Abstract
Background and Aims:
An audit was conducted between July 2017 and November 2017 to assess the adequacy of American Society of Anesthesiologists (ASA) fasting guidelines on 246 patients by means of gastric ultrasonography (USG). The relevance of this audit is that many of our patients have one or more risk factors for aspiration such as diabetes mellitus, chronic kidney disease (CKD), gastro-oesophageal reflux disease (GERD), and obesity.
Methods:
This audit was a prospective observational study which included all patients posted for surgery within the audit period. Patients were fasted according to ASA fasting guidelines. Their gastric content was assessed preoperatively using USG. The residual gastric volume was calculated using a validated formula. Statistical correlation between gastric volumes and the risk factors were analysed.
Results:
Of 246 patients, 69 (28.04%) had high residual gastric volume. We found no correlation between hours of fasting and residual gastric volume (P = 0.47). We found a linear correlation between rising body mass index and residual gastric volume (P < 0.0001). Patients with GERD had 2.3 times higher risk. The CKD patient subgroup had 24 patients (30%) with high residual gastric volume. No incidents of aspiration were noted.
Conclusion:
In our audit, we found that risk factor association has a greater effect on residual gastric volume than hours of fasting. While the current fasting guidelines are adequate for healthy individuals, they are not conclusive in patients with risk factors. Ultrasound assessment of preoperative gastric volume is an effective screening tool in patients with risk factors.
Key words: Gastrointestinal contents, fasting, risk assessment, standard, ultrasonography
INTRODUCTION
Along the evolutionary journey of anaesthesiology, Mendelson has played a major role in being the first to describe aspiration of gastric contents with fatal consequences.[1] Aspiration of gastric contents has been described to occur in one out of every 2000–3000 operations,[2] with a greater risk in parturients, obese patients, chronic liver disease, chronic kidney disease (CKD), gastroesophageal reflux disease (GERD), diabetes mellitus (DM), neuromuscular disease, patients posted for emergency procedures, and patients who have not followed fasting guidelines.[3,4,5,6]
This audit was conducted to assess the effectiveness of American Society of Anesthesiologists (ASA) fasting guidelines in our patients particularly because 31.88% of our patient population had more than one risk factor for aspiration.
METHODS
This study was conducted as a part of an internal audit for which ethics committee approval was obtained. This was a prospective observational study conducted from 1 July 2017 to 30 November 2017. Inclusion criteria were patients who were >18 years of age, ASA physical status 1–4, undergoing elective or emergency surgery, and those who had the ability to understand and were willing to participate in the study. Exclusion criteria were pregnancy, recent upper gastrointestinal bleed, or upper gastrointestinal surgery.
All patients for elective surgery were fasted according to ASA fasting guidelines, that is, 8 h after fatty solids, 6 h after a light meal, and 2 h after clear liquids. The number of hours of preoperative fasting was noted for all emergency procedures. Gastric ultrasound examination was performed after the patient was shifted into the operation theater before administration of any premedication. Assessment was performed by anaesthesiologists who have had the experience of doing more than 40 gastric ultrasound examinations as a part of their learning curve which was steadfastly supervised by the radiology team. All ultrasound examinations were done with a General Electric Venue 40 system with a curvilinear array probe with a frequency of 4 Hz.
Ultrasound assessment was initially done in the supine and right lateral decubitus (RLD) positions. The gastric antrum was identified in the sagittal plane in the epigastric region. The liver was identified at a cephalad position to the gastric antrum and the aortic pulsation was identified posteriorly. Colon was identified in the posterior and lateral quadrant. An ellipse was drawn to include the visualized gastric antrum circumferentially, including the serosa [Figure 1]. The final cross-sectional area (CSA) was taken as an average of three readings, in between two peristaltic contractions when the antrum was at rest. Qualitative assessment was done by classifying the antral content into clear, particulate, or solid. Quantitative assessment was done by the measurement of the CSA of the antrum which was then applied in the formula to arrive at the estimated gastric volume. This formula has been proved to be valid in obese and pregnant population.
Figure 1.
