Abstract
Rapid progress in tissue engineering research in past decades has opened up vast possibilities to tackle the challenges of generating tissues or organs that mimic native structures. The success of tissue engineered constructs largely depends on the incorporation of a stable vascular network that eventually anastomoses with the host vasculature to support the various biological functions of embedded cells. In recent years, significant progress has been achieved with respect to extrusion, laser, micro-molding, and electrospinning-based techniques that allow the fabrication of any geometry in a layer-by-layer fashion. Moreover, decellularized matrix, self-assembled structures, and cell sheets have been explored to replace the biopolymers needed for scaffold fabrication. While the techniques have evolved to create specific tissues or organs with outstanding geometric precision, formation of interconnected, functional, and perfused vascular networks remains a challenge. This article briefly reviews recent progress in 3D fabrication approaches used to fabricate vascular networks with incorporated cells, angiogenic factors, proteins, and/or peptides. The influence of the fabricated network on blood vessel formation, and the various features, merits, and shortcomings of the various fabrication techniques are discussed and summarized.
Keywords: 3D bioprinting, Tissue engineering, Vascularization, Extrusion, Laser-based printing, Co-axial printing
Graphical abstract
Highlights
-
•
Recent developments in direct/indirect extrusion-based fabrication techniques in vasculature formation.
-
•
Current status of laser-based, nano-scale, and biopolymer-free fabrication techniques in vascularization.
-
•
Highlights on the recent progress in micro-pattern and micro-module assemblies for vascularization.
-
•
Influence of decellularized matrix and mechanical spacer in tissue vascularization.
1. Introduction
Tissue regeneration requires the simultaneous growth of vasculature to facilitate the diffusional mass transfer of nutrients, oxygen, growth factors, biochemical signaling factors, carbon dioxide, and metabolic waste from the surroundings to cells and vice versa [1], [2]. In particular, the vascular network should reach within 100–200 µm of the tissue to avoid ischemic conditions and cell death [3]. Blood vessels with different diameters (∼ 4–300 µm) spread in a complicated fashion (i.e., fractal shapes) into tissue to exchange nutrients, gas, and metabolites to a huge cell population [4]. Capillaries in the vascular network play a vital role in the mass transfer mechanism. Therefore, tissue regeneration with scaffolds requires the incorporation of an interconnected capillary network with vessels located every 100–200 µm in all directions.
Tissue vascularization is a complex process that develops through vasculogenesis and angiogenesis in vivo [4], [5], [6]. In vasculogenesis, endothelial progenitor cells (EPCs) migrate to an ischemic site, proliferate, and differentiate to form capillary vessels, while angiogenesis occurs when new blood vessels sprout from existing ones according to a gradient of angiogenic factors [5], [7]. The blood vessels formed by either vasculogenesis or angiogenesis are eventually remodeled and mature as per the demands of specific tissues through the upregulation of various growth factors.
Taking into account the in vivo vasculature formation mechanism, a number of 3D fabrication approaches have evolved over past decades to mimic the native vascular network. Direct and indirect bioprinting approaches have proven promising for the fabrication of large 3D tissue constructs with intricate vascular networks. The use of coaxial needles in extrusion-based (EB) systems revolutionized these biofabrication techniques and resulted in the ability to print lumen-incorporated strands. Indirect biofabrication is convenient when scaffolding biopolymers demonstrate poor printability and manipulation complexity. In this regard, sacrificial biopolymers are a smart choice of scaffolding material in vascular network fabrication. In addition to EB biofabrication, several potential approaches including micro-pattern fabrication and assembly, laser-based fabrication, nano-scale fabrication, and natural matrix recellularization have evolved to generate vascular networks. Stacking 2D micropatterned substrates or micro tissue modules results in the formation of complex interconnected vascular networks. Laser-based fabrication allows both 2D and 3D fabrication in a layer-by-layer fashion in the presence of a photo mask, donor substrate, photo sensitive polymer, or photo initiator. Electrospinning, an advanced fabrication technique reported in numerous studies [8], [9], [10], [11], supports the fabrication of extracellular matrix (ECM)-like nano-scale filaments that enhance the interaction with endothelial cells (ECs). Seeding ECs into decellularized tissue matrix promotes vascularization, with the preparation process affecting the quality of the native matrix.
A review of recent progress with respect to tissue vascularization with available fabrication techniques is necessary to guide future research. Several articles have focused on the synergistic effect of cells, biopolymers, angiogenic factors, and fabrication approaches in terms of tissue vascularization. However, review articles that focus on recent progress in terms of 3D vasculature formation techniques are lacking. This article provides a brief overview of the 3D biofabrication of vascular networks with EB, laser, electrospinning, stacking of micropattern or modules, and cell sheet techniques; discusses the effect of prevascularization on the vascular network formed by the various fabrication techniques; summarizes the challenges, advantages, and shortcomings of different fabrication approaches; and proposes potential future research directions.
2. Rapid prototyping
2.1. Bio-printing vascular networks
Macro-scale tissue constructs require well connected vascular networks to ensure the viability of the large cell population embedded or seeded in the scaffold. Embedded tissue-/organ-specific cells require time to form functional tissues and, during this period, require nutrients, gas, and biomolecules to maintain metabolism, proliferation, and differentiation and enable remodeling. Therefore, researchers have attempted to form perfusable capillary networks within macro-engineered grafts seeded with different types of cells. Specifically, some researchers have used rapid prototyping (RP) or additive manufacturing techniques to form complex capillary networks with hydrogel-based, fugitive, and sacrificial ink. Extrusion- (e.g. 3D bioplotting, and inkjet printing) and laser-based (e.g. stereolithography) techniques in particular have achieved outstanding results with respect to printing vascular networks with intricate architecture.
2.1.1. Inkjet-based bioprinting
Bioprinting of cells ensures a higher cell density in the scaffold compared to post fabrication cell seeding. A number of studies conclude that higher cell densities in the scaffold promote tissue generation by secreting numerous bio-molecules. Therefore, researchers have emphasized 3D bioprinting to incorporate a huge cell population in the scaffold in a controlled and well-distributed fashion. For bioprinting mixtures of cells and hydrogels, extrusion-based RP approaches have been explored in many studies. In particular, inkjet printers and 3D bioplotters are commonly used for scaffold and vascular network printing due to some attractive features. In inkjet printing, bioink drops are dispensed layer-by-layer, while a bioplotting system extrudes continuous filaments to fabricate a predefined structure based on computer-generated (CAD) digital data. Thermal- and piezoelectric-based inkjet bioprinters can print a cell-crosslinking ion mixture at high resolution and speed on hydrogel precursors. In general, hydrogels with rapid gelation properties are used in inkjet systems to handle the high printing speed. Moreover, inkjet printers allow the deposition of multiple cell types in a controlled and organized fashion to mimic the distribution of multiple cells in native tissues. Different inkjet printing studies used alginate or its composites as a bioink and calcium chloride as a crosslinker [12], [13], [14], [15], [16], with vascular networks shown to grow after in vitro or in vivo culture. In one study, three bioinks containing canine smooth muscle cells, human amniotic fluid-derived stem cells, and bovine aortic endothelial cells, respectively, were used to print an alginate-collagen scaffold layer-by-layer using a thermal inkjet printer; vascularized, mature, and functional tissues grew when the scaffolds were implanted in vivo [17]. Likewise, bioink composed of human microvascular endothelial cells (HMVECs) and thrombin solution and dispensed on fibrinogen using an inkjet printer resulted in the alignment and proliferation of the HMVECs and the formation of a capillary-like tubular structure inside the channels [18]. Although inkjet printers are economical and have several attractive features, shortcomings including nozzle clogging, cavitation bubbles, selective ink viscosity, and cell damage during dispensing limit their use for the fabrication of vascular networks [19].
2.1.2. Extrusion-based bioprinting
Several studies have explored both indirect and direct EB techniques in fabricating intricate vascular network to date. In indirect printing, tissue-specific, cell-incorporated hydrogels are used to encapsulate a prefabricated sacrificial vascular network, followed by the removal of the sacrificial ink using an appropriate solvent. ECs are then seeded around the capillary channels using an injection/microfluidic approach. The perfusion of the capillary network with media or blood forms a monolayer of ECs around the capillaries within a couple of days that facilitates diffusional mass transfer and eventual remodeling into blood vessels.
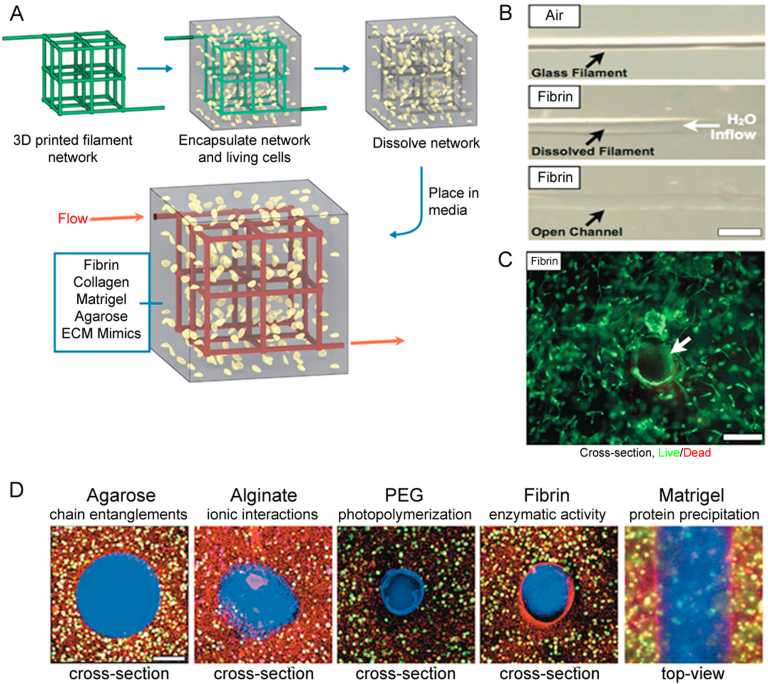
Cytocompatible sacrificial templates have been explored to avoid the use of cytotoxic organic solvents or processing conditions to eliminate the sacrificial filaments from engineered constructs [20] (Fig. 1). In one study, carbohydrate glass filaments were dispensed at 110 °C using a 3D printer to form a patterned 3D network, and then the network was encapsulated in an agarose polymer loaded with primary rat hepatocytes and fibroblast cells. The soluble sacrificial filaments were removed by the cell culture media, the vascular lumen then seeded with human umbilical vein endothelial cells (HUVECs), and the vascular network perfused with blood in vivo. This vascularized construct supported the metabolic function of primary rat hepatocytes, which maintained higher albumin secretion and urea synthesis than in gels without channels [21]. However, complexities related to high temperature dispensing, random distribution of multiple cell types, and hygroscopic behavior of carbohydrate filaments limit vascularization applications. A number of studies have investigated the efficacy of sacrificial networks with respect to generating vasculature. Rather than random incorporation of multiple types of tissue- or organ-specific cells in the matrix, capillary vessels and various types of cells were printed separately side-by-side to mimic the native tissue or organ. In one study, a 3D bioprinting technique was used to co-print fibroblast-laden GelMA, fugitive ink, and human neonatal dermal fibroblast-loaded GelMA strands layer-by-layer. The vascular network fabricated in this way was encapsulated into GelMA, and then the fugitive ink (Pluronic® F127) removed by liquifying at 4 °C. When the HUVECs were injected in the fugitive ink-removed lumen followed by gentle rocking, greater than 95% viability was achieved with a confluent EC layer identified 48 h after seeding [22].
Fig. 1.
(A) Carbohydrate glass lattice as the sacrificial structure for the creation of vascular architecture, (B) a single carbohydrate glass fiber encapsulated in a fibrin gel, (C) cross-section image of unlabeled HUVEC and 10T1/2 co-cultures (not expressing enhanced green fluorescent protein (EGFP)) encapsulated in the interstitial space of fibrin gel with perfusable channels, and (D) cross-section of cell-incorporated biomaterials (scale bars = 200µm) (reproduced with permission from [21]).
