Abstract
Background
Fermented black ginseng (FBG) is produced through several cycles of steam treatment of raw ginseng, at which point its color turns black. During this process, the original ginsenoside components of raw ginseng (e.g., Re, Rg1, Rb1, Rc, and Rb2) are altered, and less-polar ginsenosides are generated (e.g., Rg3, Rg5, Rk1, and Rh4). The aim of this study was to determine the effect of FBG on wound healing.
Methods
The effects of FBG on tube formation and on scratch wound healing were measured using human umbilical vein endothelial cells (HUVECs) and HaCaT cells, respectively. Protein phosphorylation of mitogen-activated protein kinase was evaluated via Western blotting. Finally, the wound-healing effects of FBG were assessed using an experimental cutaneous wounds model in mice.
Results and Conclusion
The results showed that FBG enhanced the tube formation in HUVECs and migration in HaCaT cells. Western blot analysis revealed that FBG stimulated the phosphorylation of p38 and extracellular signal-regulated kinase in HaCaT cells. Moreover, mice treated with 25 μg/mL of FBG exhibited faster wound closure than the control mice did in the experimental cutaneous wounds model in mice.
Keywords: ginseng, human umbilical vein endothelial cells, vascular endothelial growth factor, wound healing
1. Introduction
Healing of a wound in the human tissue can be defined as a complex process that restores not only the anatomical integrity but also function by injury [1], [2]. The phases of wound healing include inflammation, proliferation, granulation tissue formation, re-growth of epithelial tissue, and remodeling of new tissue for the recovery of tissue integrity [3], [4]. Among these, the newly formed blood vessels play a crucial role in the formation of temporary granulation tissue and supply of oxygen and nutrients in replacement of tissues [5], [6], [7]. The expressions of proangiogenic growth factors, including fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and angiopoietins, are important in wound angiogenesis [5]. VEGF is most essential in the initiation step of angiogenesis as it increases vascular permeability [8] as well as promotes endothelial cell proliferation and migration [9], [10], [11]. FGF-2, also called basic FGF, promotes endothelial cell differentiation and proliferation as a strong mitogenic factor [12], [13]. VEGF and FGF-2 act cooperatively to promote angiogenesis [14], [15], [16]. TGF-β regulates capillary tubule formation, endothelial cell migration, proliferation, and extracellular matrix deposition [17], [18], [19].
Ginseng is one of the species of genus Panax which comprises several species of slow-growing perennial plants [20]. It is considered as one of the most important herbal medicine with various health benefits arising from consumption of the root and its extracts [21], [22]. The medicinal efficacies of ginseng identified by modern science include cancer prevention, analgesia, enhanced immune function, blood pressure normalization, enhanced liver function, anti-stress, anti-oxidative, and anti-fatigue effects, improved sexual functions, improved climacteric disorder, as well as anti-aging effects [23]. The active components of ginseng are ginsenosides which have a steroid-like structure and are divided into three types according to their aglycones: protopanaxadiol (PPD), protopanaxatriol, and oleanoic acid ginsenosides [24]. Fermented black ginseng (FBG) is produced through several cycles of steam treatment of raw ginseng, at which point its color turns black [25]. During this process, the original ginsenoside components of raw ginseng (e.g., Re, Rg1, Rb1, Rc, and Rb2) are altered and less-polar ginsenosides are generated (e.g., Rg3, Rg5, Rk1, and Rh4) [26].
In this study, we aim to assess the influence of FBG, which includes various bioactive ingredients, on wound healing by in vitro and in vivo experiments and to verify the possibility of using FBG as a wound healing medical ingredient.
2. Materials and methods
2.1. Reagents and chemicals
EZ-Cytox cell viability assay kit was obtained from ITSBIO (Seoul, Korea). Fetal bovine serum (FBS) was purchased from Invitrogen Co. (Grand Island, NY, USA). Primary antibodies for p38, extracellular signal-regulated kinase (ERK), phosphorylated-p38 (p-p38), and phosphorylated-ERK (p-ERK), and secondary antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
2.2. Preparation of ginseng extract and ginsenoside
Dried powder of FBG extract was provided by GINSENG BY PHARM Co., Ltd. (Wonju, Korea), which produced FBG extract by a recently reported method [27]. In brief, black ginseng was first generated after nine cycles of repeated steaming of raw ginseng at 85°C for 8 h. The produced black ginseng extract was further fermented with Saccharomyces cerevisiae (Lallemand, Denmark) at 34°C for 25 h. PPD, which is known to be effective in wound healing, was used as a positive control and was prepared as reported previously [22], [25].