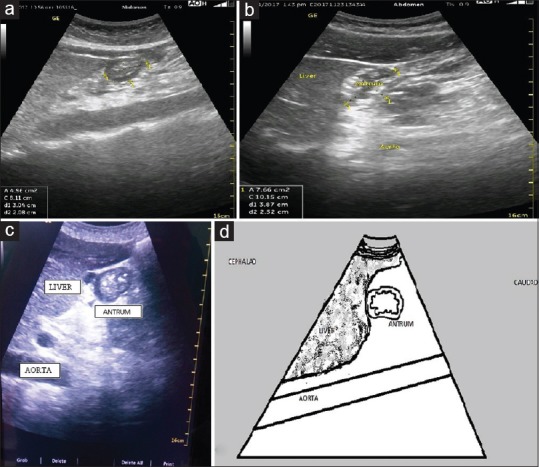
Sonoanatomy of the gastric antrum. (a and c) Solid antral contents, (b) empty antrum with gastric rugosities visible, and (d) schematic line diagram
Volume (mL) = 27.0 + 14.6 × RLD CSA -1.28 × age.
Where RLD CSA represents CSA of gastric antrum in the RLD position.
The main outcome measure was to identify those patients who were at higher risk of aspiration through both qualitative and quantitative assessment of residual gastric volume. All patients who had particulate or solid content on qualitative assessment and/or more than 1.5 mL/kg residual gastric volume on quantitative assessment were classified as having high risk for aspiration. Risk factors such as history of GERD, obesity, DM, CKD, or a combination of the above and their association with increased residual gastric volume studied.
This study was designed as an internal audit of existing practice, and therefore, a formal sample size calculation was not done. Statistical analysis was done with Statistical Packages for Social Sciences (SPSS) software version 23.0. Univariate and multivariate regression analyses were done to assess the association between the various risk factors, with risk of aspiration.
RESULTS
Of 246 patients, 164 were male and 82 were female. The average age was 52.74 ± 17.60 years, and average body mass index was 24.82 ± 6.74 kg/m2. While 212 patients (86.17%) were taken up for elective surgical procedures, 34 patients (13.83%) were taken up for emergency surgical procedures. The average number of hours of fasting was 7.75 h for elective procedures and 7.345 h for emergency procedures. No correlation was found between hours of fasting and residual gastric volume (P = 0.47). Patients who were taken up first on the elective operative list were fasted for at least 8 h after unspecified solid meal and those who were taken up subsequently during the day were fasted for 6 h after a light meal. Thus, the mean fasting duration was 7.75 h in elective surgeries.
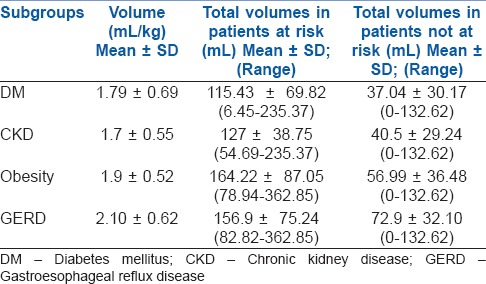
In all, 69 patients were found to be at risk for aspiration of gastric contents on qualitative or quantitative assessment. On qualitative assessment, 23 patients of these 69 had particulate matter and 2 patients had solid content. On quantitative assessment, the average gastric volume of patients who were not at risk was 0.56 mL/kg body weight. The at-risk population had an average residual volume of 1.89 mL/kg body weight. Patients with GERD presented with highest residual volume of 2.10 mL/kg body weight. Patients who had both CKD and DM presented with a residual gastric volume of 1.91 mL/kg, which is almost similar to patients with obesity. Patients with CKD presented with an average residual volume of 1.75 mL/kg body weight. Refer to Graph 1 and Table 1. The presence of even one risk factor significantly increases the residual gastric volume despite adequate hours of fasting. The average gastric volume exceeded the safe limit in all the risk factor categories studied. Statistical significance was only found for patients who had GERD and obesity. Of the 69 patients in the at-risk group, 24 (34.78%) had CKD. Although statistically insignificant (P = 0.65), these patients had gastric volume significant enough to put them at risk for aspiration. None of our patients developed any features of aspiration during the audit period.
