Abstract
Drug delivery strategies generally use inert materials, such as high molecular weight polymers, to encapsulate and control the release rate of therapeutic drugs. Diffusion governs release and depends on the ease of permeation of the polymer alongside the device thickness. Yet in applications such as osteoarthritis, the physiological constraints and limited intra-articular joint space prevent the use of large, solid drug delivery implants. Other investigators have explored the use of micro- and nanoparticle drug delivery systems. However, the small size of the systems limits the total drug that may be encapsulated and its short diffusion distance causes rapid release. Ordinarily, the extremely low diffusivity of a polymer fluid would make this an unsuitable delivery system. Our technology takes advantage of specific molecular interactions between drug and polymer, which can control the rate of release beyond diffusion. With this “affinity-based drug delivery”, we have shown that delivery rates from solid polymer can be prolonged from hours and days, to weeks and months. In this paper, we demonstrate that this affinity-based mechanism also applies to low diffusivity fluid-phase polymers. They show release rates that are substantially slower than chemically similar polymers incapable of forming those inclusion complexes. The similarity of this study’s liquid polymers to the viscoelastic fluids used in current clinical practice makes it an ample delivery system for osteoarthritic application. We confirmed the capacity of anti-inflammatory delivery of corticosteroids: hydrocortisone, triamcinolone, and dexamethasone; from both solid implants and polymer fluids. Further, we demonstrated that viscoelastic properties are widely tunable, and within the range of native synovial fluid. Lastly, we determined these polymer fluids have no impact on the differentiation of mesenchymal stem cells to cartilage and are not cytotoxic to a common cell line.
Keywords: Affinity Delivery, Viscosupplements, Osteoarthritis, Corticosteroids, Cyclodextrin, Mesenchymal Stem Cells
Introduction
If conventional knee osteoarthritis (OA) treatments fail, doctors resort to intra-articular injections to alleviate patient pain. The two forms of Federal Drug Administration (FDA) approved intra-articular injections are either symptomatic for pain and inflammation (e.g. corticosteroids), or are intended to be preventative (e.g. viscosupplements).[1] However, the high disease burden coupled with the absence of a cure cause patients to seek treatment even when research shows statistically insignificant pain reduction.[2] Intra-articular corticosteroids injections offer patients fast pain relief but have short residence times due to their low molecular weight, with a half-life shorter than 12 hours within the knee joint space. [2,3] Viscosupplements have a high residence time, due to their high molecular weight, but provide limited pain relief. [4] An ideal system would combine the residence time and delayed need for knee replacement of viscosupplements with the pain relief of corticosteroids injections.
Previous attempts of combining the aforementioned properties into a single drug delivery system by physically mixing corticosteroids and viscosupplement have not resulted in higher residence times. Despite the longer residence time of the viscosupplement component, [5,6] corticosteroids diffuse rapidly out of the mixture [2,7–9] due to the weak nonspecific interactions between the drug and the polymer. As a response, several strategies have emerged to increase the drug residence time in the knee from highly hydrated polymers, such as hyaluronic acid (HA). [10,11] Some general strategies include chemically conjugating drugs to different polymers[12], to the viscosupplement [13] and increasing the viscosity of HA by formulating it as an injectable hydrogel.[14]
Both chemical conjugations onto hyaluronic acid and hydrogel drug encapsulation delivery methods have limitations. The challenges with chemical conjugation are (1) the chemical modification may render the drug inactive, (2) not all drugs are amenable to conjugation depending on the functional groups available on the drug of interest and (3) the mechanism for release (e.g. low pH, change of temperature) may lie outside the physiological state of knee joint even in its arthritic stage. On the other hand, hydrogels are commonly used for the delivery of macromolecules such as peptides and proteins.[15,16] Engineering the sustained release of small molecules from hydrogels in the timeframes needed for OA treatment is notably more difficult. Slower release of small molecules from hydrogels can be achieved by increasing the crosslink density in the polymer. This crosslinking procedure alters the mechanical properties of the hydrogel.[14] Crosslinking may result in a formulation that does not flow through a syringe or does not meet the mechanical properties of the knee.