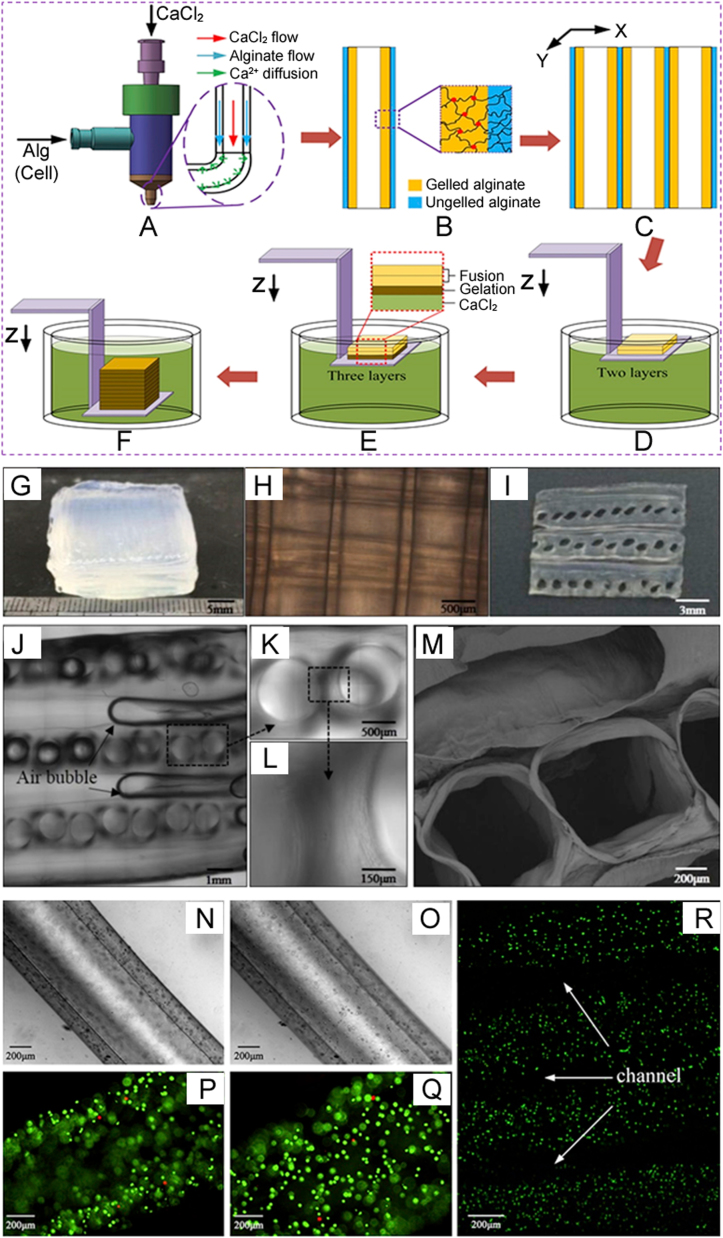
Extrusion-based systems featuring coaxial or shell/core nozzles have been used to directly print hollow fibers or filaments with a microfluidic channel to form a vascular network [23], [24]. In particular, Fig. 2 shows that the flow of ionic crosslinker (e.g. CaCl2) is maintained in the core side of a coaxial nozzle while the flow of hydrogel is maintained in the shell side [25]. The flow rheology of the hydrogel precursor and crosslinker as well as the hydrogel percentage significantly affects the geometry of the core diameter, wall thickness, and microfluidic channels. Such 3D microfluidic networks can be encapsulated into hydrogels containing multiple cell types. In general, microfluidic channels fabricated with a coaxial nozzle-extrusion system are seeded with ECs, and then perfused with culture media or blood to promote capillary blood vessel formation. Alteration of shell/core size significantly affects the ultimate strength, compressive strength, and Young's modulus of the hollow fibers [26]. In addition to seeding, ECs can also be encapsulated in the shell of the hollow channel during fabrication to generate high cell densities [27]. In some studies, tissue spheroids composed of tissue-specific cells were dispensed simultaneously in the space between two successive hollow filaments using multiple robotic arms [28]. Several studies report the efficacy of microfluidic channels fabricated with a coaxial nozzle for promoting vasculature. When human bone marrow stromal cells (hBMSCs) were seeded on the inner walls of hollow alginate-poly(vinyl alcohol) (PVA) fibers extruded from a coaxial nozzle, they showed excellent attachment and spreading after 14 days of culture [26]. Similarly, cartilage progenitor cell (CPC)-encapsulated sodium alginate has been extruded from a coaxial nozzle to form the shell of a microfluidic channel. Initially, CPC viability decreased due to the dispensing pressure, coaxial nozzle geometry, and alginate concentration, but improved significantly over 7 days of incubation [27].
Fig. 2.
Coaxial printing of scaffolds fabricated by an extrusion-based technique: (A) crosslinker diffusion while the biomaterial is extruding through the outer tube, (B) crosslinking of biomaterial, (C) deposition of numerous stands, (D–F) printing layers of scaffolds and immersing in crosslinker, and characterization of the fusion phenomenon between adjacent alginate hollow filaments: (G) macroscopic image of a cuboid scaffold containing six layers of hollow strands, (H) inverted microscopic image of longitudinal section of the scaffold, (I) macroscopic image of the scaffold cross-section, (J–L), confocal microscopic images at different magnifications showing the cross-section of the scaffold, (M) SEM image of fused filaments, with fibroblasts encapsulated in hollow alginate filaments, (N and O) microscopic images showing the lumen and wall of the hollow strands (white light), (P and Q) laser confocal images showing the lumen and wall of the hollow strands, and the live and dead cells as fluorescent green and fluorescent red, respectively, and (R) laser confocal image revealing the fused structure with channels (reproduced with permission from [29]).
To date, a wide range of natural, synthetic, and hybrid biomaterials have been used in bioprinting; however, none are free from shortcomings. Most biomaterials show uncontrolled degradation, immunogenicity, inflammation, and cytotoxicity in in vivo or in vitro applications. In some cases, biomaterials inhibit ECM secretion, distribution, and organization as well as cell-cell communication. To address these issues, researchers have investigated self-assemble approaches in which scaffold-free multicellular spheroids or filaments are extruded using a bioprinter. In such systems, sacrificial spheroids and multicellular tissue spheroids are concurrently printed layer-by-layer as per the CAD design. Upon incubation in a bioreactor, the multicellular spheroids fuse together to form single- or double-layered microvascular tubes. The fusion process is time-consuming and causes non-uniform tubular surfaces, and the fabrication of long vascular tubes is a slow process and demonstrates poor spatial resolution. To tackle these issues, RP technology has been used to bioprint cylindrical multicellular building blocks using collagen gel as a biopaper. However, vascular cells eventually integrate with the collagen rod in the fusion process, which causes complexities in the removal of the collagen gel from the vascular cell-fused hollow channels. Extruded inert agarose rods as a molding template solved the issues related to the collagen gel. When human umbilical vein smooth muscle cells and dermal fibroblasts were dispensed as a multicellular cylinder according to the CAD design, double-layered vascular walls formed 3 days after fusion [30].
2.2. Laser-based 3D printing
Laser-based bioprinting, particularly laser-guided direct writing (LGDW) and matrix-assisted pulsed laser evaporation direct writing (MAPLE DW), have been explored in different studies for 2D and 3D cell patterning [31]. This printing technique has some attractive features, including no nozzle clogging and the ability to print cells at high resolution and accuracy with high-viscosity bioink. Compared to laser-induced forward transfer (LIFT), the MAPLE DW technique uses a lower powered pulsed laser to deposit multiple cell types. In this technique, laser pulse-induced bubbles create shock waves that compel cells to move toward the collector substrate. A number of studies have used laser-based bioprinting to print patterned structures with vascular cells and observed capillary vessel formation. For example, a study using the LIFT-based cell printing technique to print HUVECs and human mesenchymal stem cells (hMSCs) in a defined pattern on a cardiac patch reported increased capillary vessel density and functional improvement of infarcted hearts [32]. Researchers have also used LGDW to print a 3D vascular network by stacking cell aggregates layer-by-layer, with a hydrogel layer placed on top of each deposited cell aggregate. LGDW-printed 3D patterned HUVEC on Matrigel™ formed elongated and tube-like structures in vitro [33]. However, shortcomings such as long fabrication time, laser-induced cell damage, and low scalability limit the application of the techniques in tissue vascularization.
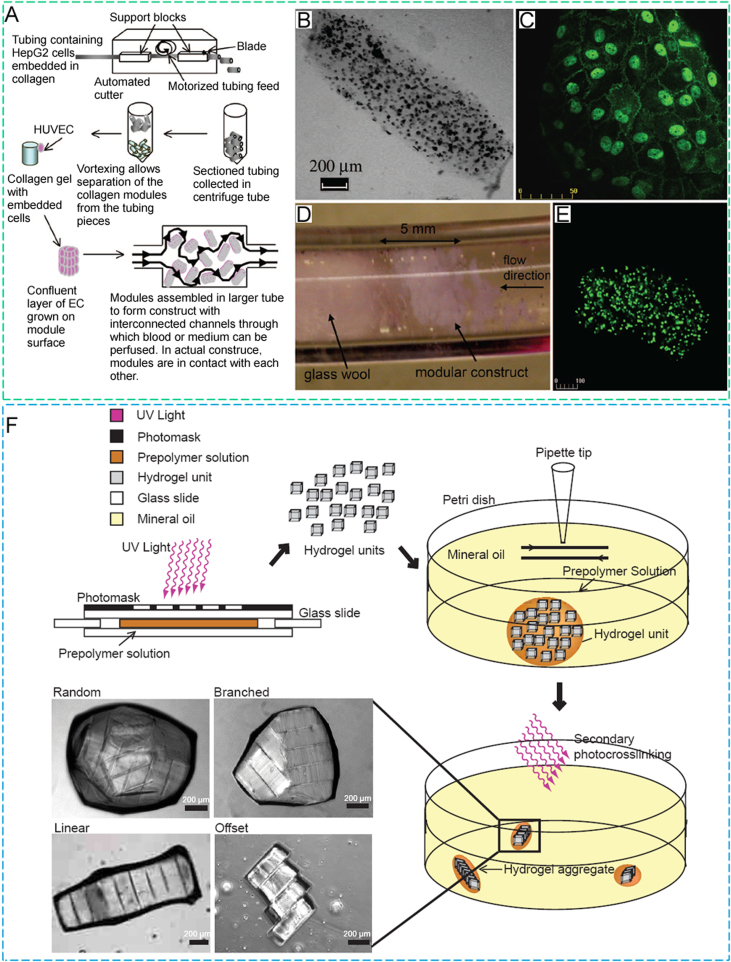
Stereolithography, a maskless photolithography, has been investigated to generate complex 3D vascular patterns with photosensitive materials [34]. In particular, digital light projection (DLP) and laser-based stereolithography have been used to print intricate architectures based on designs developed from CAD software, computer tomographic, and magnetic resonance imaging (MRI) scanned information [35]. In a DLP system, a digital mirror device containing several million tiny mirrors regulates the movement of the mirrors via a digital signal. This rotation of mirrors causes a two-dimensional pixel-pattern that is projected on the photo-curable biomaterial to obtain intricate 3D structures. In a study, a DLP chip was used to generate active and reflective dynamic photomasks as per the CAD drawing. Then the cross-sectional images of the 3D microstructure were reproduced from photomasks and the images were projected onto the methacrylate (GelMA) solution using an ultraviolet (UV) light source. When the 3D intricate pattern was seeded with HUVECs, the HUVECs formed a confluent monolayer and maintained their phenotype for 4 days following dynamic seeding [36]. Similarly, another study reported that HUVECs formed cord-like structures after 4 days of culture in a scaffold fabricated with a DLP system [37]. While this technique can print 3D structures quickly with high resolution, shortcomings such as high costs, less detailed printing for large constructs, and cytotoxicity limit the application of the DLP technique.