2.3. Analysis of ginsenosides
By using YL9100 HPLC system (Young-Lin, Anyang, Korea) fitted with a C-18, reversed-phase column (5 μm, 150 cm × 4.6 mm i.d.; YMC-Pack NH2, Kyoto, Japan) utilizing a solvent gradient system, ginsenoside samples were analyzed at a flow rate of 1 mL/min. The mobile phase consisted of 15% acetonitrile containing 5% acetic acid (solvent A) and 80% acetonitrile (solvent B). The model of evaporative light scattering detector was YL9180 (Young-Lin, Anyang, Korea). Gradient elution was carried out as follows: 0 min, 0% B; 6 min, 30% B; 18 min, 50% B; 30 min, 100% B; and 37 min, 100% B. Identification of ginsenosides was performed by the comparison of retention times with authentic samples (Table 1).
Table 1.
Comparison of contents of ginsenosides between red ginseng and fermented black ginseng (FBG)
| Sample | Content (μg/mg extract) |
||||
|---|---|---|---|---|---|
| Re | Rg1 | Rb1 | Rd | Rg3 | |
| Red ginseng | 0.016 ± 0.006 | 0.132 ± 0.012 | 0.578 ± 0.038 | 14.995 ± 1.137 | 2.190 ± 0.093 |
| FBG | 0.094 ± 0.009 | 0.047 ± 0.005 | — | — | 4.159 ± 0.106 |
FBG, fermented black ginseng.
2.4. Cell culture
Human umbilical vein endothelial cells (HUVECs) and HaCaT cells, an immortalized keratinocyte cell line from adult human skin, were purchased from ATCC (Manassas, VA, USA). HUVECs were cultured in Clonetics EGM-2 MV BulletKit (Lonza Inc., Walkersville, MD, USA) in a humidified atmosphere (5% CO2, 95% air). HaCaT cells were cultured using the media supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL), and 10% FBS at 37°C (5% CO2, 95% air). The cells were passed on reaching about 70–80% confluence. The number of cells was calculated when cells were plated according to each experimental design.
2.5. Cell viability assay
The cytotoxicity of FBG and PPD to HUVECs and HaCaT cells was assessed using the EZ-Cytox cell viability assay. In brief, cells were seeded at a density of 2 × 104 cells/mL in 96-well plates. The cells were then treated with various concentrations of FBG extract or PPD and incubated for 24 h at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After treatments, cell viability was measured according to manufacturer's instructions.
2.6. Tube formation assay
Cells were seeded (2.5 × 105 cells/mL) onto the Matrigel-coated plate. Media, with or without sample, was added. The plates were then incubated at 37°C for 24 h. After incubation, the cells were fixed with 4% paraformaldehyde, followed by staining with Mayer's hematoxylin (Muto Pure Chemicals, Tokyo, Japan). Cell morphology changes and tubular-structure formation were observed using a light microscope. The degree of tube formation was quantified by measuring the lengths of the tubes in the images captured using the ImageJ program.
2.7. Western blotting analysis
HUVECs (8 × 105 cells/mL) grown in 60 mm dishes were treated with the various concentrations of FBG extract (12.5 μg/mL and 25 μg/mL) for 24 h. Next, cell extracts were prepared using RIPA buffer (Cell Signaling, Danvers, MA, USA) that was supplemented with 1× protease inhibitor cocktail and 1mM phenyl methyl sulfonyl fluoride. Proteins (30 μg/lane) were separated by electrophoresis, transferred onto polyvinylidene fluoride membranes, and allowed to bind with epitope-specific primary and secondary antibodies. Visualization of antibody bounding was confirmed using ECL Advance Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK) and a LAS 4000 imaging system (Fujifilm, Tokyo, Japan) following instruction manual.
2.8. Cell scratch wound healing assay in HaCaT cells
The scratch wound assay was assessed by cell migration after formation of scratch in the cell monolayer. Briefly, HaCaT cells were plated in 35-mm dish at a density of 8 × 105 cells/mL. The following day, scratch wounds were formed in the HaCaT cell monolayer using a sterile pipette tip. The culture medium was changed with fresh serum-free medium and FBG (25 μg/mL) was added after washing away detached cells. After incubating for 12 h and 24 h, the wound width was measured at randomly chosen points under a light microscope equipped with a digital camera. The extent of wound closure was measured as the percentage compared to the original scratch width that had decreased at each measured time point.
