Abstract
Perfluorinated alkylate substances (PFASs) are highly persistent and may cause immunotoxic effects. PFAS-associated attenuated antibody responses to childhood vaccines may be affected by PFAS exposures during infancy, where breastfeeding adds to PFAS exposures. Of 490 members of a Faroese birth cohort, 275 and 349 participated in clinical examinations and provided blood samples at ages 18 months and 5 years. PFAS concentrations were measured at birth and at the clinical examinations. Using information on duration of breastfeeding, serum-PFAS concentration profiles during infancy were estimated. As outcomes, serum concentrations of antibodies against tetanus and diphtheria vaccines were determined at age 5. Data from a previous cohort born eight years earlier were available for pooled analyses. Prenatal exposure showed inverse associations with the antibody concentrations five years later, with decreases by up to about 20% for each two-fold higher exposure, while associations for serum concentrations at ages 18 months and 5 years were weaker. Modeling of serum-PFAS concentration showed levels for age 18 months that were similar to those measured. Concentrations estimated for ages 3 and 6 months showed the strongest inverse associations with antibody concentrations at age 5 years, particularly for tetanus. Joint analyses showed statistically significant decreases in tetanus antibody concentrations by 19% to 29% at age 5 for each doubling of the PFAS exposure in early infancy. These findings support the notion that the developing adaptive immune system is particularly vulnerable to immunotoxicity during infancy. This vulnerability appears to be the greatest during the first 6 months after birth, where PFAS exposures are affected by breast-feeding.
Keywords: Antibodies, developmental toxicity, immune functions, infancy, perfluorinated compounds, prospective study, vaccination
Introduction
Industrial chemicals are not routinely tested for immunotoxicity, but recent evidence suggests that some environmental chemicals may harm immune functions, perhaps in particular during early development of the adaptive immune system (Dietert and Dewitt 2010; Dietert 2014). Among recently confirmed immunotoxicants, the perfluorinated alkylate substances (PFASs) have been widely applied for a multitude of purposes, and due to their persistence, human PFAS exposures now occur globally (Lindstrom et al. 2011). Women eliminate PFASs via lactation (Mondal et al. 2014), and a child’s exposure in infancy is strongly affected by transfer via human milk (Liu et al. 2011; Mogensen et al. 2015b). With human milk being a main source of exposure early postnatally, serum-PFAS concentrations in infancy could potentially be estimated from the exposure level at birth and the duration of breastfeeding.
Experimental evidence so far supports the possibility that early development of the adaptive immune system may be particularly vulnerable to PFAS exposure (Dietert 2014), but the U.S. National Toxicology Program recently concluded that specific immune cell vulnerabilities and their dependence upon developmental stages are unclear so far (National Toxicology Program 2016). Unfortunately, epidemiology studies of toxicant exposures in infancy are problematic due to the difficulty in obtaining blood samples from small children.
In addition, immune function tests are not easily applied in epidemiological studies of small children. However, serum-antibody concentrations induced by routine immunizations have been recommended for this purpose (van Loveren et al. 1999) due to the advantage that a vaccination constitutes a well-defined stimulation of the adaptive immune system (Ljungman 2013). The first vaccinations in infancy stimulate the virgin immune system, and subsequent re-vaccinations trigger the primary repertoire and response as well as secondary responses (Capua et al. 2013). The immune response against specific protein antigens (such as tetanus and diphtheria toxoids) thereby reflects a variety of functions, such as antigen presentation, antigen processing, T-cell help, B-cell activation, germinal center B-cell reactivation and B-cell maturation (Schatz et al. 1998).
Recent studies relied on serum concentrations of antibodies specifically directed against vaccines that were administered in infancy (Grandjean et al. 2012; Granum et al. 2013), but none of them attempted to link the PFAS-associated decreases in antibody concentrations to past exposures during infancy. Because a sizable proportion of fully-vaccinated children may have vaccine responses that provide incomplete protection against the disease (Grandjean et al. 2012), PFAS immunotoxicity may be of substantial public health relevance in regard to the specific vaccine responses and possibly also in regard to other immune functions.
The present study extends earlier reports on inverse associations between serum concentrations of PFASs and vaccine-specific antibodies in children (Grandjean et al. 2012; Mogensen et al. 2015a), now in a new prospective birth cohort in the Faroe Islands (Cohort 5, born in 2007–2009). Serum samples from ages 18 months and five years were analyzed for PFASs, and serum-PFAS concentrations during infancy were modeled. To increase the statistical power of the study, joint statistical analyses were conducted with a previous birth cohort (Grandjean et al. 2012), where PFAS exposures were higher.
Methods
Study population
The Faroese Cohort 5 of 490 children was recruited from births at the National Hospital in Tórshavn, Faroe Islands, during 2007–2009 (Kim et al. 2014; Timmermann et al. 2017). A maternal serum sample was collected about two weeks after the expected term date. As part of the government-supported health care system, Faroese children receive vaccinations against diphtheria and tetanus at ages 3 months, 5 months, and 12 months, and a booster at age 5 years. This way, the same amount of vaccine and associated alum adjuvant were applied at the same ages. In this population, PFAS exposures are associated with marine food contamination (Weihe et al. 2008), and these substances do not occur as contaminants of local drinking water (Eriksson et al. 2013).
All cohort members were invited for clinical follow-up at ages 18 months and 5 years (before the age-5 booster), where maternal interview, physical examination of the child, and blood sampling took place. Detailed vaccination records were available, as were obstetrics information, and questionnaire information on duration of breastfeeding, past medical history and current health status. In order to increase the statistical power, this study included data from the earlier Cohort 3 (born 1997–2000), which had formed the basis of previous reports (Grandjean et al. 2012; Grandjean and Budtz-Jorgensen 2013; Mogensen et al. 2015a). Apart from being about eight years apart and therefore differing in exposure levels and profiles (median concentrations of PFHxS, PFOS, PFOA, and perfluorodecanoate (PFDA) were lower in Cohort 5, while the concentrations of perfluorononanoate (PFNA) were slightly higher compared to Cohort 3), the two cohorts are highly similar, and methods were virtually identical (Cohort 3 sampled maternal blood in late pregnancy) (Dalgard et al. 2016; Timmermann et al. 2017).
