Abstract
Ginseng has been traditionally used for several millennia in Asian countries, including Korea, China, and Japan, not only as a nourishing and tonifying agent but also as a therapeutic agent for a variety of diseases. In recent years, the various effects of red ginseng including immunity improvement, fatigue relief, memory improvement, blood circulation improvement, antioxidation, mitigation of menopausal women's symptoms, and anticancer an effect have been reported in clinical as well as basic research. Around the world, there is a trend of the rising consumption of health functional foods on the level of disease prevention along with increased interest in maintaining health because of population aging and the awareness of lifestyle diseases and chronic diseases. Red ginseng occupies an important position as a health functional food. But till now, international ginseng monographs including those of the World Health Organization have been based on data on white ginseng and have mentioned red ginseng only partly. Therefore, the red ginseng monograph is needed for component of red ginseng, functionality certified as a health functional food in the Korea Food and Drug Administration, major efficacy, action mechanism, and safety. The present red ginseng monograph will contribute to providing accurate information on red ginseng to agencies, businesses, and consumers both in South Korea and abroad.
Keywords: Health functional foods, Red ginseng, Red ginseng monograph
1. Overview
Ginseng has been traditionally used for several millennia in Asian countries, including Korea, China, and Japan, not only as a nourishing and tonifying agent but also as a therapeutic agent for a variety of diseases including immune diseases, liver diseases, and cancer. Many researchers have scientifically proven its diverse effects through in vitro studies, animal experiment models, and clinical research. The efficacy of ginseng has been described as an adaptogen (substances that enhance the “state of nonspecific resistance” in stress) activity that maintains homeostasis by normalizing the overall function of the body by nonspecifically increasing the body's resistance to external stress [1], [2], [3]. Ginseng is a plant in the family Araliaceae and the genus Panax, with the scientific name of Panax ginseng Meyer. As a perennial, it is a neutral plant. “Raw ginseng” refers to freshly harvested ginseng. Red ginseng is manufactured by steaming the fresh ginseng without peeling the roots and then drying [4]. As for red ginseng, the types and content of ginsenosides, which are the unique components of ginseng, change during the process through which raw ginseng is steamed with vapor and dried. Polysaccharides, which take up the largest share among constituent components of ginseng, likewise change physically and chemically, and the gelatinization of starch makes long-term storage possible [5], [6], [7]. The functions of red ginseng as a health functional food have been certified with the enforcement of the Korean Health Functional Foods Act in 2004. Red ginseng powder and red ginseng extract rank first as health functional food materials. Claims for the immunity improvement, fatigue relief, blood circulation improvement (by preventing blood platelet aggregation), memory improvement, antioxidation, and improvement of menopausal women's symptoms functions of Korean red ginseng (KRG) have been approved by the Korean Food and Drug Administration (KFDA) [4]. Around the world, there is a trend of the rising consumption of health functional foods on the level of disease prevention along with increased interest in maintaining health because of population aging and the awareness of lifestyle diseases and chronic diseases. Red ginseng occupies an important position as a health functional food. But till now, international ginseng monographs including those of the World Health Organization (WHO) have been based on data on white ginseng and have mentioned red ginseng only partly [8], [9], [10], [11]. Therefore, the red ginseng monograph is needed for component of red ginseng, functionality certified as a health functional food in KFDA, major efficacy, action mechanism, and safety. The present red ginseng monograph will contribute to providing accurate information on red ginseng to agencies, businesses, and consumers both in South Korea and abroad.
2. Characteristics and standards/specifications
2.1. Originating plant
The scientific name of ginseng is Panax ginseng C. A. Meyer. A compound of the Greek words pan (“all”) and axos (“cure”) in etymology, Panax means “curing all diseases”. “Ginseng” is understood as a name given because the roots of the plant resemble the human figure. The most widely used ginseng species worldwide are Korean ginseng (Panax ginseng), which is native to the Korean Peninsula and northern China, and American ginseng (Panax quinquefolius), which is native to the United States and Canada. In general, only Panax ginseng C. A. Meyer is called “Korean ginseng” or “ginseng.” Korean ginseng and American ginseng are plants of disparate species that differ in ginsenoside content patterns, and American ginseng does not contain ginsenoside Rf [5], [12]. Generally, ginseng roots aged 4 years or above are used for red ginseng because that is the historical usage. Studies have reported differences in the content and biological activity of ginsenosides according to the age of ginseng [13], [14].
2.2. Ginseng roots used as raw materials should conform to the “Ginseng Industrial Act” and should be aged more than 4 years
The dried ginseng seedlings, ginseng seedlings, dried ginseng skin, and ginseng cake cannot be used.
2.3. Crude drug names and common names
Crude drug name is ginseng radix rubra. Common names are “red ginseng,” “Korean Red Ginseng” (refers to a South Korean product produced from ginseng cultivated in the country through traditional methods), “hongsam,” and “hongshen”.
2.4. History of red ginseng production
There are records of red ginseng production in Korea from approximately a millennium ago, and red ginseng is presumed to have actually been produced from even earlier times. Red ginseng is widely known in Asia as a specialty produced for the first time worldwide in Korea by processing ginseng. As for documentary appearance of the term “red ginseng,” it is mentioned in the volumes of the Annals of the Joseon Dynasty (Joseon Wangjo Sillok) from the reign of King Jeongjo (1776–1800). The document describing the process of producing red ginseng through steaming is the Illustrated Record of the Chinese Embassy to Goryeo in the Xuanhe Era (Xuanhe Fengshi Gaoli Tujing), which was penned by Chinese envoy Xu Jing (Song Dynasty; 1091–1153) during the Goryeo Dynasty after a visit to this Korean kingdom [15]. The record describing red ginseng production methods in greater detail is the Collection of Writings by Master Sohodang (Sohodangjip), written by Gim Taeg-yeong (Joseon Dynasty; 1850–1927). Here, the author states that red ginseng is produced by washing, steaming, spreading out on bamboo colanders, and drying, with either the force of fire (the hot force that can be felt from fire) or sunlight, raw ginseng roots aged 6 years or above. As far in modern documents, the Handbook to Ginseng Management in the Empire of Korea (Han'guk Samjeong Yoram) records, raw ginseng aged 6 years or above is classified by size, directly steamed with vapor for 50–90 minutes according to the size, sufficiently dried in drying rooms, and dried in sunlight for 4–5 days [15]. Ginseng turns red as it undergoes these steaming and drying processes, thus leading it to be called “red ginseng.” The period 1908–1996 saw a state monopoly system in which ginseng was cultivated, and red ginseng was produced and sold under the government's strict management. Current red ginseng production techniques have been developed by modernizing and standardizing traditionally used red ginseng production methods.
2.5. Production methods
The red ginseng manufacturing process was registered with the International Organization for Standardization (ISO) and internationally certified in April 2017 (ISO 19610). Raw ginseng aged 4–6 years is classified according to the thickness of the taproots, washed, and cooked with vapor at 90–100°C for at least 80–100 minutes. Ginseng is then dried with hot wind at 45–55°C until the moisture content is 15.5% or below. Subsequently, it is dried in sunlight. After undergoing this manufacturing process, red ginseng takes on a light red to dark brown color.
2.6. Packaging
Red ginseng is classified into different grades according to its size, shape, and tissue compactness. It is then vacuum-packed and can-packed. Can-packing makes possible storage for over 10 years.
2.7. Characteristics
While raw ginseng contains 70% moisture, red ginseng contains 15.5% or below of moisture and has a light red to dark brown color. In the course of the steaming process, ginseng starch is gelatinized, causing an increase in tissue compactness in main roots to lateral roots. Red ginsengs are graded into Chun-sam, Ji-sam, and Yang-sam according to their firmness of rhizome, proportion of taproots to lateral roots, colors, characteristics of body tissues, etc. Red ginseng powder is obtained by pulverizing red ginseng. Red ginseng extract is produced by extracting and concentrating ginseng with water or ethyl alcohol. In the case of red ginseng water extract (red ginseng extract produced by Korea Ginseng Corporation), 75% of red ginseng taproots and 25% of rootlets and fine roots are mixed, repeatedly extracted at 85°C for 12 hours with water measuring 10–13 times the amount of ginseng, cooled, and centrifuged to eliminate insoluble materials [15]. This extract is concentrated at 50–60°C until 70–73°Brix are reached. Red ginseng water extract is a blackish brown viscous liquid with approximately 36% moisture, pH of 4.6 or below, and 70–72°Brix, with water-insoluble materials amounting to 2% or below.
2.8. Standards/specifications
The marker components of red ginseng are managed in terms of combinations of ginsenosides Rb1, Rg1, and Rg3, and red ginseng must contain 2.5–34 mg/g of these components.
2.9. Hazardous material specifications
Lead should be 5 ㎍/g or below, arsenic should be 2.0 ㎍/g or below, cadmium should be 0.3 ㎍/g or below, and mercury should be 0.2 ㎍/g or below. The number of bacteria should be 3,000 or below per 1 ml (in the case of red ginseng extract). Coliform bacteria should be negative. Ash should be 5% or below.
3. Components/chemistry
Ginseng contains saponins, which are triterpene glycosides called “ginsenosides”; proteins, peptides, and alkaloids, which are nitrogenous compounds; polyacetylene, which is a fat-soluble component; polysaccharides and other flavonoids; and fatty acids [4], [5], [15]. Ginseng contains 43 types of ginsenosides including protopanaxadiol-type ginsenosides, Rb1, Rb2, Rc, and Rd; protopanaxatriol-type ginsenosides, Re, Rf, and Rg1; and oleanane-type ginsenoside, Ro. Red ginseng changes in its principal components in the process through which raw ginseng is steamed and cooked with vapor and dried, thus differing in component patterns from both raw ginseng and white ginseng [15], [16], [17]. In the red ginseng manufacturing process, the generation not only of ginsenosides but also of arginine–fructose–glucose (AFG), maltol, and panaxytriol as well as chemical changes to polysaccharides occur [5], [6], [15]. The generation mechanism of representative red ginseng–specific components is as follows:
3.1. Ginsenosides
As for red ginseng, new ginsenosides are generated in the processes through which raw ginseng is steamed and dried so that the types of ginsenosides increase in comparison with raw ginseng (ISO, 19610). The content of originally existing hydrophilic ginsenosides (polar ginsenosides) Rg1, Re, Rb1, Rc, and Rd decreases but that of low-hydrophilia transformed ginsenosides (less polar ginsenosides) Rg2, Rh1, and Rg3 increases. The representative change mechanism of ginsenosides in the red ginseng manufacturing process is as follows:
3.1.1. Demalonylation
Malonyl-ginsenoside Rb1, malonyl-ginsenoside Rb2, malonyl-ginsenoside Rc, and malonyl-ginsenoside Rd turn into ginsenoside-Rb1, ginsenoside-Rb2, ginsenoside-Rc, and ginsenoside-Rd with the elimination of malonyl in the red ginseng manufacturing process.
3.1.2. Deacetylation
With the elimination of the acetyl group from malonyl in malonyl-ginsenosides, acetylated ginsenosides are generated. Quinquenoside R1 is generated from malonyl-ginsenoside Rb1, Rs1 is generated from malonyl-ginsenoside Rb2, and Rs2 is generated from malonyl-ginsenoside Rc.
