Abstract
Background:
Leukocyte mobilization and secretions of cytokines, chemokines, and growth factors in children during exercise are necessary biochemical signals for physiological growth and long-term cardiovascular protection. Because of glycemic instability, altered exercise responses, particularly the proinflammatory cytokine interleukin (IL)-6, may occur in type 1 diabetes mellitus (T1DM) that could influence the onset/progression of diabetic vascular complications. Relatively little is known, however, on most molecular aspects of immunomodulatory adaptation to exercise in diabetic children.
Methods:
We therefore studied 21 children (age, 13.4 ± 0.3 years; 13 boys/8 girls) with T1DM and 21 age-matched healthy controls during 30 minutes of intense and intermittent cycling exercise. Euglycemia was maintained during and for greater than 90 minutes before exercise; blood samples for IL-6 and other cytokines/chemokines were drawn before, during (every 6 minutes), and after (every 15 minutes) exercise.
Results:
In T1DM, exercise-induced IL-6 peak occurred earlier and with greater magnitude than that in controls; an exploratory analysis of additional inflammatory mediators displayed a similarly accelerated/exaggerated pattern in T1DM, including the kinetic profiles of tumor necrosis factor α, IL-4, IL-12p70, IL-17, granulocyte-monocyte colony-stimulating factor, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and eotaxin (interferon-inducible protein-10 was the only measured variable essentially indistinguishable between groups).
Conclusion:
Therefore, during intense and intermittent exercise, significant alterations in the immunologic pattern of inflammatory regulation occurred in children with T1DM as compared with healthy controls. Our findings underscore how the understanding of all the underlying molecular mechanisms is a necessary prerequisite for achieving effective use of exercise and the full manifestation of its health benefits, particularly in understudied populations such as children with T1DM who are at increased risk for cardiovascular complications.
Keywords: inflammatory cytokine, exercise, type 1 diabetes, children
■ INTRODUCTION
It has become apparent in recent years that the adaptive response to physical exercise is enormously more complex than previously thought and that an important component of this adaptation is the regulation of whole-body inflammatory status.1 Exercise induces both a short-term proinflammatory stimulation and a long-term anti-inflammatory effect, an apparently paradoxical dual response that is mediated by the coordinated activation of numerous cytokines, chemokines, and other molecular factors.2 Whereas exercise has been advocated as exerting multiple beneficial health effects, especially during childhood and adolescence, all components of the physiological adaptation to exercise must be activated correctly and simultaneously for these health effects to fully manifest, including a correct balance of proinflammatory and anti-inflammatory mediators.3
Many of the immunomodulatory molecules activated during exercise are in common with the molecular pathogenetic pathways of chronic cardiovascular disease.4 The hypothesis was therefore recently formulated—that altered inflammatory responses to exercise may be present in dysmetabolic states (obesity, diabetes, and arthritis), thereby reducing (indeed, in extreme cases, abolishing) exercise’s beneficial effects.5 Paradoxically, a reduced effectiveness of exercise may occur in patients at increased risk for long-term cardiovascular disease, who should rather try to benefit from any available cardioprotective strategy to the maximal degree. The preventive and therapeutic use of exercise should therefore be optimized in specific patient populations, a strategy that can no longer be based on empirical concepts but must derive from a deep and complete understanding of all cellular and molecular adaptive mechanisms and their possible alterations.
Children with type 1 diabetes mellitus (T1DM) represent a particularly vulnerable population to potential changes in inflammatory responses to exercise. Frequent, and often prolonged, hyperglycemia is a strong proin-flammatory stimulus for this age group (glycemic control is remarkably difficult, particularly in the peripubertal years).6 This overlaps with the normal high levels of physical activity of children, which is now especially encouraged as part of their disease management. Recent evidence suggests, however, that even the same exercise format may not be equally beneficial in different situations.5 Our laboratory has recently demonstrated, for instance, that children with T1DM may display an increased inflammatory status before and during exercise, if marked hyperglycemia had occurred 3 to 5 hours before exercise (even if, by the time they exercise, their blood glucose has returned to normal levels).7 It is currently unknown, however, if these alterations extend to other immunomodulatory mediators that are also activated during exercise. Furthermore, in addition to the absolute values of inflammatory cytokines and chemokines before and after exercise, an important component of the inflammatory adaptation is the kinetic profile of the changes of these molecules during exercise, which our laboratory has recently demonstrated as not linear in healthy children but follows a fluctuating pattern characteristic of each group of inflammatory mediators.8 It is not known whether changes in this kinetic pattern may also be present in children with T1DM.
We therefore tested the hypothesis that, when compared with healthy controls, peripubertal children with T1DM display an exaggerated interleukin (IL)-6 kinetic response during exercise. As a secondary aim, to detect possible parallel changes in the kinetic profiles of other related variables, we also performed an exploratory analysis of an additional panel of selected proinflammatory and anti-inflammatory cytokines, chemokines, and colony stimulating factors (tumor necrosis factor [TNF] α, IL-1α, IL-4, IL-12p70, IL-17, granulocyte-monocyte colony-stimulating factor [GM-CSF], IL-8, monocyte chemoattractant protein [MCP]-1, macrophage inflammatory protein [MIP]-1α, interferon-inducible protein [IP]-10, and eotaxin).
■ MATERIALS AND METHODS
Subjects
Experimental procedures were first approved by the University of California, Irvine (UCI), Institutional Review Board. All parts of the studies were performed by specialized personnel from the UCI Institute for Clinical Translational Science (ICTS). After recruitment, subjects and their respective parents/guardians signed informed assent and consent forms. Twenty-one diabetic (T1DM; age, 13.4 ± 0.3 years; 13 boys/8 girls) children and 21 healthy controls (CTRL; age, 13.9 ± 0.8 years; 8 boys/ 13 girls) were enrolled. Their demographic characteristics are listed in Table 1.
TABLE 1.
Demographic Characteristics of the 2 Experimental Groups: T1DM and CTRL
| Age, yr | Sex (M/F) | Height | Weight | BMI (Percentile) | Tanner | V̇O2max | n | |
|---|---|---|---|---|---|---|---|---|
| T1DM | 13.4 ± 0.3 | 13/8 | 165.5 ± 2.7 | 56.5 ± 2.0 | 65.4 ± 5.4 | 3.3 ± 0.2 | 38.9 ± 1.5 | 21 |
| CTRL | 13.9 ± 0.8 | 8/13 | 159.6 ± 3.0 | 55.5 ± 2.6 | 65.4 ± 4.8 | 3.4 ± 0.3 | 34.6 ± 1.6 | 21 |
Data are means ± SE.
BMI indicates body mass index; CTRL, healthy controls; T1DM, type 1 diabetes mellitus; VO2max, maximum oxygen consumption.
Preliminary Visit
At least 48 hours before the main study day, all subjects attended a preliminary visit. As confirmed by an extensive medical interview during this visit, all participants were in good health (except that T1DMs had diabetes), including the absence of recent or chronic asthma, common colds, minor injuries, and so on. Other exclusion criteria included prescription or over-the-counter medications (except insulin for T1DMs), abnormal vital signs, and inability to exercise. Subjects with T1DM were inquired about their most recent glycosylated hemoglobin (hemoglobin A1c), a clinical measure of the average plasma glucose concentration in the past 90 days, which was 7.5 ± 0.05%.
