Abstract
In the developing visual system, retinal ganglion cell (RGC) axons project from the retina to several distal retinorecipient regions in the brain. Several molecules have been implicated in guiding RGC axons in vivo, but the role of extracellular matrix molecules in this process remains poorly understood. Dystroglycan is a laminin-binding transmembrane protein important for formation and maintenance of the extracellular matrix and basement membranes and has previously been implicated in axon guidance in the developing spinal cord. Using two genetic models of functional dystroglycan loss, we show that dystroglycan is necessary for correct sorting of contralateral and ipsilateral RGC axons at the optic chiasm. Mis-sorted axons still target retinorecipient brain regions and persist in adult mice, even after axon pruning is complete. Our results highlight the importance of the extracellular matrix for axon sorting at an intermediate choice point in the developing visual circuit.
Keywords: dystroglycan, axon, optic chiasm, retina, basement membrane, extracellular matrix
Introduction
The proper function of neural circuits depends on establishing precise connectivity during development. In the mammalian visual system, the axons of retinal ganglion cells (RGCs) leave the retina and cross the optic chiasm in the ventral forebrain en route to retinorecipient areas of the brain. RGC axons terminate and form synapses in both image-forming brain regions, including the lateral geniculate nucleus (LGN) and superior colliculus (SC), and non-image forming brain regions, including the suprachiasmatic nucleus (SCN) and medial terminal nucleus (MTN) (Zhang et al., 2017).
RGC axon guidance at the optic chiasm is dependent on several extracellular cues (Herrera et al., 2017). Slit1 and Slit2 signaling through Robo receptors confines RGC axons to fasciculated tracts at the optic chiasm through surround repulsion (Plump et al., 2002). Vegf164/Nrp1, Sema3d/Nrp1 and Sema6D/NrCAM/PlxnA1 ligand/receptor complexes promote contralateral axon crossing at the chiasm (Dell et al., 2013; Erskine et al., 2011; Kuwajima et al., 2012; Sakai and Halloran, 2006). In the mouse, approximately 5% of neurons are excluded from the optic chiasm and form an ipsilateral projection (Petros et al., 2008). These ipsilateral axons are repelled by EphrinB2 expressed by optic chiasm glia, and Shh secreted transaxonally by contralateral axons (Peng et al., 2018; Williams et al., 2003). Additional molecular cues govern axonal sorting, targeting of specific retinorecipient regions, and retinotopic mapping after crossing the chiasm (Feldheim et al., 1998; Hindges et al., 2002; Osterhout et al., 2011; Schmitt et al., 2006; Su et al., 2011; Suetterlin and Drescher, 2014; Sun et al., 2015).
The extracellular matrix (ECM) also plays a critical role in axon guidance by generating both permissive and non-permissive substrates for axon growth. ECM proteins are highly enriched at basement membranes that form at the interface of neuroepithelial endfeet and the surrounding tissues. Basement membranes are highly dynamic structures that also regulate the extracellular localization of attractive and repulsive secreted cues (Dominici et al., 2017; Varadarajan et al., 2017; Wright et al., 2012; Xiao et al., 2011). The trajectory of RGC axons along the inner limiting membrane (ILM) keeps them in direct contact with the basement membrane as they exit the retina. Previous work has found that altering the composition of proteoglycans affects the trajectory of RGCs at the optic chiasm (Ichijo and Kawabata, 2001; Lin et al., 2007). However, the role of the basement membrane in regulating the trajectory of RGC axons through the optic chiasm remains poorly understood.
Dystroglycan is a highly glycosylated transmembrane protein that is critical for maintaining the structural integrity of basement membranes through its binding to ECM proteins. Mutations in dystroglycan or one of the 18 genes required for its proper glycosylation result in compromised basement membrane integrity (Manya and Endo, 2017). This can lead to dystroglycanopathy, a form of congenital muscular dystrophy that is frequently accompanied by neurodevelopmental defects that include type II lissencephaly and retinal dysplasia (Clements et al., 2017; Michele et al., 2002; Moore et al., 2002; Takeda et al., 2003; Taniguchi-Ikeda et al., 2016). Dystroglycan regulates axon guidance in the spinal cord by maintaining the basement membrane as a permissive growth substrate, and by localizing secreted Slit proteins to the floorplate. (Wright et al., 2012). Here, we identify a critical role for dystroglycan in regulating the sorting of RGC axons at the optic chiasm and show that its loss results in inappropriate innervation patterns in retinorecipient regions.
Materials and Methods
Animals
Animal procedures were approved by the OHSU Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the care and use of laboratory animals. Embryonic day 0 (e0) was the day of vaginal plug observation and postnatal day 0 (P0) was the day of birth. Animals were euthanized with CO2. Controls were ISPD+/L79*, and DGF/+;Six3Cre or DGF/+ age matched littermates. ISPDL79*/L79* (Wright et al., 2012) and DGF/F (Moore et al., 2002) mice and genotyping have been described previously. Generic cre primers were used for Six3Cre (Furuta et al., 2000) and Isl1Cre (Yang et al., 2006) genotyping.
Statistical Analysis
All analysis was performed on n≥3 mice from at least two litters. Statistics were conducted using JMP Pro 13.0 software (SAS Institute). A Student’s t-test was used to compare means of two groups, and an ANOVA with Tukey post-hoc test was used to compare means of two or more groups. A significance threshold of 0.05 was used for all statistical tests. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001.
Labeling and Analysis of the Optic Chiasm
DiI injections were performed at e13 and P0. Animals were decapitated and heads were fixed in 4% PFA at 4°C overnight. The left eye was enucleated and a crystal of DiI approximately the size of the optic disc was placed inside the optic disc. Low melting point 2.5% agarose was placed into the eyecup to hold the crystal in place. Heads were incubated at 37°C for three days at e13, one week at P0. Projections were visualized by removing the lower jaw, tongue, and palate to expose the ventral forebrain. Images were acquired on a Zeiss AxioZoom.V16.
The proportions of contralateral, ipsilateral, and contralateral retinal projections labeled with DiI at P0 were determined by image analysis in FIJI. Each individual projection was traced to determine the area of the projection. Only axons that had already exited the chiasm were included in the analysis. The area of all projections was added to determine a total axonal area projection for each chiasm image. The proportion was determined by dividing the area of each individual projection by the total axonal area projection.