3.1.3. Deglycosylation
Sugar elimination in carbon-20 (C-20) of dammarane saponins generates typical stereoisomers. First, the elimination of sugar from C-20 in Rb1, Rb2, Rc, and Rd generates 20 (S/R) Rg3 stereoisomer. 20 (S/R) Rg2 is generated from protopanaxatriol-type (PPT) ginsenoside Re, Rf is generated from 20-gluco-Rf, and 20 (S/R)-Rh1 is generated from Rg1. Then sugar is eliminated from either C-3 or C-6. Sugar elimination in C-3 of 20 (S/R) Rg3 generates 20 (S/R) Rh2, and sugar elimination in C-6 of 20 (S/R) Rg2 generates 20 (S/R)-Rh1. Sugar elimination in C-3 and C-6 of Rh2 and Rh1 generates protopanaxadiol-type (PPD) and PPT, respectively. Sugar elimination in C-20 of Rs1 and Rs2 generates 20 (S/R) Rs3. Steaming and drying in the red ginseng manufacturing process lead to sugar elimination in C-20, C-6, and C-3 of ginsenosides, thus generating diverse ginsenosides that exist in red ginseng.
3.1.4. Dehydration
Sugar elimination in C-20 of ginsenosides is followed by dehydration, and double bonds are generated in either C-20 and C-21 or C-20 and C-22, thus generating positional isomers and geometric isomers. Rg5, Rk1, and Rz1 are generated from Rg3; Rh3 is generated from Rh2; and Rs4 is generated from Rs3. F4, 20(E) F4, and Rg6 are generated from dehydration in C-20 of Rg2; Rg9, 20(Z) Rg9, and Rg10 are generated from Rf; and Rk3 and Rh4 are generated from Rh1.
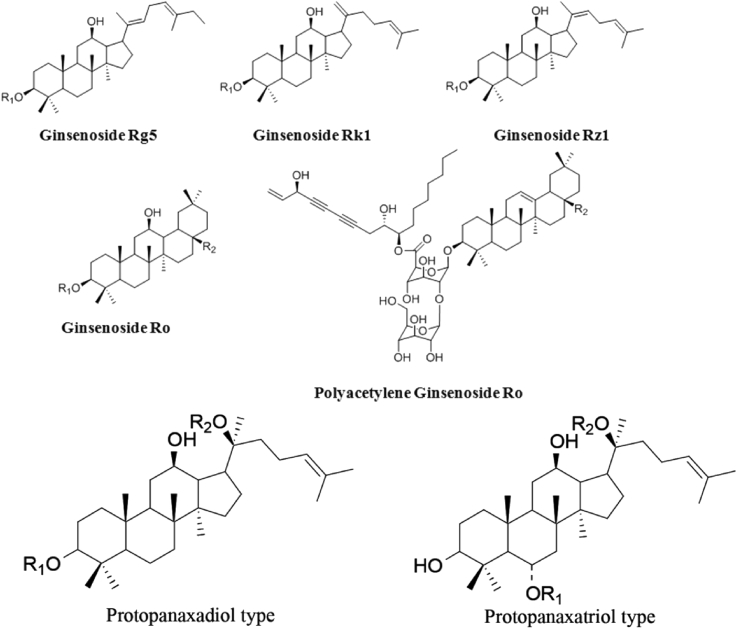
As has been explained above, because of chemical changes in the red ginseng manufacturing process, Rg3, Rh2, Rh4, and Rg5 are generated as red ginseng's unique components so that the types of ginsenosides contained in red ginseng increase in comparison with white ginseng. The types and content of transformed ginsenosides differ according to the ginseng steaming and drying conditions. Ginsenosides in red ginseng and their structures are summarized in Table 1, Table 2 and in Fig. 1.
Table 1.
Chemical structures of protopanaxadiol ginsenosides
| Type | Name | R1 (C-3) | R2 (C-20) |
|---|---|---|---|
| PPD (16 types) | Malnonylginsenoside Rb1 Malnonylginsenoside Rb2 Malnonylginsenoside Rc Malnonylginsenoside Rd Ginsenoside Ra1 Ginsenoside Ra2 Ginsenoside Ra3 Ginsenoside Rb1 Ginsenoside Rb2 Ginsenoside Rb3 Ginsenoside Rc Ginsenoside Rd Notoginsenoside R4 Koryoginsenoside R2 Neoginsenoside L1 Neoginsenoside L2 |
-glu-glu-mal -glu-glu-mal -glu-glu-mal -glu-glu-mal -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu -glu-glu |
-glu-glu- -glu-ara(pyr) -glu-ara(fur) -glu -glu-ara(pyr)-xyl -glu-ara(fur)-xyl -glu-glu-xylose -glu-glu -glu-ara(pyr) -glu-xyl -glu-ara(fur) -glu -glu-glu-xyl -glu-glu (C-25 OH) −12β-O-20(S)-ginsenoside Rg3 −12β-O-20(R)-ginsenoside Rg3 |
| Processed PPD (11 types) | Ginsenoside Rg3(S,R) Ginsenoside Rh2(S) Ginsenoside Rg5 Ginsenoside Rk1 Ginsenoside Rz1 Ginsenoside Rs1 Ginsenoside Rs2 Ginsenoside Rs3 Ginsenoside Rs4 Quinquenoside R1 |
-glu-glu -glu -glu-glu -glu-glu -glu-glu -glu-glu-ac -glu-glu-ac -glu-glu-ac -glu-glu-ac -glu-glu-ac or -glu-glu |
-H -H -H (E)C20/22 double bond -H C20/21 double bond -H (Z)C20/22 double bond -glu-ara(pyr) -glu-ara(fur) -OH -H (E)C20/22 double bond -glu-glu or -glu-glu-ac |
glu, β-D-glucopyranosyl; mal, malonyl; ara(pyr), α-L-arabinopyranosyl; ara(fur), α-L-arabinofuranosyl; xyl, β-D-xylopyranosyl; ac, acetyl.
Table 2.
Chemical structures of protopanaxatriol-type and oleanane ginsenosides in red ginseng
| Type | Name | R1 (C-6) | R2 (C-20) |
|---|---|---|---|
| PPT (11 types) | Ginsenoside Re Ginsenoside Rf Ginsenoside Rg1 Ginsenoside Rf2 Ginsenoside Rg2(S,R) Ginsenoside Rh1(S,R) 20-gluco-ginsenoside Rf Notoginsenoside R1 Koryoginsenoside R1 |
-glu-rha -glu-glu -glu -glu-rha -glu-rha -glu -glu-glu -glu-xyl -glu6-(E)-2-butenoyl |
-glu -H -glu -H (C25-OH) -H -H -glu -glu -glu |
| Processed PPT (3 types) | Ginsenoside Rg6 Ginsenoside Rh4 20(E)-ginsenoside F4 |
-glu-rha -glu -glu-rha |
-H C20/21 double bond -H (E)C20/22 double bond -H (E)C20/22 double bond |
| Oleanane (2 types) | Ginsenoside Ro Polyacetyleneginsenoside Ro |
-glucuronic acid-glu polyacetylene-glu-glu |
-glu -glu |
Glu, β-D-glucopyranosyl; rha, α-L-rhamnopyranosyl; xyl, β-D-xylopyranosyl.
Fig. 1.
Structural formulas of ginsenosides in red ginseng.
3.2. Arginine–fructose–glucose
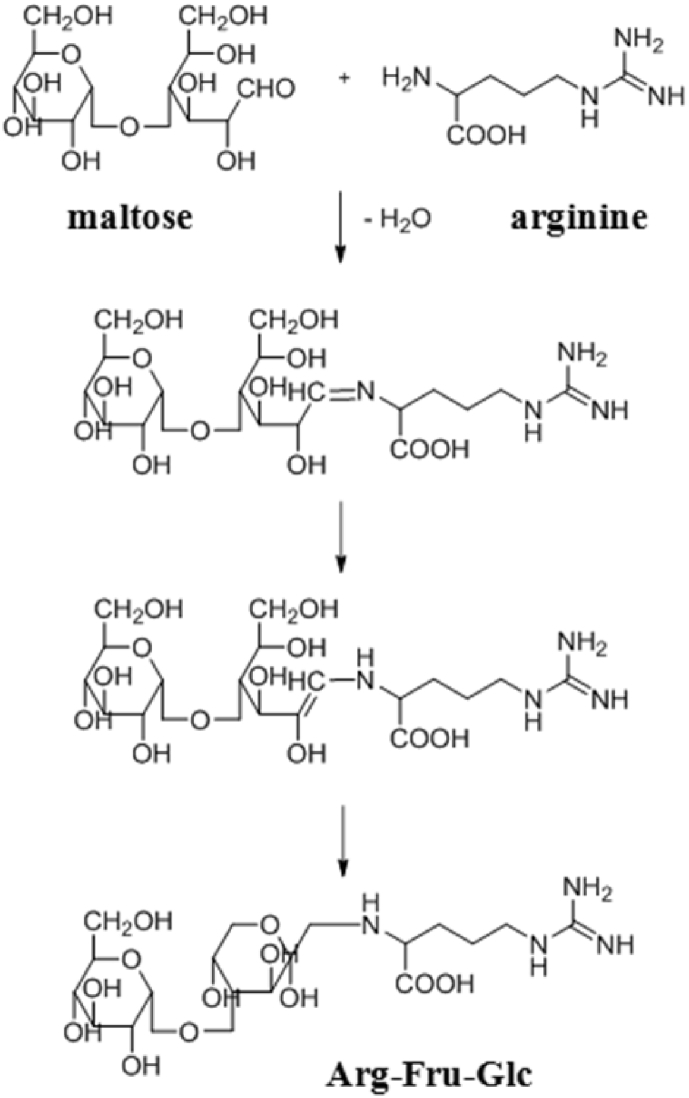
Raw ginseng contains large amounts of glucose, fructose, sucrose, and maltose, which are nutrients, and diverse amino acids such as arginine. In the red ginseng manufacturing process, because of heat, Amadori rearrangement occurs between arginine and either maltose or glucose so that AFG and arginine–fructose, which are amino sugars, are generated (Fig. 2). Materials including maltol are generated in amino sugars as the final products of the Maillard reaction [18], [19], [20]. Decreases in the content of free sugars and amino acids in red ginseng are caused by the generation of caramel coloring, which is a product of thermal degradation, following the generation of amino sugars [20].
Fig. 2.
Generation of AFG in the red ginseng manufacturing process.
3.3. Polyacetylenes
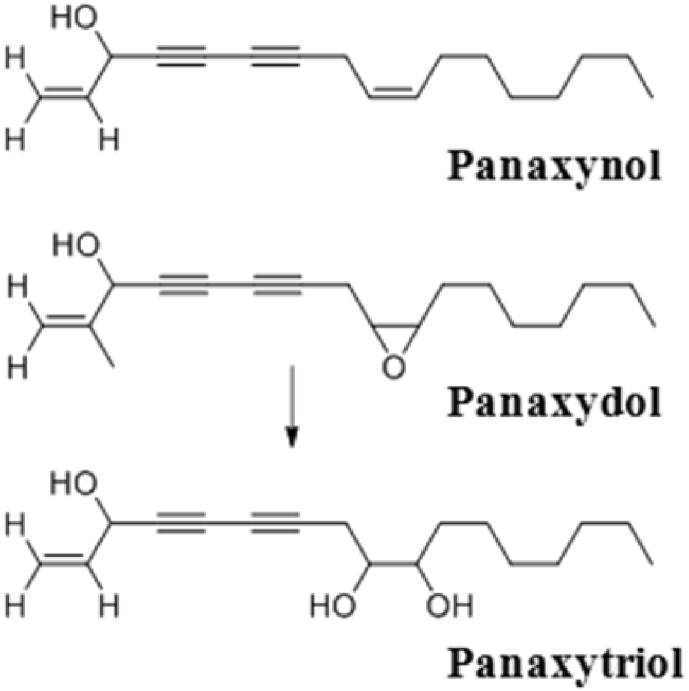
Over 20 types of polyacetylene compounds including panaxynol (heptadeca-1,9-diene-4,6-diyne-3-ol) and panaxydol (heptadeca-1-ene-9,10-epoxy-4,6-diyne-3-ol), which are fat-soluble compounds separated through petroleum ether fraction, have been found in ginseng roots. Panaxytriol (heptadeca-1-ene-9,10-dihydroxy-4,6-diyne-3-ol) exists only in red ginseng (Fig. 3), which is because the epoxy ring of panaxydol is subjected to hydrolysis due to heat [15], [21], [22].