Tanner stage was then assessed via a standard validated questionnaire9 that our study team has routinely used in past years. A high accuracy of assessing pubertal status in this way has been clearly demonstrated to be attainable because this methodology precludes interoperator variability; furthermore, it prevents anxiety that some children may experience from direct physical examination that may adversely affect the subsequent exercise protocol. For these reasons, this mode of Tanner staging has become the method of choice in many pediatric research institutions.9
Finally, a preliminary cycling exercise test was performed to evaluate individual maximal aerobic capacity (maximum oxygen consumption [V̇O2max]), which is the gold standard measurement of physical fitness. The test lasted 10 to 15 minutes and was administered by a specialized exercise physiologist from the UCI ICTS. The subjects pedaled on an electronically braked stationary cycle ergometer (Ergoline 800S; Sensors Medic, Yorba Linda, CA) initially with no resistance for approximately 2 minutes; the work rate was then raised steadily by 10 to 20 W per minute (10% of predicted maximal workload per minute according to age, sex, and body size)10 until the subjects reached their respective limit of exercise tolerance. Gas exchange was measured breath by breath with a commercial metabolic cart (Sensors Medic), and the anaerobic threshold (AT) and V̇O2max for each individual were calculated by standard methods. The AT is the point beyond which skeletal muscular work must be supplemented with anaerobic mechanisms, which is generally reached between 40% and 60% of V̇O2max.
Main Study Day
All subjects were admitted to the UCI ICTS at approximately 8 am; vital signs were repeated, and intravenous lines were started on forearms for blood draws and (in diabetic patients only) for glucose and insulin infusions.
Control Subjects
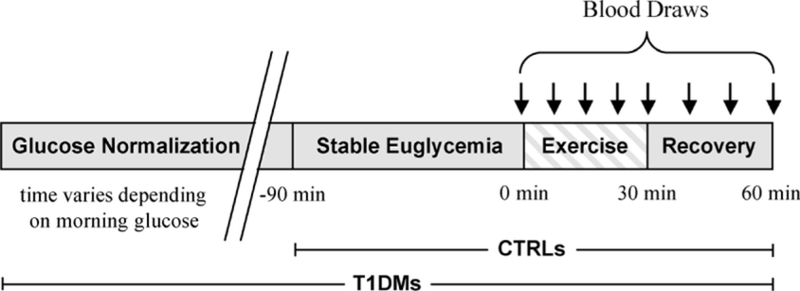
Subjects rested for 90 minutes before acquiring baseline blood samples. This rest period will be defined as t = −90 to 0 minutes (time −90 minutes was delayed by 1–2 hours in some subjects to compensate for the variability in start time that occurred in some patients with T1DM because of the time needed to stabilize their plasma glucose). At t = 0 minutes, they started pedaling on a stationary cycling ergometer at a workload halfway between the AT and V̇O2max, which is expected to generate a detectable physiological response at the systemic and molecular levels; on average, this workload corresponded to 80% of their predetermined V̇O2max. Subjects exercised for 2 minutes, followed by 1-minute rest, and repeated this intermittent pattern 10 times for a total of 30 minutes (exercise therefore ended at t = 30 minutes; Fig. 1). The intermittent cycling paradigm is a standard exercise challenge for pediatric patients at the UCI ICTS and has been successfully implemented in thousands of exercise tests in healthy children and children with a variety of clinical conditions. This exercise pattern mimics the real-life physical activities of children (intermittent stop-and-go pattern), ensures patient safety and efficiency of blood draws during the 1-minute rest interval, and permits subjects to sustain a high level of intense physical exertion (80% of V̇O2max) for a prolonged period.
FIGURE 1.
Experimental paradigm. Gray and striped bars represent nonexercise and exercise, respectively. Arrows signify time of blood sampling. Only type 1 diabetes mellitus (T1DM)s underwent this entire paradigm. The healthy control (CTRL) group bypassed the glucose normalization because they entered the study in the euglycemic range.
Blood sampling for immunoassays was performed at baseline, every 6 minutes during exercise (after the second, fourth, sixth, eight, and 10th exercise bout) and at 15 and 30 minutes after exercise (Fig. 1).
Diabetic Subjects
Upon awakening in the morning of study visit day, T1DMs immediately tested their blood glucose and called the study principal investigator to confirm insulin injection amounts, mostly in accordance to their regular prescribed regimen; subjects on multiple insulin injections only injected the rapid-acting insulin component of their regimen on the day of the visit, whereas those on insulin pumps remained on their prescribed settings.
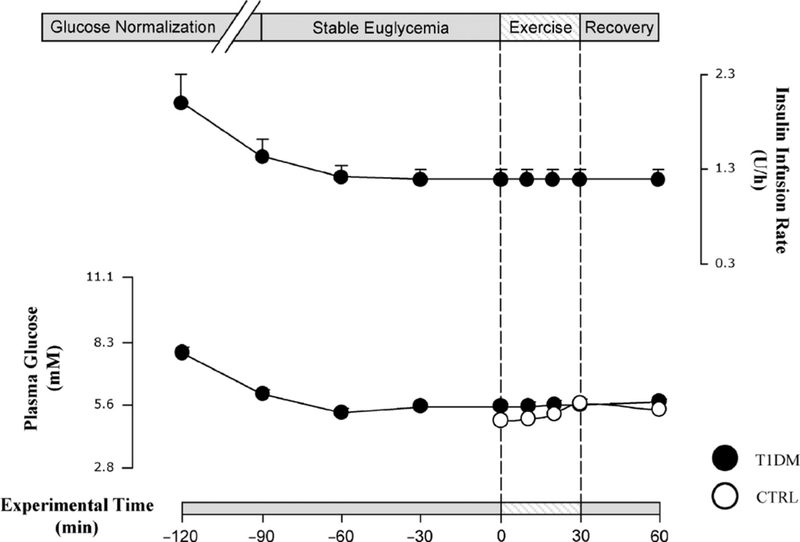
Upon arrival to the UCI ICTS, normal vital signs were confirmed, and intravenous lines were started. Thereafter, blood glucose concentrations were continuously monitored at 10- to 15-minute intervals in triplicates using a Beckman Coulter Glucose II Analyzer (Beckman Coulter, Fullerton, CA). Subjects whose initial blood glucose levels were above 8.3 mM were given an insulin infusion rate of 9.0 nmol/h for every 5.6 mM above 8.3 mM of plasma glucose; the insulin infusion was gradually tapered as their blood glucose approached euglycemia (4.4–6.1 mM). The time point when euglycemia was attained was defined as t = −90 minutes. The intravenous insulin infusion was then maintained at the minimum basal rate sufficient to sustain stable euglycemia for 90 minutes, before the start of exercise at t = 0 minute. To reproduce physiological conditions, the basal intravenous insulin infusion rate was targeted to be approximately 6.0 nmol/h or 110% of the basal rate for those who are normally using an insulin pump and remained constant from t = −90 minutes until the end of the study. Intravenous dextrose was infused as needed if, based on feedback from the repeated blood glucose measurements, a drop of plasma glucose concentration below 4.4 mM seemed impending (Fig. 2).