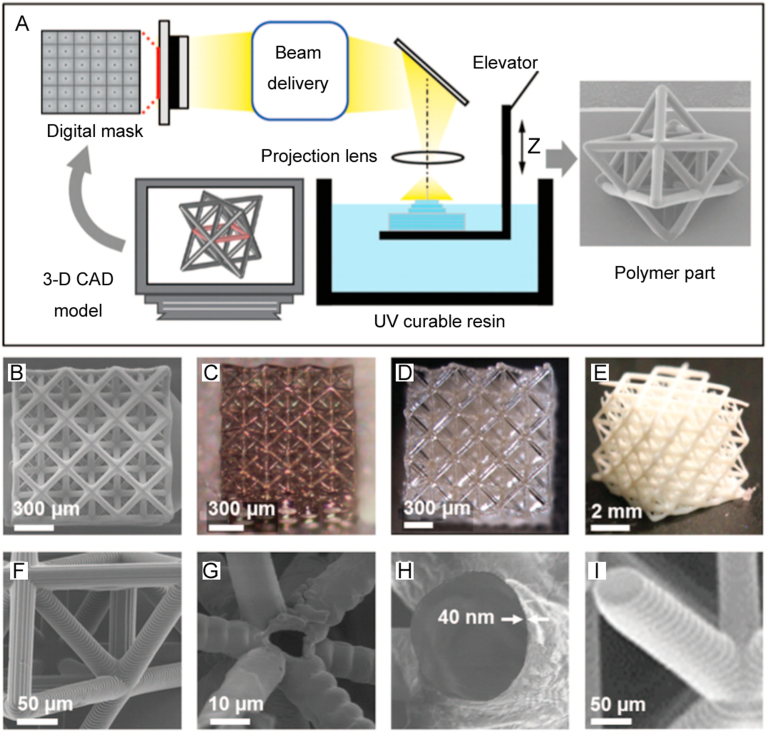
Laser-based stereolithography (LS) was developed to eliminate the requirement of a photomask and assembly of multiple 2D planar surfaces to form 3D vascular networks. Although LS is suitable for printing large and detailed vascular constructs, the lower printing speed of LS compared to the DLP technique needs to be improved [38]. In this technique, a computer-controlled ultraviolet laser beam generates a pattern on a photosensitive material as per the CAD design [39], as shown in Fig. 3. A number of researchers have printed complicated structures with LS and reported outstanding results with respect to forming vasculature. Post-fabrication seeding of ECs in the LS-printed scaffold showed improved viability, whereas incorporated cells in the photosensitive hydrogel demonstrated low viability due to laser (short wavelength)-induced cell damage. To avoid short wavelengths, researchers have employed two- or multi-photon laser systems to print intricate 3D structures with micro- or nano-scale precision [40]. Although the two- and multi-photon laser systems maintain a less harsh environment than LS during printing, use of a photo-initiator in the gelation process significantly decreases cell viability [38]. To address this issue, a multi-photon printing technique was used to fabricate 3D multi-scale patterns in soft silk protein hydrogels without using a photo-initiator; the 3D features supported the growth and penetration of human mesenchymal stem cells deep into the gel [41]. Several studies have investigated the efficacy of vascular patterns/networks printed with the LS technique with respect to promoting vascular tissue. In one study, polytetrahydrofuran diacrylate resin-based macro-scale vascular tubes and micro-scale bifurcating tubes were printed using LS and two‐photon polymerization (2PP) techniques, respectively. Acellular vascular vessels printed using this method could be seeded with ECs to form vasculature, because the grafts demonstrated good cytocompatibility and mechanical properties similar to native capillaries [42]. Similarly, when granulosa cells were seeded on an epoxy-based acellular microcapillary vascular tree printed with the 2PP technique, improved cell growth and sustained cell-cell junctions were identified in vitro [43]. These studies demonstrate that EC monolayer formation is possible in an LS-printed vascular pattern.
Fig. 3.
Fabrication of scaffolds using a computer-controlled ultraviolet laser beam: (A) projection microstereolithography (SEM image at right is an octet-truss unit cell), (B–E) octet-truss structures with different patterns and biomaterials, and (F–I) SEM images of the struts of structures in (B–E) (reproduced with permission from [44]).
3. Assembled scaffolds
3.1. Micro-patterning/microfluidics
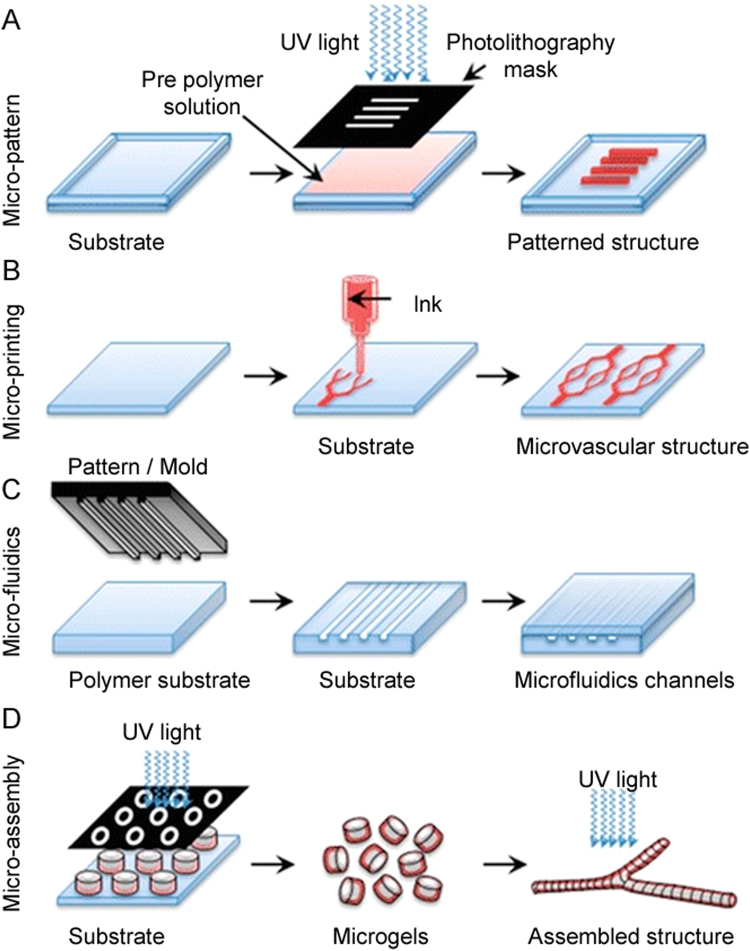
Native tissue contains macro- and micro-blood vessels that supply nutrients, oxygen, and other bio-molecules to large cell populations. A number of studies have investigated micropatterned substrates to create capillary bed-like structures that mimic native tissue as shown in Fig. 4. Biophysical factors, such as ECM stiffness, interstitial and shear flow have significant effects on capillary vessel formation in micropatterned substrates. In particular, interstitial flow regulates capillary morphogenesis [45], shear stress enhances angiogenesis [46], and material stiffness regulates the architecture of the capillary network in the developing tissue. In one study, bovine pulmonary microvascular ECs seeded in rigid collagen gels formed thick and deep vascular networks with large capillary lumens, while those seeded in a flexible hydrogel formed thin and intense networks with tiny lumen [47]. Researchers have used plasma etching [48], laser ablation [49], soft lithography [50], and replica molding [51] to generate microfluidic patterns on biocompatible substrates. Researchers have also used a direct write laser technique to prepare microchannels with various widths and depths following Murray's law to closely mimic the capillary architecture seen in vivo. Such micropatterns facilitate the uniform flow of fluid and achieve low resistance similar to that of physiological vascular systems [52].
Fig. 4.
Creation of a capillary bed-like structure mimicking native tissue: (A) fabrication of a micro-scale structure using a soft lithographic technique, (B) microprinting using conformational contact to form a pattern of ink on the surface, (C) microfluidic channels fabricated using micromolds (channels are used to form microfibers of a sacrificial substance that is then removed to form hollow fibers), and (D) complex vascularized structures fabricated using an assembly of microgels (reproduced with permission from [53]).
To date, several methods have been developed to seed vascular and tissue-specific cells on patterned substrates. In one study, ECs and mural cells (i.e. smooth muscle cells (SMCs) and pericytes) were seeded in a single-layered substrate following a microfluidic approach, and then cultured for a certain period of time to promote stable capillary formation [54]. Several studies report that microfluidics regulate the temporal and spatial distribution of cells, media, enzymes, and biomolecules within the micropattern [55]. Further, microfluidics facilitates the formation of patterned cell distribution and shear-induced endothelialization in a co-culture system [56]. Especially in a microfluidic system, circulating biomolecules and the applied gradient of shear stress into the microchannel regulate the morphology, reorganization, alignment, differentiation, and remodeling of ECs that are significant for capillary formation [57]. To regulate shear stress, researchers have applied computational microfluidics and different fluid flow patterns (e.g. laminar, pulsatile, and turbulent) that result in the formation of endothelial monolayers around the microchannels [58], [59]. Micropatterned single planar layer substrates prepared in this way are compiled to form macro 3D tissue constructs. Because the preparation and assembly of multiple layers is a time-consuming process, researchers have searched for alternative ways to fabricate 3D macro-sized substrates with microfluidic channels. Implementation of advanced technologies, such as direct write assembly (robotic deposition, fused deposition, and two-photon polymerization) [60], sacrificial material-based extrusion printing [21], modular assembly [61], omnidirectional printing (Figs. 5A-F) [62], and electrostatic discharge printing (Figs. 5G and H) [63] have demonstrated unprecedented success in generating complex 3D vascular patterns. Such complex patterns are often embedded in hydrogel-containing tissue-specific cells.
Fig. 5.
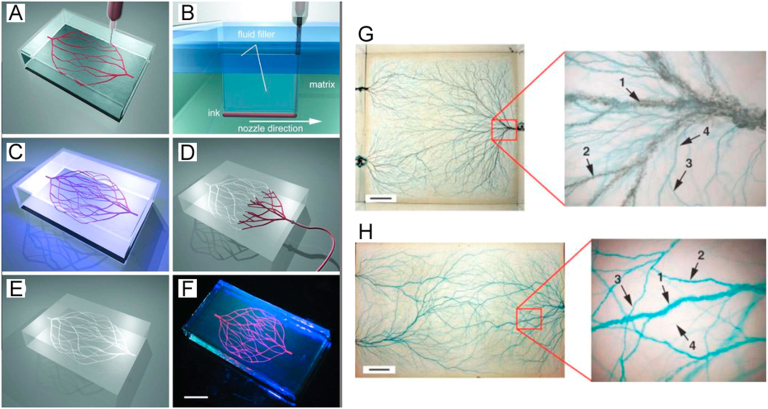
Schematics of omnidirectional and electrostatic discharge fabrication of 3D microvascular networks: (A) extrusion of a fugitive ink into a gel in a hierarchical fashion, (B) migration of fluid from capping layer to the voids generated by nozzle translational speed, (C) photopolymerization of hydrogel matrix, (D and E) microvascular channels that are created by dissolving and removing the fugitive ink under a modest vacuum, (F) fluorescent image of a 3D microvascular network (scale bar = 10 mm), (G) blue food-dye injected microvascular networks in an acrylic block with three fluidic access points (scale bar = 1 cm), and (H) branched microvascular networks embedded in a molded PLA block incorporated with a hierarchy of microchannel diameters (scale bar = 2 cm) (reproduced with permission from [62], [63]).
The success of microfluidics networks in terms of vasculature formation largely depends on the mechanical and biological properties of the biomaterial. A wide range of synthetic polymers, including poly(methylmethacrylate), poly(dimethyl siloxane), silicon, polycarbonate, polyvinyl chloride, polystyrene, poly(lactic-co-glycolic) acid (PLGA), and poly(glycerol sebacate) have been used in microfluidics [64]. However, EC monolayers grown into micropatterned synthetic substrates show poor barrier function in terms of transporting biomolecules, oxygen, and nutrients. Because patterned synthetic materials show poor biodegradation, barrier function, and biocompatibility and provoke cytotoxicity and inflammatory responses in vivo, researchers have explored a wide range of hydrogels for printing micropatterns for vasculature formation. A number of studies have used silk fibroin, Matrigel, type I collagen, and fibrin to form endothelial tubes in 3D scaffolds [65]. By nature, these hydrogels are biodegradable and biocompatible and can provide a 3D milieu for vascular network formation. In addition to the biomaterials, several parameters have been identified as potential factors in generating microvessels with microfluidics. For example, the combined effect of microfluidics and vasculogenesis from cell seeding grew perfusable and functional microvessels in a 3D fibrin gel system in vitro and showed strong barrier function and long-term stability [66]. Similarly, the collective effects of growth factors and fluid shear stress have also been investigated, with gradients of vascular endothelial growth factor (VEGF) and angiopoietin 1 (ANG-1) forming a stable and connected 3D capillary network within a type-I collagen matrix embedded with microfluidic channels [67]. The success of such a strategy led researchers to further explore the synergistic effect of multiple parameters to promote and regulate capillary formation in a 3D microfluidics-hydrogel system. Co-culture of HUVECs and human lung fibroblasts (HLFs) in fibrin gels/microfluidics systems containing VEGF and sphingosine-1-phosphate (S1P) promoted the formation of a stable capillary network with smaller lumen diameters [68]. In addition, stem cell-incorporated gels/microfluidic systems have been investigated. For example, a co-culture of HUVECs and MSCs in a collagen gel/microfluidic system reduced capillary formation, but stabilized the newly formed capillaries [69].