Fig. 3.
Generation of panaxytriol in the red ginseng manufacturing process.
3.4. Polysaccharides
The components contained in ginseng in the largest numbers are polysaccharides, which have been reported to possess immune-regulatory and anticancer an effect [23]. In red ginseng, galacturonic acid is increased by heating because esterified galacturonic acid is converted to unesterified galacturonic acid [6].
4. Health claims as a health functional food and mechanism of action
In South Korea, for materials to be certified as health functional foods, their functions must be proven with respect to or in the materials' standardization, safety, and basic and human studies. Materials must demonstrate their functions as foods through repeated intake in clinical trials, and pharmaceutical drugs must manifest their effects in patients with particular diseases. Health functional foods are evaluated for their effects through administration to either healthy individuals or semi-healthy individuals (a state in which people are neither in pain nor carrying diseases but are either easily tired or do not feel themselves to be healthy) who are not on medication in order to exclude the effects of medication. Red ginseng has been certified for its six functions as a health functional food [3]. But the mechanism of action of each of these functions has yet to be clearly elucidated. So far, the mechanism and supporting data that revealed the functionality of red ginseng in the base research are as follows (Table 3):
Table 3.
Clinical data on red ginseng as a health functional food
| Samples | Design | Participants | Daily intake/intake duration | Results | References |
|---|---|---|---|---|---|
| Red ginseng powder | Random (no placebo) | 36 stomach cancer patients and 36 colorectal cancer patients | 4.5 g/6 months |
|
[36] |
| Red ginseng extract | Random (no placebo) | 25 healthy individuals and 50 stomach cancer patients | 3 g/3 months |
|
[37] |
| Red ginseng extract | Random (no placebo) | 47 colorectal cancer patients | 3 g/3 months |
|
[38] |
| Red ginseng powder | Case study | 12,295 common cold patients | No dose |
|
[39] |
| Red ginseng powder | Random, double-blind, placebo-controlled study1) | 24 male college students | 2.5-4 g/3 weeks |
|
[43] |
| Red ginseng extract | Placebo-controlled study | 24 students majoring in physical education | 3 g/8 weeks |
|
[44] |
| Red ginseng extract | Random, double-blind, placebo-controlled study | 18 healthy men | 60 g/11 days |
|
[45] |
| Red ginseng extract | Random, double-blind, control study | 87 healthy men and women aged 20-59 years | 1.5 g, 3 g/8 weeks |
|
[52] |
| Red ginseng products | Case study | 10 red ginseng product takers and 7 non-red ginseng product takers | 1.6 g/4-5 years |
|
[53] |
| Red ginseng extract | Random, double-blind, control study | 15 healthy men | 200 mg/8 weeks |
|
[62] |
| Red ginseng powder | Random, placebo-controlled, open-label (open study) | 31 Alzheimer's disease patients aged 50 years or above taking medication | 4.5 g or 9.0 g/12 weeks |
|
[64] |
| Red ginseng powder | Double-blind, random, placebo-controlled | 15 healthy, smoking male students aged 19-31 years (smoked 20 cigarettes/day or above in the past 2 years) | 1.8 g/4 weeks |
|
[74] |
| Red ginseng extract | Random, placebo-controlled | 40 male college students | 2.7 g/3 months |
|
[47] |
| Red ginseng powder | Random, double-blind, placebo-controlled | 57 healthy drinking and smoking adults aged 20-65 years | 3 g or 6 g/8 weeks |
|
[75] |
| Red ginseng powder | Random, double-blind, placebo-controlled | 82 menopausal women aged 45-60 years | 3 g/12 weeks |
|
[76] |
| Red ginseng powder | Random, double-blind, placebo-controlled | 63 menopausal women aged 45-60 years | 3 g/12 weeks |
|
[77] |
| Red ginseng extract | Random, double-blind, placebo-controlled | 26 menopausal women with hot flashes | 0.9 g/8 weeks |
|
[78] |
| Red ginseng powder | Comparison before and after intake | 83 menopausal women | 6 g/8 weeks |
|
[79] |
| Red ginseng powder | - | 17 menopausal women | 6 g/3 months |
|
[80] |
| Red ginseng powder | - | Women with estrogen levels of 10 pg/ml or below | 6 g/30 days |
|
[81] |
ADP, adenosine diphosphate; ADAS, Alzheimer's Disease Assessment Scale; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BCAA, branched-chain amino acid; CAT, catalase; CDR, clinical dementia rating; CK, creatine kinase; DHEA, dehydroepiandrosterone; GOT, glutamic oxaloacetic transaminase; GPX, glutathione peroxidase; γ-GTP, γ-glutamyl transpeptidase; IL, interleukin; LDL, low-density lipoprotein; MDA, malondialdehyde; NK, natural killer; PT, prothrombin time; SOD, superoxide dismutase.
A randomized, double-blind, and placebo-controlled trial.
4.1. Immunity improvement
In order to elucidate immune activity of red ginseng, numerous in vitro and in vivo studies on ginsenosides, saponin fraction, and polysaccharides have been conducted. Red ginseng activates macrophages and natural killer cells [24], [25], [26], which are primarily responsible for innate immunity, thus nonspecifically possessing protective effects against external infection or hazardous materials. In addition, red ginseng increases specific immune responses by regulating the activity of immunocytes and cytokines, which act on cell-mediated and humoral immunity [27], [28], [29]. The oral administration of red ginseng extract to mice before infection with the H1N1 virus increased mouse survival rates, increased the interferon-gamma (IFN-γ) content in both the bronchi and the lungs, and decreased inflammation, thus exhibiting antiviral effects [30], [31], [32]. When orally administered to mice for a long time, red ginseng extract defended against infection with the highly pathogenic H5N1 influenza virus, thus increasing survival rates [33]. The administration of red ginseng extract before infection with the respiratory syncytial virus increased CD11c+IFN-γ+/CD11b− DC, which generates IFN-γ and CD8+IFN-γ+/CD11b−CD11c+ DC (Th1 reaction induced), thus confirming that red ginseng promotes innate immunity and adaptive immunity [34], [35]. When postsurgery gastrointestinal cancer patients were administered with either 4.5 g/day of red ginseng powder for 6 months or 3 g/day of red ginseng extract for 3 months, in comparison with the control group (placebo), the red ginseng group increased overall in numbers of CD4+, CD8+ T cells, and B cells as well as in blood interleukin (IL)-2 content. However, this group decreased blood IL-10 content, thus improving immune functions that had weakened after cancer surgery [36], [37], [38]. In a study on red ginseng intake and the incidence of the common cold, in comparison with the control group, the incidence rate decreased significantly in the group that had taken red ginseng powder [39]. In a randomized controlled trial, the administration of 3 g/day of red ginseng extract for 12 weeks to healthy individuals led to a significant decrease in the number of times that the participants caught acute respiratory diseases. These results confirm that red ginseng has protective effects against acute respiratory diseases [40]. When type 1 human immunodeficiency virus-infected patients (AIDS patients) either took medication and red ginseng together or took red ginseng alone for a long time, decrease in the numbers of CD4+ T cells was delayed, and the concentration of soluble CD8 was maintained, thus confirming that red ginseng is effective for treating the AIDS virus [41], [42].
4.2. Fatigue relief
Red ginseng decreased the accumulation of lactic acid, which is a muscle fatigue material generated after exercise tolerance has been reached, and promoted the recovery of creatine kinase (CK), which provides energy to muscles [43], [44], [45]. In addition, red ginseng mitigated the development of central fatigue by decreasing the generation of serotonin precursors, which are central fatigue materials [44], [46]. When exercise tolerance has been reached, reactive oxygen species are generated. Red ginseng increased antioxidant enzyme activity, thus mitigating physical fatigue such as muscle fatigue [47]. When the participants took 50 mg/day of red ginseng powder for 3 weeks and performed continuous running for 45 minutes on sloped treadmills at the intensity of 70% VO2 max (maximal aerobic capacity) from the second week of intake, CK and glutamic oxaloacetic transaminase liver function test figures, which are indices of muscle fatigue, decreased [43]. When high dose of red ginseng extract was taken, CK quickly recovered after exercise on treadmills, and IL-6 decreased and insulin sensitivity improved in the early stage of exercise as well [45]. When subjects took 3 g/day of red ginseng extract for 8 weeks and simultaneously performed endurance training at 60% VO2 max, their ability to perform both anaerobic and aerobic exercise was not affected, but the blood lactate concentration decreased. Red ginseng intake decreased branched-chain amino acid concentration both before and after endurance exercise and suppressed the generation of serotonin, thus mitigating central fatigue [44]. Male college students were administered with 2.7 g/day of red ginseng powder for 3 months and simultaneously subjected to regular exercise. When changes in the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPX), which are antioxidant enzymes, and the malondialdehyde (MDA) content during maximum exercise were examined, SOD and GPX activity increased and blood MDA decreased in the red ginseng group in comparison with the control group, thus demonstrating that red ginseng mitigates fatigue generated during exercise with its antioxidant [47]. Consisting of recovery effects on muscle fatigue that is generated after exercise tolerance has been reached, red ginseng's relief of physical fatigue has been certified acknowledged. The material's effects on stress and psychological fatigue will need to be proven in the future through advanced research.
4.3. Aid to blood flow through the suppression of platelet aggregation (blood circulation improvement)
Red ginseng inhibits platelet aggregation by regulating the synthesis of prostacyclin (PGI2), which has an antagonistic mechanism toward platelet aggregation, as well as thromboxane A2 (TXA2) and serotonin, which promote platelet aggregation, thus suppressing the generation of thrombi and improving blood circulation. Red ginseng extract, saponins, and ginsenosides suppress the generation of platelet-aggregating materials such as TXA2, thrombin, and serotonin. In addition, they promote the generation of PGI2, which suppresses platelet aggregation, thus inhibiting platelet aggregation [48], [49], [50], [51]. In a randomized controlled trial in which red ginseng extract was administered for 8 weeks to a low-dose group (1.5 g/day) and to a high-dose group (3.0 g/day), both consisting of healthy individuals, when platelet aggregation tests using adenosine diphosphate and collagen were conducted, red ginseng intake was found to suppress platelet aggregation significantly in both the low- and high-dose groups. However, there was no significant change to prothrombin time, which is an exogenous blood coagulation factor pathway, or to activated partial thromboplastin time (APTT), which is an endogenous blood coagulation factor pathway. In addition, the total cholesterol, high-density lipoprotein, low-density lipoprotein (LDL)-cholesterol, and neutral fat levels were not affected [52]. Healthy individuals who had steadily taken red ginseng products for 4–5 years showed more inhibition of platelet aggregation using collagen than those who had not taken such products. These individuals also exhibited extended APTT, which is an endogenous blood coagulation factor pathway [53]. When arteriosclerosis patients were administered with 9 g/day of red ginseng powder for 1 month, the generation of PGI2, which suppresses platelet aggregation, increased [54].