A viable drug eluting viscosupplement formulation must meet several criteria.[2] First, the release of drug must last for periods longer than those currently provided by physical mixtures of viscosupplements and corticosteroids. Second, the formulation must flow from an 18-22 gauge syringe, the size of syringe used to inject current viscosupplements. Third, the formulation must exhibit a tunable range of viscosities between normal synovial fluid and that of commercially available viscosupplements. Finally, the formulation must exhibit good biocompatibility with the different biological structures in the knee (e.g. synovium, cartilage, ligaments, etc).
To achieve these design specifications, we aimed to develop an injectable fluid formulation with extended corticosteroid release properties by taking advantage of the affinity-based drug inclusion complex properties of cyclodextrins. Like hyaluronic acid, cyclodextrins are composed by a sugar backbone with a ring architecture that allows for inclusion complex formation with a wide array of small molecules.[17] The diameter of the cyclodextrin ring can be varied to allow the binding of molecules of different molecular weights. Previously, we and others have shown that the inclusion of these interactions in insoluble disk polymers results in an increased drug loading,[18,19] reduced initial release burst,[19,20] and prolonged release.[21] Because of the chemical similarity between cyclodextrin and hyaluronic acid, we hypothesized that similar viscosities to viscosupplements could be achieved by titrating the crosslinking density before it forms an insoluble gel. A similar approach is currently used to produce highly viscous crosslinked HA viscosupplements.
We examined different polymer concentration conditions to test the range of viscoelastic properties capable from our formulation. We determined whether the viscous and elastic moduli were similar to native synovial fluid and commercially available viscosupplements could be attained. In addition to incorporating affinity based polymer interactions, we aimed to design the system with a viscoelasticity comparable to native synovial fluid to increase the residence time of the delivery platform itself within the intra-articular joint. The study was required to determine the capability of the designed polymer solution to fulfill the biomechanical functions in the intra-articular cavity as well as increase the residence time of the supplement in the knee cavity.[22] Studies have been performed on hyaluronan and hyaluronic acid derivatives in an attempt to understand the deformation behavior and chemical structure. [23] These studies were important for determining the capability of the designed polymer solution to fulfill the biomechanical functions in the intra-articular cavity and correlate the potential capacity of high residence time of the formulation within the cavity.[22] Also, we explored the practical aspects like the shear rates that the fluid would be subjected to during injection into the joints. Lastly, we investigated the cytotoxicity of the new formulation in a long term exposure assay of differentiating human mesenchymal stem cells (hMSC) and in a short term exposure assay on mouse cells.
Materials and Methods
Lightly epichlorohydrin crosslinked prepolymer of the different forms of cyclodextrins (CD) (α-CD, β-CD, and γ-CD) were purchased from Cyclolab (Budapest, Hungary). The chemically similar, but non-affinity prepolymer, dextran (Mr 15,000- 25,000), was purchased from Sigma-Aldrich(St. Louis, MO). All polymers were vacuum dried. The crosslinker 1,6-hexamethylene diisocyanate was obtained from Sigma-Aldrich(St. Louis, MO). For the polymer formulations all reagents were capped with nitrogen gas from Airgas(Cleveland, OH). All other materials such as solvents were from Fisher Scientific (Hampton, NH).
Binding Affinities
Prior to testing, molecular simulations were conducted to predict whether the chosen drugs (dexamethasone, hydrocortisone and triamcinolone) would form complexes with CD and to determine the strength of the binding affinity. Favorable complexes are desired for increasing the loading capacity as well as extending release. The resulting binding affinities were predicted as previously described.[18] The structure data files for the corticosteroids were obtained from PubChem then converted to Protein Data Bank, Partial Charge (Q), & Atom Type (T)) format (PDBQT) format with Open Babel and docked with the AutoDock Vina algorithm via the PyRx 0.9.4 interface.[24] The PDBQT files for the different cyclodextrins were obtained from the protein data bank repository. The results were reported as kcal/mol where more negative values are indicative of a stronger predicted drug-cyclodextrin interaction.