FIGURE 2.
On average, subjects with type 1 diabetes mellitus (T1DM) were infused with high levels of exogenous insulin to stabilize their blood glucose. The insulin infusion rate was reduced as euglycemia approached and was maintained at a constant rate of 90 minutes and through exercise and recovery periods. The healthy controls (CTRLs) required no insulin infusion because their blood glucose were physiologically in the euglycemic range before cycling. There was no difference in blood glucose concentrations between T1DMs and CTRLs during exercise and recovery. Dots are means ± SE. Black dots are T1DM; hollow dots are CTRLs.
Both T1DMs and CTRLs were permitted to ingest water ad libitum during any portion of the study. Hematocrit was checked before, immediately after, and 30 minutes after exercise for all subjects and was found to be essentially identical between the 2 groups. Therefore, except for the fact that euglycemia was maintained iatro-genically in the T1DM group, whereas CTRLs depended solely on endogenous counterregulatory responses, all aspects of the exercise protocol, including timing and amount of blood draws, were identical in both groups.
Experimental Assay
After each blood draw, approximately 1.0 mL of whole blood was placed in a preheparinized tube and immediately centrifuged. Thereafter, the supernatant was removed and stored in a separate microcentrifuge tube at a temperature of −80°C. On the day of the experimental run, the frozen plasma was thawed to room temperature and prepared for LINCOplex microbeads immunoassay (LINCO Research, St Charles, MO; catalog kit HCYTO-60K; intra-assay % coefficient of variation, 7.5–13.6; interassay %CV, 5.0–13.2; sensitivities [in picogram per milliliter]: IL-6, 0.79; TNF-α, 0.22; IL-1α, 0.63; IL-4, 2.87; IL-12p70, 0.23; IL-17, 0.25; GM-CSF, 0.23; IL-8, 0.32; MCP-1, 0.63; MIP-1α, 1.23; IP-10, 1.14; and eotaxin, 5.37), whose yields are comparable to enzyme-linked immunosorbent assay.11 Luminex 200 (Luminex Corporation, Austin, TX) was used to read the quantity of cytokine-antibody complexes reflecting concentrations of plasma cytokines. The raw data were acquired with the MasterPlex Software (Miraibio, Alameda, CA).
For a subset of subjects, ex vivo incubation of total white blood cells (WBCs) and subsequent quantification of messenger RNA (mRNA) expressions of select cytokines were also performed. Triplicate 60-μL aliquots of heparinized whole blood were placed in 8-well strip microtubes on the same study day of the study, then incubated at 37°C for 4 hours in phosphate-buffered saline, and stored at −80°C. On assay day, samples were thawed, and each 60-μL aliquot was applied to 96-well filter plates to trap leukocytes, followed by cell lyses on membranes. The resultant lysates were transferred to 96-well oligo(dT)-immobilized microplates for poly(A)+ mRNA purification and complementary DNA synthesis in the same well.12 Polymerase chain reaction primers were purchased from Primer Express (Applied Biosystems, Foster City, CA) and HYBsimulator (RNAture, Irvine, CA), whereas oligonucleotides were from IDT (Coralville, IA). Reverse transcriptase–polymerase chain reaction with SYBR green fluorescence detection was used to individually amplify each target mRNA, and cycle threshold (Ct) was determined.13 The ΔCt was then calculated by subtracting the Ct values of blood samples drawn before ex vivo incubation from the Ct of the same subject’s blood samples after the incubation for both baseline and after exercise.
Statistical Analysis
Subjects’ demographic characteristics and circulating concentrations of measured inflammatory mediators are presented as group means ± SE of the means.
Power calculations were based on prior IL-6 measurements in children from our laboratory, indicating that to detect a change of 30% or more, with an SD of 33% or lesser of the mean, a level of significance of 0.05, and an 80% power, a group size of 16 was required. The greater sample of the population for each group (21) was used to account for additional variability because of sex composition and additional exploratory analyses.
For comparison of absolute values of circulating IL-6 (at baseline, end-exercise, and 30 minutes after exercise), 2-tailed unpaired t tests were used with Bonferroni correction to compensate for multiple comparisons. Given the exploratory nature of the additional cytokine assays included in the paper, individual Bonferroni corrections were also applied to each additional variable, whereas, in the ex vivo mRNA assays, the correction was applied to the whole set of data. For comparison of exercise responses between groups, the area under the curve (AUC) of the changes over baseline of each variable were computed using all exercise time points (at 6-minute intervals); AUCs were then compared with 2-tailed unpaired t tests.
For comparison of exercise-induced peaks, only variables for which a clearly identifiable peak was detected in both experimental groups were considered. A peak was considered to be present if the mean of the individual highest values during exercise was higher than the mean baseline value. By this definition, a peak may not correspond to the highest mean value for that variable at any given time point because individual subjects may display peaks at different time points; the time to reach peak was calculated as the mean time during exercise at which each subject reached her/his highest value. For each included variable, the mean time to reach a peak was compared across groups with 2-tailed unpaired t tests. Observed differences were considered statistically significant when a P value of less than 0.05 was detected.
■ RESULTS
Subjects
There was no difference between CTRLs and T1DMs in terms of age, sex, height, weight, and Tanner (Table 1).
Plasma Glucose and Insulin Infusion for T1DMs
Several children with T1DM presenting with hyperglycemia and required intravenous insulin infusion to achieve euglycemia were admitted to the study. The time required to reach the time point t = −90 (the start of stable euglycemia), therefore, varied across subjects with T1DM. Once euglycemia was achieved, however, it was strictly maintained, with group mean values of 5.5 ± 0.1 mM throughout exercise and recovery, similar to the mean glycemic levels in control subjects (average during exercise and recovery, 5.4 ± 0.1 mM). In T1DM participants, the mean insulin infusion rates during the 90 minutes preceding exercise, and during cycling and recovery, was 1.2 U/h, a rate comparable to the physiological endogenous baseline insulin secretion rate in fasting healthy individuals (Fig. 2).
Inflammatory Mediators
Exercise-Induced Changes.