3.2. Micromodule assembly
The success of a macro-scale tissue engineered construct depends on several factors, including the availability of a mass diffusion network within 100–200 µm from the cell population, uniform distribution of multiple cell types with reasonable density, and nonthrombogenic phenotype of ECs upon integration with the host vasculature [4], [70]. To address this issue, researchers have taken a bottom-up approach to fabricate macro-scale scaffolds, and found that self-assembled micro-tissues or -modules were a possible solution for fabricating large engineered constructs, as shown in Fig. 6. When such micro-modules are loaded into large tissue constructs, the micro-dimensions of these modules facilitate the diffusion of nutrients, oxygen, and essential biomolecules to the cell population embedded in the gels [71]. To date, several methods have been investigated to generate micro-tissues or -modules. Micro-scale molding and UV crosslinking, directed self-assembly, and gravity-enforced self-assembly approaches have frequently been used to build micro-scale modules [72], [73], [74]. During preparation, tissue-specific cells are often encapsulated in the micro-modules, while a confluent layer of HUVECs is provided to coat their outer surface [75]. In some studies, the outer surface was further coated with protein molecules before EC seeding to improve the biofunctionality. Fibronectin-coated micro-collagen modules implanted in mice formed more stable, mature, and perfused capillaries 14 days postoperatively compared to collagen modules without the fibronectin coating [76]. Further, EC-coated microgels embedded with stem or progenitor cells showed impressive results with respect to stabilizing the newly formed capillary blood vessels relative to EC-coated modules; for example, implanted EC-coated collagen micro-modules containing BMSCs in the omental pouch in rats formed less leaky and more dense and mature capillaries [77].
Fig. 6.
Micromodule assembly: (A) collagen solution loaded with human hepatoma (HepG2) cells was gelled into ethylene oxide tube at 37°C for 30 min, the tube was then segmented into 2-mm length, and the collagen modules were collected after rotating in a centrifuge. Then HepG2 cell loaded modules were seeded with HUVECs, accumulated into a larger tube, and perfused with medium or blood, (B) light micrographic image of a collagen–HepG2 module without HUVECs, (C) confocal microscopic image of vascular endothelial (VE)-cadherin-stained module showing a confluent layer of HUVECs around the outer surface of module after 7 days of culture, (D) perfusion of a modular construct in a large tube with phosphate buffered saline (PBS), (E) confocal microscopic image of a collagen–HepG2–HUVEC module after 7 days of culture with HepG2 cells labeled with a Vybrant™ CFDA SE cell tracer kit, and (F) schematic diagram of the microgel assembly process (reproduced with permission from [75,79]).
A number of studies have used random packing, directed, or sequential assembly to accumulate cell-loaded micro-modules with different sizes and shapes to prepare macro-scale vascularized tissue constructs [78], [79]. In random packing, EC-coated modules are perfused with blood or culture medium that causes interconnected channel formation in the interstitial spaces of the modules. In directional assembly, shape-controlled microgels spontaneously form offset, linear, branched, or random aggregates depending on surfactant concentration, aspect ratio, agitation rate, and time [79]. The sequential assembly approach was investigated in an effort to control the organization of the microgels having internal microchannels [80]. Such microgels were prepared with photolithography and then assembled into a tubular construct where an interconnected and bifurcated structure resembling native vasculature was formed. In particular, SMCs and HUVECs were incorporated in the outer and inner layer of the microgel to form a biomimetic 3D vasculature. Variables including the thickness and diameter of the microgels, swiping speed of the needle, concentration of surfactants, and space between two successive microgels affected the length of the assembled 3D tubular construct. Although sequential assembly is economical and applicable with respect to the formation of a complex vascular network, shortcomings associated with the fabrication of thick microgels (≥ 600 µm) containing non-straight vertical cross-sections and with thin microgels (≤ 150 µm) showing poor mechanical strength limit the application of this method [81]. Overall, vascularized tissue formed with the modular approach shows poor tissue integration and capillary network formation in vivo.
4. Nano-fabrication
In the past decade, researchers have focused on fabricating nanofiber-based vascular constructs because nanofibers possess similar topographical cues to ECM [82]. Using different electrospinning techniques, random, aligned, and core/shell nanofibers have been printed and nanofiber-based matrices studied for tissue vascularization. A wide range of synthetic and natural materials, including polycaprolactone (PCL), poly(L-lactic acid) (PLLA), PLGA, poly(ethylene oxide) (PEO), PVA, chitosan, gelatin, and collagen, have been explored for nano-fabrication applications [83]. Synthetic nanofibers show more mechanical stability compared to natural fibers, but hydrophobicity, the absence of cell binding motifs, and poor biodegradability limit their applications. Conversely, natural polymeric materials are hydrophilic, and biocompatible, and possess cell binding motifs, yet poor degradation and mechanical properties are major shortcomings that need to be overcome for nano-fabrication. To address these issues, researchers have explored composites, copolymers, and hybrid biopolymers to fabricate nano-fibers and achieved some remarkable successes in tissue vascularization [84]. In some studies, random or aligned fibers were effective in terms of vasculature formation. For example, when electrospun random PCL/collagen (rPCL/Col) and aligned PCL/collagen-PEO (aPCL/Col-PEO) nanofibers were implanted in the arterio-venous loop in rats, a larger number of blood vessels and better capillary density and branching hierarchy were seen in the rPCL/Col fiber-implanted group 8 weeks postoperatively compared to the aPCL/Col-PEO group. Moreover, rPCL/Col fibers facilitated the formation of small pore sized capillaries, whereas the aPCL/Col-PEO scaffold promoted early and evenly distributed blood vessels throughout the scaffold, resulting in a shorter prevascularization time [85]. Prevascularized and aligned nanofibers can also promote vascularization. Random or aligned poly (ε-caprolactone)/cellulose electrospun nanofibers have been used to fabricate a mesh structure in a layer-by-layer fashion. HUVECs seeded onto the aligned nanofibers promoted prevascularization in vitro compared to randomly oriented nanofibers by forming capillary-like structures. When the prevacularized and aligned nanofibers were implanted subcutaneously in rats, host blood vessels penetrated deep into the nano-meshes and integrated with the vascular network [86]. In an attempt to prevent thrombosis in a small-diameter blood vessel, graded chitosan/poly ɛ-caprolactone (CS/PCL) nanofibers fabricated with sequential co-electrospinning were heparinized to immobilize VEGF. HUVECs and SMCs were cultured on the top and bottom surfaces of the graded CS/PCL nanofibers, mimicking the lumen and adventitia of blood vessels, respectively. The graded and heparinized nanofibers demonstrated outstanding anti-thrombogenic properties. Further, better HUVEC attachment, proliferation, and monolayer formation have been identified on graded CS/PCL scaffolds compared to uniform CS/PCL scaffolds [87].
5. Creation of vascular networks with mechanical spacers
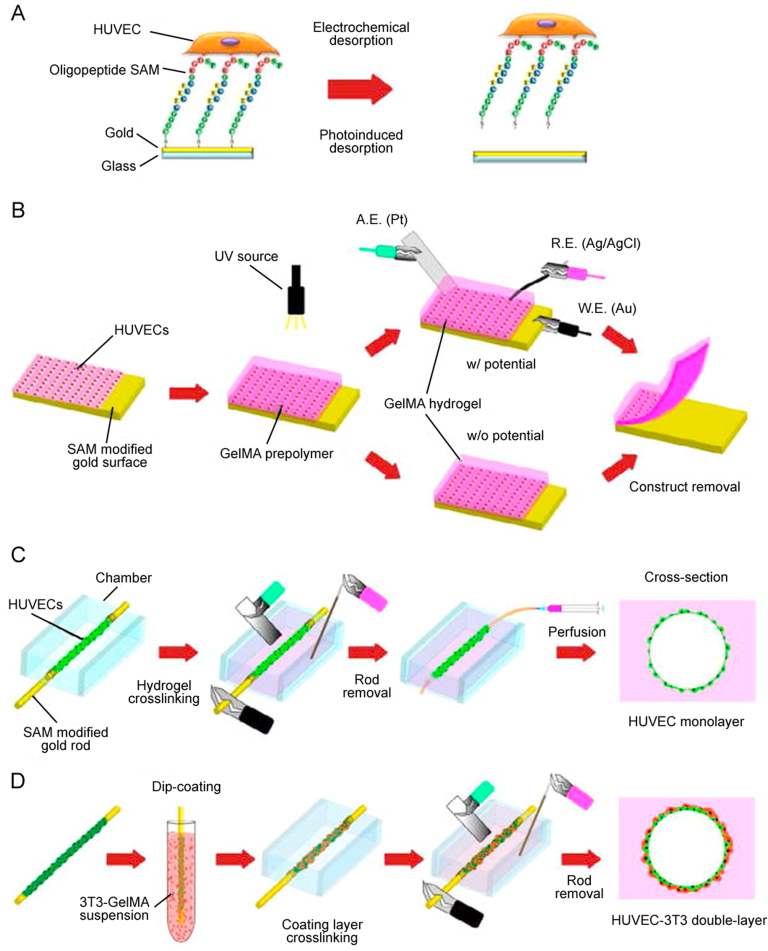
The shortcomings related to sacrificial filaments used to form microfluidic networks have led researchers to investigate alternative approaches. Incorporation of mechanical spacers into the 3D scaffold (Fig. 7) generates linear microchannels without causing any cytotoxicity. In particular, several studies have used mechanically removable spacers to create microchannels in a hydrogel matrix. In one study, linear wire arrays ranging from 152 to 787 µm in diameter were used to form microfluidic channels in a silk fibroin scaffold. Because the channels often lose uniformity after removal of the mechanical spacers, attempts have been made to introduce hollow tubes into the spacer-generated hollow channels. Hollow channels with open wall porosity, as well as silk tubes and porous silk tubes with incorporated channels were formed in the silk scaffolds, after which human arterial endothelial cells (hAECs) were seeded into the hollow channels in vitro with or without the presence of collagen-I or laminin. After 7 days of seeding, hAECs formed a nearly continuous layer around the spacer-generated hollow channel and ECM protein-loaded silk tubes [88]. Because the formation of confluent monolayers of ECs in spacer-generated hollow channels is difficult, researchers have attempted to transfer self-assembled cell layers into the hollow channel to avoid the cell seeding approach. In one study, HUVECs were seeded on oligopeptide-adsorbed micrometric gold rods to form self-assembled monolayers (SAMs), and then the SAM-attached gold rods were encapsulated in photocrosslinked GelMA. The SAMs of ECs were transferred into the GelMA by applying an electrical potential, followed by the perfusion of SAM with medium at 2 μL/min. This approach was further explored to transfer double layers of assembled cells from gold rods into GelMA to mimic native blood vessels. To form double layers of vascular cells, HUVEC-coated gold rods were dipped into GelMA solution containing NIH 3T3 fibroblast cells, and then encapsulated in the GelMA hydrogel. By applying an electrical potential, the double layers of assembled cells were transferred into the bulk gel, and then the layers were perfused with media for stabilization [31]. Although promising, mechanical spacers have not to date been able to generate complicated and branched structures that resemble native blood vessels.
Fig. 7.
Schematic of cell transfer, and vascular network formation mechanism by mechanical spacers: (A) oligopeptide modified gold surface was seeded with HUVECs, (B) HUVECs seeded on gold substrate were transferred to photocrosslinked GelMA hydrogel with or without electrical potential, (C) HUVECs coated micrometric gold rod was placed in a culture chamber and encapsulated in GelMA hydrogel, and the layer of HUVECs was transferred to GelMA by using an electrical potential. Then the rod was taken out and the hollow lumen was cultured under perfusion, and (D) HUVECs coated gold rod was dipped into 3T3 fibroblast cell loaded GelMA solution to form double-layered microvascular structures. The rod was then encapsulated in hydrogel, the double-layer of vascular cells was transferred by an electrical potential, the rod was removed, and the hollow channel was cultured under perfusion (reproduced with permission from [31]).