4.4. Aid to memory improvement
Red ginseng and ginsenosides strengthen cholinergic nerves by promoting the generation and release of acetylcholine, which has important effects on memory, thus exhibiting its effects on learning and memory [55], [56], [57]. In an animal model in which memory disorders had been induced by damages to the hippocampus, the oral administration of red ginseng extract exhibited improvement effects in learning and spatial intelligence [58]. Red ginseng powder, saponins, and ginsenosides improved memory in young mice and aged mice [59], [60] as well as in an ischemic memory disorder animal model [61]. When healthy individuals were administered with 200 mg/day of red ginseng extract for 8 weeks and subjected to memory tests such as the 3-back task and the Corsi block-tapping task, both the working memory and the subjective quality of life improved, but no increase in intelligence was confirmed [62]. When Alzheimer's disease patients receiving treatment were administered with either 4.5 g/day or 9.0 g/day of red ginseng for 12 weeks, the high-dose red ginseng group significant improved on both the Alzheimer's Disease Assessment Scale and the Clinical Dementia Rating, which are dementia measurement indices [63].
4.5. Aid to antioxidant activity
Red ginseng either decreases or eliminates the generation of free radicals by regulating the activity of antioxidant enzymes such as SOD, catalase, and GPX out of diverse factors that cause oxidative damages and strengthening the synthesis of endogenous antioxidants such as glutathione, thus decreasing oxidative damages [64], [65], [66], [67], [68], [69], [70], [71], [72], [73]. The administration of either 1.8 g/day or 3 g/day of red ginseng powder for 4 weeks to healthy smokers significantly decreased the carbonyl content of 8-OHdG and peripheral hemoglobin [74]. In a randomized controlled trial in which healthy drinking and smoking adults aged 20–65 years were administered with either 3 g/day or 6 g/day of red ginseng for 8 weeks, the tail length and mobility of DNA, which are indices of the degree of lymphocyte DNA damages, both decreased in the red ginseng group. In addition, the activity of SOD, which is an antioxidant enzyme, increased, and the activity of GPX and catalase increased as well in the high-dose group. The concentrations of both blood-oxidized LDL, which is an oxidant, and urine 8-epi prostaglandin (PG) F2α, decreased in both the low- and high-dose groups [75]. In menopausal women, the intake of 3 g/day of red ginseng powder for 12 weeks significantly increased SOD activity but did not affect blood GPX or 8-OHdG. While blood MDA decreased after red ginseng intake, there was no statistical significance in comparison with the control group [76].
4.6. Aid to menopausal women's health
In a randomized controlled trial on menopausal women's subjective symptoms such as hot flashes, insomnia, and depression, the intake of 3 g/day of red ginseng for 12 weeks improved results on both the Kupperman Index and the Menopause Rating Scale, which are internationally certified survey evaluation methods that comprehensively evaluate menopausal symptoms. While the total cholesterol and LDL-cholesterol decreased significantly, the estrogen content was not affected [77]. In menopausal women administered with either 0.9 g/day (8 weeks) or 6 g/day (30 days) of red ginseng, the frequency of the occurrence of hot flashes, which constitute a menopausal symptom, decreased [78], [79]. In women with menopausal symptoms who had taken red ginseng, the stress hormone ratio (cortisol/DHES-A) became similar to that of women without menopausal symptoms, and red ginseng mitigated menopausal stress and decreased tissue-type plasminogen activator inhibitor type 1, thus improving blood circulation [80], [81]. Red ginseng improved lowered sexual functions in menopausal women as well [82]. In human studies on menopausal women, red ginseng mitigated menopausal symptoms but did not affect the content of hormones such as serum estrogen and prolactin [77], [79], [82]. These results imply that red ginseng has no side effects or risks, unlike hormone replacement therapy, which involves a high risk of the development of breast cancer due to hormone increase. In addition, red ginseng can improve the risk of cardiovascular disease due to a decrease in estrogen in menopausal women.
5. Other effects
Besides its functions as a health functional food, diverse effects of red ginseng have been elucidated in both cells and animals and have been proven in clinical trials as well. In recent years, based on clinical research, the effects of ginseng including red ginseng have been evaluated through systematic examinations and meta-analyses of the improvement of blood glucose levels [83], health of menopausal women [84], erectile dysfunction (ED) [85], and anticancer an effect [86]. The major effects of red ginseng, besides those functions certified by the KFDA, and recent clinical studies related to quality of life are as follows:
5.1. Improvement of blood glucose levels
The antidiabetic effect of red ginseng has been reported. When type 2 diabetes patients with good regulation of blood glucose were administered with 6 g/day of red ginseng powder for 12 weeks and measured for blood glucose, the glycemic index was decreased and insulin sensitivity was increased in the red ginseng group in comparison with the control group [87]. When subjects with fasting blood glucose and postprandial blood glucose levels slightly higher than normal levels or those recently diagnosed with type 2 diabetes were administered with 5 g/day of red ginseng powder for 12 weeks and subjected to oral glucose tolerance tests, insulin and C-peptide levels were decreased, and the blood glucose area under the curve tended to have decreased in the red ginseng group. However, there were no changes to glycated hemoglobin (HbA1c) [88]. Reay et al have reported that 200 mg/day of red ginseng extract administered for 8 weeks to healthy individuals did not affect the HbA1c or insulin content [89]. Reeds et al have reported that when participants with glucose tolerance or those recently diagnosed with type 2 diabetes were administered with 3 g/day of red ginseng for 2 weeks and then with 8 g/day of the same material for 2 weeks, there was no effect on glucose tolerance, pancreatic B cells' functions, or insulin sensitivity [90]. Researchers are mutually inconsistent in findings on blood glucose improvement of red ginsengs because of differences in the intake, intake duration, and participant’s health status and blood glucose-related index. In the future, it will be necessary for clinical studies to study numerous individuals based on a research design that satisfies health functional food standards.
5.2. Anticancer
Red ginseng has been reported to suppress angiogenesis and cancer metastasis and to act on signaling pathways related to anticancer activity. Rg3, Rh2, Rg5, Rs4 (acetylated Rg5), Rg1, Rf, and PPD were found to block cell cycles or apoptosis through caspase-activating signaling [91], [92], [93], [94], [95], [96], [97], [98]. Red ginseng, ginsenoside, and acidic polysaccharides showed anticarcinogenic effects in carcinogenesis involving inflammation through diverse pathways including the suppression of cyclooxygenase-2 (COX-2), inducible nitric oxide (iNOS), and nuclear factor-kappa B (NF-κB) activity and the elimination of reactive oxygen species [99], [100], [101] and showed anticancer-assisted effect when it was combined with an anticancer drug [102], [103], [104], [105], [106], [107]. In the results of both cohort studies and case-control studies conducted to determine the effects of the intake of ginseng and red ginseng on the development of cancer, the intake of ginseng products including red ginseng was found to decrease the relative risk of developing cancer. In addition, the risk of developing stomach cancer, lung cancer, ovarian cancer, laryngeal cancer, esophageal cancer, and pancreatic cancer decreased as the frequency and duration of the intake of red ginseng and ginseng products increased [108], [109]. To determine the effects of red ginseng on the development of cancer, chronic atrophic gastritis patients were administered with 1 g/week of red ginseng extract powder for 3 years and subjected to a tracking survey for 8 years [110]. While the relative reduction of risk of developing cancer had no statistical significance in the red ginseng group in comparison with the control group, this risk did decrease significantly among men in the red ginseng group. In this research, as in epidemiological surveys [108], [109], red ginseng was found likewise to exhibit effects of nonspecifically preventing the development of cancer in men.
5.3. Men's health
Ginsenosides promoted the generation of nitric oxide (NO) in endothelial cells and nerves around blood vessels in the corpora cavernosa penis and increased the sensitivity of the smooth muscles of blood vessels to NO [111], [112]. Red ginseng extract relaxed the smooth muscles of the corpora cavernosa penis in vitro and increased the pressure inside rats' corpora cavernosa penis [113]. Rg1 generated NO in endothelial cells in a glucocorticoid receptor-dependent way [114] and increased NO release and cyclic guanosine monophosphate (GMP) accumulation in mice's corpora cavernosa penis [115]. In seven clinical studies in which randomized controlled trials were conducted on patients with psychogenic, vasculogenic, or any other type of ED, the red ginseng intake was 1.8 g/day, 2.7 g/day, or 3 g/day, and the intake duration was 4-12 weeks. When these seven studies were subjected to meta-analysis, in six of them, red ginseng was effective for all types of ED and had significant effects on psychogenic ED and sexual functions [116]. When 1.5 g/day of red ginseng powder were administered for 12 weeks to varicocele patients and the effects red ginseng on spermatozoa's functions were studied, the numbers, motility, and shapes of spermatozoa improved in the red ginseng group in comparison with the control groups, which had either received or had not received varicectomy [117].
5.4. Sleep time, mouth dryness, and hair loss
Red ginseng intake extended the total sleep time and either increased sleep efficiency or extended stage 2 and stage 3 sleep [118], [119]. In a study of mouth dryness patients, red ginseng improved mouth dryness in the secondary analysis of menopausal women out of the participants [120]. Red ginseng intake for 24 weeks increased both hair density and hair thickness in both male-pattern hair loss and female-pattern hair loss patients [121].
6. Effects in traditional Korean medicine
The history of the use of ginseng for medicinal purposes in Asia goes back several millennia. The foremost Chinese pharmacological text, Shennong's Classic of Herbal Medicine (Shennong Bencaojing; ca. 100 BC) describes the pharmacological use of ginseng for the first time [122]. It is recorded that ginseng is a life-preserving drug and therefore leads to no harm even when it is consumed in large quantities and over long periods. In traditional Korean medicine, red ginseng is used for indicators such as ginseng. Possessing both sweet and slightly bitter flavor and warm qi (vital force or vital energy), ginseng enters the acupuncture meridians of the spleen, lungs, and heart. It is used to arouse energy, to fortify qi in the spleen and the lungs, to produce bodily fluids, to quench thirst, to stabilize the mind, and to increase wisdom. The spleen and the lungs in traditional Korean medicine are unrelated to the anatomical organs of the same names in Western medicine [122]. Used for cases including fatigue due to deficient qi, loss of appetite, diarrhea, shortness of breath, weak pulse, diabetes, febrile diseases, forgetfulness, insomnia, and ED, ginseng is a representative restorative for invigorating qi [123]. A drug preparation consisting solely of ginseng, the deshentang (“ginseng-only decoction”) has been used for the symptoms of mental and physical fatigue and the utter lack of energy. In traditional Korean medicine, ginseng has been used as a component of multiple-ingredient drug preparations rather than as a single-ingredient drug preparation. It has been used either to yield additional or synergistic actions with other crude drugs mixed into multiple-ingredient drug preparations or to decrease the side-effects of crude drugs that possess high therapeutic mechanism but are toxic and to increase these drugs' efficacy.