Labeling and Analysis of Retinorecipient Brain Regions
Cholera Toxin Subunit B (CTB) injections to label anterograde axonal projections were performed at P0 or P1 for P2 analysis, at P0-P4 for P10 analysis, and at P26 for P28 analysis. Mice between P0-P4 were anesthetized on ice, and mice aged P26 were anesthetized with isofluorane. Glass capillary needles were backfilled with CTB conjugated to Alexa-488 or Alexa-555. The right eye was injected with CTB-488 and the left eye was injected with CTB-555. Injections were performed using a picospritzer injecting for 15 milliseconds at 30 PSI. At P2 or P10, brains were removed and drop fixed in 4% PFA at 4°C overnight. At P28, animals were perfused, and brains were post-fixed in 4% PFA at 4°C overnight. Whole mount images of the superior colliculus were obtained prior to fixation on a Zeiss AxioZoom.V16. After fixation, brains were washed in PBS for 30 minutes, embedded in 2.5% low melting point agarose, and sectioned on a vibratome at 150 μm. Sections were incubated in DAPI overnight and mounted using Fluoromount medium. Brain sections were imaged on a Zeiss Axio Imager M2 upright microscope equipped with an ApoTome.2. Medial sections of the LGN and SC, and ventrally situated sections of the optic chiasm that included the early optic tract were analyzed. Analysis of superior colliculus misprojections (Figure 5E) was conducted on whole mount colliculus images by tracing the regions with inappropriate ipsilateral projections, adding to determine total area, and dividing by the area of the superior colliculus. At P28, the superficial area of the SC visible without removing the cortex was used for analysis.
Figure 5: Abnormal RGC innervation of the SC persists into adulthood in DGF/-;Six3Cre mice.
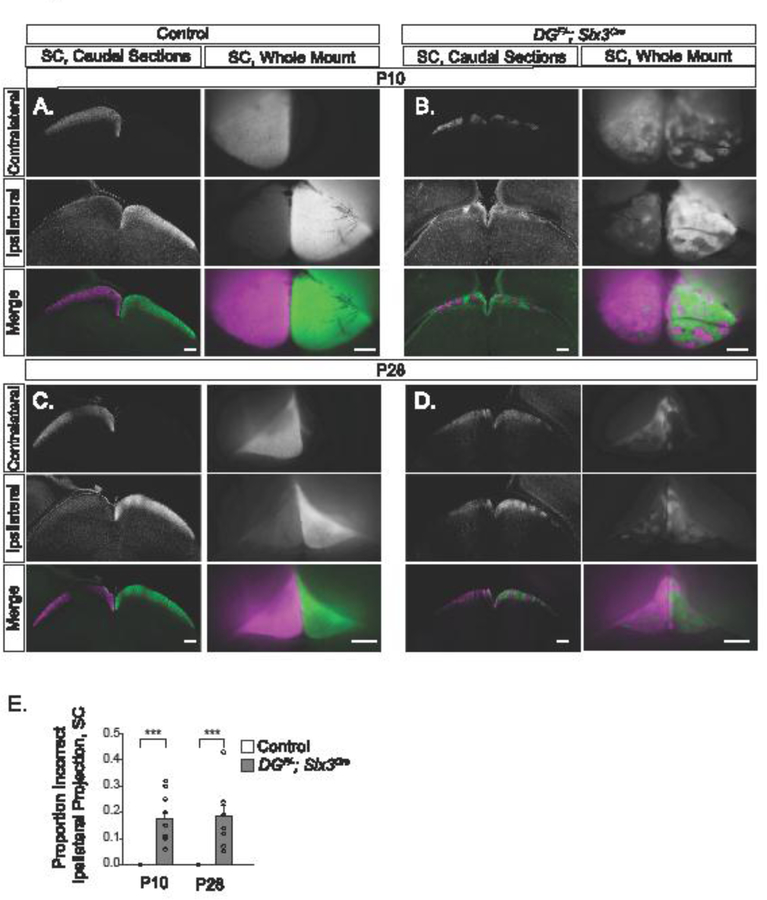
(A) CTB analysis of SC sections and whole mounts at P10 show segregation of projections from contralateral and ipsilateral retinas in controls. (B) CTB analysis in DGF/-;Six3Cre mice at P10 reveal innervation from the ipsilateral retina that prunes into discrete patches. (C) At P28, control SC CTB injections in sections and whole mounts show segregation of projections from the contralateral and ipsilateral retina. (D) Analysis of CTB labeled axons in the SC at P28 in DGF/-;Six3Cre show that inappropriate projections from the ipsilateral retina remain after eye opening. (E) In DGF/-;Six3Cre mice, approximately 20% of the axons in the SC at P10 and P28 inappropriately arise from the ipsilateral retina (ANOVA, Tukey HSD post hoc test, p<0.0001, n=7control and 6 mutant SCs at P10, n=4 control and 4 mutant SCs at P28). Scale bars: Sections, 200μm; Whole mount, 500μm.
A threshold-dependent analysis method was used to determine the proportion of contralateral and ipsilateral projections to the dLGN based on previously described methods (Jaubert-Miazza et al., 2005). Briefly, the LGN was thresholded in FIJI to determine the pixels corresponding to either contralateral signal or ipsilateral signal. Threshold values were kept constant between corresponding contralateral and ipsilateral images. The total area of pixels in each image, and the overlap between the two areas, was measured.
A threshold-independent method was utilized to confirm results and analyze segregation between contralateral and ipsilateral projections (Torborg and Feller, 2004). We applied a rolling ball filter of 200 pixels to the LGN to subtract background. The logarithm of the intensity ratio (R) was calculated for each pixel to compare the intensity of ipsilateral (FI) and contralateral (FC) fluorescence, where R = log10(FI/FC). This generates a distribution of R-values for each LGN image. We then computed the variance for each R-distribution. Smaller variances reflect greater overlap, and less segregated axonal input, whereas larger variances reflect decreased overlap and more segregated axonal input.
Brains containing cre-dependent fluorescent markers used to define regions of cre recombination in retinorecipient brain regions were processed the same way as brains injected with CTB.