Graph 1.
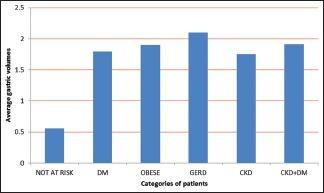
Average gastric volumes (mL/kg) in various categories of patients
Table 1.
Gastric volumes in various subgroups
Upon analysis of the other risk factors, 27.8% of obese patients versus 11.4% of nonobese patients were at risk of aspiration (P < 0.007). Upon univariate regression analysis between BMI and gastric contents, a strong linear association was found in all the patients in our study (P = 0.0003). This association was even stronger in the obese patient subgroup (P < 0.0001). Refer to Graph 2. Patients with higher BMI were 1.07 times more at risk for aspiration.
Graph 2.
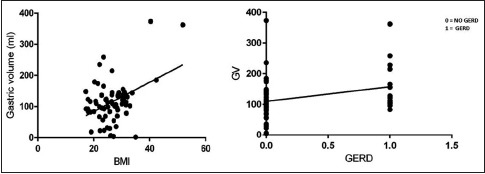
Correlation of body mass index and gastroesophageal reflux disease with residual gastric volumes
We also found that there is a positive association between GERD and risk of aspiration (P < 0.037). In the study, 28.2% of patients with GERD versus 14.1% patients without GERD were at risk of aspiration. Patients with GERD were 2.3 times more at risk for aspiration. DM (P = 0.8933), CKD (P = 0.5996), and number of hours of fasting (P = 0.47) did not show any association with risk of aspiration in our study. Multivariate regression analysis was performed for risk factors of age, GERD, and obesity. The results are displayed in Table 2.
Table 2.
Multivariate regressional analysis with age, body mass index, and gastroesophageal reflux disease as independent variables

DISCUSSION
It has been a standard anaesthetic practice in the precedent decades to fast patients posted for surgery for a said period of time and then proceed with anaesthesia under the assumption that adequate gastric emptying has occurred. It is imperative to change this hitherto acceptable practice, as the anaesthetic mortality related to aspiration of gastric contents is 9%.[7] Our data questions the validity of fasting duration in predicting the adequacy of gastric emptying.
Ultrasonography (USG) has become an indispensible tool in the evolution of anaesthetic practice after having been extensively explored over the past few decades. It has evolved to become a point of care standard, the importance of which cannot be overemphasized.[8,9] Ultrasound assessment of gastric contents is a relatively recent addition to the anaesthetic practice which has seen an upsurge only in the past decade. Bedside ultrasound examination has emerged as a fast, reliable, easily reproducible, and noninvasive tool to assess the gastric contents of a patient posted for surgery.[10,11,12,13] It presents itself as a skill that can be mastered easily, and prompt acquisition of this skill can be noted after as few as 33 supervised USG examinations.[13] The clinical utility of gastric ultrasound apart from risk stratification of patients is its reliability in clinical decision-making, especially in emergency situations or with a patient who has not followed fasting guidelines.[14] It has been proven to be as reliable as gastric scintigraphy for volume assessment which is known to be the gold standard for gastric content assessment.[15] The formula that we have used for estimation of gastric volume has been tested in various populations[10] and found to provide a reasonably accurate estimate, with a 95% confidence interval.[4] The mathematical model used has already been validated in morbidly obese patients and parturients.[4,16] There have been varying opinions on what amount of gastric volume constitutes risk for aspiration. Earlier, a volume of >0.8 mL/kg was considered as high risk for aspiration.[11] However, the more prevalent opinion states that a residual gastric volume of >1.5 mL/kg[12] puts a patient at a high risk for aspiration. We have chosen the latter to evaluate our results. Qualitative analysis of the gastric contents has been widely described and discussed.[17]
The purpose of this audit was to assess the adequacy of standard ASA fasting guidelines in our special population with multiple risk factors. The fasting guidelines, despite extensive revisions, have failed to provide clarity on whether they are equally applicable in all comorbidities.[18] We are entering an era of outpatient surgeries where patients are admitted just before surgery and discharged just after surgery. It is also imperative that our patients be safe, comfortable, and pain-free. Accordingly, modern anaesthesia practice has come a long way from strict fasting overnight to allowing clear fluids until 2 h before surgery.