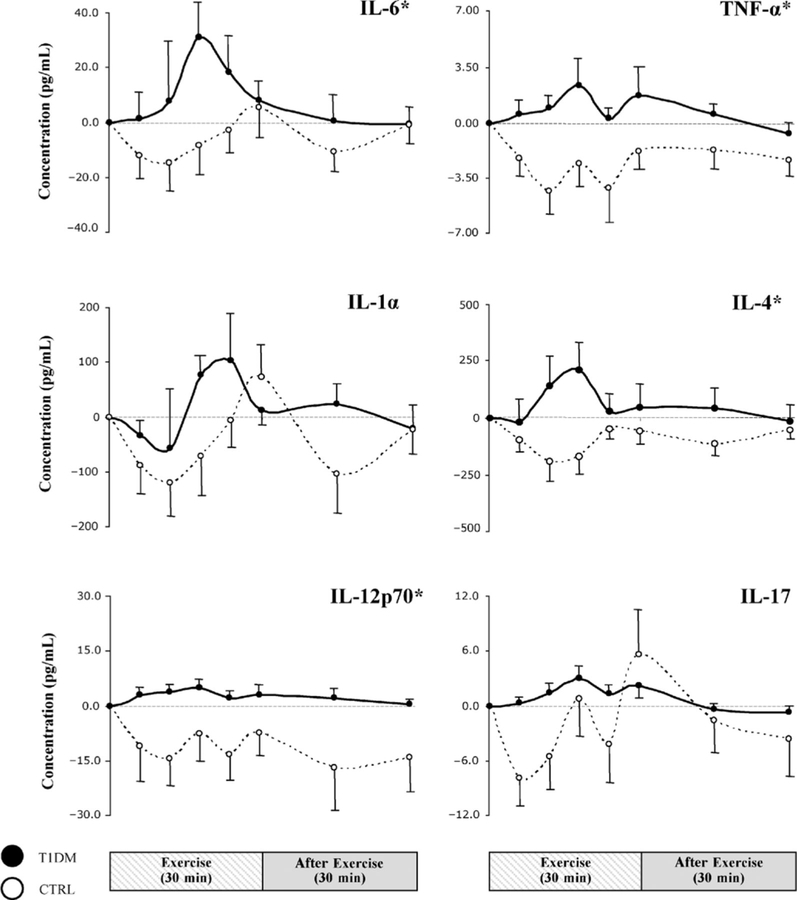
In the T1DM group, IL-6 increased rapidly during exercise (Fig. 3), with a mean maximum increment over baseline of 31 ± 12 pg/mL by 18 minutes into the protocol; this was in sharp contrast with control subjects, in which IL-6 remained stable (indeed slightly below pre-exercise values) for most of the exercise time and only exceeded baseline levels at end-exercise at a mere 5 ± 7 pg/mL.
FIGURE 3.
Striped and gray bars represent exercise (t = 0–30 minutes) and recovery (t = 30–60 minutes), respectively. Dots are means ± SE. *P < 0.05. CTRL indicates healthy controls; IL, interleukin; IP, interferon-inducible protein; T1DM, type 1 diabetes mellitus; TNF-α, tumor necrosis factor-α.
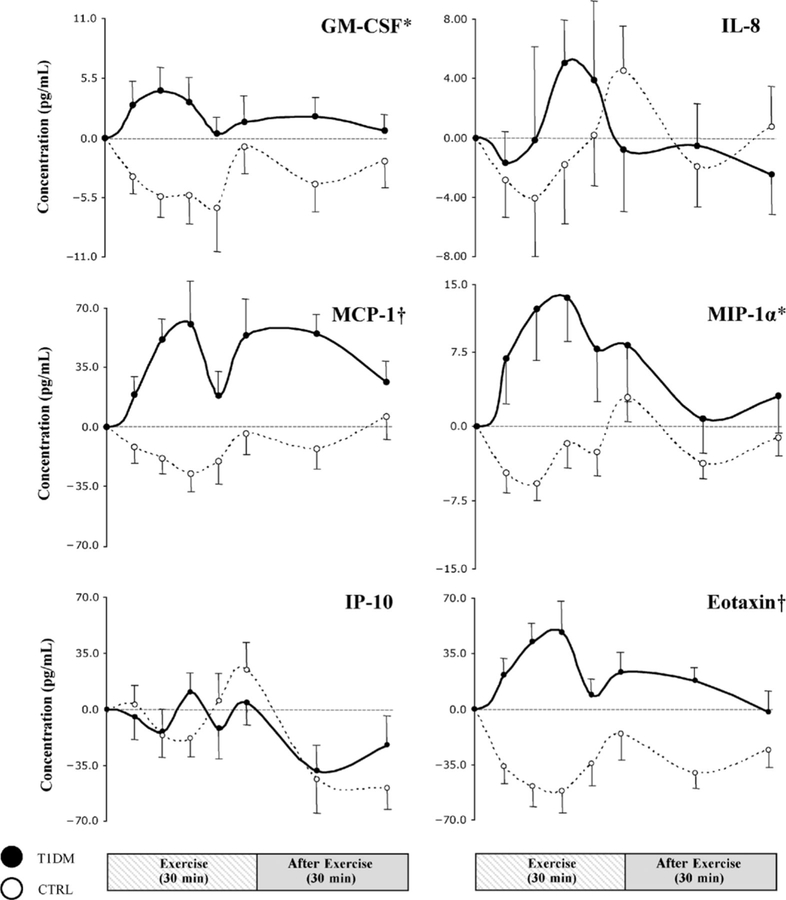
Our exploratory analysis of the kinetic profiles of a number of additional inflammatory factors revealed that the difference across groups in the pattern of exercise-induced changes, observed for IL-6, persisted in most of the remaining measured variables. Children with T1DM generally displayed greater and more rapidly activated responses (Figs. 3 and 4). In the control group, the initial response during the first 10 to 15 minutes of exercise was a generalized decreased in cytokine concentrations; this was followed by a rebound during the second half of exercise, such that by end-exercise, IL-1α, IL-17, IL-8, MIP-1α, and IP-10 had exceeded baseline values, whereas TNF-α, IL-4, IL-12p70, GM-CSF, MCP-1, and eotaxin remained below pre-exercise levels. In contrast, for T1DMs, most mediators did not display initial reductions (TNF-α, IL-1α, IL-4, IL-12p70, IL-17, GM-CSF, MCP-1, MIP-1α, and eotaxin), resulting in significantly greater levels indicated by AUCs during exercise for TNF-α, IL-4, IL-12p70, GM-CSF, MCP-1, MIP-1α, and eotaxin as compared with those for controls. Interferon-inducible protein 10 was the only variable with profiles graphically and statistically indistinguishable between the 2 groups.
FIGURE 4.
Striped and gray bars represent exercise (t = 0–30 minutes) and recovery (t = 30–60 minutes), respectively. Dots are means ± SE. *P< 0.05; † P< 0.001, type 1 diabetes mellitus (T1DM)s vs healthy controls (CTRLs) during exercise. GM-CSF indicates granulocyte monocyte colony-stimulating factor; IL, interleukin; IP, interferon-inducible protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein.