2.9. Wound healing effect in an experimental cutaneous wounds model in mice
Animal testing regulations were approved by the animal research and ethics committee of Gachon University. Male ICR mice (age, 5 wk) were purchased from Orient Bio Co., Ltd. (Seongnam, Korea) and were allocated into three experimental groups. Under light anesthesia with ethyl ether, a 5-mm full-width excisional skin wound was made in the shaved back of each mouse. Then, each wound in mice was treated with phosphate-buffered saline (PBS) containing 0.5% dimethyl sulfoxide (DMSO) or FBG (25 μg/mL) dissolved in PBS containing 0.5% DMSO daily. The sample was treated with 10 μL of the solution once a day. The wound images of mice were captured on 0 d, 2 d, 4 d, 6 d, and 8 d using a digital camera. The quantification of wound healing was estimated by calculation of the remained area of wound at 0 d, 2 d, 4 d, 6 d, and 8 d after formation of wounds for each group. ImageJ software was used for the calculation of wound-size measurements. Briefly, the calculation of percentage wound closure was presented as follows: [(area of original wound (Day 0) – area of actual wound at 0 d, 2 d, 4 d, 6 d, and 8 d)/area of original wound (Day 0)] × 100.
2.10. Statistical analysis
Statistical significance was assessed using analysis of variance, followed by multiple comparison analysis with a Bonferroni adjustment. A p value of less than 0.05 was considered to be statistically significant.
3. Results and discussion
Wound healing progresses through the consecutive and synergistic remodeling phases that lack or overlap temporal distinction, including phases of inflammation, wound contraction, re-epithelialization, and maturation. The inflammatory phase occurs in a few days after wounding, and inflammatory cells such as macrophages and leukocytes are the major participants [28], [29]. During the healing process that provides the delivery of nutrients and oxygen, angiogenesis is the most critical step. Angiogenesis is modulated by the expression of various vascular growth factors and modulators [30]. In this paper, we have demonstrated that FBG enhances angiogenesis in vitro and wound healing in vivo.
3.1. Analysis of ginsenosides in FBG extract
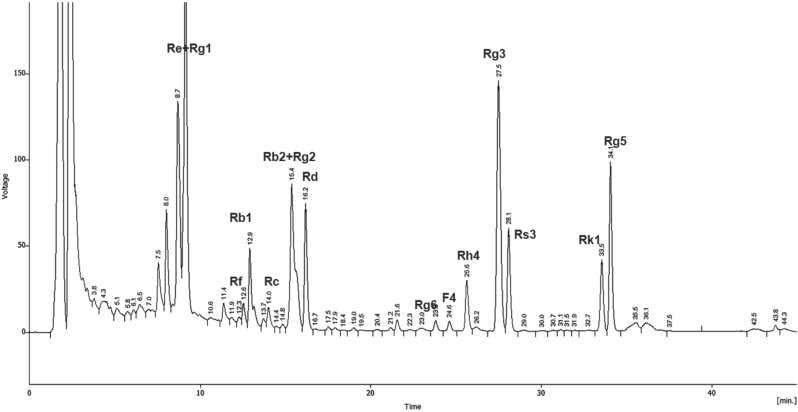
The HPLC profile of FBG extract is illustrated in Fig. 1. A quantitative analysis revealed that FBG contains ginsenoside Re (0.09 mg/g), Rg1 (0.05 mg/g), and Rg3 (4.16 mg/g).
Fig. 1.
Representative HPLC chromatogram of fermented black ginseng (FBG). Ginsenoside samples were analyzed using HPLC system fitted with a C-18 column utilizing a solvent gradient system. Identification of ginsenosides was performed by comparison of retention times with authentic samples.
3.2. Effects of the FBG and PPD on cell viability
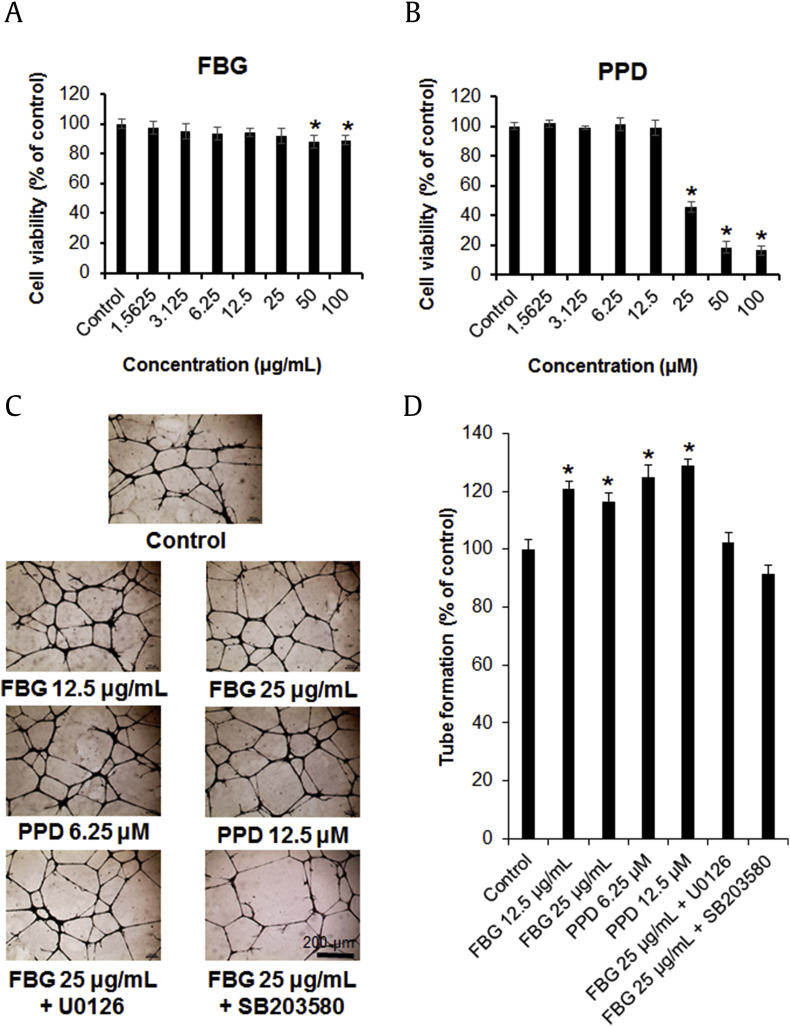