[18] This has been found to effectively combat irritability, hunger, and anxiety in the perioperative period without increasing the risk of aspiration in patients without additional risk factors.[19] It is known that increasing the number of hours of preoperative fasting maybe counterproductive in terms of patient morbidity, namely, glycaemic control and hydration. Hence, we propose that all patients be screened routinely by means of a gastric ultrasound and additional measures be taken for patients who are at risk for aspiration. Specific goal-directed therapy with gastric-acid-neutralizing agents and measures to decrease the gastric contents will prove beneficial. Studies have shown that preoperative ranitidine or pantoprazole 1 h prior to surgery significantly reduces gastric volume and pH.[20] Although the role of prokinetics in healthy patients to improve gastric emptying has been established, their role in patients with gastroparesis is unclear, although some centers do report a improvement in gastrointestinal symptoms in patients with gastroparesis with use of prokinetics.[21]
The incidence of delayed gastric emptying in the general population after an 8-h fast is 3.5%.[22] The results of our study point to a very high incidence (28.04%) of patients who were potentially at risk for aspiration. The likely explanation for the above is that 31.88% of patients had multiple risk factors. A detailed risk factor analysis is provided in Table 3. Despite the presence of higher residual gastric volume and multiple risk factors, no patient had any aspiration of gastric contents. This may be explained by the fact that the majority of the procedures were elective (86.17%) and were performed under regional anaesthesia (43%). Even the patients posted for emergency surgery were fasted for at least 6 h for a light meal, with three exceptions.
Table 3.
Detailed risk factor analysis of number of risk factors
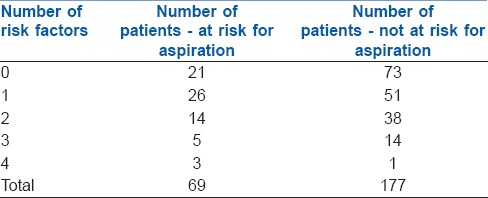
The cardinal question which then arises is the identification of the high-risk population and devising an appropriate plan for mitigation of risk when encountered with an adequately fasted patient with a high residual gastric volume. The plan of anaesthesia may be modified on a case-to-case basis especially in emergency procedures, patients who are obese, have a history of GERD, DM, and CKD.
According to the World Health Organization guidelines, any patient with a BMI >30 kg/m2 was considered obese.[23,24] In our study, we had 33 patients with a BMI >30 kg/m2 and five patients with a BMI >35. The average gastric volume in this group was 1.9 mL/kg body weight and there was a significant linear relationship between increasing BMI and gastric volume (P = 0.0003). This presents as a particularly worrisome situation in the face of sharply rising worldwide incidence of childhood and adult obesity.[25]
We had 43 patients who had history suggestive of GERD. They had significant residual gastric volume averaging at 2.10 mL/kg body weight. Incidence of gastric aspiration in patients with this specific risk factor ranges from 7.7% to 16%.[26] This was the group with the highest residual gastric volume out of all the risk factors studied. Therefore, it is essential that patients with established reflux disease receive preemptive measures routinely.
In our study population of 246 patients, 100 were diabetics and 27 had significant residual gastric volume averaging at 1.79 mL/kg. DM has been recognized to cause gastroparesis with an incidence ranging from 9.9% to 76%.[27] There a few reports on the use of metoclopramide in diabetic patients posted for surgery with benefit.[27]
Gastroparesis has been described classically as a part of the symptomatology of CKD due to uremia, autonomic neuropathy, and chronic inflammation associated with other comorbidities.[28] Some reports have questioned the applicability of the current fasting guidelines in patients with CKD.[29] A study done on patients with CKD reported that 16.6% of patients had significant gastric volume after 6 h of fasting.[29] Another study reported a prevalence of delayed gastric emptying in CKD to be as high as 34%.[28] Both these studies were conducted on outpatients coming for haemodialysis. In our audit, which was conducted on patients posted for surgery, 34.78% of patients with CKD had significant residual gastric volume averaging at 1.75 mL/kg body weight. It must be noted that one in three patients with CKD remain at risk for aspiration. What is interesting to note is that patients who had a combination of DM and CKD had a higher average residual gastric volume of 1.91 mL/kg body weight.