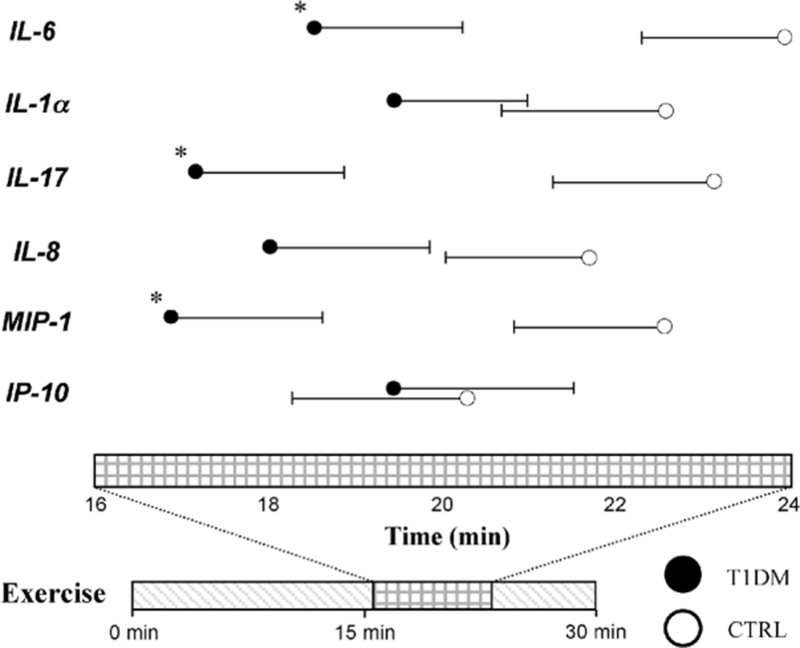
The striking difference between T1DM individuals and controls in exercise-induced changes in cytokine concentrations was not limited to the direction of these changes (decrease in controls, rapid increase in T1DM) but also extended to how rapidly these changes occurred. We defined a peak to be present for a given variable if its mean peak concentration was greater than baseline levels (note that the peak concentration for a given variable is the mean of individual peak values that may have occurred at different time points in different subjects and is therefore different from the highest group mean recorded at any given time point). By this definition, peaks were observed in both groups for IL-1α, IL-6, IL-17, IL-8, MIP-1, and IP-10; interestingly, for all 6 molecules, the peak was reached earlier in T1DMs than in controls (Fig. 5). Whereas the peaks for all 6 variables in T1DMs occurred before the earliest peak in controls, statistical significance in the time difference was only observed in IL-6, IL-17, and MIP-1α (P = 0.028, 0.023, and 0.026, respectively).
FIGURE 5.
Circles are mean times for cytokines to reach peaks, and bars are SE. Only inflammatory mediators that reach above baseline average value during exercise in Figures 2 and 3 are depicted with peaks. *P < 0.05. CTRL indicates healthy controls; IL, interleukin; IP, interferon-inducible protein; MIP, macrophage inflammatory protein; T1DM, type 1 diabetes mellitus.
Absolute Systemic Concentrations.
At baseline, systemic concentrations of TNF-α, IL-12p70, IL-17, GM-CSF, MCP-1, MIP-1α, IP-10, and eotaxin were significantly lower in patients with T1DM as compared with controls and, with the exception of IL-12p70 and IP-10, remained significant after Bonferroni correction (Table 2). These differences were attenuated at peak exercise (only IL-12p70, IL-17, and GM-CSF remaining significantly different across groups) and reappeared after 30 minutes of postexercise recovery. Conversely, IL-1α, IL-4, IL-6, and IL-8 were not different across groups throughout the study; interestingly, the first 3 of these cytokines were previously observed to be the most sensitive to prior hyperglycemic changes in children with T1DM.
TABLE 2.
Absolute Concentrations of Inflammatory Mediators for the 2 Experimental Groups
| Baseline | End-EXE |
30 min After Exercise |
|
|---|---|---|---|
| IL-6 | |||
| CTRL | 162.7 ± 23 | 168.1 ± 22 | 161.7 ± 21 |
| T1DM | 144.8 ± 38 | 152.7 ± 39 | 144.3 ± 37 |
| TNF-α | |||
| CTRL | 20.3 ± 4.3 | 18.4 ± 3.6 | 17.9 ± 3.7 |
| T1DM | 6.4 ± 1.4 | 8.0 ± 2.7 | 5.6 ± 1.4 |
| IL-1α | |||
| CTRL | 1105 ± 244 | 1178 ± 261 | 1081 ± 43 |
| T1DM | 557 ± 194 | 569 ± 199 | 535 ± 165 |
| IL-4 | |||
| CTRL | 1053 ± 193 | 996 ± 196 | 999 ± 184 |
| T1DM | 1384 ± 479 | 1438 ± 498 | 1383 ± 453 |
| IL-12p70 | |||
| CTRL | 65.9 ± 25.0 | 58.5 ± 21.0 | 51.6 ± 16.0 |
| T1DM | 7.9 ± 2.3 | 10.9 ± 2.7 | 8.4 ± 2.1 |
| IL-17 | |||
| CTRL | 48.6 ± 12.3 | 54.2 ± 13.2 | 45.0 ± 10.3 |
| T1DM | 6.2 ± 1.5 | 8.6 ± 2.4 | 5.4 ± 1.5 |
| GM-CSF | |||
| CTRL | 34.2 ± 6.6 | 33.3 ± 5.6 | 32.1 ± 5.0 |
| T1DM | 10.7 ± 2.8 | 12.2 ± 2.9 | 11.4 ± 2.8 |
| IL-8 | |||
| CTRL | 54.4 ± 7.8 | 58.9 ± 8.0 | 55.1 ± 6.9 |
| T1DM | 34.6 ± 10.0 | 33.8 ± 9.7 | 32.1 ± 8.5 |
| MCP-1 | |||
| CTRL | 170.3 ± 17 | 165.9 ± 15 | 176.0 ± 15 |
| T1DM | 103.4 ± 19 | 155.7 ± 37 | 124.0 ± 19 |
| MIP-1α | |||
| CTRL | 51.7 ± 6.1 | 54.8 ± 7.5 | 50.6 ± 6.0 |
| T1DM | 29.1 ± 4.7 | 38.0 ± 6.0 | 32.5 ± 4.9 |
| IP-10 | |||
| CTRL | 274.4 ± 26 | 298.5 ± 32 | 224.7 ± 24 |
| T1DM | 195.7 ± 27 | 199.9 ± 25 | 172.4 ± 19 |
| Eotaxin | |||
| CTRL | 237.0 ± 24 | 221.0 ± 28 | 210.8 ± 20 |
| T1DM | 108.4 ± 34 | 127.0 ± 34 | 99.0 ± 33 |
Data are means ± SE. Units are in picogram per milliliter.
CTRL indicates healthy controls; End-EXE, end-exercise; GM-CSF, granulocyte monocyte colony-stimulating factor; IL, interleukin; IP, interferon-inducible protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; T1DM, type 1 diabetes mellitus; TNF-α, tumor necrosis factor-α.
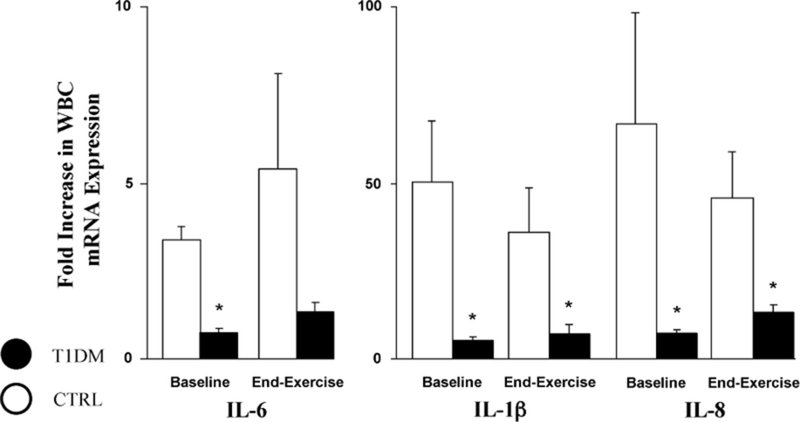
Leukocyte Gene Expression.