Proliferation of cells such as endothelial cells and fibroblasts is essential for effective wound healing [11]. Therefore, we first investigated the effects of various concentrations of FBG and PPD on the viability of HUVECs using an MTT assay. As shown in Fig. 2A, FBG reduced the viability of HUVECs slightly in a treatment dose-dependent manner. The cell viability (percent of control) ranged from 97.2 ± 2.56% (1.5625 μg/mL FBG) to 89.02 ± 1.49% (100 μg/mL FBG). At 100 μg/mL, FBG inhibited the viability of HUVECs by 10.98%. Compared with no effect with treatment of ≤ 25 μg/mL of FBG on HUVEC proliferation, cell viability was slightly decreased with the treatment of 50–100 μg/mL of FBG. As shown in Fig. 2B, the proliferation of HUVECs was reduced by PPD in a treatment dose-dependent manner. The cell viability (percent of control) ranged from 102.02 ± 1.79% (1.5625 μM PPD) to 16.34 ± 1.92% (100 μM PPD). PPD inhibited the viability of HUVECs by 83.66% at 100 μM concentration. Treatment with ≤ 12.5 μM PPD exerted no effect on HUVEC viability, whereas treatment with 25–100 μM inhibited its cell viability.
Fig. 2.
The effects of fermented black ginseng (FBG) and protopanaxadiol (PPD) on human umbilical vein endothelial cell (HUVEC) proliferation and tube formation. (A) Cells were treated with FBG in various concentrations (1.5625–100 μg/mL) or with 0.5% dimethyl sulfoxide (DMSO) only (control) for 24 h, followed by evaluation of cell viability by EZ-Cytox assay. (B) Cells were treated with PPD in various concentrations (1.5625–100 μM) or with 0.5% DMSO only (control) for 24 h, followed by evaluation of cell viability by EZ-Cytox assay. (C) Photographs of tube formation of HUVECs with or without FBG (12.5 μg/mL and 25 μg/mL) or PPD (6.25 μM and 12.5 μM) after 24 h. The inhibitor concentrations were 5 μM for U0126 and SB203580. (D) The relative lengths of tubes were quantified using ImageJ software. *p < 0.05 compared to the control value. FBG, fermented black ginseng; PPD, protopanaxadiol.
3.3. Effects of FBG and PPD on tube formation in HUVECs
The effect of non-toxic concentrations of FBG and PPD was tested on tube formation in HUVECs. FBG (doses of 12.5 μg/mL and 25 μg/mL) and PPD (doses of 6.25 μM and 12.5 μM) were used to test their effects on the tube formation in HUVECs (Figs. 2C and 2D). As shown in Fig. 2D, treatment with FBG and PPD increased tube formation, as estimated by the number of branching points. FBG (12.5 μg/mL) and PPD (12.5 μM) increased the tube formation by 20.3% and 29.53%, respectively, compared with the control cells. The phosphorylation of p38 and ERK has been suggested to be critical for tube formation. To determine the involvement of the pathways, the cells were treated with specific inhibitors of p38 (SB203580) and ERK (U0126). These inhibitors suppressed the enhancement of tube formation in the presence of FBG (Fig. 2C and 2D).