6. Scaffolds with natural architecture
Tissue-specific acellular matrix contains biological, structural, functional, and topographical cues that promote vascular tissue growth through the upregulation of signaling pathways, phenotype, mechano-transduction, and differentiation and proliferation of the repopulated vascular cells [89], [90]. Different studies have prepared decellularized vascular tissue with chemical (acid, base, detergent, hypotonic and hypertonic solutions), biological (e.g. trypsin, dispase, etc.), or physical (e.g. temperature, pressure, electroporation, agitation, pressure gradient, etc.) agents in consideration of tissue properties (e.g. size, lipid content, density, thickness, cellularity, etc.). However, such processes often disrupt the ultrastructure of ECM, remove ECM and growth factors, and provoke immune responses [91], [92], [93]. A number of studies have successfully grown functional macro blood vessels with decellularized matrix. For example, in one study a recellularized tissue-engineered vessel with autologous EPCs prevented clotting and intimal hyperplasia for 30 days in a porcine model [94]. When decellularized rat iliac arteries seeded with ECs were implanted in the abdominal aorta of rats, native vessel-like structures were observed at 3 months [95]. Similarly, the inner layer of decellularized pig arteries were repopulated with human ECs and implanted in the pigs as iliac artery substitutes; the inner layer of the recellularized matrix was covered by ECs, and no thrombi formation was reported 70 days postoperatively [96]. Apart from EC seeding, the success of decellularized matrix vascularization largely depends on the implementation of physical stimuli. For example, decellularized rat hearts were repopulated with cardiac cells or ECs, and then cultured in a bioreactor maintaining simulated cardiac physiology over 28 days. In both larger and smaller coronary vessels, EC layer formation was identified 7 days after recellularization. Interestingly, cell-seeded heart tissue showed contraction and expansion under electrical stimulation 8 days after cell seeding [97]. Decellularized cadaveric lungs containing intact internal structures (e.g. perfusable vascular network, airways, and alveolar geometry) were seeded with ECs and then cultured in a bioreactor simulating developmental physiological conditions. When such lungs were transplanted and perfused by the recipient's blood circulation in vivo, gas exchange was observed up to 6 h after extubation. However, the success of this approach largely depends on the use of progenitor cells, prolonged in vitro and in vivo culture, and an ideal postoperative ventilation regimen for the regenerated lungs [98].
7. Biopolymer-free fabrication
7.1. Cell aggregates as bioink
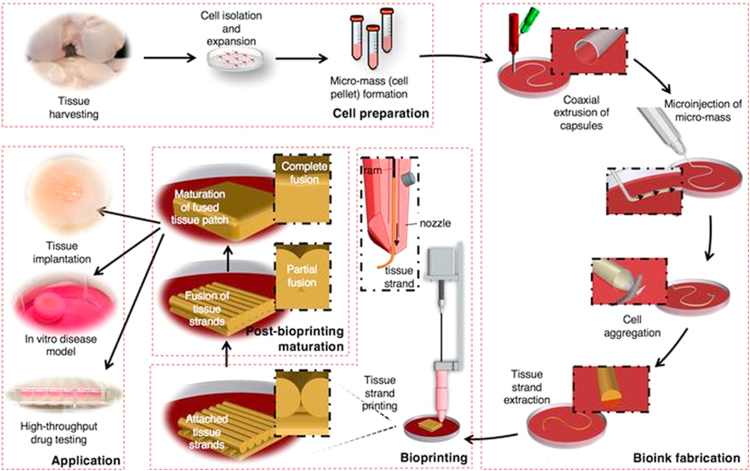
In vivo, tissues or organs develop due to the self-assembly and self-organization of multiple cell types without the influence of scaffolds. Although it was established decades ago that tissue regeneration requires engineered constructs, several complexities related to the scaffolding biomaterial have led researchers in recent years to harness the in vivo mechanism of tissue regeneration. A number of studies report that bioprinted cell aggregates form pre-designed tissue constructs through self-organization and tissue fusion [99]. Cell aggregates composed of single cell or multiple cell types can be bio-printed as cell pellets, tissue spheroids, or tissue filaments (Fig. 8). Tissue spheroids must be prepared in a controlled fashion to avoid clogging and cell damage in the bioprinting process [100]. In contrast, bioprinting of cell pellets ensures high cell densities and does not cause nozzle clogging or cell damage. However, bio-dispensing of cell pellets requires a supportive hydrogel in which they can accumulate, organize, and fuse to form tissue [101]. Tissue strands can also be formed by injecting cell pellets into tubular molds prepared with a co-axial nozzle system. In one study, injected cell pellets in permeable alginate capsules organized and fused to form tissue strands in an in vitro culture. Tissue strands were loaded into a custom-made bioplotter and then extruded layer-by-layer to form a pre-defined 3D structure [102]. These cell aggregate approaches can be applied to form tissue with vascular networks.
Fig. 8.
Schematic elucidating the concept of tissue printing using tissue strands as a new bioink (reproduced with permission from [102]).
7.2. Rolled up cell sheet
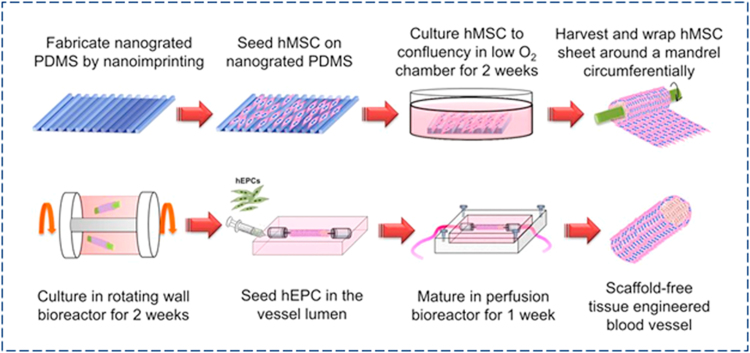
In recent years, a number of studies have printed cell aggregates instead of 3D tissue scaffolds to create vascularized functional tissue, as scaffolding material inhibits cell-cell interactions and causes various complexities including inflammation, cytotoxicity, and tissue remodeling. In the cell sheet technique, tissue-specific cells are cultured on a temperature-responsive material to create a cell-ECM matrix. To form vascularized tissue, a single monolayer sheet of ECs can be stacked with cell sheets of interest in a sandwich fashion or vascular cells can be co-cultured with tissue-specific cells to form vascularized multiple cell sheets. As an illustration, Fig. 9 shows an overall experimental scheme for fabricating an hMSC-based scaffold-free tissue engineered blood vessels. In general, cell sheets can be attached and detached from a temperature-responsive culture dish incubating at either a higher (37 °C) or lower temperature (< 25 °C). Nanometer-scale coating of poly(N-isopropylacrylamide) on polystyrene tissue culture surfaces facilitates attachment and detachment of endothelial cells and hepatocytes by shifting from hydrophobic to hydrophilic conditions at temperatures below 25 °C [103]. Vascularized cell sheets prepared in this way can be compiled to form thick tissue. For instance, triple-layered cell sheets were produced from co-culture of endothelial and cardiac cells overlaid on a resected tissue containing a vascular bed and perfused in a bioreactor. After 3 days of perfusion culture, ECs formed luminal capillaries throughout the cardiac sheet upon connection with the vascular bed. The triple-layered vascularized cardiac tissues prepared in this way could beat and were transplantable. For increased tissue thickness, a six-layered cell sheet was prepared by compiling either two triple-layered or six single-layered cell sheets together and then the stack overlaid on the vascular bed. The combined two-triple layered sheets created thicker cardiac tissues with greater cell density compared to the compiled six-layer sheets due to improved cell viability and vascularization [104]. Further, functional and vascularized tissue was obtained with 12 stacked cell sheets, indicating the efficacy of this approach to create vascularized thick tissue in vitro. Multiple transplantation of thin (~ 80 µm) cardiac cell sheets in vivo has also been found effective with respect to developing thick (~ 1 mm) vascularized tissues. In one study, cell sheets grown from neonatal rat cardiomyocytes were repeatedly transplanted into rats at 1-, 2-, or 3-day intervals. This poly-surgical approach created a thick (~ 1 mm), well-vascularized, and perfused myocardium tissue [105]. Although vascularized tissue formation is possible with cell sheets, poor mechanical strength and tissue integrity of stacked cell sheets are major challenges that require further research.
Fig. 9.
Vasculature formation with cell sheets: scaffold-free fabrication approach of hMSC-based tissue engineered blood vessel (TEBV), incorporation of human EPC in the vessel lumen, and culture and maturation of TEBV in a perfusion bioreactor (reproduced with permission from [106]).
8. Scaffold pre-vascularization
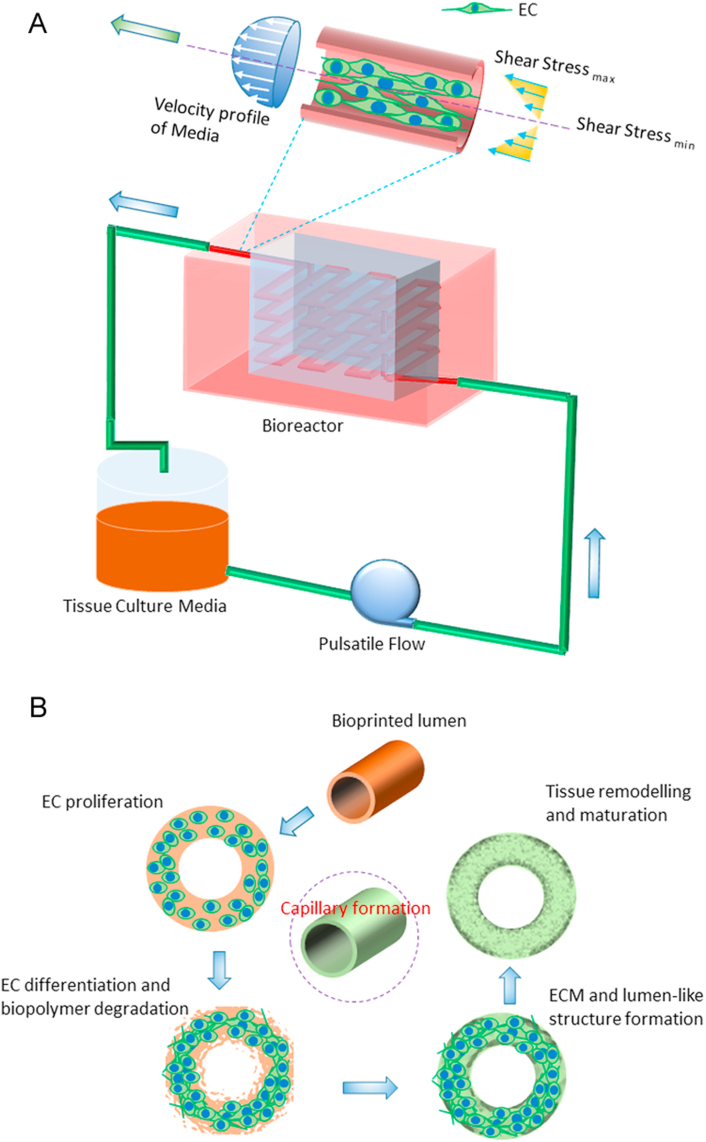
A macro-scale tissue engineered construct containing a large cell population requires a sufficient supply of nutrients, oxygen gas, and biomolecules to maintain the metabolic activity, viability, and proliferation of embedded/seeded cells. In vitro, tissue scaffolds featuring a microfluidic network can be perfused with culture media to supply the necessary elements to the large cell population. However, the situation becomes complicated when scaffolds are implanted in vivo. Extrinsic vascular networks develop in the tissue construct by angiogenesis and vasculogenesis mechanisms that take time. This time delay can cause ischemia in the large embedded cell population in a scaffold, and reduce cell viability by triggering apoptosis or necrosis. To address this issue, researchers have incorporated a microfluidic network into the tissue graft and then formed a monolayer of ECs by seeding (Fig. 10). Such microfluidic network-embedded grafts were then sutured with host arteries/veins during in vivo implantation. Because thrombopoiesis-induced restenosis is often seen in tiny blood vessels, nutrient supply throughout the scaffold might be hindered due to the blockage of the microfluidic network in vivo. Therefore, different studies have adapted several techniques to prevascularize the engineered construct, and then seed the graft with multiple cell types. In particular, scaffolds loaded with microvessel fragments, angiogenic factors, or vascular cells (ECs, SMCs) have been cultured in vitro or in vivo (e.g. arterio-venus loop) to form vascular networks prior to tissue-specific cell seeding [107], [108]. The prevascularized grafts take less time to inosculate with host vasculature, and thus support tissue growth, modeling, and integration. For example, when prevascularized collagen grafts seeded with fibroblasts, keratinocytes, and ECs were transplantated into mice, it took only 4 days to anastomose with host capillaries. In contrast, non-prevascularized scaffolds took 14 days to perfuse with native blood vessels [109]. In a co-culture system, prevascularization of tissue grafts largely depends on media composition, cell seeding technique, and ratio of multiple cell types. In a multiculture system, tri-culture of myoblasts, fibroblasts, and endothelial cells at certain ratios enhances capillaries compared to co-culture of myoblasts and endothelial cells after 4 weeks of culture [110]. Furthermore, the tri-culture graft incubated with VEGF grew more blood vessels compared to those incubated with PDGF after 2 weeks of in vitro culture. While these cell-incorporated scaffolds achieved significant success in terms of prevascularization, formation of rapid, dense, mature, and functional capillary beds remains challenging. Apart from in vitro prevascularization, scaffolds incorporating microvessel fragments have been used as prevascularized constructs. In fact, these ECM matrices contain tissue-specific bio-chemical and topographic cues that regulate numerous cell-functions to form functional capillary beds. Implanted collagen gel containing microvessel fragments in immunocompromised mice formed neovessels with lumen by day 11 and a mature functional microvascular bed by day 28 [111]. Although promising, allogeneic or xenogeneic microvessel fragments can cause immunological complexities in the host body. The success of in vitro prevascularization largely depends on the artificial physiological conditions applied during tissue culture. A number of studies maintained dynamic culture conditions including pulsation, variable flow rate, and dynamic pressure to form vascular networks. For example, cyclic mechanical strain and stress for 8 weeks promoted the proliferation, alignment, and collagen production of rabbit aortic SMCs seeded onto poly(L-lactide-co-caprolactone) (PLCL) scaffolds [112]; dynamic sequential seeding of aortic SMCs and ECs onto poly(glycolic acid) (PGA) scaffolds and biomechanical stimulation for 25 days enhanced capillary formation and ECM deposition [113]; and a controlled hypoxic environment influenced cells to secrete VEGF, which promoted vascularization in vitro [114]. However, modulation of oxygen concentration in a co-culture system can alter the differentiation of stem cells into different lineages [115]. Moreover, bidirectional flow in the biaxial bioreactor of a co-culture system (e.g. EPCs and MSCs) significantly reduces hypoxia-induced VEGF expression by eliminating the oxygen gradient. Thus, blood vessel formation is hindered in a bidirectional flow system compared to static culture and unidirectional flow systems [116].