The study protocol was approved by the Faroese ethical review committee and by the institutional review board at Harvard T.H. Chan School of Public Health. Written informed consent was obtained from all mothers.
Immunotoxicant exposures
The PFAS concentrations were measured in all serum samples by online solid-phase extraction followed by high-pressure liquid chromatography with tandem mass spectrometry (Haug et al. 2009; Grandjean et al. 2012). The analyses quantified the five major PFAS, i.e., perfluorohexanesulfonic acid (PFHxS), PFOA, PFOS, PFNA, and PFDA. Within-batch and between-batch imprecision levels (coefficients of variation) for all five PFASs were < 3% and 5–6%, respectively, in agreement with excellent results obtained in regular comparisons organized by the German Society of Occupational Medicine.
As developmental exposure to polychlorinated biphenyls (PCBs) can also reduce vaccine responses (Heilmann et al. 2010), this study also measured maternal serum-PCB concen-trations and used the sum of the three major congeners as an indicator of the total PCB exposure (Grandjean et al. 2012).
Vaccine antibodies
Focus was maintained on tetanus and diphtheria, as these toxoid vaccines trigger immune system responses involving both T- and B-cells (Schatz et al. 1998). Serum concentrations of IgG antibodies were again measured by the Danish vaccine producer (Statens Serum Institut [SSI], Copenhagen, Denmark) using an enzyme-linked immunosorbent assay for tetanus (Hendriksen et al. 1988), while diphtheria antibodies were measured using a standard Vero cell-based neutralization assay employing two-fold dilutions (Miyamura et al. 1974). For both assays, calibration was performed using international and local standard antitoxins. We used the cut-off limit of 0.1 IU/mL for both vaccine antibodies, as recommended by the SSI, to determine whether antibody concentrations could be considered protective.
Statistical methods
Due to skewed distributions, antibody concentrations and serum-PFAS concentrations were log-transformed (base 2) before entering the models. We first applied standard multiple regression analyses, where log-transformed PFAS concentrations at different ages were included as independent variables one at a time. More advanced models relied on structural equations (Mogensen et al. 2015a). All analyses were adjusted for age and sex. Joint models were developed for the combined data that also included the data from Cohort 3 (Grandjean et al. 2012), where cohort identity was included as an additional covariate that was allowed to interact with sex, age and PFAS concentrations. Effects were expressed as the relative change in the antibody concentration associated with a doubling in the PFAS concentration. In sensitivity analyses, it was tested whether cesarean section (N = 63) played a role, and likewise possible effects of prenatal PCB exposure and duration of breastfeeding were considered.
Because previous results on Cohort 3 showed that PFAS concentrations in childhood depended on the duration of breastfeeding (in months) (Mogensen et al. 2015b), early postnatal serum-PFAS concentrations were modeled from their dependence on the duration of breastfeeding in a piece-wise linear model:
| (1) |
where log PFASi,a is the log-transformed serum concentration of child i at age a, while exclusivei,a, partiali,a, and nomilki,a indicate the number of months that the child was exclusively breastfed, partially breastfed, and not breastfed at all by age a. The variable Ui accounts for within-child correlation, i.e., the fact that a child above the mean at one time-point will also tend to be higher at other time points. The last term εi,a is a random measurement error. So, at birth, the log transformed serum concentration is given by the intercept (μ + Ui), which is allowed to be specific for each child. During the period of exclusive breastfeeding, the log-transformed concentration is assumed to change by a slope of α. Likewise, during partial breastfeeding the slope is β, and after weaning, the slope is γ. This model was fitted to the data from the two cohorts separately, and a joint model was then fitted under the assumption that breastfeeding effects were homogeneous while allowing the serum-PFAS concentration (μ) to depend on cohort. This part of the model allows, in principle, a prediction of the serum-PFAS concentration at any given age, based on the breastfeeding history up to that age. Estimated concentrations for age 18 months were then compared with measured serum concentrations at that age.
A second part of the structural equation model links the exposure to the vaccine antibody concentration at age 5 using a linear regression similar to the standard models used for observed exposures, i.e.,
| (2) |
where age60i is the child’s age (months) at the 5-year examination. In this model, we estimated the effect of a doubling of the exposure at ages 3, 6, and 12 months. As in the regression analyses, the calculations allowed for differences between cohorts by allowing the intercept and the effects of the covariates (age and sex) to depend on cohort. We focused on the two major PFASs that showed the strongest associations for prenatal exposures.
The calculations were carried out for the two cohorts separately and jointly. As maternal serum in Cohort 5 was not obtained at childbirth, sensitivity analyses were carried out to assess whether the number of days between parturition and blood sampling for PFAS analysis affected the estimated relation between breastfeeding and the child’s PFAS-concentrations. This was done by changing model (1) for the concentration at birth (a = 0) so that it also depended on the interval (days) between childbirth and blood sampling, as well as a covariate indicating whether the child was breastfed, and an interaction term. This way, allowance was made for the possibility that maternal serum-PFAS concentrations measured post parturition may have been affected by changes in distribution volume or transfer of PFASs from mother to child via lactation. The algorithm also allowed for a possible beneficial effect of breastfeeding on the child’s antibody concentration, as an additional covariate in model (2) indicated whether the child had been breastfed for at least 6 months.