3.4. Effects of FBG on angiogenic protein expression in HUVECs
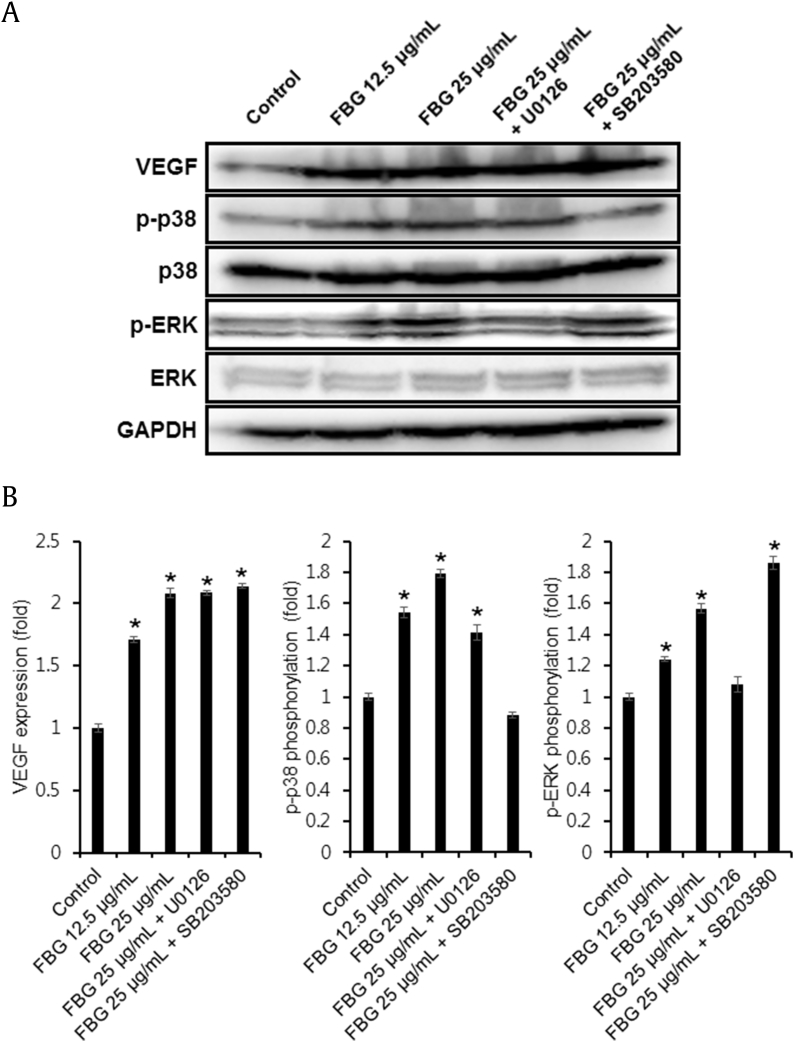
The activation of p38 and ERK mitogen-activated protein kinases (MAPKs) signaling proteins are known to be involved in the main signaling pathways for migration and proliferation of endothelial cells [31], [32]. Therefore, we investigated the effects of FBG on the phosphorylation of ERK and p38 MAPKs. Western blotting analysis was performed to explore the effect of FBG on the activation of MAPK signaling pathway in HUVECs (Fig. 3A). As quantified in Fig. 3B, the Western blot analysis showed that the levels of VEGF (1.71 ± 0.02 and 2.08 ± 0.03 fold at 12.5 μg/mL and 25 μg/mL FBG, respectively), p-p38 (1.54 ± 0.03 and 1.79 ± 0.02 fold at 12.5 μg/mL and 25 μg/mL FBG, respectively), and p-ERK (1.24 ± 0.01 and 1.57 ± 0.03 fold at 12.5 μg/mL and 25 μg/mL FBG, respectively) were markedly increased in the cells treated with FBG as compared to the non-treated control cells. The phosphorylation of p38 stimulated with FBG was completely inhibited by SB203580 (Fig. 3B). In addition, the treatments of specific inhibitors for ERK (U0126) decreased the phosphorylation of ERK. However, the phosphorylation of ERK by treatment with FBG was upregulated in the presence of SB203580 (Fig. 3B).
Fig. 3.
(A) The effect of fermented black ginseng (FBG) on protein expressions of vascular endothelial growth factor, p38, p-p38, extracellular signal-regulated kinase (ERK), and phosphorylated-ERK (p-ERK) in human umbilical vein endothelial cells (HUVECs). (B) Western blotting results show the levels of p-p38, p38, p-pERK, and ERK in HUVECs treated with FBG at a various concentrations for 24 h. The inhibitor concentrations were 5 μM for U0126 and SB203580. Whole cell lysates (30 μg) were electrophoresed by SDS-PAGE, transferred onto polyvinylidene fluoride membranes, and treated with the indicated antibodies. The targeted proteins were then visualized using ECL detection reagents. *p < 0.05 compared to the control value. ERK, extracellular signal-regulated kinase; FBG, fermented black ginseng; p-ERK, phosphorylated-extracellular signal-regulated kinase; VEGF, vascular endothelial growth factor.