Fig. 10.
Mechanism of blood vessel formation and maturation within a scaffold cultured in a bioreactor: (A) effect of shear stress on ECs and (B) step-wise demonstration of blood vessel formation by ECs in a scaffold.
In vitro tissue prevascularization requires well-connected and porous scaffolds, microfluidic networks, complex bioreactors, and culture media; however, the long-term fate of the capillary bed formed remains unknown. To address this issue, researchers have paid attention to in vivo prevascularization of engineered constructs. In this approach, cell/angiogenic factor-loaded tissue constructs are implanted into a temporary site within the host body, and then transplanted into a specific location. In the case of angiogenic factor-embedded scaffolds, angiogenic sprouting takes place due to the controlled release of VEGF. Because angiogenic factors demonstrate short half-lives in vivo, autologous cells are incorporated in the scaffold to express VEGF. In particular, scaffolds are initially implanted close to an arteriovenous loop in the host body to promote vasculature [117]. When a well-perfused capillary bed is formed within the matrix after a certain time, scaffolds are explanted, seeded with multiple cell types, and then transplanted into the target location. In one study, an FGF-2-enriched Matrigel chamber was implanted around the epigastric pedicle of a diabetic mouse. The Matrigel was replaced by highly vascularized adipose tissue after 21 days of culture. Seeding of pancreatic islets into the prevascularized chambers in diabetic mice significantly reduced the blood glucose levels, indicating the better survival and function of islets in the prevascularized chamber compared to non-prevascularized chambers [118]. Likewise, neonatal cardiac cells seeded onto prevascularized cardiac patches and transplanted onto infarcted rat hearts enhanced cardiac function after 28 days [107].
9. Summary and future research directions
The success of tissue and organ regeneration largely depends on the formation of a mature and well-perfused vascular network within the developing tissue. To date, significant progress has been achieved in the printing of vascular constructs (Table 1).
Table 1.
Fabrication of vascular networks using different techniques for tissue engineering applications.
| Fabrication technique | Biopolymer | Scaffold geometry | Embedded cells and other factors | In vitro/In vivo | Study period | Results of vascularization | Ref. |
|---|---|---|---|---|---|---|---|
| Ink-jet printing (HP DeskJet 550C printer) | Alginate-collagen | 3D pie construct (∼ 7 mm in diameter), and rectangular samples (2.5 cm × 0.5 cm × 0.3 cm) | Canine smooth muscle cells, human amniotic fluid-derived stem cells, and bovine aortic endothelial cells | In vivo: pie shaped scaffolds were implanted subcutaneously into the backs of outbred athymic nude mice | Up to 18 weeks | Vascularized, mature, and functional tissues | [17] |
| Ink-jet printing (HP Deskjet 500 printer and HP 51626A cartridges) | Fibrinogen and thrombin | Around 9 mm × 1.8 mm rectangular scaffolds | HMVECs | In vitro: The patterns were then cultured at 37 °C with 5% CO2 | Up to 21 days | Cells were seen to align, proliferate, and form a capillary-like tubular structure inside the channels | [18] |
| Extrusion-based printing | Carbohydrate glass encapsulated in the agarose, alginate, fibrin, Matrigel, and poly(ethylene glycol)-based hydrogel | Rectangular structure (20 mm ×10 mm × 2.4 mm); varying filament diameters (150–750 µm) | Primary rat hepatocytes, fibroblast cells, and HUVECs | HUVECs were seeded in the micro-lumen by injection method; channels were perfused with blood in vivo | Varying by experiment (up to 9 days) | Supported the metabolic function of primary rat hepatocytes by maintaining higher albumin secretion and urea synthesis than gels without channels | [21] |
| Extrusion-based printing | GelMA and fugitive ink (Pluronic F127) | 200–300 µm thick | Human neonatal dermal fibroblasts, 10T1/2 fibroblast, and HUVECs | In vitro | Up to 7 days | HUVECs showed greater than 95% viability and formed a confluent layer around the lumens after 48 h | [22] |
| Extrusion-based printing | Alginate-PVA | Inner diameter of channels: 150–450 µm; | hBMSC | In vitro | 14 days | Excellent attachment and spreading of hBMSCs on the outer and inner walls of the hollow fibers | [26] |
| Strand diameter: 400–1190 µm | |||||||
| Laser-based printing | Polyester urethane urea (PEUU) | Two layers of HUVECs were printed following orthogonal grid pattern with 900 µm grid-line distance; two layers of hMSCs were printed at right angles with 600 µm side length between the HUVEC lines; 300 µm thick cardiac patch made of PEUU was sliced to circles of 8 mm diameter | HUVECs and hMSCs | In vitro and in vivo, patches were transplanted to the infarcted zone of rat hearts after ligation of left anterior descending coronary artery | 8 weeks | An increased capillary vessel density and functional improvement of infarcted hearts was reported | [32] |
| Laser-based printing: projection stereolithography | GelMA | 3D rectangular scaffolds (5 mm × 5 mm × 1 mm) | HUVECs | In vitro | 7 days | Even distribution and proliferation of the HUVECs in the scaffolds caused high cell density and confluency as well as improved biological functionality | [36] |
| Laser-based printing: stereolithography, DLP technique | GelMA and poly(ethylene glycol) diacrylate (PEDGA) | Different micro-structured wells including stepwise, spiral, embryo-like and flower-like wells | HUVECs and NIH-3T3 fibroblast | In vitro | 4 days of culture | Scaffolds enhanced cell-cell interactions and multicellular organizations; HUVECs aligned around the boundary of the fabricated geometry and formed cord-like structures | [37] |
| Laser-based printing: stereolithography (LS and 2PP techniques) | Polytetrahydrofuran ether-diacrylate | Tubes and branched tubular structure with a diameter smaller than 2 mm | Human dermal fibroblasts | In vitro | 48 h after cell seeding | Grafts demonstrated good cytocompatibility, and mechanical properties similar to native capillaries | [42] |
| Laser-based printing: stereolithography (2PP technique) | Photosensitive organically modified ceramics | Epoxy-based acellular microcapillary vascular tree with μm features | Granulosa cells | In vitro | Up to 4 days | Improved cell growth and sustained cell-cell junctions | [43] |
| Micro-patterning/micro-fluidics | Collagen hydrogels of various stiffness | Hydrogel-based scaffold | Bovine pulmonary microvascular ECs | In vitro | – | ECs formed thicker and deeper vascular networks in the rigid gel than in the flexible gel; the lumen size of the capillaries grew in the rigid gel was larger than in the flexible gel | [47] |
| Micromodule assembly | Collagen modules and fibronectin-coated collagen modules | Micromodule with 760 µm internal diameter | HUVECs | In vivo and in vitro; modules were injected subcutaneously on the back of mice using 18 gauge needles and implanted through a micropipette in a subcutaneous pocket | In vivo: 7, 14, and 21 days; In vitro: 42 days | Coated collagen modules had more stable, mature, and perfused capillaries than sole collagen modules | [76] |
| Nano-fabrication | Random PCL/collagen and aligned PCL/collagen-PEO nanofibers | Average thickness, pore size, and filament diameter of randomly spun scaffolds are 300 µm, 1.2 µm, and 250 nm ± 73 nm, respectively | – | In vivo: implantation inside the arterio-venous loop in rats (male Lewis) | 8 weeks | A larger number of blood vessels, capillary density, and branching hierarchy were observed in random vs. aligned nanofibers | [85] |
| Vascular network by mechanical spacer | Silk fibroin, collagen-I, and laminin | Linear wire array ranging from 152 to 787 µm in diameter | hAECs | In vitro | 7 days | Cells formed a nearly continuous layer around the spacer-generated hollow channel and ECM protein-loaded silk tubes | [88] |
| Scaffolds with natural architecture | Decellularized rat iliac arteries | – | ECs | In vivo: implantation in the abdominal aorta of rats | 3 months | Native vessel-like structure was observed | [95] |
| Custom-made bioplotter and co-axial printer | Tubular alginate capsules and cell aggregate as bioink | Around 8-cm long tissue strands, 3 mm × 3 mm tissue patch | Primary chondrocytes | In vitro: a bovine in vitro cartilage defect model (square chondral defects) | up to 4 weeks | A significant amount of cartilage ECM was found around tissue strands over time, and this approach can be used to form vascularized tissue | [102] |
| Molding | Alginate, Matrigel | Diameter, thickness, and average pore size of cardiac patch were 5 mm, 2 mm, and 100 µm, respectively | Neonatal rat cardiac cells | In vitro culture, in vivo pre-vascularization of cardiac patch onto rat omentum, and transplantation onto the infarcted rat hearts | 28 days post- transplantation | Cardiac patch showed structural and electrical integration with native tissue as well as prevented dilatation and ventricular dysfunction of rat heart | [107] |
In recent decades, technological advancements in 3D bioprinting systems have made it possible to print cell aggregates, tissue strands, or cell/GF-loaded biomaterials layer-by-layer as per a predefined geometry developed by CAD software. In particular, inkjet- and 3D-bioplotting-based techniques have been recurrently used in different studies [119,120]. Each technique has advantages and drawbacks. For example, 3D inkjet printers are cheap and fast but can result in cell damage and clogged nozzles while 3D bioplotting is suitable for printing intricate networks with sacrificial materials but at the expense of printing resolution. Recently, bioplotting with a coaxial nozzle has attracted significant attention due to the ability to print capillary strands with lumen. Using microfluidics, EC monolayers can be developed inside the hollow strands. Over the last decade, the rapid advancement of laser technology has brought significant changes to 3D scaffold printing in terms of accuracy and resolution. The LGDW and MAPLE DW methods, as nozzle-free techniques, can handle high-viscosity bioink and pattern 3D vascular cells with high printing resolution and accuracy. Unfortunately, research in this direction has not advanced far because these techniques are time-consuming and cause significant cell damage during fabrication. In contrast, DLP and LS methods are comparatively biocompatible and capable of printing intricate architecture. The DLP technique can print 3D capillary networks promptly with high resolution, but the technique is costly, compromises details for a large construct, and causes resin cytotoxicity. In contrast, the LS approach (particularly two- or multi-photon laser systems) is capable of printing complicated 3D vascular patterns with details, although at a lower printing speed compared to the DLP technique. Nonetheless, the effect of the laser on cell damage remains controversial, and both LS and DLP techniques are only applicable to photo-crosslinkable hydrogels.