Immunohistochemistry
Embryos used for immunohistochemistry were decapitated and fixed in 4% PFA at 4°C overnight. Tissue was washed in PBS for 30 minutes and equilibrated in 15% sucrose at 4°C overnight. Tissue was flash frozen in embedding media and sectioned at 20 μm on a cryostat. Sections were blocked with PBS containing 2% Normal Donkey Serum and 0.2% Triton for 30 minutes, and incubated in antibody diluted in this solution at 4°C overnight. Tissue was washed for 30 minutes and incubated in secondary antibody diluted in a PBS solution containing 2% Normal Donkey Serum for 2–4 hours. Tissue was incubated in PBS containing DAPI for 10 minutes, washed in PBS for 20 minutes, and mounted using Fluoromount medium. Sections were imaged on a Zeiss Axio Imager M2 upright microscope equipped with an ApoTome.2. Sections through the optic chiasm (Figures 1 and 2) were taken from the central chiasm. Preparation of P0 retinas (Supplemental Figure 2) was performed as previously described (Clements et al., 2017). Antibody information is contained in the Key Resource Table.
Figure 1: Glycosylated dystroglycan is required for axon guidance at the optic chiasm.
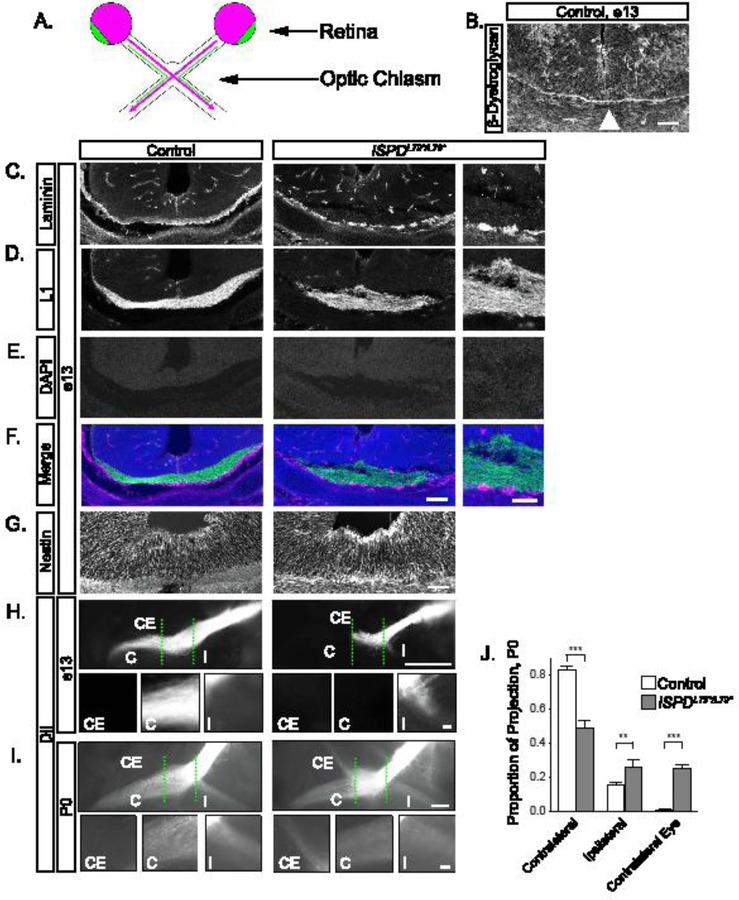
(A) Schematic of RGC axon crossing at the optic chiasm, with contralateral projections in purple, ipsilateral projections in green. (B) At e13, β- Dystroglycan is enriched at the basement membrane in the ventral forebrain (arrowhead). (C-F) Sections at e13 reveal RGC axons (L1, D, green, F) in direct proximity to the basement membrane (laminin, C, purple, F) in controls (left panels). The basement membrane is discontinuous in ISPDL79*/L79* mutants (right panels), and axons are defasciculated and enter the ventral forebrain. DAPI reveals that the cellular architecture around the chiasm is normal (E). (G) The endfeet of Nestin+ midline glia at the optic chiasm appear disorganized in ISPDL79*/L79* mutants compared to controls. (H) At e13, DiI labeled RGC axons form an exclusively contralateral projection at the optic chiasm (dashed green lines) in controls (left). Axons in ISPDL79*/L79* mutants fail to advance though the chiasm and form an abnormal ipsilateral projection (right). (I) At P0, axons in controls make a dominant contralateral and minor ipsilateral projection (left). In ISPDL79*/L79* mutants, axons project contralaterally, ipsilaterally, and to the contralateral eye (right). (J) Quantification of RGC axon trajectories indicate that mutant axons have a reduced contralateral projection (ANOVA, Tukey HSD post hoc test, p<0.01, n=12 control, 6 mutant chiasms). C denotes contralateral projection, I denotes ipsilateral projection, and CE denotes contralateral eye projection. Scale bars: B, 50μm; C-F, 100μm; C-F insets, 50μm; G, 50μm; H, I, 200μm; H insets, 10μm; I insets, 50μm.
Figure 2: Conditional deletion of dystroglycan results in RGC axon guidance defects at the optic chiasm.
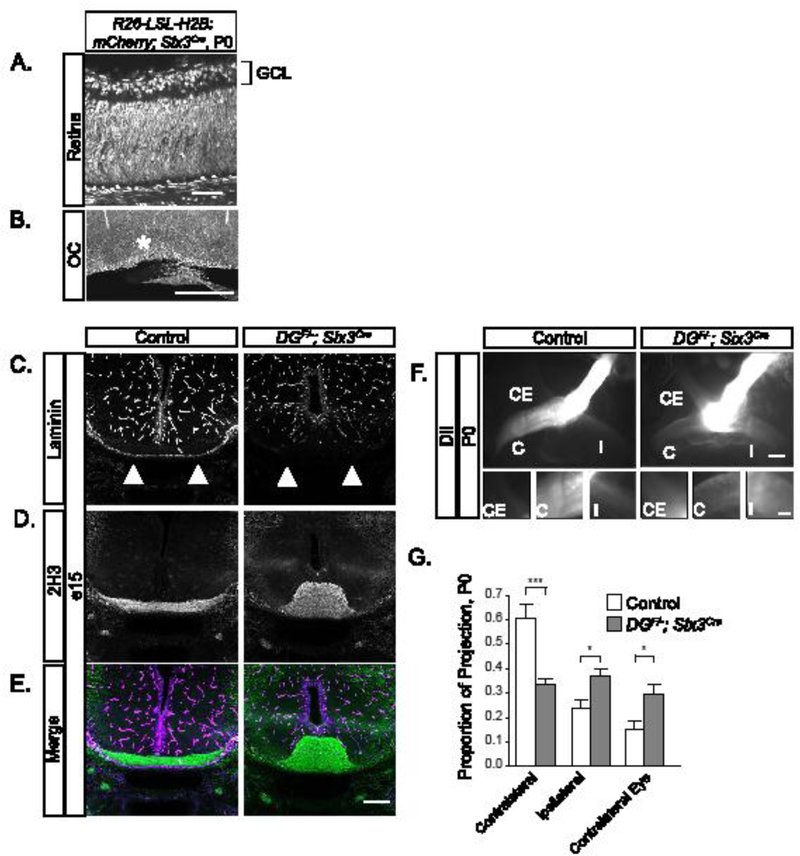
(A-B) Sections from Rosa26-lox-stop- lox-H2B:mCherry;Six3Cre mice show recombination throughout the retina (A) and in cells lining the optic chiasm at P0. (C-E) Sections through the ventral forebrain at e15 reveal a loss of laminin staining at the chiasm (C, E purple) and axons (2H3) grow inappropriately into the ventral forebrain (D, E green) in DGF/-;Six3Cre mice. (F) RGC axons in DGF/-;Six3Cre mutants stall at the optic chiasm and have reduced projections into the contralateral and ipsilateral optic tracts. (G) Quantification of RGC axon trajectories indicate that mutant axons have a decreased contralateral projection and increased ipsilateral and contralateral eye projections compared to controls (ANOVA, Tukey HSD post hoc test, p<0.01, n=4 control, 3 mutant chiasms). C denotes contralateral projection, I denotes ipsilateral projection, and CE denotes contralateral eye projection. Scale bars: A, 50 μm; B, 500μm; C-F, 200μm; F insets, 50μm.