3.5. Effects of FBG on cell scratch wound healing and proliferation in HaCaT cells
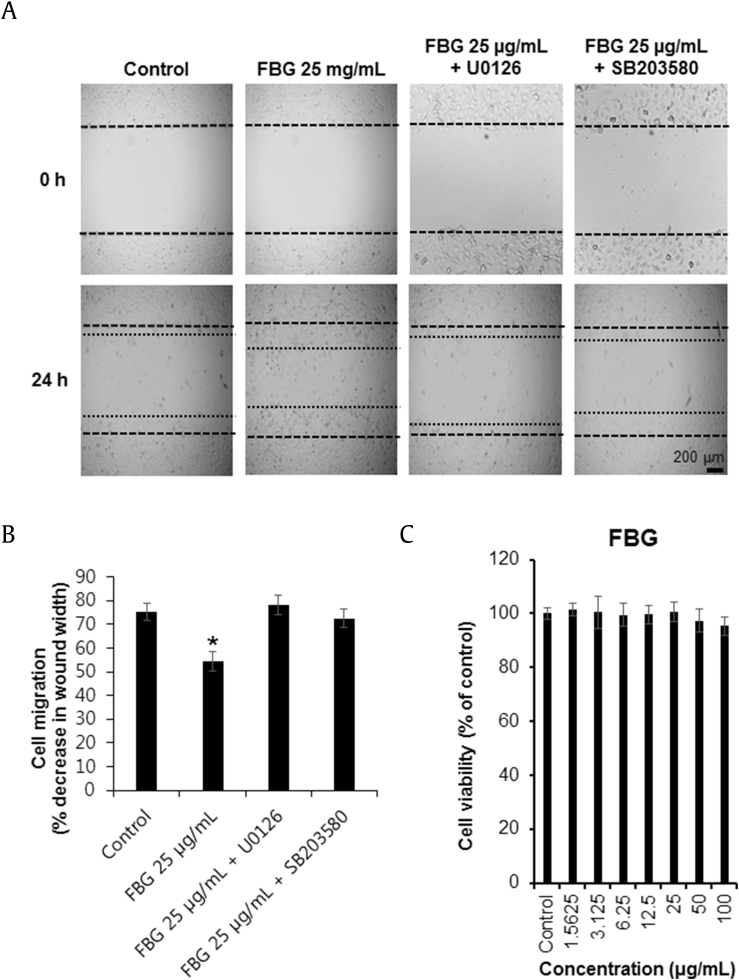
The effect of FBG on the scratch wound healing in HaCaT cells was tested using a cell-based wound healing model in which scratch wounds were formed in the HaCaT cell monolayer (Fig. 4A). The repaired percentage of scarification in the presence of 25 μg/mL of FBG was 45.8% at 24 h (Fig. 4B). However, various concentrations of FBG had no toxic effects on HaCaT cell proliferation (Fig. 4C). Scratch wounds in the culture containing FBG was inhibited with U0126 and SB203580 (Figs. 4A and 4B). From these findings, we concluded that U0126 and SB203580 suppress the phosphorylation of ERK and p38, respectively, resulting in an apparent decrease of wound healing.
Fig. 4.
The effects of fermented black ginseng (FBG) on scratch wound healing and proliferation in HaCaT cells. (A) Scratch wound healing was evaluated using a cell-scratch wound healing assay in HaCaT cells. Images from the same area were captured 0 h and 24 h after wound infliction. The inhibitor concentrations were 5 μM for U0126 and SB203580. (B) Scratch wound healing of FBG-treated HaCaT cells was detected using the scratch wound healing assay. Quantification of migration was calculated as a percentage of the wound closure. (C) Cells were treated with FBG in a various concentrations (1.5625–100 μg/mL) or with 0.5% dimethyl sulfoxide only (control) for 24 h, followed by evaluation of cell viability by EZ-Cytox assay. *p < 0.05 compared to the control value. FBG, fermented black ginseng.