Regression analyses were conducted using SAS version 9.4 (SAS Institute, Inc. Cary, NC), while structural equation models were fitted using the R software, the lava package.
Results
A total of 381 children (77% of 495 cohort members) participated in the age-5 examinations, and 370 (97%) of these had also participated at age 18 months. The characteristics of the 349 children who provided sufficient serum for analyses at age 5 and the 275 children who additionally provided sufficient serum for analyses at age 18 months are shown in Table 1.
Table 1.
Characteristics of children who contributed serum antibody concentrations at the two follow-up examinations in Cohort 5.
| Age 18 months (N=275) | Age 5 years (N=349) |
|
|---|---|---|
| n (%) | n (%) | |
| Sex, girls | 139 (50.6) | 173 (49.6) |
| Median (25; 75 percentile) | Median (25; 75 percentile) | |
| Age (months) | 18.5 (18.1; 18.9) | 60.4 (59.9; 60.9) |
| PFOS (ng/ml) | 7.1 (4.5; 10.0) | 4.7 (3.5; 6.3) |
| PFOA (ng/ml) | 2.8 (2.0; 4.5) | 2.2 (1.8; 2.8) |
| PFHxS (ng/ml) | 0.2 (0.1; 0.4) | 0.3 (0.2; 0.4) |
| PFNA (ng/ml) | 1.0 (0.6; 1.5) | 1.1 (0.8; 1.6) |
| PFDA (ng/ml) | 0.3 (0.2; 0.4) | 0.3 (0.2; 0.5) |
| Tetanus (IU/ml) | 1.5 (0.5; 3.0) | 0.1 (0.1; 0.3) |
| Diphteria (IU/ml) | 1.6 (0.8; 3.2) | 0.1 (0.1; 0.3) |
| Duration of exclusive breastfeeding (mo) | 5 (4; 6) | |
| Duration of mixed breastfeeding (mo) | 4 (2; 7) |
PFOS was by far the most prevalent PFAS in Cohort 5 with a median age-5 serum concentration of 4.7 ng/ml. PFOS and PFOA concentrations decreased from age 18 months to age 5 years (Table 1). Moderate correlations were observed between the concentrations at ages 18 months and 5 years of the same PFAS (r up to 0.7 after log transformation), though somewhat stronger between the different PFASs at each age (r up to 0.9 at age 18 months and up to 0.8 at age 5 years). The close correlations prevented meaningful adjustment for concomitant PFAS exposures. In regard to the antibody concentrations, they showed clear decreases from age 18 months to the (pre-booster) examination at age 5 years (Table 1). At age 5, 152 (44%) children had antibody concentrations lower than the protective level of 0.1 IU/mL for diphtheria and 126 (36%) for tetanus.
Multiple regression analyses showed inverse associations between serum-PFAS concentrations and antibody concentrations in most analyses (Table 2). In Cohort 5, the strongest inverse associations were found for tetanus, especially in regard to prenatal PFAS exposures. For all three sets of serum analyses, PFOA showed the strongest associations with lower tetanus antibody concentrations at age 5. Little difference occurred between the cohorts, and joint analyses again revealed clear inverse associations for PFOA. In regard to prenatal exposures, both PFOS and PFOA showed clear inverse associations with antibody levels, and inclusion of the interval between childbirth and maternal blood sampling in Cohort 5 did not affect the results. Cross-sectional comparisons in the joint analysis at age 5 showed inverse associations also for PFNA and PFDA, though in regard to diphtheria only. The PCB concentration in maternal pregnancy serum correlated poorly with the child’s serum-PFAS concentrations (r between 0.0 and 0.3), similar to previous results (Grandjean et al. 2012), and adjustment for developmental PCB exposure had no appreciable effect on the associations for the PFASs. Likewise, adjustment for cesarean section did not substantially affect the results.
Table 2.
Change (in percent) of the pre-booster serum-antibody concentrations at age 5 years associated with a doubling of the serum concentration of major PFASs at three different times of examination.
| Cohort 5 | Cohort 3 | Joint | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Change | 95% Cl | p | Change | 95% Cl | p | Change | 95% Cl | p | |
| Tetanus | |||||||||
| At birth | |||||||||
| PFOS | −10.84 | −28.34; 10.94 | 0.30 | −10.09 | −31.78; 18.51 | 0.45 | −10.55 | −24.63; 6.16 | 0.20 |
| PFOA | −22.25 | −35.25; −6.63 | 0.007 | −10.46 | −28.04; 11.41 | 0.32 | −17.59 | −28.38; −5.17 | 0.007 |
| PFHxS | −11.31 | −21.72; 0.49 | 0.060 | −6.30 | −15.05; 3.34 | 0.19 | −8.24 | −15.05; −0.89 | 0.029 |