Polymer formulation
To engineer a viscous and fluid formulation of cyclodextrins, we hypothesized that stopping the isocyanate cyclodextrin curing reaction at its solution gel transition point could yield a polymer that retained its affinity release behavior with rheological properties (e.g. viscosities and moduli) within the ranges of native synovial fluid and current viscosupplements. The liquid polymer was synthesized as follows. A total of 1.5g of prepolymer was massed into a 20mL scintillation vial, covered with a kimwipe held in place by a rubber band and then dried in a vacuum oven for at least four hours. After drying, the polymer was dissolved in 6mL of methyl sulfoxide, a stir bar was added, then the vial was capped with nitrogen gas and finally sealed from ambient air. The scintillation vial was submerged in a 55°C preheated light mineral oil bath subjected to magnetic stirring. After allowing the contents within the vial to equilibrate to the temperature of the oil bath (approximately 15 minutes), the respective amount of crosslinker 1,6-hexamethylene diisocyanate (HDI) shown in table 1 were added. The vial was recapped with nitrogen, sealed with an extra layer of parafilm, and submerged in the oil bath. Every 15 minutes after the addition of crosslinker, a tilt test was performed for the visual indication of the viscous state of the solution. The reaction was stopped when the solution started to coat the walls of the scintillation vial and was resistant to flow. The vial was then filled with 16ml water and vortexed. The vial was left to rotate on a rotisserie overnight to ensure thorough mixing for complete HDI consumption. The next morning, the liquid polymer was pipetted into a regenerated cellulose dialysis bags with a molecular weight cutoff of less than 6500 Da. The polymers were washed in 2 Liters of deionized water for two hours, followed by another cycle of 2 Liters of deionized water for another 2 hours and finally by an overnight wash in 2 Liters of deionized water. The next day, the samples were transferred into 50mL tubes, frozen to −80°C overnight, and lyophilized for three days until completely dry.
Table 1.
Polymer synthesis conditions
| Polymer | Crosslinker Volume (μ1) |
Time (minutes) |
|---|---|---|
| α-CD | 97.5 | 1260 |
| β-CD | 42.0 | 1213 |
| γ-CD | 21.0 | 120 |
| Dextran | 214.0 | 200 |
For the solid polymer disks the formulation was prepared as previously described. [25] Briefly, 1 gram of prepolymer of either Beta, Gamma or Dextran was dissolved in 4 ml of dimethyl sulfoxide(DMSO) to form a prepolymer solution of 25% (w/v). The polymer solution was stirred until completely dissolved. After the prepolymer solution is completely dissolved 154 microliters of 1,6-hexamethylene diisocyanate are added and the reaction is heated at 70 °C until the polymer solidifies. The resulting polymer film is cut with an 8mm circular punch and the resulting polymer disks are washed first in 500 ml of DMSO overnight, then in a second wash of 500ml of equal volumetric parts of DMSO-deionized water overnight and finally in a third wash of 500ml of deionized water for 3 days.