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| 2H3 (neurofilament) | Developmental Studies Hybridoma Bank |
AB_531793 |
| β-Dystroglycan | Developmental Studies Hybridoma Bank |
AB_2618140 |
| CTB, Alexa Fluor 488 Conjugate | Life Technologies | n/a |
| CTB, Alexa Fluor 555 Conjugate | Life Technologies | n/a |
| Collagen IV | Southern Biotech | AB_2082646 |
| L1 | Millipore | AB_2133200 |
| Laminin | Sigma | AB_477163 |
| Nestin | Developmental Studies Hybridoma Bank |
AB_2235915 |
| Perlecan | Millipore | AB_10615958 |
| Zic2 | Millipore | AB_1977437 |
Results and Discussion
Dystroglycan is required for axon sorting at the optic chiasm
In the mouse, approximately 95% of RGC axons cross at the optic chiasm (Petros et al., 2008). The contralateral projection initially forms at e13 (Figure 1A, purple), followed by the ipsilateral projection (Figure 1A, green) at e14-e17 (Petros et al., 2008). At e13, dystroglycan protein is enriched in the basement membrane of the ventral forebrain where RGC axons cross to form the optic chiasm (Figure 1B). We therefore examined RGC axon trajectories in ISPDL79*/L79* mutant mice, in which dystroglycan cannot bind to ECM proteins including laminin due to defective glycosylation. In control sections at e13, laminin staining is continuous along the ventral forebrain (Figure 1 C, F), with fasciculated bundles of RGC axons (Figure 1 D, F) growing in direct contact with the laminin. In contrast, laminin in the basement membrane is fragmented and discontinuous, and RGC axons appear defasciculated in ISPD79*/79* mutants at e13 (Figure 1 C–F). Additional basement membrane proteins such as perlecan and collagen IV are also discontinuous at the chiasm in mutants at e13 (Supplemental Figure 1A–D). The endfeet of midline radial glia in in ISPDL79*/L79* mutants appear abnormally swelled (Figure 1G), possibly reflecting their detachment from the fragmenting basement membrane. Otherwise, there are no obvious structural defects in the chiasm of ISPDL79*/L79* mutants (Figure 1E).
In control mice, RGC axons labeled with intraretinal DiI at e13 extended through the optic chiasm to form an exclusively contralateral projection (Figure 1H). In contrast, in ISPDL79*/L79* mutants, axons stalled at the chiasm, failed to project into the contralateral optic tract, and formed a premature projection into the ipsilateral optic tract (Figure 1H). By P0, many axons in ISPDL79*/L79* mutants progressed through the chiasm, but only 50% of RGC axons enter the contralateral optic tract, with the remainder of the axons inappropriately projecting into the ipsilateral optic tract and contralateral optic nerve (Figure 1I, J, ANOVA, Tukey HSD post hoc test, p<0.01). Based on these results, we conclude that RGC axons fail to enter and sort appropriately at the optic chiasm in ISPDL79*/L79* mutants.
To confirm that the basement membrane degeneration and axon sorting phenotypes in ISPDL79*/L79* mutants are due to loss of functional dystroglycan, we generated DGF/-;Six3Cre mice to delete dystroglycan in both the retina and ventral forebrain (Figure 2A, B), (Furuta et al., 2000). We observed a complete loss of laminin staining along the ventral forebrain by e15 in DGF/-;Six3Cre mice (Figure 2C, E). The increased severity of this phenotype relative to e13 ISPDL79*/L79* mutants is consistent with a progressive degeneration of the basement membrane in the absence of functional dystroglycan. RGC axons stalled and misrouted at the optic chiasm (Figure 2D, E, F), forming a large ectopic bundle in DGF/-;Six3Cre mice. Axons labeled with DiI in DGF/-;Six3Cre mice at P0 (Figure 2F) show that some axons do exit the chiasm, with an increased proportion routing ipsilaterally and to the contralateral eye compared to controls (Figure 2G, ANOVA, Tukey HSD post hoc test, p<0.01). The intensity of the projections is also fainter, likely representing early RGC loss (Clements et al., 2017). These results verify that RGC axon guidance defects at the optic chiasm of ISPDL79*/L79* mutants are due to the loss of functional dystroglycan.
The disruption of basement membrane integrity in ISPDL79*/L79* and DGF/;Six3Cre mutants suggests a potential mechanism for axonal misrouting at the optic chiasm. However, we also considered the possibility that dystroglycan could function cell-autonomously in RGCs. To test this, we deleted dystroglycan specifically in RGCs using Isl1Cre, (Supplemental Figure 2A), (Pan et al., 2008). DiI labeling did not reveal any differences in RGC axon crossing between controls and DGF/-;Isl1Cre mutants at P0 (Supplemental Figure 2B), suggesting that dystroglycan is not required within RGC axons to navigate the optic chiasm. We also examined whether RGCs were mis-specified in the absence of dystroglycan. Zic2+ marks a population of neurons that are generated in the ventrotemporal retina at e14.5–18.5 that normally project ipsilaterally (Herrera et al., 2003). At e13, when we first detected inappropriate projections in ISPDL79*/L79* mutants, there were no Zic2+ RGCs, similar to controls (Supplemental Figure 3A, B). At e16, when the normal ipsilateral projection should be specified, the Zic2+population appeared normal in mutants (Supplemental Figure 3C, D). Therefore, dystroglycan is not required for the proper specification of ipsilaterally projecting RGCs, and does not function within RGC axons for their proper sorting at the optic chiasm.