Circulating absolute WBC counts were similar across groups throughout the study (total WBC × 103: T1DMs, at 5.4 ± 0.2, 8.2 ± 0.3, and 5.1 ± 0.3 at baseline, end-exercise, and 30 minutes after exercise, respectively; controls, at 5.8 ± 0.3, 9.2 ± 0.5, and 5.6 ± 0.3 at baseline, end-exercise, and 30 minutes after exercise, respectively). In parallel, WBC subtypes, including neutrophils, lymphocytes, and monocytes, were also equivalent between T1DM and CTRLs. Therefore, the observed difference in systemic cytokine levels between groups was unlikely due to changes in leukocyte numbers but possibly to changes in secretory activities by leukocytes. When WBCs, collected from a subset of our T1DMs and control subjects both at rest and at end-exercise, underwent 4 hours of unstimulated ex vivo incubation (Fig. 6), the fold increase of mRNA levels for IL-1β, IL-6, and IL-8, was markedly and significantly reduced in T1DMs as compared with controls (IL-6: before, 0.7 ± 0.2 vs 3.4 ± 1.4; end, 1.3 ± 0.4 vs 5.4 ± 2.7; IL-1β: before, 5 ± 1 vs 50 ± 17; end, 7 ± 3 vs 36 ± 13; IL-8: before, 7 ± 1 vs 67 ± 32; end, 13 ± 2 vs 46 ± 13; interferon γ: before, 5 ± 1 vs 50 ± 17; end, 7 ± 3 vs 36 ± 13; Fas ligand: before, 5 ± 1 vs 50 ± 17; end, 7 ± 3 vs 36 ± 13); no difference was observed across groups in terms of mRNA expression of interferon γ and Fas ligand.
FIGURE 6.
Leukocytic mRNA productions of interleukin (IL)-6, IL-1β, and IL-8 after 4 hours of ex vivo incubation in healthy controls (CTRLs) and patients with type 1 diabetes mellitus (T1DM)s at baseline and after 30 minutes of intense and intermittent cycling. Significantly lower basal and end-exercise levels of gene expression for all 3 cytokines were observed in T1DMs. *P < 0.05 between CTRLs and T1DMs. WBC indicates white blood cell.
■ DISCUSSION
In this study, we report the kinetic profile of the proinflammatory cytokine IL-6 during and after exercise in a group of 21 children with T1DM and 21 healthy controls. Although baseline IL-6 values were similar across groups, exercise resulted in a significantly greater and faster IL-6 surge in T1DMs. We also performed an exploratory analysis of the kinetic profiles of 11 additional key immunomodulatory cytokines and chemokines. For most of these factors, children with T1DM displayed an exaggerated exercise response, similar to that observed for IL-6. Interestingly, this increased exercise response occurred despite a widespread (and somewhat unexpected) reduction in pre-exercise cytokine concentrations in the T1DM group.
In addition to its action as an acute-phase reactant and proinflammatory signaling protein, IL-6 is also known to exert a role in energy substrate regulation (glucose production and lipolysis) on the hepatic and adipose tissues.14–16 Indeed, whereas part of the systemic IL-6 derives from leukocyte secretion, the majority is of skeletal muscle origin, especially during exercise and skeletal muscular contractions.17,18 Evidence of IL-6 plasma elevations after various forms of physical activity have, in fact, been consistently documented in both adults and children.1,7,19,20 In contrast with the acute proinflammatory surge associated with individual exercise bouts, the long-term effects of exercise are generally considered anti-inflammatory,21 suggesting that exercise-induced IL-6 elevation must be part of a more complex adaptive mechanism. Several cytokines with predominantly antiinflammatory activity, such as IL-1 receptor antagonist, soluble TNF-receptor, and IL-10, have been shown to increase after long-term exercise training,22 resulting in improved overall anti-inflammatory status, as indicated by reduced circulating C-reactive protein,23 which is an independent prognostic marker for cardiovascular disease and mortality.24,25 Although the relation between short- and long-term effects of physical activity on immunomodulatory factors is still incompletely understood, growing evidence suggests that a dysregulated acute response of IL-6 and other key cytokines to exercise could conceivably disrupt its anti-inflammatory benefits. This may indeed be the case for the children with T1DM from this study in whom, despite blood glucose normalization for at least 90 minutes before exercise, plasma IL-6 increased immediately during cycling and reached a significantly higher and earlier peak than that in healthy control children. However, in healthy controls, IL-6 had actually decreased below baseline for the first half of exercise, indicating that the physiological exercise response may include protective mechanisms aimed at reducing an early proinflammatory activation; these mechanisms seemed disrupted for children with T1DM.
Because IL-6 is part of a complex interacting network of proinflammatory and anti-inflammatory factors that are often simultaneously activated in response to stress, we also ran an exploratory analysis of the kinetic behavior of 11 additional key immunomodulatory factors. The choice of the individual molecules included in this panel was driven by the general purpose of obtaining a good representation of different classes of mediators (proinflammatory and anti-inflammatory cytokines, chemokines, colony-stimulating factors) while limited by the inability of the used technique at the time of assay to reliably measure certain factors (IL-1β, for instance, a natural choice for this type of panel, could not be included for this reason). The tested molecules in children with T1DM maintained the general pattern of elevated and accelerated proinflammatory activation when compared with controls, as similarly observed for IL-6. This general pattern, however, differed across variables. Among the molecules with predominant proinflammatory action, for instance, the cytokines IL-1α and IL-17,26,27 the neutrophil chemotactic factor IL-828 and the chemokine MIP-1α29 resembled the IL-6 profile, with a clearly discernible exercise-induced peak in both subjects groups, although generally smaller and occurring later in controls than in T1DM. On the other hand, TNF-α (a “classic” proinflammatory cytokine),30 GM-CSF (polypeptide involved in leukocyte differentiation and proliferation),31 and MCP-1 and eotaxin (both of which are proinflammatory chemokines)28,32 displayed a diametrically opposed profile in the 2 groups, with an early and sustained increase in T1DM and an overall suppression in controls throughout exercise and recovery. Similarly, IL-4 and IL-12p70 profiles were also markedly different across groups during exercise, but during recovery in both groups, values reverted to baseline levels. Interestingly, IP-10 (also known as the proinflammatory chemokine CXCL10, which is implicated in the evolution of autoimmune diseases including T1DM33,34) was the only studied molecule with virtually identical kinetic profiles between the 2 groups. Although prior work to date has failed to clearly establish a correlation between physical activity and the progression of autoimmune disease,35,36 the long-term cardioprotective effects of exercise are empirically well-documented in chronic inflammatory conditions such as systemic lupus erythematosus, rheumatoid arthritis, and diabetes. Furthermore, beneficial effects of exercise relevant to T1DM, in addition to decreasing microvascular and macrovascular diseases, include the maximization of physical growth,37 preservation of overall endothelial integrity, improved glycemic and lipid control,38 lowering of overall concenrations of proinflammatory factors,39 and reduction of morbidity and mortality from various vascular diseases.40 The physiological significance of our observations in the context of T1DM, therefore, resides in the notion that the many benefits derived from exercise may vary broadly based on several factors (including altered inflammatory responses) and that a necessary prerequisite to optimize these beneficial effects is a complete understanding of all underlying molecular mechanisms. Along this line of thought, our data confirm the presence of a complex regulation of the inflammatory response to exercise, in which clusters of functionally related factors undergo common alteration patterns in T1DM. Because these alterations may contribute to the onset/progression of diabetic cardiovascular complications, their correction represents a logical target for optimization of exercise effects through a careful design of appropriate individually adjusted physical activity protocols.