In vitro drug release
We carried different drug delivery experiments to test the hypothesis that a fluid formulation of our affinity polymer could maintain the extended release kinetics of the insoluble disk version. For all the liquid polymer experiments, the system was loaded independently with different corticosteroids (dexamethasone, triamcinolone and hydrocortisone) and their release over time tracked by spectroscopy. In the first release experiment, we investigated the role that varying the cyclodextrin cavity size (α-CD, β-CD, and γ-CD) and varying the ratio of the cyclodextrin pockets (1mg/ml Polymer, 100mg/ml Polymer) to corticosteroid (1mg Total) molecules had on the release kinetics of the different corticosteroids. Three replicates were used for each of the 18 conditions. To load the liquid polymers a drug solution is prepared and used to dissolve the polymer to its target concentration. For example, to prepare 1 replicate of the gamma cyclodextrin polymer at 1mg/ml with hydrocortisone, 3 mg of polymer are measured onto a 15ml tube. To load the 3mg of polymer a total of 3ml of a 0.333mg/ml hydrocortisone solution are pipetted onto the tube which are then transferred to the regenerated carboxymethyl cellulose dialysis bag. This effectively creates a 1mg total drug dose per replicate containing 1mg of polymer. Similarly, for the 100mg/ml polymer condition a total of 300mg of polymer are measured and loaded with 3ml of a 0.333mg/ml hydrocortisone solution. Each separate corticosteroid drug solution consisted of 0.333mg/ml of each individual drug dissolved in 1M PBS supplemented with 0.025%(w/v) sodium dodecyl sulfate (SDS) at pH 7.4. The drug loaded polymer dialysis bags were submerged in a 15ml release solution consisting of 1M phosphate buffered saline (pH 7.4) supplemented with 0.025%(w/v) SDS. At every time point, a 1mL sample was taken for the quantification of drug and the rest of the release solution in the scintillation vial was replaced with 15ml of fresh release solution. The aliquoted samples were frozen at −80°C until later analysis by UV Spectroscopy with an H1 BIOTEK reader (Biotek, Winooski, VT) in a 96 well quartz plate (Hellma GmbH & Co., Müllheim, Germany). The wavelengths used for detection were 250nm for hydrocortisone, 240nm for triamcinolone, and 235nm for dexamethasone. The release kinetic experiments were carried at room temperature for the 18 conditions with 3 replicates per condition.
In the second experiment, we studied the effect that varying temperature and crosslinking state of the polymer had on the release kinetics. Due to their superior capacity to elongate drug release, of the initial 18 experimental groups, only those containing γ-CD were chosen; since these samples could provide the best slope contrast if release was faster at higher temperatures as expected from Eyring theory. To understand the role of the crosslinker, the liquid polymer were compared to starting uncrosslinked prepolymer solutions of each one of the polymer types at the same concentrations as the first experiment (100mg/mL, 1mg/mL) and the same drug loading (1mg corticosteroid per repeat). All release were carried at 37°C for this experiment. Release from disks was carried in a similar manner as described above with some modifications. Each polymer disk was loaded with 1mg of each corticosteroid in DMSO. After loading, the insoluble polymer disks were left to air dry for three days in a chemical fume hood. After three days, the polymers were placed in 1ml of release solution in 1.5ml centrifuge tubes with sampling as described above at 37°C. Release for this experiment was carried with the β-CD, γ-CD and dextran polymers. Data for all the release experiments is presented as the average percent of drug release over time with their corresponding standard deviations. Final corticosteroid release amounts at the end of the experiments are included in the supplementary materials.
Rheology
Rheology tests were conducted to determine the viscoelastic behavior of the polymer solutions under an applied deformation. All experiments were performed on a Paar Physica MCR 501 (Anton Paar, TX). Temperature equilibration was achieved by a CTD 450 oven setup using nitrogen for convection. The geometry of choice was a 55mm parallel plate in which experiments were conducted at a gap of 0.85mm between the plates, requiring about 2mL of sample per run. Oscillatory amplitude sweeps were performed prior to the sweeps at a frequency of 1 Hz within 0.01- 100 strain% range. Oscillatory frequency sweeps from 0.1Hz to 100Hz were followed at an appropriate strain of 4%, which falls within the linear viscoelastic region.
All oscillatory rheometry experiments were performed at 37°C, normal human body temperature. Following necessary cleaning, the fixture and oven were thoroughly equilibrated at the desired temperature before loading the polymer samples. The nitrogen flow was kept to a minimal to avoid skin effects arising from evaporation. Having displayed the most desired release profiles amongst considered polymer systems, oscillatory shear rheology was performed on polymer systems constituting γ-CD at three concentrations: 350mg/mL, 225mg/mL and 200mg/mL. Response to oscillatory deformation was obtained by plotting viscous and elastic moduli and damping function of material. Dynamic viscosity was measured and presented. Necessary fittings and appropriate correction factors have been incorporated and their equations are included in the supplementary materials. To demonstrate feasibility of injection the polymers we carried a flow experiment consisting of passing 1 ml of a solution of 225mg/ml of liquid polymer fluid through a syringe of internal diameter 0.51mm over a period of 10 seconds.