Based on these findings, we conclude that glycosylated dystroglycan is required within the optic chiasm for the growth and sorting of RGC axons. Previous work has shown that heparan sulfate proteoglycans (HSPGs) are modulators of axon guidance signaling, and that deletion of the heparin sulfotransferases Hs2st and Hs5st1 affects Slit/Robo-dependent RGC axon guidance at the optic chiasm (Conway et al., 2011; Hussain et al., 2006; Pratt et al., 2006). While it is likely that the loss of dystroglycan affects the distribution of secreted axon guidance cues at the chiasm, the defects we observe in ISPDL79*/L79* and DGF/-; Six3Cre mutants are far more severe than those observed following deletion of heparin sulfotransferases or canonical axon guidance cues such as Slit. Therefore, we propose that the predominant role for dystroglycan is to maintain the basement membrane as a permissive growth substrate for RGC axons at the optic chiasm.
Axon mistargeting in retinorecipient regions due to loss of dystroglycan
After exiting the optic chiasm, RGC axons project to retinorecipient regions in the brain. While ISPDL79*/L79* mutants die perinatally, DGF/-;Six3Cre mutants survive to adulthood, allowing us to examine how the loss of dystroglycan affects the final pattern of RGC innervation in the brain. Importantly, Six3Cre does not drive a significant level of recombination in the LGN or SC (Supplemental Figure 4), allowing us to examine how inappropriate sorting at the chiasm affects retinorecipient targeting.
To visualize axons, we performed dual-color Cholera Toxin Subunit B (CTB) injections. At P2, the majority of axons in the optic tract immediately posterior to the optic chiasm originate from the contralateral retina. However, axons from both the contralateral and ipsilateral retina were bilaterally distributed in the optic tract in DGF/-;Six3Cre mutants (Figure 3A). In the dLGN of controls (Figure 3B), axons from the contralateral retina (purple) innervate the outer shell, and axons from the ipsilateral retina (green) innervate the inner core. In stark contrast, axons show overlapping innervation from the contralateral and ipsilateral retinas throughout the entire dLGN in dystroglycan mutants (Figure 3B). Although axons are still actively undergoing eye-specific segregation at P2, threshold-independent analysis indicates that projections have greater overlap in mutants than controls (Figure 3F, t-test, p=0.038) (Torborg and Feller, 2004).
Figure 3: Retinal ganglion cell axons target retinorecipient regions in DGF/-; Six3Cre mice.
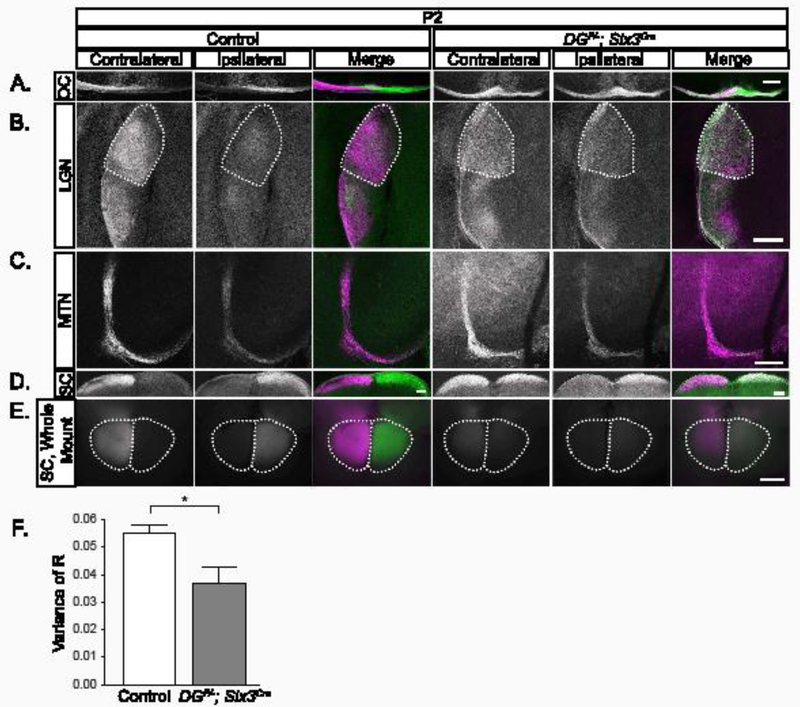
(A) CTB labeled axons at the optic chiasm show eye-specific segregation in controls at P2 (left panels) and overlapping projections in DGF/-; Six3Cre chiasms (right panels). (B) Axons from the contralateral retina (purple) innervate the dorsal and lateral LGN, while axons from the ipsilateral retina (green) are restricted to LGN core regions in controls (left) at P2. Axons from the contralateral and ipsilateral retinas overlap across the entirety of the LGN in DGF/- ;Six3Cre mutants. (C) In the MTN, controls and DGF/-;Six3Cre mutants receive innervation from both the contralateral retina (left), and the ipsilateral retina (D, E) In sections (D) and whole mount views of the SC (E), controls (left) are innervated by the contralateral retina. In DGF/-;Six3Cre mice (right), there is innervation from both contralateral and ipsilateral retina across the entire SC. (F) Threshold-independent analysis of LGN projections (B) is measured by the variance of R. Smaller values indicate less segregation and greater axonal overlap, whereas larger values indicate higher segregation and less axonal overlap. The analysis shows a significantly greater axonal overlap in the dLGN in mutants than controls (t-test, p=0.038, n=3 control, 3 mutant). Dotted lines indicate dLGN. Scale bar 200μm.