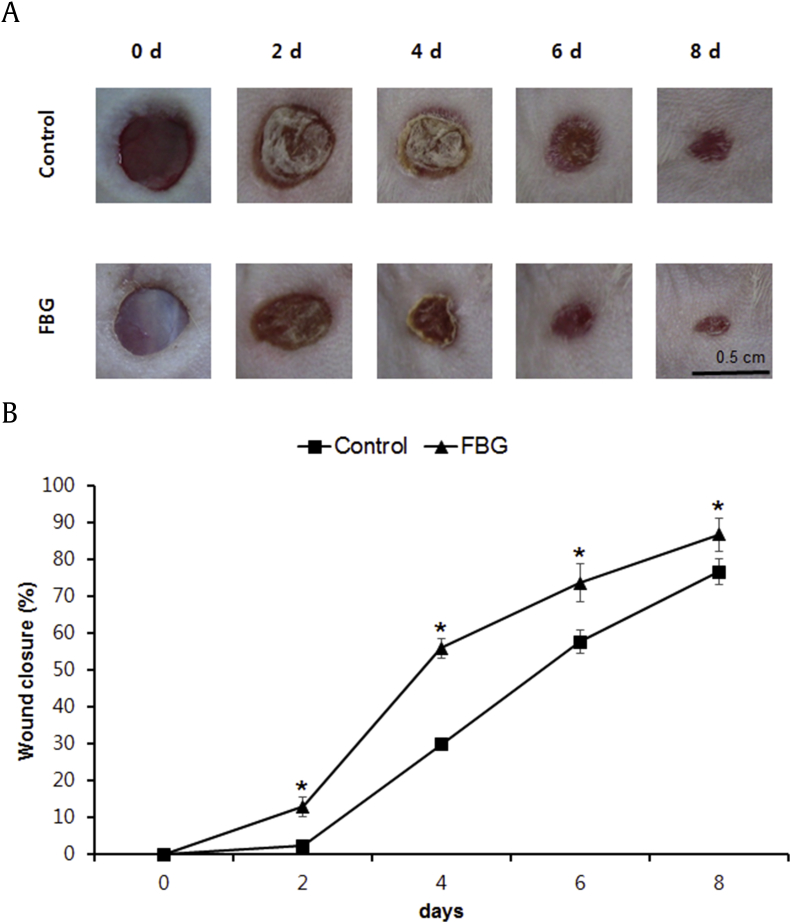
3.6. Effects of FBG on an experimental cutaneous wounds model in mice
Considering that FBG has significant effects in promoting migration and tube formation of HUVECs and HaCaT cells, we further employed an experimental cutaneous wounds model in mice to verify whether topical application of FBG may modify the healing of dermal wounds. As shown in Fig. 5A, the 25 μg/mL FBG-treated group exerted accelerated wound healing compared with the control mice. The enhancement of wound healing became apparent from 2 d after initiation of treatment and became the most evident after 4 d. As shown in quantitative results, treatment with 25 μg/mL of FBG exhibited a statistically significant effect on wound closure at 2 d, 4 d, 6 d, and 8 d after treatments compared with control mice (Fig. 5B).
Fig. 5.
The effects of fermented black ginseng (FBG) on wound healing in an experimental cutaneous wounds model in mice. (A) Comparison in wound closure between two groups (mice treated with 25 μg/mL FBG and the control mice). Representative photographs for the wounds at Day 0, Day 2, Day 4, Day 6, and Day 8 after treatments. (B) Quantitative analysis for wound size in the FBG-treated groups compared with that of the control group after 2 d. *p < 0.05 compared to the control value. FBG, fermented black ginseng.
From the cell-based assays, it is evident that FBG increased the HUVEC proliferation, tube formation, and cell migration of HaCaT cells. FBG exerted significant angiogenic potential, which is associated with phosphorylation of ERK and p38. Furthermore, in vivo assays showed that FBG accelerates the process of wound healing in experimental cutaneous wounds model in mice. Based on these data, we conclude that FBG is a potent promoter of wound repair.
Funding/support
This work was supported by the Korea Institute of Oriental Medicine Undergraduate Research Program (C16010) and the Korea Institute of Science and Technology institutional program (2Z04230).
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Su-Nam Kim, Email: snkim@kist.re.kr.
Ki Sung Kang, Email: kkang@gachon.ac.kr.