7. Precautions regarding intake
Precautions regarding the intake of ginseng when taking pharmaceutical drugs (antidiabetic agents and anticoagulants) are mentioned in “Drug interaction”. Contraindications to red ginseng were not known. The WHO monographs and the German Commission E state, “There are no contraindications regarding ginseng.” [8], [11]. While there is no clinical research on ginseng intake during pregnancy, the plant has no effect whatsoever on teratogenicity and mothers in animals. Although the effects of ginseng intake on mothers and newborns during lactation have not been proven, traditional Korean medicine has prescribed the plant to women in cases of mental and physical weakness during pregnancy as well as childbirth and postpartum care. Consultation with physicians is necessary for the intake of ginseng by pregnant and lactating women. While the effects of ginseng on children have not been proven, traditional Korean medicine has used prescriptions containing ginseng for children's growth. On sale are products that appropriately adjust adult intake to children's growth stages.
8. Daily intake and dosage
The determination of the intake of ginseng as a health functional food is based on both the traditional intake of ginseng as a food and effective doses in clinical research results. The most frequent ginseng doses in traditional Korean medicine have been 2.7–4.5 g, and the most frequent red ginseng powder dose in research conducted in the past 10 years has been 3 g, found in nine cases [124]. In data on the “fatigue relief and immunity improvement” functions, red ginseng was in the powder form and amounted to 0.5–5 g, with the daily intake of 3–80 mg consisting of combinations of Rb1 + Rg1 + Rg3. As for red ginseng extract, with the 3 g of red ginseng water extract reviewed in the “aid to blood flow through suppression of platelet aggregation” function as the standard, combinations of marker components from the marker component specifications of red ginseng water extract amounted to 2.4–23 mg. The intake for the “memory improvement” and “antioxidation” functions likewise was determined with red ginseng extract and powder as the standard. Red ginseng's functions regarding “fatigue relief and immunity improvement” were approved up to the dose of 80 mg so that the doses for the “aid to blood flow through suppression of platelet aggregation (blood circulation improvement),” “memory improvement,” and “antioxidation” functions were determined to be in the range of 2.4–80 mg. As for the “aid to menopausal women's health” function, the daily intake was determined as 24–80 mg.
9. Intake duration
Ginseng has been used from over several millennia, and red ginseng is recorded to have been produced and used from over 1,000 years but is presumed to have been used from even earlier periods. Traditional Korean medicine has classified ginseng as a safe crude drug ingredient, lacking toxicity even when taken for a long time and extending human life. Based on clinical research, the National Institutes of Health (NIH) in the United States have cautioned that the intake of ginseng for 12 weeks or below is safe, but long-term intake of the plant may not be safe [125]. They have stated that this is because hormone-like effects of ginseng may make long-term use harmful. According to the WHO monographs, in research on ginseng extract, ginseng components did not interact with either estrogen receptors in mature rats' uteri or progesterone receptors in human uterine muscles, and ginseng extract was found not to affect female hormones or male hormones in clinical research [8]. Red ginseng did not affect female hormones or male hormones in not only animal studies but also clinical research [77], [79], [82], [117], [126].
10. Safety/adverse events
10.1. Preclinical safety
Red ginseng extract is presumed to have no-observed-adverse-effect levels (NOAEL) of 2,000 ㎎/㎏ or above in acute and subacute toxicity tests using animals and did not exhibit genetic toxicity in bacterial reverse mutation tests using microorganisms. Up to the dose of 2,000 mg/kg, the samples did not affect or cause fetal mortality rates, external abnormalities, abnormalities in the internal organs and skeletons, sex ratios, or surviving fetus body weight in embryo–fetal development tests. Abnormalities such as miscarriages, preterm deliveries, and dystocia were not observed in combined fecundity and maternal function tests, and no differences whatsoever were observed between the control group and all sample groups in the pregnancy duration, birth rates, sex ratios, numbers of survivors, mortality rates, survival rates on the fourth day of nursing, or weaning rates. There was no effect whatsoever on motor skills or learning/memory related to the next generation's body growth and reproductive functions such as fertility rates and fetal growth, thus showing that red ginseng does not cause reproductive or developmental toxicity.
10.1.1. Single and repeated dose toxicity
When Institute of Cancer Researc (ICR) mice were administered with up to 5,000 mg/kg, which is the limit dose for single oral administration, of red ginseng water extract and observed for 14 days, no abnormalities were found in not only dead animals but also general symptoms, body weight changes, feed/water intake, autopsy findings, and histopathological findings [127]. The repeated oral administration of either 50–2,000 mg/kg of red ginseng water extract or 500, 1000, or 2000 ㎎/㎏ of red ginseng extract (produced by the Korea Ginseng Corporation) to female and male mice for 28 days did not lead to animal deaths or abnormalities in general symptoms caused by the administration of the test specimens during the test period; significant differences in body weight changes, feed/water intake, biochemical blood tests, or organ weight measurements; or unusual findings in the results of gross autopsies [127], [128]. Based on the results above, NOAEL are presumed to be 2,000 ㎎/㎏ or above. When 0.625 g/kg, 1.25 g/kg, or 2.5 g/㎏ of red ginseng powder was mixed into feed and fed to animals for 1–6 months, no significant differences were observed in body weight changes, organ weight changes, biochemical blood test results, or histopathological test results [129].
10.1.2. Genetic toxicity
Up to the maximum dose of 5,000 ㎍/plate, red ginseng extract (produced by the Korea Ginseng Corporation) did not lead to the existence of metabolic activation systems in the Salmonella (TA98 and TA100) and E. coli WP2 or to bacterial reverse mutation when metabolic activation systems existed [130].
10.1.3. Reproductive/developmental toxicity
In doses of 20–2,000 mg/kg, freeze-dried powder of red ginseng water extract (containing 0.61 ± 0.12% and 0.90 ± 0.17% of Rb1 and Rg1, respectively) was orally administered every day to male mice for 63 days before mating, to female mice from 14 days before mating to the last stage of pregnancy, and to female mice undergoing combined fecundity and maternal function tests from 2 weeks before cohabitation to pregnancy, delivery, and lactation [131], [132]. When generations F0 and F1 were observed for clinical symptoms, body weight changes, water/feed intake, estrus, sex hormones in the blood, and organ weight, there were no unusual changes, and the production, motility, and denaturation rates of spermatozoa as well as the number of spermatozoa in the epididymides did not change. There were neither abnormalities in implantation rates and fetal mortality rates nor external abnormalities in embryo–fetal development tests. In addition, there were no abnormalities in the internal organs or skeletons of surviving fetuses obtained through autopsies in the last stage of pregnancy. As for the effects on the next generation, there were no differences from the control group in neonatal survival rates, growth-related indices, reflex functions, and learning/memory [131], [132]. When 0.625–2.5 g/㎏ of red ginseng powder was mixed into feed and fed to animals for 6 months, no abnormalities were observed in the blood biochemistry, organ weight, histopathological test results, or appearances of generations F1 and F2 [129].
10.2. Adverse events
Ginseng roots (Panax ginseng) have grounds for a long history of use and are medicinal ingredients or foods that have been consumed safely without serious side-effects. As for adverse effects, both German Commission E and Expanded Commission E monographs state, “none known.” [9], [11]. As for adverse events involving ginseng, insufficient information on the types and content of ginseng makes evaluation of adverse events difficult. Ginseng Abuse Syndrome has been reported to develop in cases in which abnormally excessive doses (15 g/day or above) of ginseng have been taken [8], [133]. A systematic literature review evaluating safety in randomized controlled trials of red ginseng, white ginseng, fermented ginseng, and black ginseng administered to healthy individuals not on medication in the past 10 years (2005–2014) was recently published [124]. Out of a total of 44 studies, 30 studies were on the Korean Red Ginseng, thus taking up the largest share. Twelve studies reported side-effects, 14 studies mentioned no side-effects whatsoever, and three studies reported the absence of any side-effect. Adverse effects reported after red ginseng intake consisted of symptoms such as indigestion, diarrhea, headaches, insomnia, palpitations, hot flashes, and mouth dryness, which were identically observed in the placebo control group as well and whose occurrence rates were the same for the placebo control group and the red ginseng group. While many of the studies used small numbers of participants and did not report safety in detail, there were no significant differences between the placebo group and either the red ginseng group or the ginseng group in the frequency and symptoms of adverse events. The degree of adverse events was light, and the symptoms disappeared once (red) ginseng intake was temporarily suspended. With respect to cases in which red ginseng increased body heat (the sense of heat caused by an increase in qi) in Chinese subjects, according to the results of research on safety following the intake of red ginseng, white ginseng, or American ginseng by South Korean and Chinese individuals, there were no differences in general symptoms or adverse events by ginseng type or subject nationality [124]. After taking 3 g/day of white ginseng, red ginseng, or American ginseng for 35 days, healthy individuals were proven by the results of blood and biochemical tests to be unaffected and did not experience any adverse event [134]. It has been reported that, after menopausal women took ginseng, breast pain increased in seven cases and vaginal bleeding and sexual desire increased in one case [8]. Although a study showing improvements in menopausal women's sexual functions reported two cases of vaginal bleeding in the red ginseng group [82], other studies on menopausal women did not report any adverse event [77], [78], [79], [80], [81]. It is unlikely for vaginal bleeding in the red ginseng group to have been due to hormone changes because red ginseng does not affect hormone levels or estrogen receptors.
10.3. Drug interaction
Many in vitro studies have been conducted on the effects of individual ginsenosides or red ginseng extract on cytochrome P 450 (CYP 450) enzyme and drug delivery systems (Table 4). The saponin fraction of red ginseng suppressed CYP2E1 [135], but the effects of individual ginsenosides on CYP activity were found not to be identical (Table 4). Rg3, Rh1, and Rh2 or intestinal metabolome compounds K and PPT, which are aglycones, have been reported to have greater effects in inhibiting CYP activity than ginsenosides originally existing in ginseng [136], [137], [138], [139], [140]. When healthy individuals took 3 g/day of red ginseng extract (produced by the Korea Ginseng Corporation) for 2 weeks, CYP2C19 and CYP2D6 activity levels were not affected; CYP1A2, CYP2C9, and CYP3A4 activity levels were weakly suppressed but had no clinical significance; and P-glycoprotein activity was not affected [141] (Table 4). When taken by heart valve transplant patients for 6 weeks, red ginseng extract (1 g/day) did not affect warfarin's anticoagulation [142]. Based on these results, it can be shown that there is no interaction between red ginseng and warfarin. While precautions regarding the intake of red ginseng together with anticoagulants take into consideration the possibility of a delay in blood coagulation due to the material's suppression of platelet aggregation, healthy individuals' intake of red ginseng extract for 8 weeks was found to suppress platelet aggregation but not to affect blood coagulation systems such as prothrombin time and APTT [52]. When the research above is summarized, according to in vitro studies and clinical studies on effects of red ginseng on CYP and uridine-5'-diphosphate (UDP) metabolism and drug delivery systems such as P-glycoprotein, red ginseng neither interacted with drug metabolism and drug delivery systems nor affected metabolism and efficacy of warfarin. Precautions regarding the combined intake of red ginseng and antidiabetic agents can be understood as concerning the material's reported blood glucose effects [87], [88], which can cause low blood glucose, rather than its effects on the metabolism or absorption of antidiabetic agents.
Table 4.