In controls, the SC receives innervation primarily from the contralateral retina with a small patch of innervation originating from the ipsilateral retina in the rostromedial SC. In sections taken from the caudal SC of control mice, axons originate from the contralateral retina (Figure 3D, E). In DGF/-;Six3Cre mutants, axons correctly target the SC, but overlapping projections from the contralateral and ipsilateral retina span the entire mediolateral extent of the SC (Figure 3D, E). RGC axons in DGF/-;Six3Cre mutants also innervate the MTN in the non-image forming visual system (Figure 3C).The reduced intensity of CTB labeled RGC projections in the LGN, SC, and MTN (Figure 3E) likely reflects increased apoptotic death of RGCs in DGF/-;Six3Cre mutants (Clements et al., 2017). Thus, RGC axons specifically innervate retinorecipient areas of the brain and do not project into inappropriate brain regions in DGF/-;Six3Cre mutants. However, there is increased innervation originating from the ipsilateral retina seen in the LGN and SC in DGF/-;Six3Cre mutants, despite the normal specification of the Zic2+ population. This indicates that a population of Zic2 negative RGCs that would normally cross the optic chiasm are instead projecting to ipsilateral retinorecipient regions.
In the LGN and SC, RGC axons undergo refinement prior to eye opening to form specific laminar and retinotopic organization (Godement et al., 1984; Jaubert-Miazza et al., 2005). This refinement is mediated by retinal waves, Eph/Ephrin signaling, and caspase-dependent pruning (Butts et al., 2007; Feldheim et al., 1998; Hindges et al., 2002; McLaughlin et al., 2003; Simon et al., 2012). We have previously shown that retinal waves are preserved in DGF/- ;Six3Cre mice (Clements et al., 2017; Sato et al., 2008). To determine whether the inappropriate innervation of the LGN and SC by axons originating from the ipsilateral retina observed at P2 were eliminated by axon pruning, we performed CTB injections prior to eye opening at P10, and at P28, when eye-specific axon segregation is complete (Furman and Crair, 2012). Surprisingly, while pruning does occur after P2, axons from the ipsilateral retina that have inappropriately invaded contralateral retinorecipient regions persist into adulthood.
At both P10 and P28 in DGF/-;Six3Cre mutants (Figure 4A, B), we observe axons from both ipsilateral and contralateral retinas intermingled within the optic chiasm. In the LGN of DGF/-;Six3Cre mutants, (Figure 4C, D), axons fail to prune in a manner that forms a “contralateral shell” and “ipsilateral core”. Rather, axons from the ipsilateral retina are present in patches distributed throughout the entire LGN. To determine if the appropriate proportion of contralateral and ipsilateral axons is maintained in the dLGN, we measured the area occupied by contralateral, ipsilateral, and overlapping axons. We observed a significant increase in the proportion of ipsilateral axons in DGF/-;Six3Cre mutants at P10 (Figure 4E, p=0.04, t-test), whereas the proportion of contralateral and overlapping projections do not show any difference (Figure 4E, p>0.05, t-test). Axons in the dLGN continued pruning between P10 and P28, as the proportion of projections from the ipsilateral retina was not different between DGF/-;Six3Cre mutants and controls at P28 (Figure 4E, p>0.05, t-test). Using a threshold- independent method to measure segregation between projections from the contralateral and ipsilateral retina, we found that despite axons from the ipsilateral retina innervating inappropriate regions of the dLGN shell, normal eye- specific segregation still occurs (Figure 4F, p>0.05, t-test).
Figure 4: Abnormal RGC innervation of the optic chiasm and LGN persists into adulthood in DGF/-;Six3Cre mice.
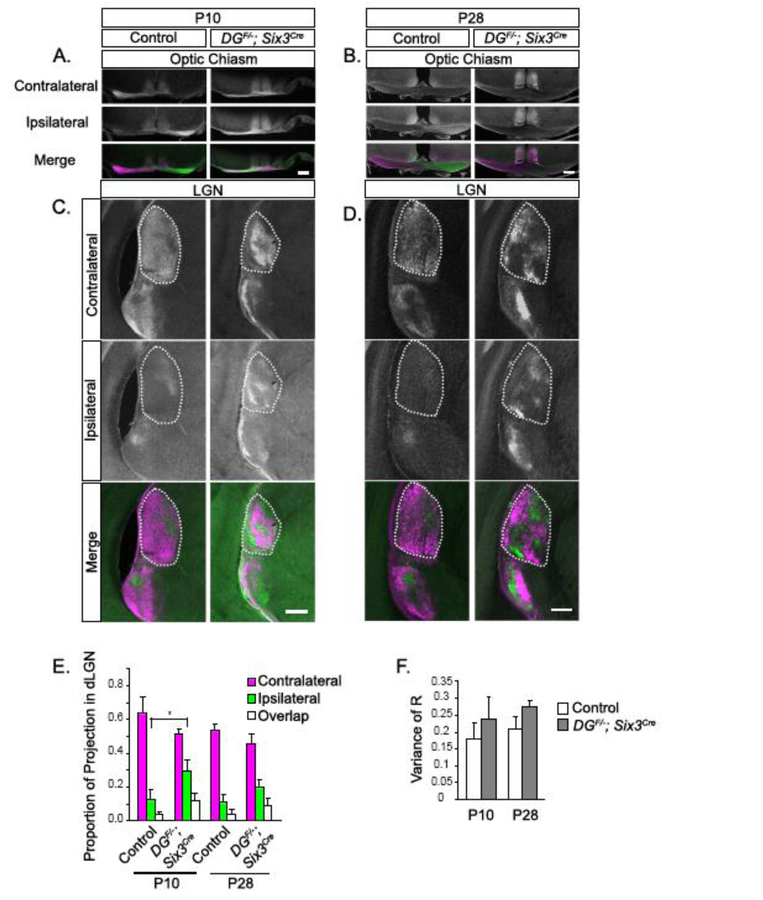
(A, B) CTB labeling reveals overlap between axons from the contralateral and ipsilateral retina at the optic chiasm in DGF/-;Six3Cre mice at P10 (A) that persists through eye opening. (C, D) CTB labeling in the LGN shows that axons do not prune into the appropriate contralateral shell/ipsilateral core arrangement. (E) Threshold-dependent measurement of the total area of contralateral, ipsilateral, and overlapping projections in the dLGN show that the amount of ipsilateral innervation is significantly greater in DGF/-;Six3Cre mice at P10 (p=0.04, t-test, n=4 controls, 4 mutants at P10, n=4 controls, 4 mutants at P28), but not different from controls at P28. (F) Threshold-independent analysis of the dLGN as measured by the variance of R does not show a difference in contralateral/ipsilateral segregation between controls and mutants at P10 or P28 (t test, p>0.05, n=4 controls, 4 mutants at P10, n=4 controls, 4 mutants at P28). Dotted lines indicate dLGN. Scale bars: 200μm.
