 13.Borland LM, Sereika SM, Woelfel SK, Saitz EW, Carrillo PA, Lupin JL, et al. Pulmonary aspiration in pediatric patients during general anesthesia: Incidence and outcome. J Clin Anesth. 1998;10:95–102. doi: 10.1016/s0952-8180(97)00250-x. [DOI] [PubMed] [Google Scholar]
- 14.Cubillos J, Tse C, Chan VW, Perlas A. Bedside ultrasound assessment of gastric content: An observational study. Can J Anaesth. 2012;59:416–23. doi: 10.1007/s12630-011-9661-9. [DOI] [PubMed] [Google Scholar]
- 15.Näslund E, Bogefors J, Grybäck P, Jacobsson H, Hellström PM. Gastric emptying: Comparison of scintigraphic, polyethylene glycol dilution, and paracetamol tracer assessment techniques. Scand J Gastroenterol. 2000;35:375–9. doi: 10.1080/003655200750023930. [DOI] [PubMed] [Google Scholar]
- 16.Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi: 10.1093/bja/aeu151. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar D, Mehta D. A comparative study of volume and P H of gastric fluid after ingestion of water and sugar-containing clear fluid in children. Indian J Anaesth. 2007;51:117–20. [Google Scholar]
- 18.Schmitz A, Kellenberger C, Lochbuehler N, Fruehauf M, Klaghofer R, Fruehauf H, et al. Effect of different quantities of a sugared clear fluid on gastric emptying and residual volume in children. Surv Anesthesiol. 2012;56:301–2. doi: 10.1093/bja/aer497. [DOI] [PubMed] [Google Scholar]
- 19.Hussain R, Nazeer T, Aziz N, Ali M. Effects of fasting intervals on gastric volume and pH. Pak J Med Health Sci. 2011;5:582–6. [Google Scholar]
- 20.De Schoenmakere G, Vanholder R, Rottey S, Duym P, Lameire N. Relationship between gastric emptying and clinical and biochemical factors in chronic haemodialysis patients. Nephrol Dial Transplant. 2001;16:1850–5. doi: 10.1093/ndt/16.9.1850. [DOI] [PubMed] [Google Scholar]
- 21.Hirata ES, Mesquita MA, Alves Filho G, Camargo EE. Gastric emptying study by scintigraphy in patients with chronic renal failure. Rev Bras Anestesiol. 2012;62:39–47. doi: 10.1016/S0034-7094(12)70101-0. [DOI] [PubMed] [Google Scholar]
- 22.Kruisselbrink R, Arzola C, Jackson T, Okrainec A, Chan V, Perlas A, et al. Ultrasound assessment of gastric volume in severely obese individuals: A validation study. Br J Anaesth. 2017;118:77–82. doi: 10.1093/bja/aew400. [DOI] [PubMed] [Google Scholar]