Effects of ginseng on drug-metabolizing enzymes and drug delivery systems
| Samples | Study type | Metabolisms | Transporters | References |
|---|---|---|---|---|
| Ginsenoside Rd | In vitro (R) | CYP3A4 (↓), CYP2D6 (↓), CYP2C19 (↓), CYP2C9 (↓) | [137] | |
| Ginsenoside Rg3 | In vitro (H) | UGT1A7, UGT1A8, UGT2B7, UGT2B15: (↓) | [138] | |
| Ginsenosides Rc and Rf | In vitro (R) | CYP2C9 (↑), CYP3A4 (↑) | [139] | |
| Ginsenoside F1 | In vitro (H) | CYP3A4 (↓), CYP2D6 (↑) |
P-gp (-) | [139] |
| Compound K | In vitro (H) | CYP2C9 (↓) | [138] | |
| PPD and PPT | In vitro (H) | CYP2C9 (↓), CYP3A4 (↓) | [136] | |
| Ginsenoside Rh2 (S) |
In vitro (H) In vivo (R) |
CYP2A6 (↓), CYP2C9 (↓), CYP3A4 (↓) | P-gp (↓) | [136], [138] |
| Red ginseng extract (Korea Ginseng Corporation) | 14 healthy men (aged 20–55 years) | CYP2C19 (-), CYP2D6 (-), CYP1A2 (↓), CYP2C9 (↓), CYP3A4 (↓) | P-gp (-) | [141] |
H, human liver microsome; R, rat liver microsome; (-), no effects; (↓), inhibition; (↑), stimulation.
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Brekhman I.I., Dardymov I.V. New substances of plant origin, which increase non-specific resistance. Ann Rev Pharmacol. 1968;8:419–430. doi: 10.1146/annurev.pa.09.040169.002223. [DOI] [PubMed] [Google Scholar]
- 2.Patela S., Raufb A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed Pharmacother. 2017;85:120–127. doi: 10.1016/j.biopha.2016.11.112. [DOI] [PubMed] [Google Scholar]
- 3.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Food and Drug Safety of the Republic of Korea: Health Functional Food Code (Ministry of Food and Drug Safety Notification, revised 12/21/2016).
- 5.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 6.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287e298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiaoa L., Zhanga X., Wanga M., Li B., Liua Z., Liu S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohydrate Polymers. 2014;114:567–573. doi: 10.1016/j.carbpol.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO), Radix Ginseng . World Health Organization; Genova, Switzerland: 2010. WHO Monographs on medicinal plants commonly used in the Newly Independent States (NIS) pp. 141–160. [Google Scholar]
- 9.Blumenthal M. Theime; Austin, TX: 2003. The ABC clinical guide to herbs; pp. 211–225. [Google Scholar]
- 10.Panax ginseng Monograph. Altern Med Rev. 2009;14:172–176. [PubMed] [Google Scholar]
- 11.Blumenthal M., Goldberg A., Brinkmann J. Integrative Medicine Communications; Austin, TX: 2000. Herbal medicine: expanded commission E monographs; pp. 170–177. [Google Scholar]
- 12.Wang Y., Choi H.K., Brinkmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): a review. J Chromatogr A. 2015;1426:1–15. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y.C., Li G., Jiang C., Yang B., Yang H.J., Xu H.Y., Huang L.Q. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules. 2014;19:1781–1799. doi: 10.3390/molecules191117381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan S.M., Luo J.G., Huang F., Kong L.Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J Pharm Biomed Anal. 2014;89:76–82. doi: 10.1016/j.jpba.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwark Y.S. Characterization of Korean Red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:382–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CZ, Anderson S, Du W, He TC, Yuan CS. Red ginseng and cancer treatment. Chinese J Nat Med 216;14:7–16 [DOI] [PubMed]
- 17.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Kim G.N., Lee J.S., Song J.H., Ch Oh, Kwon Y.I., Jang H.D. Heat processing decreases Amadori products and increases total phenolic content and antioxidant activity of Korean Red ginseng. J Med Food. 2010;13:1478–1484. doi: 10.1089/jmf.2010.1076. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura Y., Zheng Y., Takaku T., Kameda K., Okuda H. Isolation and physiological activities of new amino acid derivatives from Korean Red ginseng. Korean J Ginseng Sci. 1994;18:204–211. [Google Scholar]
- 20.Matsuura Y., Hirao Y., Yoshida S., Kunihiro K., Fuwa T., Kasai R., Tanaka O. Study on Red ginseng: new ginsenosides and a note on the occurrence of maltol. Chem Pharm Bull. 1984;32:4674–4677. [Google Scholar]
- 21.Yuo C.R., Yong J.J., Popovich D.G. Isolation and characterization of bioactive polyacetylenes Panax ginseng Meyer roots. J Pharmaceut Biomed Anal. 2017;139:148–155. doi: 10.1016/j.jpba.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. Chemical studies on crude drug precession. I. On the constituents of ginseng radix rubra (1) Yakugaku Zasshi. 1983;103:612. [PubMed] [Google Scholar]
- 23.Zhang X., Yu L., Bi H., Li X., Ni W., Han H., Li N., Wang B., Zhou Y., Tai G. Total fractionation and characterization of the water soluble polysaccharides isolated from Panax ginseng C.A. Meyer. Carbohydr Polym. 2009;77:542–552. [Google Scholar]
- 24.Kim K.H., Jang S.A., Kim K.S., Park S., Park H.J., Lee S.J., Pyo S., Sohn E.H. Effects of non-saponin red ginseng components (NSRG) on functions of macrophages isolated from young and aged mice. J Ginseng Res. 2009;33:177–182. [Google Scholar]
- 25.Im J.K., Cho I.Y., Min K.Y., Lee H.Y., Kim S.J., Park Y.J., Lew B.J., Kim S.W., Joo I.W. Comparative study of natural killer cell activity after Red ginseng medication on rat. Korean J Orient Int Med. 2008;29:1075–1082. [Google Scholar]
- 26.Kim Y.S., Park K.M., Shin H.J., Song K.S., Nam K.Y., Park J.D. Anticancer activities of Red ginseng acidic polysaccharide by activation of macrophages and natural killer cells. Yakhak Hoeji. 2002;46:113–119. [Google Scholar]
- 27.Lee B., Heo H., OH S Lew J. Comparison study of Korean and Chinese ginsengs on the regulation of lymphocyte proliferation and cytokine production. J Ginseng Res. 2008;32:250–256. [Google Scholar]
- 28.Jang S.K., Kim J.H., Chung Y.S., Ahn D.C., Kang M.J., Lee D.G., Kim S.H. An experimental study on the effect of immunopotential and the anticancer effect of Red ginseng extract. Korean J Ginseng Sci. 1994;18:151–159. [Google Scholar]
- 29.Lee H.Y., Lee H. Stimulatory effect of Korean Red ginseng extract on the proliferation and cellular activity of lymphocytes. Korean J Ginseng Sci. 1998;22:60–65. [Google Scholar]
- 30.Yoo D.G., Kim M.C., Park M.K., Song J.M., Quan F.S., Park K.M., Cho Y.K., Kang S.M. Protective Effect of Korean Red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 2012;15:855–862. doi: 10.1089/jmf.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J.Y., Kim H.J., Kim H.J. Effect of oral administration of Korean Red ginseng on influenza A (H1N1) virus infection. J. Ginseng Res. 2011;35:104–110. [Google Scholar]
- 32.Xu M.L., Kim H.J., Choi Y.R., Kim H.J. Intake of Korean Red ginseng extract and saponin enhances the protection conferred by vaccination with inactivated influenza A virus. J Ginseng Res. 2012;36:396–402. doi: 10.5142/jgr.2012.36.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park E.H., Yum J., Ku K.B., Kim H.M., Kang Y.M., Kim J.C., Kim J.A., Kang Y.K., Seo S.H. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res. 2014;38:40–46. doi: 10.1016/j.jgr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.S., Lee Y.N., Lee Y.T., Hwang H.S., Kim K.H., Ko E.J., Kim M.C., Kang S.M. Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication. Nutrients. 2015;7:1021–1036. doi: 10.3390/nu7021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.S., Ko E.J., Hwang H.S., Lee Y.N., Kwon Y.N., Kim M.C., Kang S.M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int J Mol Med. 2014;34:183–190. doi: 10.3892/ijmm.2014.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh S.O., Jeung C.H., Cho M.Y., Soon G.S. The effect of Red ginseng for postoperative immune response in gastrointestinal carcinoma. Korean J Ginseng Sci. 1988;22:32–42. [Google Scholar]
- 37.Suh S.O., Kim J., Cho M.Y. Prospective study for Korean Red ginseng extract as an immune modulator following a curative gastric resection in patients with advanced gastric cancer. J Ginseng Res. 2004;28:104–110. [Google Scholar]
- 38.Suh S.O., Boo Y.J., Park J.M., Kim J. Prospective study for Korean Red ginseng extract as an immune modulator following a curative surgery in patients with advanced colon cancer. J Ginseng Res. 2007;31:54–59. [Google Scholar]
- 39.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean Red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 40.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., Kwon D.Y., Ha K.C., Park E.O., Lee N. Preventive effect of Korean Red ginseng for acute respiratory illness: a randomized and double-blind clinical trial. J Korean Med Sci. 2012;27:1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho Y.K., Sung H., Lee H.J., Joo C.H., Cho G.J. Long-term intake of Korean Red ginseng in HIV-1-infected patients: development of resistance mutation to zidovudine is delayed. Int Immunopharmacol. 2001;1:1295–2305. doi: 10.1016/s1567-5769(01)00061-3. [DOI] [PubMed] [Google Scholar]
- 42.Sung H., Kang S.M., Lee M.S., Kim T.G., Cho Y.K. Korean Red ginseng slows depletion of CD4 T cells in human immunodeficiency virus type 1-infected patients. Clin Dian Lab Immunol. 2005;12:497–501. doi: 10.1128/CDLI.12.4.497-501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H.D., Nho H.S., Khil J.H. The effect of Korean Red ginseng supplement on CK, GOT, peak torque, and ROM after strenuous downhill running. Korean J Sport Sci. 2004;15:53–62. [Google Scholar]
- 44.Yoon S.J., Kim K.H., Kim C.J., Park H.C., Kang K.H., Kim M.J., Kang S.M., Kwak U.H., Kim H.J. Effects of Red ginseng supplementation on aerobic · anaerobic performance, central and peripheral fatigue. J Ginseng Res. 2008;32:210–219. [Google Scholar]
- 45.Jung H.L., Kwak H.E., Kim S.S., Kim Y.C., Lee C.D., Byurn H.K., Kang H.Y. Effects of Panax ginseng supplementation on muscle damage and inflammation after uphill treadmill running in humans. Am J Chin Med. 2011;39:441–450. doi: 10.1142/S0192415X11008944. [DOI] [PubMed] [Google Scholar]
- 46.Min Y.K., Chung S.H., Lee J.S., Kim S.S., Shin H.D., Lim B.V., Shin M.C., Jang M.H., Kim E.H., Kim C.J. Red ginseng inhibits exercise-induced increase in 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in dorsal raphe of rats. J Pharmacol Sci. 2003;93:218–221. doi: 10.1254/jphs.93.218. [DOI] [PubMed] [Google Scholar]