Based on an understanding of physiology, researchers have reached a common agreement that micro-scale capillary networks are needed to maintain the viability of large cell populations incorporated in engineered constructs. Different fabrication techniques have been applied to form capillaries and ECs have been seeded in the micro-lumen using microfluidic approaches. Plasma etching, laser ablation, soft lithography, and replica molding have been frequently used to generate patterned vascular networks on a single layer planar surface. Although capillary bed-embedded 3D tissue constructs can be constructed by stacking multiple single layers, assembling complexities and the requirements of long processing time limit their application. However, significant success in 2D capillary formation has been achieved when advanced computational approaches have been applied to manipulate the shear stress, medium circulation, and bio-molecule distribution in micro-lumen seeded with ECs and mural cells. The success in 2D has been further translated to 3D microfluidic systems to promote EC monolayer formation on the lumen. To achieve a self-assembled capillary network rather than a predesigned one, EC-coated micromodules containing tissue-specific cells have been loaded into engineered constructs and then perfused with medium in a bioreactor. Unfortunately, this approach grows vascular networks that are dissimilar to those of native tissue and demonstrate poor tissue integration. In recent years, the introduction of omni-directional printing has enabled the fabrication of native tissue-like capillary networks instead of 3D periodic lattice structures. This approach needs further improvement as inefficient removal of pluronic acid can significantly reduce EC viability. Similarly, highly branched or fractal-like structures have been generated in plastic materials with high energy electron beam irradiation. This approach is suitable for post EC seeding, while tissue-specific cell incorporation in the plastic material requires further study. Linear channels with varying diameter and linear 3D patterns have also been generated, with double layers of vascular cells transferred in the hydrogel using mechanical spacers. However, the capillary vessels formed by mechanical spacers are not continuous and cannot mimic the native vascular network.
To date, the formation of micro-capillary beds in developing tissue remains challenging. ECM-cell interactions are essential in vasculogenesis and angiogenesis processes, where ECM features a specific geometry close to that of nano-filaments. Therefore, researchers have recently conducted several experiments to form micro- and macro-blood vessels using nanofiber-based engineered constructs. Structures similar to native blood vessels containing lumen and adventitia have been grown by seeding cells on nanofiber-made tubular structures. Likewise, micro-capillaries have been successfully grown in implanted nano-filaments.
Efforts have also been made to vascularize tissue with decellularized matrix, which contains all of the necessary biological and biophysical cues for capillary formation. Because the approach is related to the decellularization and recellularization methods, destruction of proteins, difficulties in cell removal and seeding, and aggravation of immunological complexities are quite common outcomes. However, several studies have reported outstanding results in terms of vascular network formation and research is continuing to address the shortcomings of this approach.
While artificial structures have shown substantial progress with respect to generating vasculature, the long-term fate of such vascularized tissue remains controversial. The tissue forming mechanism in the embryonic stage suggests that cells are capable of creating their own matrix, and incorporation of foreign material in the tissue significantly impedes cell-cell interactions. Inspired by the theory, several studies have recently been conducted based on the scaffold-free approach. In recent times, macro-scale blood vessels have been grown successfully by dispensing cell aggregates and sacrificial material, and micro-patterned structures have been printed using tissue strands. Likewise, capillary vessel-embedded cell sheets are prepared and outstanding blood perfusion has been reported in vivo. Repeated implantation in multiple surgeries has been found to enhance the development of vascularized thick tissue with thin cell sheets. The mechanical stability of such self-assembled structures is a major issue that needs to be further addressed.
One of the major problems of culturing macro-scale tissue constructs is the development of tissue ischemia, which triggers necrosis and apoptosis in the large embedded cell population in the scaffold. In this regard, the strategy of adding cells to the in vitro or in vivo prevascularized construct has proven effective in terms of obtaining better cell viability compared to non-prevascularized constructs. The requirement of multiple surgeries for prevascularization and for biodegradable filaments need to be addressed to make the approach applicable to vasculature formation. The future success of this approach requires improvements in terms of bioreactor design, culture and conditioning, cell seeding, microfluidics, and the need for multiple surgeries.
It is anticipated that a combined inkjet and 3D bioplotter bioprinting system will eventually emerge as a smart approach for printing cell aggregates, tissue strands, and capillary network simultaneously to form a complex vascularized tissue or organ.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by the Natural Sciences and Engineering Research Council of Canada [NSERC RGPIN-2014-05648].
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
M.D. Sarker, Email: mas921@mail.usask.ca.
Xiongbiao Chen, Email: xbc719@mail.usask.ca.
References
- 1.Nomi M., Atala A., De Coppi P. Principals of neovascularization for tissue engineering. Mol. Aspects Med. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 2.Sarker M., Naghieh S., McInnes A.D. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol. J. 2018;13:e1700635. doi: 10.1002/biot.201700635. [DOI] [PubMed] [Google Scholar]
- 3.Frerich B., Lindemann N., Kurtz-Hoffmann J. In vitro model of a vascular stroma for the engineering of vascularized tissues. Int. J. Oral Maxillofac. Surg. 2001;30:414–420. doi: 10.1054/ijom.2001.0130. [DOI] [PubMed] [Google Scholar]
- 4.Sarker M., Chen X.B., Schreyer D.J. Experimental approaches to vascularisation within tissue engineering constructs. J. Biomater. Sci. Polym. Ed. 2015;26:683–734. doi: 10.1080/09205063.2015.1059018. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J., D’Amore P.A. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 6.Risau W., Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 7.Soker S., Machado M., Atala A. Systems for therapeutic angiogenesis in tissue engineering. World J. Urol. 2000;18:10–18. doi: 10.1007/pl00007070. [DOI] [PubMed] [Google Scholar]
- 8.Naghieh S., Foroozmehr E., Badrossamay M. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: mechanical and biological assessment. Mater. Des. 2017;133:128–135. [Google Scholar]
- 9.Naghieh S., Badrossamay M., Foroozmehr E. Combination of PLA micro-fibers and PCL-gelatin nano-fibers for development of bone tissue engineering scaffolds. Int. J. Swarm Intell. Evol. Comput. 2017;06:1–4. [Google Scholar]
- 10.Kharaziha M., Fathi M.H., Edris H. Tunable cellular interactions and physical properties of nanofibrous PCL-forsterite: gelatin scaffold through sequential electrospinning. Compos. Sci. Technol. 2013;87:182–188. [Google Scholar]
- 11.Hosseini Y., Emadi R., Kharaziha M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater. Chem. Phys. 2017;194:356–366. [Google Scholar]
- 12.Naghieh S., Karamooz-Ravari M.R., Sarker M. Influence of crosslinking on the mechanical behavior of 3D printed alginate scaffolds: experimental and numerical approaches. J. Mech. Behav. Biomed. Mater. 2018;80:111–118. doi: 10.1016/j.jmbbm.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Sarker M., Chen X.B. Modeling the flow behavior and flow rate of medium viscosity alginate for scaffold fabrication with a three-dimensional bioplotter. J. Manuf. Sci. Eng. 2017;139:081002. [Google Scholar]
- 14.Izadifar Z., Chang T., Kulyk W.M. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng. Part C Methods. 2016;22:173–188. doi: 10.1089/ten.TEC.2015.0307. [DOI] [PubMed] [Google Scholar]
- 15.You F., Wu X., Zhu N. 3D printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016;2:1200–1210. doi: 10.1021/acsbiomaterials.6b00258. [DOI] [PubMed] [Google Scholar]
- 16.Sarker M., Izadifar M., Schreyer D. Influence of ionic crosslinkers (Ca2+/Ba2+/Zn2+) on the mechanical and biological properties of 3D bioplotted hydrogel scaffolds. J. Biomater. Sci. Polym. Ed. 2018;29:1126–1154. doi: 10.1080/09205063.2018.1433420. [DOI] [PubMed] [Google Scholar]
- 17.Xu T., Zhao W., Zhu J.-M. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34:130–139. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Cui X., Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 19.Yanagawa F., Sugiura S., Kanamori T. Hydrogel microfabrication technology toward three dimensional tissue engineering. Regen. Ther. 2016;3:45–57. doi: 10.1016/j.reth.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naghieh S., Karamooz Ravari M.R., Badrossamay M. Numerical investigation of the mechanical properties of the additive manufactured bone scaffolds fabricated by FDM: the effect of layer penetration and post-heating. J. Mech. Behav. Biomed. Mater. 2016;59:241–250. doi: 10.1016/j.jmbbm.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Miller J.S., Stevens K.R., Yang M.T. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolesky D.B., Truby R.L., Gladman A.S. 3D bioprinting of vascularized, heterogeneous cell‐laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Yu Y., Chen H. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication. 2013;5:025004. doi: 10.1088/1758-5082/5/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naghieh S., Sarker M., Izadifar M. Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications – a brief review. J. Mech. Behav. Biomed. Mater. 2018;78:298–314. doi: 10.1016/j.jmbbm.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Yu Y., Ozbolat I.T. Direct bioprinting of vessel-like tubular microfluidic channels. J. Nanotechnol. Eng. Med. 2013;4:020902. [Google Scholar]
- 26.Luo Y., Lode A., Gelinsky M. Direct plotting of three‐dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. Adv. Healthc. Mater. 2013;2:777–783. doi: 10.1002/adhm.201200303. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y., Zhang Y., Martin J.A. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013;135:91011. doi: 10.1115/1.4024575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozbolat I.T., Yu Yin. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans. Biomed. Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 29.Gao Q., He Y., Fu J.Z. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Norotte C., Marga F.S., Niklason L.E. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadr N., Zhu M., Osaki T. SAM-based cell transfer to photopatterned hydrogels for microengineering vascular-like structures. Biomaterials. 2011;32:7479–7490. doi: 10.1016/j.biomaterials.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaebel R., Ma N., Liu J. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32:9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 33.Nahmias Y., Schwartz R.E., Verfaillie C.M. Laser‐guided direct writing for three‐dimensional tissue engineering. Biotechnol. Bioeng. 2005;92:129–136. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 34.Melchels F.P., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 35.Mankovich N.J., Samson D., Pratt W. Surgical planning using three-dimensional imaging and computer modeling. Otolaryngol. Clin. North Am. 1994;27:875–889. [PubMed] [Google Scholar]
- 36.Gauvin R., Chen Y.-C., Lee J.W. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33:3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang A.P., Qu X., Soman P. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 2012;24:4266–4270. doi: 10.1002/adma.201202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torgersen J., Qin X., Li Z. Hydrogels for two‐photon polymerization: a toolbox for mimicking the extracellular matrix. Adv. Funct. Mater. 2013;23:4542–4554. [Google Scholar]
- 39.Lee J.W., Ahn G., Kim D.S. Development of nano-and microscale composite 3D scaffolds using PPF/DEF-HA and micro-stereolithography. Microelectron. Eng. 2009;86:1465–1467. [Google Scholar]
- 40.Xing J.F., Zheng M.L., Duan X.M. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015;44:5031–5039. doi: 10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 41.Applegate M.B., Coburn J., Partlow B.P. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc. Natl. Acad. Sci. USA. 2015;112:12052–12057. doi: 10.1073/pnas.1509405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer W., Engelhardt S., Novosel E. Soft polymers for building up small and smallest blood supplying systems by stereolithography. J. Funct. Biomater. 2012;3:257–268. doi: 10.3390/jfb3020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ovsianikov A., Schlie S., Ngezahayo A. Two‐photon polymerization technique for microfabrication of CAD‐designed 3D scaffolds from commercially available photosensitive materials. J. Tissue Eng. Regen. Med. 2007;1:443–449. doi: 10.1002/term.57. [DOI] [PubMed] [Google Scholar]