Because at least a fraction of circulating cytokines arise from WBC secretion, altered exercise-induced leukocytosis could be held responsible for part of the observed exercise-induced differences across experimental groups.41 In our study, however, the changes in total WBC, WBC subtype percentages, and hematocrit were identical across groups, indicating that differences in the circulating mass of activated leukocytes and fluid shifts likely had no effect on the reported observations. On the other hand, because resting pre-exercise systemic concentrations of most analyzed cytokines were lower in the T1DM group, we hypothesized that constitutive WBC mRNA expression of key immunomodulatory factors could also be reduced in this group, a notion confirmed by our finding of markedly lower WBC gene expressions of IL-1β, IL-6, and IL-8. We believe that this dichotomy between exaggerated inflammatory responses to stress and reduced baseline inflammatory status of the diabetic child represents an interesting parallel with the paradoxical coexistence of increased chronic inflammation and impaired immune competence (delayed wound healing and increase susceptibility to infections) in diabetes.
An intriguing observation in this study was that, in children with T1DM, despite their exaggerated inflammatory exercise response, baseline concentrations of several inflammatory mediators were significantly lower than those in controls. We have previously reported that, in children with T1DM exposed to a similar exercise challenge, both pre-exercise and postexercise IL-6 varied based on prior glycemia: concentrations were similar to controls if hyperglycemia had not occurred for several hours before exercising but were markedly elevated with hyperglycemia 3 to 4 hours before exercise. These findings are consistent with previous reports of elevated acute-phase reactants during hyperglycemia in T1DM, type 2 diabetes, and healthy controls.6,42–44 Although whether diabetes per se affects basal levels of inflammatory markers is unclear,45 this body of work supports the concept that hyperglycemic fluctuations are responsible for transient—albeit possibly prolonged—elevations of proinflammatory cytokines (indeed, we have recently reported that IL-1α, IL-4, and IL-6 were elevated in a group of children with T1DM during hyperglycemia and remained elevated for several hours after hyperglycemia was corrected6). It could therefore be expected that with ideal glycemic control, cytokine levels should not differ from healthy controls, this, at least, with insulin dosages equivalent to physiological endogenous insulin production. The reality of diabetic management, however, indicates that circulating insulin concentrations are consistently higher in well-controlled T1DM than in healthy controls.46 Because insulin has well-established anti-inflammatory effects47–49; suppression of several inflammatory mediators during these conditions may not be surprising. Indeed, in our study, patients displayed insulin concentrations approximately 50% greater than those in controls, despite careful adjustments of insulin infusion at the minimal levels necessary to sustain euglycemia. Although it may be argued that this represents an iatrogenic effect rather an intrinsic characteristic of diabetes, it reflects the real-life status of a well-controlled subject with T1DM (very much like the reported greater incidence of hypoglycemia with tight glycemic control) and may have an impact on overall immunologic competence with conceivable contributions to delayed wound healing and reduced defense capability against certain types of infection. Along the same line of thought, it may be argued that the extremely tight glycemic control obtained during the study may mask the real average inflammatory status present in T1DM, with unrealistically low baseline concentrations of inflammatory markers. The fact that despite this acute, possibly insulin-induced, suppression of inflammation, the actual proinflammatory response to exercise still seems markedly enhanced in T1DM, which further strengthens, in our opinion, the significance of our results.
It should be noted that the sex composition was somewhat different across our experimental groups, with a higher (but not significantly) male-female ratio in our controls. We believe that this difference have affected only minimally, if at all, our main results because the different kinetic patterns of exercise responses were very consistent within each group, independent of sex or fitness level. However, we acknowledge that this difference may have been, in part, responsible for the observed element of random variability in individual cytokine levels within groups. In general, the understanding of gender effects on cytokine responses to exercise (or cytokine concentrations in general) in children is still far from complete. A few investigators have noted that gender and menstruation in young adult humans have inconsequential influences on several widely recognized inflammatory mediators (IL-6, TNF-α, IL-1, and C-reactive protein).50–52 In addition, reports on the influence of V̇O2max on IL-6 is conflicting; some have noted an inverse relationship between the two, whereas others have observed no interaction.53 As each of these factors may alter cytokines and exercise responses in an apparently complex manner, future studies powered to specifically examine each concern will be needed to define their effects.
In terms of the assays used in this study (ie, the multiplex conjugated beads technique), a note of caution should be issued in data interpretation, especially concerning absolute values of reported variables. This technique has numerous unique characteristics (very small plasma volume requirement, low cost, and very low per variable assay time) that render it especially suitable for studies requiring measurement of numerous variables over multiple time points with small sample volumes but is also known to provide absolute concentration values markedly higher than previously reported with different techniques, including enzyme-linked immunosorbent assay. We therefore encourage the reader to interpret our results in terms of kinetic changes of reported variables over time, understanding that comparison of absolute concentrations with previously published work may not be applicable.
In conclusion, our results indicate that, in a group of well-controlled children with T1DM, the IL-6 response to an intense exercise challenge was significantly amplified compared with age-matched healthy controls; a similarly amplified response was also observed in most several other immunomodulatory factors. This occurred despite the detection of relatively lower basal concentrations of most measured variables in children with T1DM that was possibly due to the anti-inflammatory effect of exogenous insulin administration. Given the critical role played by proinflammatory and anti-inflammatory mediators in the onset and progression of diabetic vascular complications, our results emphasize the necessity to gain a better understanding of all molecular processes associated with physical activity in type 1 diabetes.
■ ACKNOWLEDGMENT
The authors thank the staff of all branches of the UCI ICTS.
This research was supported by National Institutes of Health Grants M01-RR00827-28 and K-23 RR018661-01 and by the Juvenile Diabetes Research Foundation Grant 11-2003-332.