- 47.Kim S.S., Ha S.H., Yoo J.H. The effects of long term submaximal exercise and Red ginseng administration on the antioxidant enzymes and lipid peroxidation during maximal exercise. J Coaching Development. 2006;8:233–242. [Google Scholar]
- 48.Lee S.H., Park C.W., Lee I.R., Han B.H. Effect of ginseng saponin on the biosynthesis of prostaglandins. Korean J Ginseng Sci. 1989;13:202–210. [Google Scholar]
- 49.Yu J.Y., Jin Y.R., Lee J.J., Chung J.H., Noh J.Y., You S.H., Kim K.N., Im J.H., Lee J.H., Seo J.M. Antiplatelet and antithrombotic activities of Korean Red ginseng. Arch Pharm Res. 2006;29:898–903. doi: 10.1007/BF02973912. [DOI] [PubMed] [Google Scholar]
- 50.Park K.M., Rhee M.H., Park H.J. Panaxadiol and panaxatriol from Panax ginseng C. A. Meyer inhibit the synthesis of thromboxane A2 in adrenaline-stimulated human platelet aggregations. Korean J. Ginseng Sci. 1994;18:44–48. [Google Scholar]
- 51.Yamamoto K., Hirai A., Tamura Y., Yoshida S. In vitro and in vivo effect of ginseng saponins, major components of Korean Red ginseng on human platelet aggregation and arachidonic acid metabolism. J Med Pharmaceu Soc WAKAN-YAKU. 1988;5:184–190. [Google Scholar]
- 52.Shin K.S., Lee J.J., Jin Y.R., Yu J.Y., Park E.S., Im J.H., You S.H., Oh K.W., Lee M.K., Wee J.J. Effect of Korean Red ginseng extract on blood circulation in healthy volunteers: a randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2007;31:109–116. [Google Scholar]
- 53.Lee J.H., Park H.J. Effects of intaking of Red ginseng products on human platelet aggregation and blood lipids. J Ginseng Sci. 1998;22:173–180. [Google Scholar]
- 54.Hirai A. Proc '99 Korea-Japan Ginseng Sym. Korea Ginseng Research Institute, Seoul, Korea. 1999. Studies on the mechanisms of anti-platelet and anti-atherosclerotic effects of Korean Red ginseng: focusing on arachidonic acid cascade; pp. 16–31. [Google Scholar]
- 55.Benishin C.G., Lee R., Wang L.C., Liu H.J. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacol. 1991;42:223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 56.Benishin C.G. Action of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem Int. 1992;21:1–5. doi: 10.1016/0197-0186(92)90061-u. [DOI] [PubMed] [Google Scholar]
- 57.Salim K.N., McEwen B.S., Cha H.M. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Mol Brain Res. 1997;47:177–182. doi: 10.1016/s0169-328x(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 58.Nishijo H., Uwano T., Zhong Y.M., Ono T. Proof of the mysterious efficacy of ginseng: basic and clinical trials: effects of Red ginseng on learning and memory deficits in an animal model of amnesia. J Pharmacol. 2004;95:145–152. doi: 10.1254/jphs.fmj04001x3. [DOI] [PubMed] [Google Scholar]
- 59.Zhao H., Li Q., Zhang Z., Pei X., Wang J., Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 60.Lee Y., OH S. Administration of Red ginseng ameliorates memory decline in aged mice. J Ginseng Res. 2015;39:250–256. doi: 10.1016/j.jgr.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen T.C., Yoshimura H., Matsuda S., Lim J.H., Sakanaka M. Ginseng root prevents learning disability and neuronal loss in gerbils with 5-minute forebrain ischemia. Acta Neuropathol. 1996;91:15–22. doi: 10.1007/s004010050387. [DOI] [PubMed] [Google Scholar]
- 62.Kennedy D.O., Reay J.L., Scholey A.B. Effects of 8 weeks administration of Korean Panax ginseng extract on the mood and cognitive performance of healthy individuals. J Ginseng Res. 2007;31:34–43. [Google Scholar]
- 63.Heo J.H., Lee S.T., Chu K.C., Oh N.J., Park H.J., Shim J.Y., Kim M. An open-label trial of Korean Red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur J Neurology. 2008;15:865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 64.Lee J.K., Kim N.Y., Han Y.N., Choi J. Effects of pretreated Korean Red ginseng on carbon tetrachloride and galactosamine-induced hepatotoxicity in rats. J Ginseng Res. 2003;27:1–10. [Google Scholar]
- 65.Lee J.K., Han Y.N., Kim N.Y., Choi J. The therapeutic effects of Korean Red ginseng on carbon tetrachloride and galactosamine-induced hepatotoxicity in rats. J Ginseng Res. 2003;27:1–6. [Google Scholar]
- 66.Kim Y.S., Kim Y.H., Noh J.R., Cho E.S., Park J.H., Son H.Y. Protective effect of Korean Red ginseng against aflatoxin B1-induced hepatotoxicity in rat. J. Ginseng Res. 2011;35:243–249. doi: 10.5142/jgr.2011.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdel-Aziem S.H., Hassan A.M., Abdel-Wahhab M.A. Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutation Res. 2011;723:65–71. doi: 10.1016/j.mrgentox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Seong G.S., Chun S.G., Chang C.C. Hepatoprotective effects of White and Red ginseng extracts on acetaminophen-induce hepatotoxicity in mice. J Ginseng Res. 2005;29:131–137. [Google Scholar]
- 69.Park M.S., Cho E.J., Lee S.K., Lee E.J., Lee D.S., Lee K.H., Jeon B.H. Korean Red ginseng protects oxidative injury caused by lead poisoning. J Ginseng Res. 2010;34:132–137. [Google Scholar]
- 70.Kim D.J., Chang C.C. The effects of red ginseng extract on antioxidant enzyme activities and lipid peroxidation of the kidney in γ-postirradiated mice. Korean J Ginseng Sci. 1994;18:25–31. [Google Scholar]
- 71.Lee T.K., Johnke R.M., Allison R.R., O’Brien K.F., Dobbs J., Jr. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 72.Kang K.S., Kim H.Y., Pyo J.S., Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–754. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 73.Kim Y.K., Guo Q., Packer L. Free radical scavenging activity of Red ginseng aqueous extracts. Toxicol. 2002;172:149–156. doi: 10.1016/s0300-483x(01)00585-6. [DOI] [PubMed] [Google Scholar]
- 74.Lee B.M., Lee S.K., Kim H.S. Inhibition of oxidative DNA damage, 8-OHdG, and carbonyl contents in smokers treated with antioxidants (vitamin E, vitamin C, b-carotene and Red ginseng) Cancer Lett. 1998;132:219–227. doi: 10.1016/s0304-3835(98)00227-4. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.Y., park J.Y., Kang H.J., Kim O.Y., Lee J.H. Beneficial effects of Korean Red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: a randomized, double-blind, placebo-controlled trial. Nut J. 2012;11:47–58. doi: 10.1186/1475-2891-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seo S.K., Hong Y., Yun B.Y., Chon S.J., Jung Y.S., Park J.H., Cho S.H., Choi Y.S., Lee B.S. Antioxidative effects of Korean Red ginseng in postmenopausal women: a double-blind randomized controlled trial. J Ethnopharmacol. 2014;154:753–757. doi: 10.1016/j.jep.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 77.Kim S.Y., Seo S.K., Choi Y.M., Jeon Y.E., Lim K.J., Cho S.H., Choi Y.S., Lee B.S. Effects of Red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: a double-blind randomized controlled trial. Menopause. 2012;19:461–466. doi: 10.1097/gme.0b013e3182325e4b. [DOI] [PubMed] [Google Scholar]
- 78.Kim H.S., Yoon Y.J., Lee J.M., Lee C.H., Jang J.B., Lee K.S., Cho J.H. A Clinical Study on the effect of Red ginseng for postmenopausal hot flushes. J Oriental Obstetrics Gynecol. 2009;22:132–139. [Google Scholar]
- 79.Ogita S. Clinical effectiveness of Korea ginseng on climacteric disturbances and its possible mechanism of action. Korean J Ginseng Sci. 1990;14:162–166. [Google Scholar]
- 80.Kikuchi Y., Tode T., Hirata J., Nakata H., Kita T. Clinical usefulness of Korean Red ginseng in postmenopausal women with severe climacteric disturbance. J Ginseng Res. 2003;27:98–102. [Google Scholar]
- 81.Tode T., Kikuchi Y. Effect of Korean Red ginseng on psychological functions in patients with severe climacteric syndromes: a comprehensive study from the viewpoint of traditional KAMPO-medicine and western medicine. J Ginseng Res. 2003;27:110–114. [Google Scholar]
- 82.Oh K.J., Chae M.J., Lee H.S., Hong H.D., Park K. Effects of Korean Red ginseng on sexual arousal in menopausal women: placebo-controlled, double-blind crossover clinical study. J Sex Med. 2010;7:1469–1477. doi: 10.1111/j.1743-6109.2009.01700.x. [DOI] [PubMed] [Google Scholar]
- 83.Gui Q., Xu Z., Xu k, Yang Y. The efficacy of ginseng-related therapies in type 2 diabetes mellitus. Medicine. 2016;96:e2584. doi: 10.1097/MD.0000000000002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee H.W., Choi J., Lee Y.J., Kil K.J., Lee M.S. Ginseng for managing menopausal woman’s health. A systematic review of double-blind, randomized, placebo-controlled trials. Medicine. 2016;95:e4914. doi: 10.1097/MD.0000000000004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jang D.J., Lee M.S., Shin B.C., Lee Y.C., Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin X., Che D., Zhang Z., Yan H., Jia Z., Jia X. Ginseng consumption and risk of cancer: a meta-analysis. J Ginseng Res. 2016;40:268–277. doi: 10.1016/j.jgr.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean Red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 88.Bang H., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean Red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17:128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reay J.L., Scholey A.B., Milne A., Fenwick J., Kennedy D.O. Panax ginseng has no effect on indices of glucose regulation following acute or chronic ingestion in healthy volunteers. Br J Nutr. 2009;101:1673–1678. doi: 10.1017/S0007114508123418. [DOI] [PubMed] [Google Scholar]
- 90.Reeds D.N., Patterson B.W., Okunade A., Holloszy J.O., Polonsky K.S., Klein S. Ginseng and ginsenoside Re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care. 2011;34:1071–1076. doi: 10.2337/dc10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sin S., Kim S.Y., Kim S.S. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. Int J Oncolog. 2012;41:1669–1674. doi: 10.3892/ijo.2012.1604. [DOI] [PubMed] [Google Scholar]
- 92.Ota T., Maeda M., Odashima S., Ninomiya-Tsuji J., Tatsuka M. G1 phase-specific suppression of the Cdk2 by ginsenoside Rh2 in cultured murine cells. Life Sci. 1996;60:PL39–PL44. doi: 10.1016/s0024-3205(96)00608-x. [DOI] [PubMed] [Google Scholar]
- 93.Wang C.Z., Zhang Z., Wan J.Y., Zhang C.F., Anderson S., He X., Yu C., He T.C., Qi L.W., Yuan C.S. Protopanaxadiol, an active ginseng metabolite, significantly enhances the effects of fluorouracil on colon cancer. Nutrients. 2015;7:799–814. doi: 10.3390/nu7020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee K.Y., Lee Y.H., Park J.H., Lee S.K. Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase activity via up-regulating p21Cip/WAF1 and down-regulating cyclin E in SK-HEP-1 cells. Anticancer Res. 1997;17:1067–1072. [PubMed] [Google Scholar]