References
- 1.Kim D.W., Lee S.H., Shin M.J., Kim K., Ku S.K., Youn J.K., Cho S.B., Park J.H., Lee C.H., Son O. PEP-1-FK506BP inhibits alkali burn-induced corneal inflammation on the rat model of corneal alkali injury. BMB Rep. 2015;48:618–623. doi: 10.5483/BMBRep.2015.48.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Won K.J., Lee H.M., Rhim H., Kim H.C., Nah S.Y. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: involvement of lysophosphatidic-acid receptors and vascular-endothelial-growth-factor signaling. J Ginseng Res. 2016;40:325–333. doi: 10.1016/j.jgr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watelet J.B., Bachert C., Gevaert P., van Cauwenberge P. Wound healing of the respiratory mucosa: a review. Am J Rhinol. 2002;16:77–84. [PubMed] [Google Scholar]
- 4.Hosemann W., Wigand M.E., Gode U., Langer F., Dunker I. Normal wound healing of the paranasal sinuses: clinical and experimental investigations. Eur Arch Otorhinolaryngol. 1991;248:390–394. doi: 10.1007/BF01463560. [DOI] [PubMed] [Google Scholar]
- 5.Li J., Zhang Y.P., Kirsner R.S. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 6.Lee C.S., Ghim J., Song P., Suh P.G., Ryu S.H. Loss of phospholipase D2 impairs VEGF-induced angiogenesis. BMB Rep. 2016;49:191–196. doi: 10.5483/BMBRep.2016.49.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S.W., Lee K.S., Lee J.H., Kang H.J., Lee M.J., Kim H.Y., Park K.I., Kim S.L., Shin H.K., Seo W.D. Suppression of Akt-HIF-1α signaling axis by diacetyl atractylodiol inhibits hypoxia-induced angiogenesis. BMB Rep. 2016;49:508–513. doi: 10.5483/BMBRep.2016.49.9.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keck P.J., Hauser S.D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D.T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 9.Senger D.R., Ledbetter S.R., Claffey K.P., Papadopoulos-Sergiou A., Peruzzi C.A., Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 10.Baek S.H., Kim K.I. Regulation of HIF-1α stability by lysine methylation. BMB Rep. 2016;49:245–246. doi: 10.5483/BMBRep.2016.49.5.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nissen N.N., Polverini P.J., Koch A.E., Volin M.V., Gamelli R.L., DiPietro L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 12.Schweigerer L., Neufeld G., Friedman J., Abraham J.A., Fiddes J., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 13.Montesano R., Vassalli J.D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepper M.S., Ferrara N., Orci L., Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 15.Goto F., Goto K., Weindel K., Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993;69:508–517. [PubMed] [Google Scholar]
- 16.Ha J.M., Baek S.H., Kim Y.H., Jin S.Y., Lee H.S., Kim S.J., Shin H.K., Lee D.H., Song S.H., Kim C.D. Regulation of retinal angiogenesis by phospholipase C-β3 signaling pathway. Exp Mol Med. 2016;48:e240. doi: 10.1038/emm.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E.Y., Moses H.L. Transforming growth factor beta 1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol. 1990;111:731–741. doi: 10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madri J.A., Pratt B.M., Tucker A.M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988;106:1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankar S., Mahooti-Brooks N., Bensen L., McCarthy T.L., Centrella M., Madri J.A. Modulation of transforming growth factor beta receptor levels on microvascular endothelial cells during in vitro angiogenesis. J Clin Invest. 1996;97:1436–1446. doi: 10.1172/JCI118565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Case M.A., Flinn K.M., Jancaitis J., Alley A., Paxton A. Declining abundance of American ginseng (Panax quinquefolius L.) documented by herbarium specimens. Biol Conserv. 2007;134:22–30. [Google Scholar]
- 21.Kong B.R., Park M.J., Min J.W., Kim H.B., Kim S.H., Kim S.Y., Yang D.C. Physicochemical characteristics of white, fermented and red ginseng extracts. J Ginseng Res. 2008;32:238–243. [Google Scholar]
- 22.Park J.Y., Choi P., Kim H.K., Kang K.S., Ham J. Increase in apoptotic effect of Panax ginseng by microwave processing in human prostate cancer cells: in vitro and in vivo studies. J Ginseng Res. 2016;40:62–67. doi: 10.1016/j.jgr.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C. A. Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 24.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han M.S., Han I.H., Lee D., An J.M., Kim S.N., Shin M.S., Yamabe N., Hwang G.S., Yoo H.H., Choi S.J. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J Ginseng Res. 2016;40:135–140. doi: 10.1016/j.jgr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roh S.S., Park J.H. The effects of ginseng radix preparata extract on anti-thrombotic activity. J East-West Med. 2008;33:47–61. [Google Scholar]
- 27.Bak M.J., Jeong W.S., Kim K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int J Mol Med. 2014;34:1516–1522. doi: 10.3892/ijmm.2014.1972. [DOI] [PubMed] [Google Scholar]
- 28.Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 29.Watelet J.B., Demetter P., Claeys C., Cauwenberge P., Cuvelier C., Bachert C. Wound healing after paranasal sinus surgery: neutrophilic inflammation influences the outcome. Histopathology. 2006;48:174–181. doi: 10.1111/j.1365-2559.2005.02310.x. [DOI] [PubMed] [Google Scholar]
- 30.Plouet J., Schilling J., Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. Embo J. 1989;8:3801–3806. doi: 10.1002/j.1460-2075.1989.tb08557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin E.Y., Kim S.Y., Kim E.G. c-Jun N-terminal kinase is involved in motility of endothelial cell. Exp Mol Med. 2001;33:276–283. doi: 10.1038/emm.2001.45. [DOI] [PubMed] [Google Scholar]
- 32.Lee J., Song J., Kwon E.S., Jo S., Kang M.K., Kim Y.J., Hwang Y., Bae H., Kang T.H., Chang S. CTHRC1 promotes angiogenesis by recruiting Tie2-expressing monocytes to pancreatic tumors. Exp Mol Med. 2016;48:e261. doi: 10.1038/emm.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]