In sections from the caudal SC of controls at P10 and P28, axons originate from the contralateral retina (Figure 5A, C). In DGF/-;Six3Cre mutants, clusters of axons from the ipsilateral retina are present throughout the mediolateral extent of the SC (Figure 5B, D). Analysis of whole mount SC at P10 and P28 reveals that approximately 20% of SC innervation in DGF/-;Six3Cre mutants originates from the ipsilateral retina and persists into adulthood (ANOVA, Tukey HSD post hoc test, p<0.0001, Figure 5E). Therefore, the inappropriate axons originating from the ipsilateral retina that are distributed throughout the SC at P2 undergo pruning into discrete clusters, resulting in their segregation from projections from the contralateral retina.
We also analyzed innervation of non-image forming retinorecipient regions. The SCN, which normally receives input from both retinas, continued to receive bilateral innervation at P10 and P28 (Supplemental Figure 5A, 6A). The optic tract in controls contains a dominant bindle of axons from the contralateral retina that segregate from the smaller bundle of axons from the ipsilateral retina (Supplemental Figure 5B, 6B). The axons from the ipsilateral retina are consistently lateral with respect to axons from the contralateral retina (Sitko et al., 2018). In DGF/-;Six3Cre mutants, the optic tract appear to have an almost equal number of axons originating from the contralateral and ipsilateral retina at both P10 and P28 (Supplemental Figure 5B, 6B). These axons are intermingled without showing the normal pattern of eye-specific segregation. The optic tract in DGF/-;Six3Cre mutants was also markedly thinner than controls at P28, presumably due to increased RGC apoptosis previously described in these mice (Clements et al., 2017). The MTN, which is normally dominated by innervation from the contralateral retina, appears to be equally innervated by both retinas in dystroglycan mutants (Supplemental Figure 5C, 6C).
We can draw three conclusions from the CTB labeling data. First, in contrast to other optic chiasm guidance models, such as HSPG and Slit/Robo mutants, RGC axons in dystroglycan mutants only innervate retinorecipient regions and do not mis-project into other areas of the brain. Second, the increased axonal overlap that we initially observe at P2 in both the dLGN and SC of DGF/-;Six3Cre mutants undergoes pruning, resulting in complete segregation of axons from contralateral and ipsilateral retinas at later stages. This pruning presumably relies on spontaneous activity in the form of retinal waves, as much of it occurs by P10, prior to eye opening. Third, despite the segregation of axons from the contralateral and ipsilateral retinas, axons from the ipsilateral retina that innervate inappropriate regions of the dLGN shell and SC fail to undergo elimination and persist into adulthood.
These findings raise interesting questions of how an abnormal persistence of innervation from the ipsilateral retina might affect downstream components of the visual circuit in DGF/-;Six3Cre mutants. First, how does the presence of RGC axons from the ipsilateral retina in the shell of the dLGN affect the formation of ocular dominance columns in cortical V1? Second, does the abnormal pattern of RGCs from the ipsilateral retina affect other axons innervating the same structures? Two recent studies show that retinogeniculate and corticothalamic axons may rely on one another for correct innervation of the LGN (Seabrook et al., 2013; Shanks et al., 2016). Finally, what is the impact of persistent inappropriate ipsilateral innervation on visual perception? Clues to these questions may come from mice lacking Ten-M3, which have an ipsilateral projection that extends abnormally into the “contralateral” region of the dLGN that is subsequently transmitted to the visual cortex by geniculocortical projections, resulting in defects in visually-mediated tasks (Leamey et al., 2007). This suggests that abnormal innervation of the LGN and SC observed in DGF/-;Six3Cremutants is likely transmitted to the visual cortex, resulting in inappropriate binocular information arising from a mismatch of inputs. Unfortunately, is since dystroglycan required for transmission at photoreceptor synapses, we are unable to test these questions in DGF/-;Six3Cre mutants (Sato et al., 2008).
Conclusions
In summary, we show that dystroglycan plays a critical role in regulating the guidance and sorting of RGC axons at the optic chiasm. The loss of basement membrane integrity in the ventral forebrain of ISPDL79*/L79* and DGF/- ;Six3Cre mutants coincident with the arrival of RGC axons at the chiasm suggests that this is the underlying cause of axonal misrouting. In DGF/-;Six3Cre mutants, mis-sorted ipsilateral RGC axons innervate inappropriate regions of retinorecipient areas through adulthood. Coupled with the requirement for dystroglycan at photoreceptor synapses and its role in regulating neuronal migration and dendritic lamination in the retina, our results provide insight into potential mechanisms that underlie visual impairments in patients with dystroglycanopathy.
Supplementary Material
Highlights.
Dystroglycan is required for maintaining the basement membrane in the optic chiasm
Axons stall and sort inappropriately at the optic chiasm in Dystroglycan mutants
Retinorecipient areas receive increased innervation from the ipsilateral retina
Inappropriate ipsilateral innervation persists through adulthood
Acknowledgements
We would like to thank Chinfei Chen and Genelle Rankin for providing software and assistance performing threshold-independent analysis of LGN innervation and members of the Wright laboratory for insightful discussion and advice throughout the course of this study. This study was funded by the National Institutes of Health Grant R01-NS091027 (K.M.W.), The Whitehall Foundation (K.M.W.), The Medical Research Foundation of Oregon (K.M.W.), National Science Foundation Graduate Research Fellowship Program (R.C.), and LaCroute Neurobiology of Disease Fellowship (R.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Butts DA, Kanold PO, and Shatz CJ (2007). A Burst-Based “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity- Dependent Refinement. PLoS Biology 5, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements R, Turk R, Campbell KP, and Wright KM (2017). Dystroglycan Maintains Inner Limiting Membrane Integrity to Coordinate Retinal Development. J Neurosci 37, 8559–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CD, Howe KM, Nettleton NK, Price DJ, Mason JO, and Pratt, (2011). Heparan sulfate sugar modifications mediate the functions of slits and other factors needed for mouse forebrain commissure development. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 1955– 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell AL, Fried-Cassorla E, Xu H, and Raper JA (2013). cAMP-induced expression of neuropilin1 promotes retinal axon crossing in the zebrafish optic chiasm. J Neurosci 33, 11076–11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, and Chedotal A (2017). Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 545, 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, and Ruhrberg C (2011). VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron 70, 951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisén J, Lu Q, Barbacid M, and Flanagan JG (1998). Topographic Guidance Labels in a Sensory Projection to the Forebrain. Neuron 21, 1303–1313. [DOI] [PubMed] [Google Scholar]
- Furman M, and Crair MC (2012). Synapse maturation is enhanced in the binocular region of the retinocollicular map prior to eye opening. J Neurophysiol 107, 3200–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, and Oliver GC (2000). Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26, 130– 132. [PubMed] [Google Scholar]