There is a paucity of literature on ultrasonographic assessment of residual gastric volume in the perioperative setting in patients with risk factors such as DM, CKD, or GERD. Most studies have been conducted on groups of patients ranging from as small a group as six to as large as 200 patients.[11,23] The prevalence of CKD in our patients was 32.52%. The overall prevalence of CKD in India ranges from 15% to 17% according to various studies.[30] Thus, there is a palpable need for more studies in this particular technique of risk assessment.
There are some limitations of this study. It was basically designed as an audit to assess the adequacy of our institutional fasting guidelines. Therefore, all patients who presented for surgery during this period were included in the audit without any formal calculation of sample size. Further research on this topic is warranted in larger groups of patients and in the form of randomised controlled trials. With more studies in this field, there is perhaps an exciting pathbreaking potential to change the existing practice where surgeries may be decided based on gastric ultrasound and guidelines may include this modality as an assessment tool.
CONCLUSION
While standard fasting guidelines are adequate in healthy patients without any risk factors, they are not conclusive in patients with additional risk factors. We propose that gastric ultrasound needs to become a standard of care, particularly in patients with additional risk factors for gastroparesis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 2.Nason KS. Acute intraoperative pulmonary aspiration. Thorac Surg Clin. 2015;25:301–7. doi: 10.1016/j.thorsurg.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jay L, Zieleskiewicz L, Desgranges FP, Cogniat B, Pop M, Boucher P, et al. Determination of a cut-off value of antral area measured in the supine position for the fast diagnosis of an empty stomach in the parturient: A prospective cohort study. Eur J Anaesthesiol. 2017;34:150–7. doi: 10.1097/EJA.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 4.Kruisselbrink R, Arzola C, Jackson T, Okrainec A, Chan V, Perlas A, et al. Ultrasound assessment of gastric volume in severely obese individuals: A validation study. Br J Anaesth. 2017;118:77–82. doi: 10.1093/bja/aew400. [DOI] [PubMed] [Google Scholar]
- 5.Smith G, Ng A. Gastric reflux and pulmonary aspiration in anaesthesia. Minerva Anestesiol. 2003;69:402–6. [PubMed] [Google Scholar]
- 6.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Lienhart A, Auroy Y, Péquignot F, Benhamou D, Warszawski J, Bovet M, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105:1087–97. doi: 10.1097/00000542-200612000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Jain PN, Ranganathan P. Ultrasound in anaesthesia. Indian J Anaesth. 2007;51:176–83. [Google Scholar]
- 9.Gupta PK, Gupta K, Dwivedi AN, Jain M. Potential role of ultrasound in anesthesia and intensive care. Anesth Essays Res. 2011;5:11–9. doi: 10.4103/0259-1162.84172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruisselbrink R, Arzola C, Endersby R, Tse C, Chan V, Perlas A, et al. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;121:46–51. doi: 10.1097/ALN.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 11.Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–92. doi: 10.1097/ALN.0b013e31820dee48. [DOI] [PubMed] [Google Scholar]
- 12.Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi: 10.1093/bja/aeu151. [DOI] [PubMed] [Google Scholar]
- 13.Arzola C, Carvalho JC, Cubillos J, Ye XY, Perlas A. Anesthesiologists' learning curves for bedside qualitative ultrasound assessment of gastric content: A cohort study. Can J Anaesth. 2013;60:771–9. doi: 10.1007/s12630-013-9974-y. [DOI] [PubMed] [Google Scholar]
- 14.Alakkad H, Kruisselbrink R, Chin KJ, Niazi AU, Abbas S, Chan VW, et al. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: A prospective case series. Can J Anaesth. 2015;62:1188–95. doi: 10.1007/s12630-015-0449-1. [DOI] [PubMed] [Google Scholar]