| PFNA | −7.11 | −26.59; 17.53 | 0.54 | 11.16 | −8.46; 34.98 | 0.29 | 3.36 | −11.02; 20.07 | 0.67 |
| PFDA | −8.40 | −26.27; 13.79 | 0.43 | −2.45 | −18.39; 16.61 | 0.79 | −4.90 | −17.14; 9.15 | 0.47 |
| 18 months | |||||||||
| PFOS | −7.027 | −21.63; 10.30 | 0.40 | −8.05 | −55.26; 89.01 | 0.82 | −7.08 | −21.29; 9.70 | 0.39 |
| PFOA | −16.31 | −29.04; −1.31 | 0.034 | −19.24 | −59.75; 62.05 | 0.55 | −16.47 | −28.84; −1.96 | 0.028 |
| PFHxS | −2.616 | −10.08; 5.47 | 0.51 | −5.18 | −51.71; 86.19 | 0.88 | −2.65 | −10.05; 5.36 | 0.50 |
| PFNA | −6.981 | −21.10; 9.67 | 0.39 | −33.79 | −64.36; 23.01 | 0.19 | −9.04 | −22.43; 6.65 | 0.24 |
| PFDA | −5.780 | −23.56; 16.13 | 0.58 | −14.47 | −56.88; 69.66 | 0.65 | −6.55 | −23.47; 14.09 | 0.50 |
| 60 months | |||||||||
| PFOS | −9.076 | −28.10; 14.98 | 0.43 | −11.86 | −29.79; 10.65 | 0.28 | −10.52 | −24.00; 5.35 | 0.18 |
| PFOA | −25.26 | −42.63; −2.64 | 0.031 | −13.28 | −31.34; 9.54 | 0.23 | −18.75 | −31.79; −3.21 | 0.020 |
| PFHxS | −4.432 | −21.26; 15.99 | 0.65 | −6.29 | −17.45; 6.38 | 0.32 | −5.74 | −15.22; 4.81 | 0.27 |
| PFNA | −10.31 | −24.39; 6.40 | 0.21 | −5.87 | −21.67; 13.12 | 0.52 | −8.28 | −19.06; 3.940 | 0.18 |
| PFDA | −1.756 | −16.73; 15.91 | 0.83 | −13.55 | −26.18; 1.24 | 0.071 | −8.11 | −18.03; 3.01 | 0.15 |
| Diphtheria | |||||||||
| At birth | |||||||||
| PFOS | −14.00 | −31.59; 8.11 | 0.20 | −38.64 | −54.07; −18.04 | 0.001 | −24.47 | −36.90; −9.60 | 0.002 |
| PFOA | −18.93 | −33.16; −1.66 | 0.033 | −16.24 | −33.43; 5.40 | 0.13 | −17.82 | −29.11; −4.74 | 0.009 |
| PFHxS | −3.33 | −15.28; 10.30 | 0.61 | −6.41 | −15.62; 3.80 | 0.21 | −5.25 | −12.66; 2.79 | 0.19 |
| PFNA | 4.79 | −18.21; 34.27 | 0.71 | −14.82 | −30.55; 4.47 | 0.12 | −7.38 | −20.89; 8.43 | 0.34 |
| PFDA | −3.54 | −23.19; 21.15 | 0.76 | −21.73 | −35.09; −5.63 | 0.010 | −14.86 | −26.33; −1.60 | 0.029 |
| 18 months | |||||||||
| PFOS | 17.55 | −0.84; 39.34 | 0.062 | −21.21 | −61.54; 61.40 | 0.51 | 15.07 | −2.49; 35.79 | 0.096 |
| PFOA | 4.19 | −11.76; 23.02 | 0.63 | 30.49 | −35.31; 163.21 | 0.46 | 5.44 | −10.28; 23.92 | 0.52 |
| PFHxS | 7.85 | −0.38; 16.76 | 0.062 | −12.42 | −55.25; 71.43 | 0.70 | 7.54 | −0.60; 16.35 | 0.070 |
| PFNA | 24.43 | 5.72; 46.45 | 0.009 | −35.28 | −64.95; 19.48 | 0.16 | 19.18 | 1.72; 39.62 | 0.030 |
| PFDA | 25.52 | 2.00; 54.48 | 0.032 | −22.87 | −60.92; 52.24 | 0.45 | 20.42 | −1.29; 46.90 | 0.067 |
| 60 months | |||||||||
| PFOS | 17.17 | −8.66; 50.31 | 0.21 | −16.02 | −34.01; 6.87 | 0.16 | −1.34 | −17.05; 17.34 | 0.88 |
| PFOA | 18.31 | −10.72; 56.78 | 0.24 | −6.84 | −27.26; 19.30 | 0.57 | 3.38 | −14.16; 24.50 | 0.73 |
| PFHxS | 4.26 | −15.12; 28.08 | 0.69 | 4.98 | −8.25; 20.13 | 0.48 | 4.77 | −6.40; 17.26 | 0.42 |
| PFNA | −8.85 | −23.95; 9.25 | 0.32 | −17.70 | −32.30; 0.03 | 0.050 | −13.06 | −23.86; −0.72 | 0.039 |
| PFDA | −8.99 | −23.63; 8.46 | 0.29 | −15.96 | −28.91; −0.66 | 0.042 | −12.71 | −22.66; −1.48 | 0.028 |
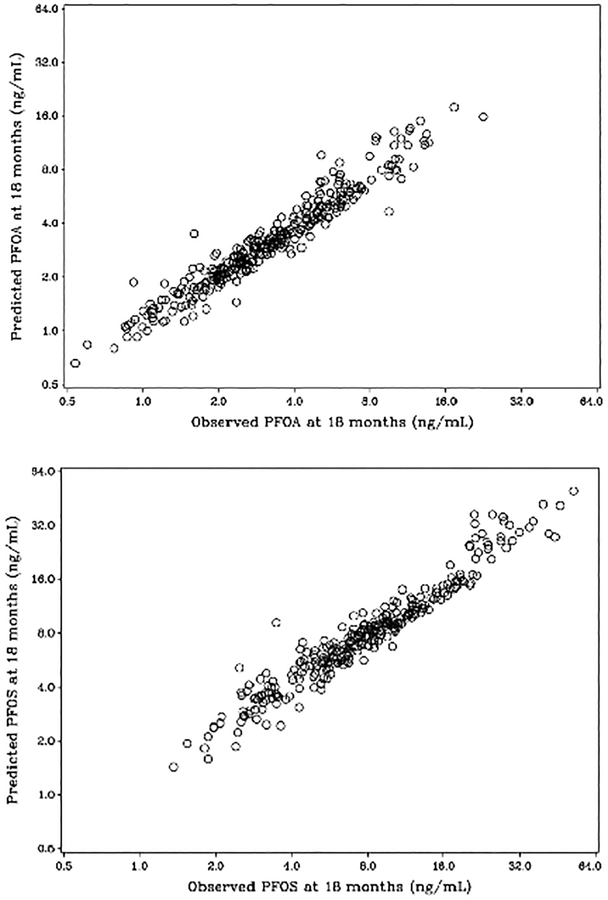
Duration of breastfeeding is closely associated with the child’s postnatal PFAS-concentrations (Table 3), even for PFHxS, but differences between cohorts 3 and 5 were minor. Serum-PFAS concentrations during infancy were then estimated on the basis of breastfeeding duration. Estimated serum-PFAS concentrations extended to age 18 months correlated closely with the measured serum concentrations in Cohort 5 at this age (Figure 1), thereby supporting the validity of the calculations. The predicted concentrations of PFOS and PFOA in early infancy tended to be inversely associated with the antibody concentrations at age 5 (Table 4), most clearly for PFOA in regard to tetanus, where a doubled exposure led to a decrease in the antibody concentration of about 30% at age 5 years. Tendencies were similar in the two cohorts, whether cohort-specific or based on the joint model that assumed identical relationships between breastfeeding and PFAS exposure in the two cohorts. The results presented in Table 4 are adjusted for duration of breastfeeding, but the direct effects of breastfeeding on antibody concentrations tended to be weakly negative, and the impact on the regression coefficients for the PFASs was minor.