- Godement P, Salaun J, and Imbert M (1984). Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol 230, 552–575. [DOI] [PubMed] [Google Scholar]
- Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, and Mason CA (2003). Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell 114, 545–557. [DOI] [PubMed] [Google Scholar]
- Herrera E, Erskine L, and Morenilla-Palao C (2017). Guidance of retinal axons in mammals. Semin Cell Dev Biol [DOI] [PubMed]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, and O’Leary D (2002). EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron 35, 475–487. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Piper M, Fukuhara N, Strochlic L, Cho G, Howitt JA, Ahmed Y, Powell AK, Turnbull JE, Holt CE, et al. (2006). A molecular mechanism for the heparan sulfate dependence of slit-robo signaling. The Journal of biological chemistry 281, 39693–39698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo H, and Kawabata I (2001). Roles of the telencephalic cells and their chondroitin sulfate proteoglycans in delimiting an anterior border of the retinal pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 9304–9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, and Guido W (2005). Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22, 661–676. [DOI] [PubMed] [Google Scholar]
- Kuwajima T, Yoshida Y, Takegahara N, Petros TJ, Kumanogoh A, Jessell TM, Sakurai T, and Mason C (2012). Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 74, 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamey CA, Merlin S, Lattouf P, Sawatari A, Zhou X, Demel N, Glendining KA, Oohashi T, Sur M, and Fassler R (2007). Ten_m3 regulates eye-specific patterning in the mammalian visual pathway and is required for binocular vision. PLoS biology 5, e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Wang J, Chan CK, and Chan SO (2007). Effects of exogenous hyaluronan on midline crossing and axon divergence in the optic chiasm of mouse embryos. The European journal of neuroscience 26, 1–11. [DOI] [PubMed] [Google Scholar]
- Manya H, and Endo T (2017). Glycosylation with ribitol-phosphate in mammals: New insights into the O-mannosyl glycan. Biochimica et biophysica acta 1861, 2462–2472. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, and O’Leary DD (2003). Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, et al. (2002). Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417–422. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. (2002). Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418, 422–425. [DOI] [PubMed] [Google Scholar]
- Osterhout JA, Josten N, Yamada J, Pan F, Wu SW, Nguyen PL, Panagiotakos G, Inoue YU, Egusa SF, Volgyi B, et al. (2011). Cadherin-6 mediates axon-target matching in a non-image-forming visual circuit. Neuron 71, 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, and Gan L (2008). ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development (Cambridge, England) 135, 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Fabre PJ, Dolique T, Swikert SM, Kermasson L, Shimogori T, and Charron F (2018). Sonic Hedgehog Is a Remotely Produced Cue that Controls Axon Guidance Trans-axonally at a Midline Choice Point. Neuron 97, 326–340.e324. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, and Mason CA (2008). Retinal axon growth at the optic chiasm: to cross or not to cross. Annual review of neuroscience 31, 295– 315. [DOI] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, and Tessier-Lavigne M (2002). Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron 33, 219–232. [DOI] [PubMed] [Google Scholar]
- Pratt T, Conway CD, Tian NM, Price DJ, and Mason JO (2006). Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 6911–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JA, and Halloran MC (2006). Semaphorin 3d guides laterality of retinal ganglion cell projections in zebrafish. Development 133, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, et al. (2008). Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nature neuroscience 11, 923–931. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, and Zou Y (2006). Wnt- Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature 439, 31–37. [DOI] [PubMed] [Google Scholar]
- Seabrook TA, El-Danaf RN, Krahe TE, Fox MA, and Guido W (2013). Retinal Input Regulates the Timing of Corticogeniculate Innervation. The Journal of Neuroscience 33, 10085–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks JA, Ito S, Schaevitz L, Yamada J, Chen B, Litke A, and Feldheim DA (2016). Corticothalamic Axons Are Essential for Retinal Ganglion Cell Axon Targeting to the Mouse Dorsal Lateral Geniculate Nucleus. The Journal of Neuroscience 36, 5252–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O’Leary DDM, Hannoush RN, and Tessier-Lavigne M (2012). A Caspase Cascade Regulating Developmental Axon Degeneration. The Journal of Neuroscience 32, 17540–17553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitko AA, Kuwajima T, and Mason CA (2018). Eye-specific segregation and differential fasciculation of developing retinal ganglion cell axons in the mouse visual pathway. The Journal of comparative neurology 526, 1077–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Haner CV, Imbery TE, Brooks JM, Morhardt DR, Gorse K, Guido W, and Fox MA (2011). Reelin is required for class-specific retinogeniculate targeting. J Neurosci 31, 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetterlin P, and Drescher U (2014). Target-independent EphrinA/EphA- mediated axon-axon repulsion as a novel element in retinocollicular mapping. Neuron 84, 740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, Dhande OS, Noda M, Huberman AD, Nathans J, and Kolodkin AL (2015). Functional Assembly of Accessory Optic System Circuitry Critical for Compensatory Eye Movements. Neuron, 1–14. [DOI] [PMC free article] [PubMed]
- Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, et al. (2003). Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet 12, 1449–1459. [DOI] [PubMed] [Google Scholar]
- Taniguchi-Ikeda M, Morioka I, Iijima K, and Toda T (2016). Mechanistic aspects of the formation of α-dystroglycan and therapeutic research for the treatment of α-dystroglycanopathy: A review. Molecular Aspects of Medicine 51, 115–124. [DOI] [PubMed] [Google Scholar]
- Torborg CL, and Feller MB (2004). Unbiased analysis of bulk axonal segregation patterns. J Neurosci Methods 135, 17–26. [DOI] [PubMed] [Google Scholar]
- Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, Eltzschig H, Kania A, Novitch BG, and Butler SJ (2017). Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 94, 790–799.e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, and Henkemeyer M (2003). Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39, 919–935. [DOI] [PubMed] [Google Scholar]
- Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, and Ginty DD (2012). Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron 76, 931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, and Baier H (2011). Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell 146, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, and Evans S (2006). Isl1Cre reveals a common Bmp pathway in heart and limb development. Development (Cambridge, England) 133, 1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kolodkin AL, Wong RO, and James RE (2017). Establishing Wiring Specificity in Visual System Circuits: From the Retina to the Brain. Annu Rev Neurosci 40, 395–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