REFERENCES
- 1.Nemet D, Oh Y, Kim H-S, et al. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002;110:681–689. [DOI] [PubMed] [Google Scholar]
- 2.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DM, Nemet D, Galassetti P. Exercise, stress, and inflammation in the growing child: from the bench to the playground. Curr Opin Pediatr. 2004;16:286–292. [DOI] [PubMed] [Google Scholar]
- 4.Lindmark E, Diderholm E, Wallentin L, et al. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–2113. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DM, Radom-Aizik S, Schwindt C, et al. Dangerous exercise: lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol. 2007;103: 700–709. [DOI] [PubMed] [Google Scholar]
- 6.Rosa JS, Flores RL, Oliver SR, et al. Sustained IL-1α, IL-4, and IL-6 Elevations following correction of hyperglycemia in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:9–16. [DOI] [PubMed] [Google Scholar]
- 7.Galassetti PR, Iwanaga K, Pontello AM, et al. Effect of prior hyperglycemia on IL-6 responses to exercise in children with type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290:E833–E839. [DOI] [PubMed] [Google Scholar]
- 8.Rosa JS, Oliver SR, Flores RL, et al. Kinetic profiles of 18 systemic pro- and anti-inflammatory mediators during and following exercise in children. J Pediatr Endocrinol Metab. 2007;20:1293–1305. [DOI] [PubMed] [Google Scholar]
- 9.Petersen AC, Crockett L, Richards M, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DM, Weiler-Ravell D, Whipp BJ, et al. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–634. [DOI] [PubMed] [Google Scholar]
- 11.duPont NC, Wang K, Wadhwa PD, et al. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsuhashi M, Cooper A, Ogura M, et al. Oligonucleotide probe design—a new approach. Nature. 1994;367:759–761. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuhashi M, Tomozawa S, Endo K, et al. Quantification of mRNA in whole blood by assessing recovery of RNA and efficiency of cDNA synthesis. Clin Chem. 2006;52: 634–642. [DOI] [PubMed] [Google Scholar]
- 14.Steensberg A, van Hall G, Osada T, et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Febbraio MA, Hiscock N, Sacchetti M, et al. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53:1643–1648. [DOI] [PubMed] [Google Scholar]
- 16.Lyngso D, Simonsen L, Bulow J. Interleukin-6 production in human subcutaneous abdominal adipose tissue: the effect of exercise. J Physiol. 2002;543:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen BK, Akerstrom TCA, Nielsen AR, et al. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. [DOI] [PubMed] [Google Scholar]
- 19.Drenth JP, Van Uum SH, Van Deuren M, et al. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. J Appl Physiol. 1995;79:1497–1503. [DOI] [PubMed] [Google Scholar]
- 20.Galassetti PR, Iwanaga K, Crisostomo M, et al. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatric Diabetes. 2006;7:16–24. [DOI] [PubMed] [Google Scholar]
- 21.Das UN. Anti-inflammatory nature of exercise. Nutrition. 2004;20:323–326. [DOI] [PubMed] [Google Scholar]
- 22.Steensberg A, Fischer CP, Keller C, et al. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. [DOI] [PubMed] [Google Scholar]
- 23.Lakka TA, Lakka H-M, Rankinen T, et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur Heart J. 2005;26:2018–2025. [DOI] [PubMed] [Google Scholar]
- 24.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
- 25.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Int Med. 2002;252:283–294. [DOI] [PubMed] [Google Scholar]
- 26.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. [DOI] [PubMed] [Google Scholar]
- 27.Koenders MI, Joosten LAB, van den Berg WB. Potential new targets in arthritis therapy: interleukin (IL)–17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis. 2006;65:iii29–iii33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. [DOI] [PubMed] [Google Scholar]
- 29.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. [DOI] [PubMed] [Google Scholar]
- 30.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. [DOI] [PubMed] [Google Scholar]
- 31.Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol. 2005;25:405. [DOI] [PubMed] [Google Scholar]
- 32.Cook EB, Stahl JL, Graziano FM. Eotaxin: what we know, and what we would like to know. Allergy Asthma Proc. 1998;19:253–255. [DOI] [PubMed] [Google Scholar]
- 33.Jha P, Sohn J-H, Xu Q, et al. The complement system plays a critical role in the development of experimental autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2006;47:1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christen U, Von Herrath MG. IP-10 and type 1 diabetes: a question of time and location. Autoimmunity. 2004;37:273. [PubMed] [Google Scholar]
- 35.Ayan C, Martin V. Systemic lupus erythematosus and exercise. Lupus. 2007;16:5. [DOI] [PubMed] [Google Scholar]
- 36.de Jong Z, Vlieland TP. Safety of exercise in patients with rheumatoid arthritis. Curr Opin Rheum. 2005;17:177. [DOI] [PubMed] [Google Scholar]
- 37.Giannini C Role of physical exercise in children and adolescents with diabetes mellitus. J Pediatr Endocrinol. 2007;20:173. [DOI] [PubMed] [Google Scholar]
- 38.Valerio G, Spagnuolo MI, Lombardi F, et al. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2007;17:376–382. [DOI] [PubMed] [Google Scholar]
- 39.Geffken DF, Cushman M, Burke GL, et al. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153: 242–250. [DOI] [PubMed] [Google Scholar]
- 40.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. [DOI] [PubMed] [Google Scholar]
- 41.Nagatomi R The implication of alterations in leukocyte subset counts on immune function. Exerc Immunol Rev. 2006;12:54. [PubMed] [Google Scholar]
- 42.Targher G, Zenari L, Bertolini L, et al. Elevated levels of interleukin-6 in young adults with type 1 diabetes without clinical evidence of microvascular and macrovascular complications. Diabetes Care. 2001;24:956–957. [DOI] [PubMed] [Google Scholar]
- 43.de Rekeneire N, Peila R, Ding J, et al. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. 2006;29:1902–1908. [DOI] [PubMed] [Google Scholar]
- 44.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 45.Shankar A, Klein R, Klein BEK, et al. Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol. 2007;166:393–402. [DOI] [PubMed] [Google Scholar]
- 46.Forst T, Forst S, Strunk K, et al. Impact of insulin on microvascular blood flow and endothelial cell function in the postprandial state in patients with type 1 diabetes. J Diabetes Complicat. 2005;19:128–132. [DOI] [PubMed] [Google Scholar]
- 47.Aljada A, Ghanim H, Saadeh R, et al. Insulin inhibits NF-kB and MCP-1 expression in human aortic endothelial cells. J Clin Endocrinol Metab. 2001;86:450–453. [DOI] [PubMed] [Google Scholar]
- 48.Hansen TK, Thiel S, Wouters PJ, et al. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–1088. [DOI] [PubMed] [Google Scholar]
- 49.Viardot A, Grey ST, Mackay F, et al. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells towards a T-helper 2 phenotype. Endocrinology. 2006;148:346–353. [DOI] [PubMed] [Google Scholar]
- 50.Ertan T, Keskek M, Kilic M, et al. Effects of gender difference in early cytokine levels in trauma patients. Bratislavské Lékarské Listy. 2007;108:128–132. [PubMed] [Google Scholar]
- 51.Chiu KM, Arnaud CD, Ju J, et al. Correlation of estradiol, parathyroid hormone, interleukin-6, and soluble interleukin-6 receptor during the normal menstrual cycle. Bone. 2000;26:79–85. [DOI] [PubMed] [Google Scholar]
- 52.Makinoda S, Mikuni M, Sogame M, et al. Erythropoietin, granulocyte-colony stimulating factor, interleukin-1b and interleukin-6 during the normal menstrual cycle. Int J Gynecol Obstet. 1996;55:265–271. [DOI] [PubMed] [Google Scholar]
- 53.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]