Table 3.
Percentage change in serum-PFAS concentrations per month during exclusive breastfeeding, partial and none.
| Cohort 5 | Cohort 3 | Joint | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | nursing | change | 95% Cl | p | change | 95% Cl | p | change | 95% Cl | p |
| PFOS | exclusive | 14.76 | (13.13, 16.41) | <0.001 | 21.03 | (17.02, 25.19) | <0.001 | 15.74 | (14.24, 17.26) | <0.001 |
| mixed | 6.32 | (5.29, 7.37) | <0.001 | 3.26 | (0.32, 6.29) | 0.030 | 5.99 | (5.00, 6.98) | <0.001 | |
| none | −0.07 | (−0.50, 0.36) | 0.75 | −0.71 | (−1.77, 0.35) | 0.19 | −0.2 | (−0.60, 0.19) | 0.31 | |
| PFOA | exclusive | 16.69 | (14.82, 18.60) | <0.001 | 24.05 | (19.35, 28.93) | <0.001 | 17.78 | (16.05, 19.54) | <0.001 |
| mixed | 7.22 | (6.04, 8.41) | <0.001 | 5.08 | (1.58, 8.71) | 0.004 | 7.01 | (5.88, 8.14) | <0.001 | |
| none | 0.8 | (0.30, 1.29) | 0.002 | 1.31 | (0.04, 2.59) | 0.043 | 0.77 | (0.31, 1.23) | 0.001 | |
| PFHxS | exclusive | 23.91 | (19.83, 28.12) | <0.001 | −2.95 | (−8.46, 2.88) | 0.31 | 20.56 | (16.68, 24.56) | <0.001 |
| mixed | 2.25 | (−0.07, 4.61) | 0.057 | −11.54 | (−16.72, −6.04) | <0.001 | 2.1 | (−0.19, 4.45) | 0.073 | |
| none | −7.91 | (−8.83, −6.98) | <0.001 | −16.82 | (−18.70, −14.90) | <0.001 | −7.48 | (−8.38, −6.56) | <0.001 | |
| PFNA | exclusive | 13.77 | (12.05, 15.51) | <0.001 | 20.15 | (15.82, 24.65) | <0.001 | 14.55 | (12.96, 16.16) | <0.001 |
| mixed | 8.55 | (7.43, 9.69) | <0.001 | 4.61 | (1.37, 7.95) | 0.005 | 8.17 | (7.09, 9.25) | <0.001 | |
| none | 0.91 | (0.45, 1.37) | <0.001 | −1.29 | (−2.44, −0.12) | 0.030 | 0.66 | (0.24, 1.09) | 0.002 | |
| PFDA | exclusive | 11.5 | (9.83, 13.19) | <0.001 | 14.51 | (8.98, 20.32) | <0.001 | 11.45 | (9.85, 13.08) | <0.001 |
| mixed | 9.64 | (8.51, 10.78) | <0.001 | 5.03 | (0.73, 9.51) | 0.021 | 9.49 | (8.38, 10.61) | <0.001 | |
| none | 4.21 | (3.73, 4.69) | <0.001 | 0.29 | (−1.24, 1.86) | 0.71 | 4.05 | (3.58, 4.51) | <0.001 | |
Figure 1.
Serum concentrations of (A) PFOA and (B) PFOS at age 18 months, as calculated from neonatal concentration and the duration of breastfeeding (vertical scale) and compared to the measured concentration (horizontal scale).
Table 4.
Effect of doubling in predicted serum concentration of PFOA and PFOA at different ages (in months) with adjustment for differences in dependence on breastfeeding in the two cohorts.