hMSC culture and staining
To determine the cytocompatibility of the fluid formulation for possible future patient treatment, the polymer was added to hMSC cells and tested for cell viability using an MTS assay against NIH 3T3 cells. Primary hMSC cultures were obtained under an approved institutional review board protocol from consented donors by the Tissue Procurement Facility of University Hospitals of Cleveland through a collaboration with the Case Western Reserve University (CWRU) Skeletal Research Center and the CWRU Center for Multimodal Evaluation of Engineered Cartilage. Cells were maintained at 37°C and 5% CO2 in low glucose Dulbecco's Modified Eagle's Medium (DMEM-LG) (Thermo Fisher, Waltham, MA) supplemented with 10% hMSC qualified fetal calf serum. After the second passage, cells were re-suspended in basal pellet medium (BPM) consisting of high glucose DMEM (DMEM-HG), 0.1 μM dexamethasone, 1% ITS+ (Becton Dickinson), 1mM sodium pyruvate, 120μM ascorbic acid-2 phosphate, 100μM non-essential amino acids (Thermo Fisher Scientific, MA) at 1.25* 106 cells/mL. For each well, 200μl (0.25* 106 cells) were pelleted in a non-adherent polypropylene conical bottom 96 well plate. After centrifugation (1640rpm, Beckman for 5 min), BPM was removed and replaced with chondrogenic pellet medium (CPM) (BPM supplemented with 10ng/mL TGF-β1), CPM supplemented with cyclodextrin polymer, CPM with cyclodextrin monomer, or CPM with dextran. On day 1, pellets were detached from sidewalls of the wells by gentle pipetting. Media was removed and replaced with fresh CPM with or without polymer/monomer cyclodextrins every 2-3 days.
Viability assay
To check cell viability after polymer exposure we used the reagent tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] MTS assay, NIH 3T3 cells were grown in culture media consisting of DMEM supplemented with 10% (v/v) FBS and 1% (w/v) Penicillin/Streptomycin. The cells were trypsinized, seeded onto a 96 well plate at 5000 cells/well, and allowed to attach overnight. The next day, the cells were exposed to 100 ul of different concentrations of γ-CD liquid polymer (100.00, 50.00, 25.00, 12.500, 6.25, 3.12, 1.56, 0.78, 0.39, 0.19, 0.10 mg/ml)in culture media. After 48 hours of polymer incubation, 100μL of MTS (Promega, WI) solution was used. After 2 hours of incubation with the reagent, absorbance was measured at 640nm in a H1 spectrophotometer. For each treatment, 8 wells were used and the result are reported against the percent signal against the lowest polymer concentration in culture media.
Statistical Analysis
All calculations were made using the R software for statistical programming. For the cumulative plots, the mean and standard deviation summary statistics are provided. For the theoretical calculations, the values with the lowest root mean squared distances were selected after inspection for physical correctness. The rheological studies were plotted and analyzed in Origin.