- 44.Zheng X., Lee H., Weisgraber T.H. Ultralight, ultrastiff mechanical metamaterials. Science (80-) 2014;344:1373–1377. doi: 10.1126/science.1252291. [DOI] [PubMed] [Google Scholar]
- 45.Ng C.P., Helm C.-L.E., Swartz M.A. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc. Res. 2004;68:258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Ueda A., Koga M., Ikeda M. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am. J. Physiol. Circ. Physiol. 2004;287:H994–H1002. doi: 10.1152/ajpheart.00400.2003. [DOI] [PubMed] [Google Scholar]
- 47.Yamamura N., Sudo R., Ikeda M. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng. 2007;13:1443–1453. doi: 10.1089/ten.2006.0333. [DOI] [PubMed] [Google Scholar]
- 48.Weston D.F., Smekal T., Rhine D.B. Fabrication of microfluidic devices in silicon and plastic using plasma etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001;19:2846–2851. [Google Scholar]
- 49.Hsieh Y.-K., Chen S.-C., Huang W.-L. Direct micromachining of microfluidic channels on biodegradable materials using laser ablation. Polymers. 2017;9:242. doi: 10.3390/polym9070242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen A.S., Hao N., O'shea E.K. High-throughput microfluidics to control and measure signaling dynamics in single yeast cells. Nat. Protoc. 2015;10:1181–1197. doi: 10.1038/nprot.2015.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He J., Mao M., Liu Y. Fabrication of nature‐inspired microfluidic network for perfusable tissue constructs. Adv. Healthc. Mater. 2013;2:1108–1113. doi: 10.1002/adhm.201200404. [DOI] [PubMed] [Google Scholar]
- 52.Lim D., Kamotani Y., Cho B. Fabrication of microfluidic mixers and artificial vasculatures using a high-brightness diode-pumped Nd: YAG laser direct write method. Lab Chip. 2003;3:318–323. doi: 10.1039/b308452c. [DOI] [PubMed] [Google Scholar]
- 53.Peak C.W., Cross L., Singh A. Microscale Technol. Cell Eng. Springer; 2016. Microscale technologies for engineering complex tissue structures; pp. 3–25. [Google Scholar]
- 54.Myers D.R., Sakurai Y., Tran R. Endothelialized microfluidics for studying microvascular interactions in hematologic diseases. J. Vis. Exp. JoVE. 2012 doi: 10.3791/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takayama S., Ostuni E., LeDuc P. Laminar flows: subcellular positioning of small molecules. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- 56.Kaji H., Camci-Unal G., Langer R. Engineering systems for the generation of patterned co-cultures for controlling cell–cell interactions. Biochim. Biophys. Acta. 2011;1810:239–250. doi: 10.1016/j.bbagen.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tardy Y., Resnick N., Nagel T. Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler. Thromb. Vasc. Biol. 1997;17:3102–3106. doi: 10.1161/01.atv.17.11.3102. [DOI] [PubMed] [Google Scholar]
- 58.Shao J., Wu L., Wu J. Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress. Lab Chip. 2009;9:3118–3125. doi: 10.1039/b909312e. [DOI] [PubMed] [Google Scholar]
- 59.Song J.W., Gu W., Futai N. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 2005;77:3993–3999. doi: 10.1021/ac050131o. [DOI] [PubMed] [Google Scholar]
- 60.Therriault D., White S.R., Lewis J.A. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2003;2:265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 61.Chen A., Pan T. Three-dimensional fit-to-flow microfluidic assembly. Biomicrofluidics. 2011;5:46505. doi: 10.1063/1.3670368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu W., DeConinck A., Lewis J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011;23:H178–H183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 63.Huang J., Kim J., Agrawal N. Rapid fabrication of bio-inspired 3D microfluidic vascular networks. Networks. 2009;77845:3567–3571. [Google Scholar]
- 64.Tsao C.-W. Polymer microfluidics: simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines. 2016;7:225. doi: 10.3390/mi7120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker B.M., Trappmann B., Stapleton S.C. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip. 2013;13:3246–3252. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S., Lee H., Chung M. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 67.Shin Y., Jeon J.S., Han S. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip. 2011;11:2175–2181. doi: 10.1039/c1lc20039a. [DOI] [PubMed] [Google Scholar]
- 68.Whisler J.A., Chen M.B., Kamm R.D. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng. Part C Methods. 2014;20:543–552. doi: 10.1089/ten.tec.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto K., Tanimura K., Mabuchi Y. The stabilization effect of mesenchymal stem cells on the formation of microvascular networks in a microfluidic device. J. Biomech. Sci. Eng. 2013;8:114–128. [Google Scholar]
- 70.Kim B.S., Putnam A.J., Kulik T.J. Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol. Bioeng. 1998;57:46–54. [PubMed] [Google Scholar]
- 71.Corstorphine L., Sefton M.V. Effectiveness factor and diffusion limitations in collagen gel modules containing HepG2 cells. J. Tissue Eng. Regen. Med. 2011;5:119–129. doi: 10.1002/term.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dean D.M., Napolitano A.P., Youssef J. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;21:4005–4012. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 73.Kelm J.M., Djonov V., Ittner L.M. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12:2151–2160. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- 74.Yeh J., Ling Y., Karp J.M. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 75.McGuigan A.P., Sefton M.V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl. Acad. Sci. USA. 2006;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooper T.P., Sefton M.V. Fibronectin coating of collagen modules increases in vivo HUVEC survival and vessel formation in SCID mice. Acta Biomater. 2011;7:1072–1083. doi: 10.1016/j.actbio.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chamberlain M.D., Gupta R., Sefton M.V. Bone marrow-derived mesenchymal stromal cells enhance chimeric vessel development driven by endothelial cell-coated microtissues. Tissue Eng. Part A. 2011;18:285–294. doi: 10.1089/ten.tea.2011.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGuigan A.P., Leung B., Sefton M.V. Fabrication of cells containing gel modules to assemble modular tissue-engineered constructs. Nat. Protoc. 2006;1:2963–2969. doi: 10.1038/nprot.2006.443. [DOI] [PubMed] [Google Scholar]
- 79.Du Y., Lo E., Ali S. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nichol J.W., Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter. 2009;5:1312–1319. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du Y., Ghodousi M., Qi H. Sequential assembly of cell-laden hydrogel constructs to engineer vascular-like microchannels. Biotechnol. Bioeng. 2011;108:1693–1703. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He W., Ma Z., Teo W.E. Tubular nanofiber scaffolds for tissue engineered small‐diameter vascular grafts. J. Biomed. Mater. Res. Part A. 2009;90:205–216. doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 83.Smith I.O., Liu X.H., Smith L.A. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:226–236. doi: 10.1002/wnan.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M. Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29:4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 85.Klumpp D., Rudisile M., Kühnle R.I. Three‐dimensional vascularization of electrospun PCL/collagen‐blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. A. 2012;100:2302–2311. doi: 10.1002/jbm.a.34172. [DOI] [PubMed] [Google Scholar]
- 86.Cui L., Li J., Long Y. Vascularization of LBL structured nanofibrous matrices with endothelial cells for tissue regeneration. RSC Adv. 2017;7:11462–11477. [Google Scholar]
- 87.Du F., Wang H., Zhao W. Gradient nanofibrous chitosan/poly ɛ-caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials. 2012;33:762–770. doi: 10.1016/j.biomaterials.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 88.Wray L.S., Rnjak-Kovacina J., Mandal B.B. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–9224. doi: 10.1016/j.biomaterials.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Badylak S.F., Taylor D., Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 91.Yang M., Chen C., Wang X. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009;91:354–361. doi: 10.1002/jbm.b.31409. [DOI] [PubMed] [Google Scholar]
- 92.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu R.-H., Wang Y.-C., Liu S.-P. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23:621–630. doi: 10.3727/096368914X678382. [DOI] [PubMed] [Google Scholar]
- 94.Quint C., Kondo Y., Manson R.J. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dall’Olmo L., Zanusso I., Di Liddo R. Blood vessel-derived acellular matrix for vascular graft application. Biomed. Res. Int. 2014;2014:685426. doi: 10.1155/2014/685426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pellegata A.F., Dominioni T., Ballo F. Arterial decellularized scaffolds produced using an innovative automatic system. Cells Tissues Organs. 2015;200:363–373. doi: 10.1159/000439082. [DOI] [PubMed] [Google Scholar]
- 97.Ott H.C., Matthiesen T.S., Goh S.-K. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 98.Ott H.C., Clippinger B., Conrad C. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 99.Jakab K., Norotte C., Damon B. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. Part A. 2008;14:413–421. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 100.Marga F., Neagu A., Kosztin I. Developmental biology and tissue engineering. Birth Defects Res. C Embryo Today. 2007;81:320–328. doi: 10.1002/bdrc.20109. [DOI] [PubMed] [Google Scholar]
- 101.Owens C.M., Marga F., Forgacs G. Biofabrication and testing of a fully cellular nerve graft. Biofabrication. 2013;5:045007. doi: 10.1088/1758-5082/5/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y., Moncal K.K., Li J. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 2016;6:28714. doi: 10.1038/srep28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Okano T., Yamada N., Okuhara M. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297–303. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 104.Sekine H., Shimizu T., Sakaguchi K. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shimizu T., Sekine H., Yang J. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 106.Jung Y., Ji H., Chen Z. Scaffold-free, human mesenchymal stem cell-based tissue engineered blood vessels. Sci. Rep. 2015;5:15116. doi: 10.1038/srep15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dvir T., Kedem A., Ruvinov E. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA. 2009;106:14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hiscox A.M., Stone A.L., Limesand S. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng. Part A. 2008;14:433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 109.Tremblay P., Hudon V., Berthod F. Inosculation of tissue‐engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 110.Levenberg S., Rouwkema J., Macdonald M. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 111.Shepherd B.R., Chen H.Y., Smith C.M. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler. Thromb. Vasc. Biol. 2004;24:898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 112.Jeong S.I., Kwon J.H., Lim J.I. Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic PLCL scaffolds. Biomaterials. 2005;26:1405–1411. doi: 10.1016/j.biomaterials.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 113.Williams C., Wick T.M. Perfusion bioreactor for small diameter tissue-engineered arteries. Tissue Eng. 2004;10:930–941. doi: 10.1089/1076327041348536. [DOI] [PubMed] [Google Scholar]
- 114.Shweiki D., Itin A., Soffer D. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 115.Xu N., Liu H., Qu F. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp. Mol. Pathol. 2013;94:33–39. doi: 10.1016/j.yexmp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y., Teoh S.H., Chong M.S. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng. Part A. 2013;19:893–904. doi: 10.1089/ten.TEA.2012.0187. [DOI] [PubMed] [Google Scholar]
- 117.Cronin K.J., Messina A., Knight K.R. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast. Reconstr. Surg. 2004;113:260–269. doi: 10.1097/01.PRS.0000095942.71618.9D. [DOI] [PubMed] [Google Scholar]
- 118.Hussey A.J., Winardi M., Han X.-L. Seeding of pancreatic islets into prevascularized tissue engineering chambers. Tissue Eng. Part A. 2009;15:3823–3833. doi: 10.1089/ten.TEA.2008.0682. [DOI] [PubMed] [Google Scholar]
- 119.Naghieh S., Sarker M., Karamooz-Ravari M. Modeling of the mechanical behavior of 3D bioplotted scaffolds considering the penetration in interlocked strands. Appl. Sci. 2018;8:1422. [Google Scholar]
- 120.Sarker M.D., aghieh S., McInnes A.D. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018 doi: 10.1016/j.pneurobio.2018.07.002. [DOI] [PubMed] [Google Scholar]