| Vaccine / Exposure |
Cohort 5 | Cohort 3 | Joint | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Age | change | 95% Cl | p | change | 95% Cl | p | change | 95% Cl | p |
| Tetanus | ||||||||||
| PFOS | 3 | −20.03 | (−38.88,4.62) | 0.10 | −24.75 | (−53.37,21.42) | 0.24 | −21.16 | (−37.60,−0.38) | 0.046 |
| 6 | −13.13 | (−30.52,8.62) | 0.22 | −27.74 | (−46.26,−2.86) | 0.031 | −18.81 | (−32.06,−2.99) | 0.022 | |
| 12 | −6.58 | (−22.62,12.78) | 0.48 | −16.92 | (−32.17,1.77) | 0.073 | −11.50 | (−22.91,1.60) | 0.083 | |
| PFOA | 3 | −32.63 | (−46.72,−14.82) | 0.001 | −23.03 | (−48.45,14.94) | 0.20 | −30.35 | (−43.20,−14.59) | 0.001 |
| 6 | −24.81 | (−38.43,−8.19) | 0.005 | −28.17 | (−45.08,−6.07) | 0.016 | −26.06 | (−37.01,−13.20) | <0.001 | |
| 12 | −16.92 | (−30.11,−1.25) | 0.035 | −17.75 | (−32.01,−0.51) | 0.044 | −17.31 | (−27.25,−6.02) | 0.004 | |
| Diphtheria | ||||||||||
| PFOS | 3 | −0.90 | (−24.02,29.26) | 0.95 | −54.36 | (−73.91,−20.16) | 0.006 | −15.30 | (−33.08,7.21) | 0.17 |
| 6 | 3.35 | (−17.16,28.95) | 0.77 | −27.40 | (−47.47,0.34) | 0.052 | −7.32 | (−22.79,11.25) | 0.42 | |
| 12 | 3.65 | (−13.90,24.78) | 0.71 | −2.70 | (−22.31,21.85) | 0.81 | 1.12 | (−12.38,16.69) | 0.88 | |
| PFOA | 3 | −12.36 | (−30.61,10.69) | 0.27 | −31.05 | (−56.20,8.54) | 0.11 | −16.87 | (−32.35,2.15) | 0.079 |
| 6 | −6.42 | (−23.28,14.14) | 0.51 | −16.46 | (−37.69,12.02) | 0.23 | −9.73 | (−23.41,6.39) | 0.22 | |
| 12 | −3.88 | (−18.97,14.02) | 0.65 | 2.63 | (−16.87,26.69) | 0.81 | −1.28 | (−13.54,12.72) | 0.85 |
Discussion
The present prospective study aimed at exploring the role of PFAS exposure in infancy in regard to responses to routine childhood immunizations in terms of antibody concentrations measured at age 5 years, i.e., four years after the most recent (third) vaccination. While greater vulnerability to immunotoxicants is suspected during early maturation of the adaptive immune system, previous studies have not been able to elucidate this possibility, as blood samples are difficult to collect from healthy infants. While serum-PFAS concentrations tend to be quite stable in adults (Zhang et al. 2013), the concentrations in children are much more variable, possibly because of age-dependent changes in exposure sources (Kato et al. 2009; Lindstrom et al. 2011). Accordingly, our results showed only modest correlations between serum-PFAS concentrations at birth and at ages 18 months and 5 years, likely due to differences in the transfer via breast-feeding (Mogensen et al. 2015b). For several industrial chemicals, breast milk is known to serve as an exposure pathway (Grandjean and Jensen 2004; Stefanidou et al. 2009); our observation that serum-PFAS concentrations in infants increase substantially with the duration of breast-feeding is in accordance with this notion.
In order to estimate PFAS concentrations over time, single-compartment toxicokinetic models have been successfully applied, e.g., to calculate past serum-PFOA concentrations after cessation of exposure (Seals et al. 2011) and serum-PFAS profiles in children exposed from breastfeeding (Verner et al. 2016). Thus, the present study included both PFAS concentrations measured in age 18-month blood samples, and the study also utilized information on the duration of exclusive breastfeeding to calculate profiles of serum-PFAS concentrations during infancy.
Such calculations assume that each child received the same daily amount of milk in regard to body weight and that the transfer was constant throughout the duration of exclusive breastfeeding without major differences in age-dependent changes in the distribution volume. Partial breastfeeding was taken into account, but seemed to be of negligible importance. Despite the reservations, the validity of the calculations is supported by the close correlation of observed and calculated serum concentrations at age 18 months (Figure 1). Nonetheless, the estimated serum-PFAS concentrations in infancy likely involve some random imprecision, thus causing a potential bias toward the null (Grandjean and Budtz-Jorgensen 2010) and thereby a possible underestimation of the impact of PFAS exposures at infancy on adaptive immune system functions. The close correlation seen in Figure 1 suggests that this bias is small.
As a further consideration, while breastfeeding may transfer toxicants to the infant, it is considered advantageous to the developing immune system (Kramer et al. 2007). However, although almost all studies on benefits from breastfeeding have ignored any possible bias from effects in the opposite direction from immunotoxicants transferred via human milk (Grandjean and Jensen 2004; Grandjean et al. 2010), thereby potentially leading to residual negative confounding (Choi et al. 2008). As before (Grandjean et al. 2012), we were unable to detect any important benefit on the two antibody concentrations associated with the duration of exclusive breast-feeding.
In agreement with our previous studies (Grandjean et al. 2012; Mogensen et al. 2015a), the present study confirmed the inverse associations with prenatal exposures for both toxoid antibodies, and our findings now extend these observations by adding information on exposures during early childhood. Associations for diphtheria and tetanus differ somewhat, perhaps because the diphtheria toxoid is considered a weaker antigen than tetanus and because the associations may depend on relative levels of exposure as well as possible differences in age-dependent vulnerability. Maintenance of the antibody concentrations in serum may be a vulnerable target, but previous studies have also documented an inverse association of serum-PFAS concentrations with the short-term response to vaccinations both in children and in adults (Grandjean et al. 2012; Looker et al. 2014; Kielsen et al. 2016). Overall, these findings are in accordance with immunotoxic effects demonstrated in laboratory models (National Toxicology Program 2016) including in vitro studies of human immune cells (Corsini et al. 2012). Still, the detailed mechanisms involved are unclear at present.