- 15.Darwiche G, Björgell O, Thorsson O, Almér LO. Correlation between simultaneous scintigraphic and ultrasonographic measurement of gastric emptying in patients with type 1 diabetes mellitus. J Ultrasound Med. 2003;22:459–66. doi: 10.7863/jum.2003.22.5.459. [DOI] [PubMed] [Google Scholar]
- 16.Arzola C, Perlas A, Siddiqui NT, Carvalho JC. Bedside gastric ultrasonography in term pregnant women before elective cesarean delivery: A prospective cohort study. Anesth Analg. 2015;121:752–8. doi: 10.1213/ANE.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 17.Cubillos J, Tse C, Chan VW, Perlas A. Bedside ultrasound assessment of gastric content: An observational study. Can J Anaesth. 2012;59:416–23. doi: 10.1007/s12630-011-9661-9. [DOI] [PubMed] [Google Scholar]
- 18.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126:376–93. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 19.Subrahmanyam M, Venugopal M. Perioperative fasting: A time to relook. Indian J Anaesth. 2010;54:374–5. doi: 10.4103/0019-5049.71021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Memiş D, Turan A, Karamanlioglu B, Saral P, Türe M, Pamukçu Z, et al. The effect of intravenous pantoprazole and ranitidine for improving preoperative gastric fluid properties in adults undergoing elective surgery. Anesth Analg. 2003;97:1360–3. doi: 10.1213/01.ANE.0000086895.64140.B3. [DOI] [PubMed] [Google Scholar]
- 21.Silvers D, Kipnes M, Broadstone V, Patterson D, Quigley EM, McCallum R, et al. Domperidone in the management of symptoms of diabetic gastroparesis: Efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. DOM-USA-5 study group. Clin Ther. 1998;20:438–53. doi: 10.1016/s0149-2918(98)80054-4. [DOI] [PubMed] [Google Scholar]
- 22.Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–7. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 23.Kivimäki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2:e277–85. doi: 10.1016/S2468-2667(17)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barak N, Ehrenpreis ED, Harrison JR, Sitrin MD. Gastro-oesophageal reflux disease in obesity: Pathophysiological and therapeutic considerations. Obes Rev. 2002;3:9–15. doi: 10.1046/j.1467-789x.2002.00049.x. [DOI] [PubMed] [Google Scholar]
- 25.Parikh NI, Pencina MJ, Wang TJ, Lanier KJ, Fox CS, D'Agostino RB, et al. Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med. 2007;120:242–50. doi: 10.1016/j.amjmed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Hardy JF. Large volume gastroesophageal reflux: A rationale for risk reduction in the perioperative period. Can J Anaesth. 1988;35:162–73. doi: 10.1007/BF03010658. [DOI] [PubMed] [Google Scholar]
- 27.Jellish WS, Kartha V, Fluder E, Slogoff S. Effect of metoclopramide on gastric fluid volumes in diabetic patients who have fasted before elective surgery. Anesthesiology. 2005;102:904–9. doi: 10.1097/00000542-200505000-00007. [DOI] [PubMed] [Google Scholar]
- 28.De Schoenmakere G, Vanholder R, Rottey S, Duym P, Lameire N. Relationship between gastric emptying and clinical and biochemical factors in chronic haemodialysis patients. Nephrol Dial Transplant. 2001;16:1850–5. doi: 10.1093/ndt/16.9.1850. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Liu L, Wang CY, Choi SW, Yuen VM. A pilot study of ultrasound evaluation of gastric emptying in patients with end-stage renal failure: A comparison with healthy controls. Anaesthesia. 2017;72:714–8. doi: 10.1111/anae.13869. [DOI] [PubMed] [Google Scholar]
- 30.Singh AK, Farag YM, Mittal BV, Subramanian KK, Reddy SR, Acharya VN, et al. Epidemiology and risk factors of chronic kidney disease in India – Results from the SEEK (Screening and early evaluation of kidney disease) study. BMC Nephrol. 2013;14:114. doi: 10.1186/1471-2369-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]