- 95.Kim S.E., Lee Y.H., Park J.H., Lee S.K. Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepatoma K-HEP-1 cells. Eur J Cancer. 1999;35:507–511. doi: 10.1016/s0959-8049(98)00415-8. [DOI] [PubMed] [Google Scholar]
- 96.Wang H.N., Zuo G.W., Li C.L. Effects of ginsenoside Rb1, Rg1 on proliferation of leukemia K562 cells. J Clin Rehabilit Tissue Engineering Res. 2009;13:7829–7832. [Google Scholar]
- 97.Shangguan W.J., Li H., Zhang Y.H. “Induction of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in human osteosarcoma MG-63 cells through the mitochondrial pathway. Oncol Rep. 2014;31:305–313. doi: 10.3892/or.2013.2815. [DOI] [PubMed] [Google Scholar]
- 98.Kim S.E., Lee Y.H., Park J.H., Lee S.K. Ginsenoside-Rs3, a new diol-type ginseng saponin, selectively elevates protein levels of p53 and p21(WAF1) leading to induction of apoptosis in SK-HEP-1 cells. Anticancer Res. 1999;19:487–491. [PubMed] [Google Scholar]
- 99.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean Red ginseng water extract. J Ethnopharm. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Mochizuki M., Yoo Y.C., Matsuzawa K., Sato K., Saiki I., Tono-oka S., Samukawa K., Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of Red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 101.Sur Y.J., Na H.K., Lee J.Y., Keum Y.S. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(suppl):S38–S41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shin H.J., Kim Y.S., Kwak Y.S., Song Y.B., Kim Y.S., Park J.D. Enhancement of antitumor effects of paclitaxel (taxol) in combination with Red ginseng acidic polysaccharide (RGAP) Planta Med. 2004;70:1033–1038. doi: 10.1055/s-2004-832643. [DOI] [PubMed] [Google Scholar]
- 103.Liu T., Huang Y., Cui D., Huang X., Mao S., Ji L., Song H., Yi C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009;9:250. doi: 10.1186/1471-2407-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Q., Kang X., Zhao W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophysic Res Comm. 2006;342:824–828. doi: 10.1016/j.bbrc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 105.Choi C., Kang G., Min Y. Reversal of P-glycoprotein mediated multidrug resistance by protopanaxatriol ginsenosides from Korean Red ginseng. Planta Med. 2003;69:235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- 106.Kim S.M., Lee S.Y., Yuk D.Y. Inhibition of NF-??B by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res. 2009;32:755–765. doi: 10.1007/s12272-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 107.Baek S.H., Piao X.L., Lee U.J., Kim H.Y., Park J.H. Reduction of cisplatin-induced nephrotoxicity by ginsenosides isolated from processed ginseng in cultured renal tubular cells. Biol Pharm Bull. 2006;29:2051–2055. doi: 10.1248/bpb.29.2051. [DOI] [PubMed] [Google Scholar]
- 108.Yun T.K. Panax ginseng-a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 109.Yun T.K. Experimental and epidemiological evidence on nonorgan specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 110.Yun T.K., Zheng S., Choi S., Cai S.R., Lee Y.S., Liu X.Y., Cho K.J., Park K.Y. Non-organ-specific preventive effect of long-term administration of Korea Red ginseng extract on incidence of human cancers. J Med Food. 2010;13:489–494. doi: 10.1089/jmf.2009.1275. [DOI] [PubMed] [Google Scholar]
- 111.Chen X., Lee T.J. Ginsenosides-induced nitric oxide mediated relaxation of the rabbit corpus cavernosum. Br J Pharmacol. 1995;115:15–18. doi: 10.1111/j.1476-5381.1995.tb16313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy L.L., Lee T.J. Ginseng, sex behavior, and nitric oxide. Ann N Y Acad Sci. 2002;962:372–377. doi: 10.1111/j.1749-6632.2002.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 113.Choi Y.D., Rha K.H., Choi H.K. In vitro and in vivo experimental effect of Korean Red ginseng on erection. J Urol. 1999;162:1508–1511. [PubMed] [Google Scholar]
- 114.Leung K.W., Cheng Y.K., Mak N.K., Chan K.K.C., Fan T.P.D., Wong R.N. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–3216. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 115.Wang X., Chu S., Qian T., Chen J., Zhang J. Ginsenoside Rg1 improves male copulatory behavior via nitric oxide/cyclic guanosine monophosphate pathway. J Sex Med. 2010;7:743–750. doi: 10.1111/j.1743-6109.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 116.Jang D.J., Lee M.S., Shin B.C., Lee Y.C., Ernst E. Red ginseng for treating erectile dysfunction; a systematic review. Br J Clin Pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park H.J., Choe S., Paek N.C. Effects of Korean Red ginseng on semen parameters in male infertility patients: a randomized, placebo-controlled, double-blind clinical study. Chin J Integr Med. 2016;22:490–495. doi: 10.1007/s11655-015-2139-9. [DOI] [PubMed] [Google Scholar]
- 118.Lee S.A., Kang S.G., Lee H.J., Jung K.Y., Kim L. Effect of Korean Red ginseng on sleep: a randomized, placebo-controlled trial. Sleep Med Psychophysiol. 2010;17:85–90. [Google Scholar]
- 119.Han H.J., Kim H.Y., Choi J.J., Ahn S.Y., Lee S.H., Oh K.W., Kim S.Y. Effects of Red ginseng extract on sleeping behaviors in human volunteers. J Ethnopharmacol. 2013;149:597–599. doi: 10.1016/j.jep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Park J.W., Lee B.J., Bu Y., Yeo I., Kim J., Ryu B. Effects of Korean Red ginseng on dry mouth: a randomized, placebo-controlled trial. J Ginseng Res. 2010;34:183–191. [Google Scholar]
- 121.Kim J.H., Yi S.M., Choi J.E., Son S.W. Study of the efficacy of Korean Red ginseng in the treatment of androgenic alopecia. J Ginseng Res. 2009;33:223–228. [Google Scholar]
- 122.Huang K.C. CRC Press; Boca Raton: 1999. The pharmacology of Chinese herbs. [Google Scholar]
- 123.Pharmacopoeia of the People's Republic of China, volume I. Chinese Pharmacopoeia Commission; Beijing (CN): 2005. [Google Scholar]
- 124.Kim Y.S., Woo J.Y., Han C.K., Chang I.M. Safety analysis of Panax ginseng in randomized clinical trials: a systematic review. Medicines. 2015;2:106–126. doi: 10.3390/medicines2020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ginseng, Panax: MedlinePlus. U. S. National Library of Medicine: http://www.nlm.nih.gov/medlineplus/druginfo/natural/1000.html?print=y.
- 126.de Andrade E., de Mesquita A.A., Claro Jde A., de Andrade P.M., Ortiz V., Paranhos M., Srougi M. Study of the efficacy of Korean Red ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9:241–244. doi: 10.1111/j.1745-7262.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 127.Lim S.H., Shin S., Jang J.Y., Byun S.K., Lee Y.E., Park D., Jeon J.H., Lee Y.K., Lee D.M., Lee S. Single- and repeated-dose toxicities of Korean Red ginseng extract in mice. J Vet Med Biotechnol. 2005;6:187–202. [Google Scholar]
- 128.Park S.J., Lim K.H., Noh J.H., Jeong E.J., Kim Y.S., Han B.C., Lee S.H., Moon K.S. Subacute oral toxicity study of Korean Red ginseng extract in Sprague-Dawley rats. Toxicol Res. 2013;29:285–292. doi: 10.5487/TR.2013.29.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong S.K., Lee S.J., Kim Y.S., Jeon B.S., Lim C.H. Pro 4th Int Ginseng Sym. Korea Ginseng and Tobacco Research Institute, Daejeon, Korea. 1984. Studies of the safety of Korean ginseng ingested as food substance. [Google Scholar]
- 130.Jo S.K., Yook H.S., Byun M.W. Genoxoticolocy safety of the gamma-irradiated Korean red ginseng in vitro. J Korean Soc Food Nut. 1996;25:491–496. [Google Scholar]
- 131.Shin S., Jang J.Y., Park D., Yon J.M., Baek I.J., Hwang B.Y., Nam S.Y., Yun Y.W., Joo S.J., Kim K.Y. Korean Red ginseng extract does not cause embryo-fetal death or abnormalities in mice. Birth Defects Res (part B) 2010;89:78–85. doi: 10.1002/bdrb.20224. [DOI] [PubMed] [Google Scholar]
- 132.Lim S.H., Shin S., Jang J.Y., Choi B., Byun S.K., Lee Y.E., Park D., Jeon J.H., Nam S.Y., Yun Y.W. National Institute of Food and Drug Safety Evaluation; Korea: 2005. Reproductive and developmental toxicity study on Korea Red ginseng extract in mice. The Annual report of KNTP. [Google Scholar]
- 133.Siegel R.K. Ginseng abuse syndrome; problems with the panacea. J Am Med Assoc. 1979;241:1641–1645. [PubMed] [Google Scholar]
- 134.Kim D.H., Xu Y.H., Kim Y.C., Bang K.W., Kim J.U., Cha S.W., He Z.M., He H., Jang I.B., Zhang L.X. Clinical study on food evaluation of Panax ginseng. Korean J Medicinal Crop Sci. 2015;23:185–189. [Google Scholar]
- 135.Kim H.J., Chun Y.J., Park J.D., Kim S.I., Roh J.K., Jeong T.C. Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by Red ginseng saponin through cytochrome P450 inhibition. Planta Med. 1887;63:415–418. doi: 10.1055/s-2006-957724. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y., Zhang J.W., Li W., Ma H., Sun J., Deng M.C. Yang Ling. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicol Sci. 2006;91:356–364. doi: 10.1093/toxsci/kfj164. [DOI] [PubMed] [Google Scholar]
- 137.Henderson G.L., Harkey M.R., Gershwin M.E., Hackman R.M., Stern J.S., Stresser D.M. Effects of ginseng components on c-DNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 1999;65:PL209–PL214. doi: 10.1016/s0024-3205(99)00407-5. [DOI] [PubMed] [Google Scholar]
- 138.Fang Z.Z., Cao Y.F., Hu C.M., Hong M., Sun X.Y., Ge G.B., Liu Y., Zhang Y.Y., Yang L., Sun H.Z. Structure–inhibition relationship of ginsenosides towards UDP-glucuronosyltransferases (UGTs) Toxicol Appl Pharmacol. 2013;267:149–154. doi: 10.1016/j.taap.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 139.Liu Y., Ma H., Zhang J.W., Deng M.C., Yang L. Influence of ginsenoside Rh1 and F1 on human cytochrome p450 enzymes. Planta Med. 2006;72:126–131. doi: 10.1055/s-2005-873197. [DOI] [PubMed] [Google Scholar]
- 140.Zhang J., Zhou F., Wu X., Gu Y., Ai H., Zheng Y., Li Y., Zhang X., Hao G., Sun J. 20(S)-Ginsenoside Rh2 noncompetitively inhibits p-glycoprotein in vitro and in vivo: A case for herb-drug interactions. Drug Metabol Dis. 2010;38:2179–2187. doi: 10.1124/dmd.110.034793. [DOI] [PubMed] [Google Scholar]
- 141.Kim D.S., Kim Y., Jeon J.Y., Kim M.G. Effect of red ginseng on cytochrome P450 and P-glycoprotein activities in healthy volunteers. J Ginseng Sci. 2016;40:375–381. doi: 10.1016/j.jgr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee Y.H., Lee B.K., Choi Y.J., Yoon I.K., Chang B.C., Gwak H.S. Interaction between warfarin and Korean Red ginseng in patients with cardiac valve replacement. Int J Cardiol. 2010;145:275–276. doi: 10.1016/j.ijcard.2009.09.553. [DOI] [PubMed] [Google Scholar]