The adaptive immune system is at first dominated by TH2 responses, and TH1 responses mature during infancy, thereby allowing proper responses to routine immunizations (Romagnani 2014). In regard to TH2-related allergy, increased odds of asthma in children were reported at elevated PFAS exposures (Dong et al. 2013), although this finding has not been replicated (Humblet et al. 2014). Our own studies (i.e., Timmermann et al. 2017) suggested that serum-PFAS concentrations at age 5 years were associated with increased odds of asthma only among the children who had not yet been vaccinated against measles, mumps, and rubella (MMR), while the association was reversed among MMR-vaccinated children. Although inhibition of antibody responses, perhaps associated with increased risk of allergy development, could represent a change in the TH1/TH2 balance (Dong et al. 2011), the relative role of the immune system components is complex. The lack of clear evidence on PFAS-associated allergy may in part be due to uncontrolled and variable allergen exposures and the absence of outcome variables that are as well-defined and standardized as are vaccine-induced antibodies.
Our previous results show that an insufficient vaccination response may be remedied at least in part by an additional booster (Grandjean et al. 2016). However, while these deficient antibody responses do not represent an irreparable insult, the boosters may not remedy any additional immune dysfunctions due to PFAS exposures. Among possible consequences of insufficient responses to childhood immunizations could be an increased risk of infectious diseases other than those that the vaccines aim to protect against. Thus, increased incidence of common cold and gastroenteritis in 3-year-olds were recorded in relation to higher maternal serum PFOA concentrations during pregnancy (Granum et al. 2013), and a greater incidence of infection was also linked with lower vaccine antibody concentrations (Pennings et al 2016). Similarly, the incidence of disease with high fever in small children was positively associated with the mother’s serum-PFAS concentrations in early pregnancy (Dalsager et al. 2016). While serum concentrations of PFOS and PFOA during pregnancy were apparently not associated with the total hospitalization rate for infectious diseases in school-age children (Fei et al. 2010), quality concerns regarding the serum-PFAS analyses in this cohort (Bach et al. 2015) questions the validity of this report.
The National Toxicology Program recently concluded that both PFOS and PFOA are presumed immune hazards for humans (National Toxicology Program 2016), while acknowledg-ing that the epidemiological studies are unable to attribute PFAS associations to individual compounds. PFAS exposures in the Faroes are quite similar to those in the United States (CDC 2015), but inter-correlations between serum-PFAS concentrations prenatally and at different ages make it difficult to determine accurately the possible age-dependent roles of individual PFASs in regard to immune function outcomes.
Conclusions
As PFASs are excreted in human milk, serum concentrations in infancy can be estimated from serum analyses at birth and the duration of exclusive breastfeeding. Both prenatal exposures and estimated serum levels in early infancy showed clear inverse associations with antibody concentrations against two toxoid vaccines at age 5 years, while associations with PFAS concentrations at ages 18 months and 5 years were weaker. Given that the adaptive immune system undergoes crucial maturation during infancy, the results support the high vulnerability of the developing immune system. Thus, the present study extends the evidence on PFAS-associated deficient antibody responses in children and emphasizes that infants may need particular protection as a highly vulnerable sub-population.
Acknowledgments
Funding
This work was supported by the National Institute of Environmental Health Sciences (ES012199) and the Danish Environmental Protection Agency as part of the environmental support program DANCEA (Danish Cooperation for Environment in the Arctic).
Footnotes
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Bach C, Henriksen T, Bossi R, Bech B, Fuglsang J, Olsen J, Nohr E. 2015. Perfluoroalkyl acid concentrations in blood samples subjected to transportation and processing delay. PLoS One 10:e0137768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua T, Katz J, Bocchini J. 2013. Update on adolescent immunizations: Selected review of U.S. recommendations and literature. Curr. Opin. Pediatrics 25:397–406. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control). 2015. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA:Centers for Disease Control and Prevention. [Google Scholar]
- Choi A, Cordier S, Weihe P, Grandjean P. 2008. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit. Rev. Toxicol 38:877–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Sangiovanni E, Avogadro A, Galbiati V, Viviani B, Marinovich M, Galli C, Dell’Agli M, Germolec D. 2012. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol 258:248–255. [DOI] [PubMed] [Google Scholar]
- Dalgard C, Petersen M, Steuerwald U, Weihe P, Grandjean P. 2016. Umbilical cord serum 25-hydroxyvitamin D concentrations and relation to birthweight, head circumference and infant length at age 14 days. Paediatr. Perinat. Epidemiol 30:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Høst A, Grandjean P, Jensen T. 2016. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4 years among 359 children in the Odense Child Cohort. Environ. Intl 96:58–64. [DOI] [PubMed] [Google Scholar]
- Dietert R, and DeWitt J. 2010. Developmental immunotoxicity (DIT): The why, when, and how of DIT testing. Meth. Mol. Biol 598:17–25. [DOI] [PubMed] [Google Scholar]
- Dietert R 2014. Developmental immunotoxicity, perinatal programming, and non-communica-ble diseases: Focus on human studies. Adv. Med 2014:867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Liu M, Wang D, Zheng L, Liang Z, Jin Y. 2011. Sub-chronic effect of perfluoro-octanesulfonate (PFOS) on the balance of Type 1 and Type 2 cytokines in adult C57Bl/6 mice. Arch Toxicol 85:1235–1244. [DOI] [PubMed] [Google Scholar]
- Dong G, Tung K, Tsai C, Liu M, Wang D, Liu W, Jin Y, Hsieh W, Lee Y, Chen P. 2013. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ. Health Perspect 121:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Karrman A, Rotander A, Mikkelsen B, Dam M. 2013. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environ. Sci. Pollution Res. Intl 20:7940–7948. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin J, Lipworth L, Olsen J. 2010. Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ. Res 110:773–777. [DOI] [PubMed] [Google Scholar]
- Grandjean P, and Jensen A. 2004. Breastfeeding and the weanling’s dilemma. Am. J. Public Health 94:1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, and Budtz-Jorgensen E. 2010. An ignored risk factor in toxicology: Total imprecision of exposure assessment. Pure Appl. Chem 82:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Poulsen L, Heilmann C, Steuerwald U, Weihe P. 2010. Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environ. Health Perspect 118:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Andersen E, Budtz-Jorgensen E, Nielsen F, Mølbak K, Weihe P, Heilmann C. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, and Budtz-Jorgensen E. 2013. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ. Health 12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen U, Budtz-Jørgensen E. 2016. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ. Health Perspect (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug L, Namork E, Stølevik S, Thomsen C, Aaberge I, van Loveren H, Løvik M, Nygaard U. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol 10:373–379. [DOI] [PubMed] [Google Scholar]
- Haug L, Thomsen C, Becher G. 2009. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J. Chromatogr 1216:385–393. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Jorgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. 2010. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ. Health Perspect 118:1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen C, Gun J, Nagel J, Kreeftenberg J. 1988. The toxin binding inhibition test as a reliable in vitro alternative to the toxin neutralization test in mice for the estimation of tetanus antitoxin in human sera. J. Biol. Stand 16:287–297. [DOI] [PubMed] [Google Scholar]
- Humblet O, Diaz-Ramirez L, Balmes J, Pinney S, Hiatt R. 2014. Perfluoroalkyl chemicals and asthma among children 12–19 years of age: NHANES (1999–2008). Environ. Health Perspect 122:1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Calafat A, Wong L, Wanigatunga A, Caudill S, Needham L. 2009. Poly-fluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ. Sci. Technol 43:2641–2647. [DOI] [PubMed] [Google Scholar]
- Kielsen K, Shamim Z, Ryder L, Nielsen F, Grandjean P, Budtz-Jørgensen E, Heilmann C. 2016. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J. Immunotoxicol 13:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Choi A, Ha E, Pedersen L, Nielsen F, Weihe P, Hong YC, Budtz-Jørgensen E, Grandjean P. 2014. Effect of hemoglobin adjustment on the precision of mercury concentra-tions in maternal and cord blood. Environ. Res 132:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Mazer B, and Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group. 2007. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: Cluster randomised trial. BMJ 335:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom A, Strynar M, Libelo E. 2011. Polyfluorinated compounds: past, present, and future. Environ. Sci. Technol 45:7954–7961. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Liu Y, Chan H, Zhao Y, Cai Z, Wu Y. 2011. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ. Intl 37:1206–1212. [DOI] [PubMed] [Google Scholar]
- Ljungman P 2013. Vaccination of immunocompromized hosts In: Vaccines, 6th Edition (Plotkin S, and Offit P, Eds.). Amsterdam: Elsevier, pp. 1243–1256. [Google Scholar]
- Looker C, Luster M, Calafat A, Johnson V, Burleson G, Burleson F, Fletcher T. 2014. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci 138:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura K, Nishio S, Ito A, Kono R 1974. Micro-cell culture method for determination of diphtheria toxin and anti-toxin titres using VERO cells. I. Studies on factors affecting the toxin and antitoxin titration. J. Biol. Stand 2:189–201. [DOI] [PubMed] [Google Scholar]
- Mogensen U, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015a. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ. Health 14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen U, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015b. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ. Sci. Technol 49:10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Weldon R, Armstrong B, Gibson L, Lopez-Espinosa M, Shin H, Fletcher T. 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ. Health Perspect 122:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program (NTP). 2016. Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid (PFOA) or Perfluorooctane Sulfonate (PFOS) Available at: http://ntp.niehs.nih.gov/pubhealth/hat/noms/pfoa/index.html [accessed 17 May 2017].
- Pennings J, Jennen D, Nygaard U, Namork E, Haug L, van Loveren H, Granum B. 2016. Cord-blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. J Immunotoxicol 13:173–180. [DOI] [PubMed] [Google Scholar]
- Romagnani S 2014. T-cell sub-populations. Chem. Immunol. Allergy 100:155–164. [DOI] [PubMed] [Google Scholar]
- Schatz D, Ellis T, Ottendorfer E, Jodoin E, Barrett D, Atkinson M. 1998. Aging and the immune response to tetanus toxoid: diminished frequency and level of cellular immune reactivity to antigenic stimulation. Clin. Diagn. Lab. Immunol 5:894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals R, Bartell S, Steenland K. 2011. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ. Health Perspect 119:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidou M, Maravelias C, Spiliopoulou C. 2009. Human exposure to endocrine disruptors and breast milk. Endocr. Metab. Immune Disord. Drug Targets 9:269–276. [DOI] [PubMed] [Google Scholar]
- Timmermann C, Budtz-Jorgensen E, Jensen T, Osuna C, Petersen M, Steuerwald U, Nielsen F, Poulsen L, Weihe P, Grandjean P. 2017. Association between perfluoroalkyl substance exposure and asthma and allergic disease in children as modified by MMR vaccination. J. Immunotoxicol 14:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loveren H, Germolec D, Koren H, Luster M, Nolan C, Repetto R, Smith E, Vos J, Vogt R. 1999. Report of the Bilthoven Symposium: Advancement of epidemiological studies in assessing the human health effects of immunotoxic agents in the environment and the workplace. Biomarkers 4:135–157. [DOI] [PubMed] [Google Scholar]
- Verner M, Ngueta G, Jensen E, Fromme H, Völkel W, Nygaard U, Granum B, Longnecker M. 2016. A simple pharmacokinetic model of prenatal and postnatal exposure to perfluoroalkyl substances (PFASs). Environ. Sci. Technol 50:978–986. [DOI] [PubMed] [Google Scholar]
- Weihe P, Kato K, Calafat A, Nielsen F, Wanigatunga A, Needham L, Grandjean P. 2008. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ. Sci. Technol 42:6291–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin J. 2013. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ. Sci. Technol 47:10619–10627. [DOI] [PubMed] [Google Scholar]