Results
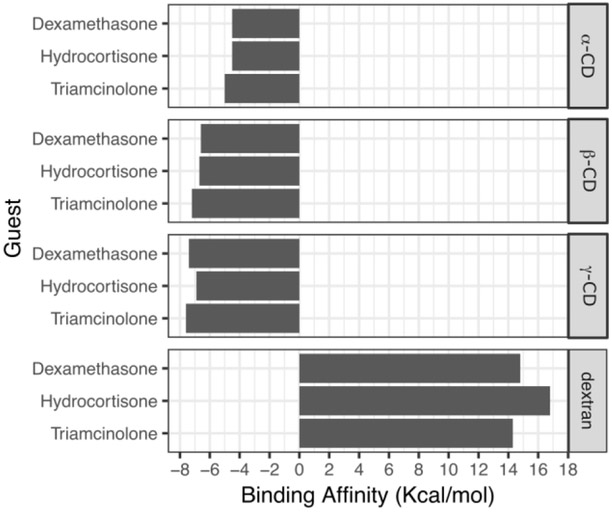
Affinity delivery relies on a high density of molecular interactions mediated by a host structure which must be present at higher fractions than the diffusing drug, in this case the corticosteroids. The extent at which affinity is able to slow the transport of molecules outside the polymer depends on several variables such as the physical dimensions of the device, drug to host ratio and to a large extent on the strength of binding affinity the host molecule has towards the diffusing guest molecule. To calculate the binding affinities between the different polymer vehicles and the different corticosteroids and determine whether sufficient interaction existed to warrant drug delivery studies, we used PyRx, a virtual drug screening software which estimates a theoretical binding affinity by calculating the minimal binding energy of 5 different contributions (bond stretching, bond rotation, bond ionic interactions, van der Waals and hydrophobic) on a per molecule basis. We use the Vina version of the software which provides a rapid calculation of the binding affinity without affecting the predictive performance according to Trott. [[26]] The results of those calculations are shown in Figure 1. The more negative the binding affinity, the stronger the affinity; values lower than −6kcal/mol were accepted as a strong affinity. The docking simulations predicted that dextran would show the weakest drug to polymer interaction. Dextran was intentionally chosen as a non-affinity control because of its similar chemical composition to the cyclodextrin derivatives without the ring architecture. For dextran, the positive binding affinity indicated that the corticosteroids are not likely to form stable drug-polymer complexes to achieve sustained release. In contrast, all cyclodextrins demonstrated favorable binding energies for all corticosteroids. In general, as the cavity size increased so did the strength of the binding affinity. The larger cyclodextrins (β-CD, γ-CD) showed larger affinities than the smallest. Of all the polymer types, γ-CD exhibited the strongest predicted binding affinity to all the corticosteroids. From all the drugs, triamcinolone showed the strongest binding affinities against all cyclodextrin types.
Figure 1.
Predicted strength of binding affinities of different cyclodextrins and dextrans to the different corticosteroids performed with Autodock Vina. Lower values represent a stronger binding affinity between the cyclodextrin and the respective corticosteroid. A cyclodextrin diameter dependant increase in affinity is predicted from the docking simulations. Triamcinolone is predicted to bind the strongest with all polymer types.
In vitro release
For long term pain relief, corticosteroid delivery should be sustained, since the anti-inflammatory effect rapidly disappears after removal of drug, and should last as long as possible since OA is a lifelong condition where frequent drug injections are undesired.[2] We conducted different release experiments to test if the affinity interactions provided by the different cyclodextrin pocket sizes slowed the rate of corticosteroid delivery from our polymer system. We carried drug delivery from solid polymer disks from the largest two cavity sizes since these were predicted to exhibit the strongest binding interaction. This experiment allowed us to rapidly observe the drug-polymer behavior. Cumulative release of the different corticosteroids from these insoluble polymer disks are shown in Figure 2. Corticosteroid release from the polymer disks showed slower release kinetics when delivery was from the cyclodextrin polymers. Saturation occurred at earlier times in the absence of cyclodextrin pockets since all the dextran conditions were depleted of drug before 4 days of delivery. In contrast, corticosteroid release from the cyclodextrin polymers lasted longer than any of the dextran group conditions with the longest release lasting for up to 10 days. The cyclodextrin containing drug release exhibited greater variability in drug release, as well as a characteristic plateau at 100 hours that may reflect polymer network swelling from the disk structure.
Figure 2.
Corticosteroid release from solid affinity polymers (β-CD, blue; γ-CD,green) and non-affinity control polymer (Dextran, gray). Dextran shows classic diffusion based burst in the first three days of release for all corticosteroids. Affinity polymers showed a slower release which validates its further exploration as potential candidates for formulation as an injectable liquid polymer for the intra-articular cavity of corticosteroids.
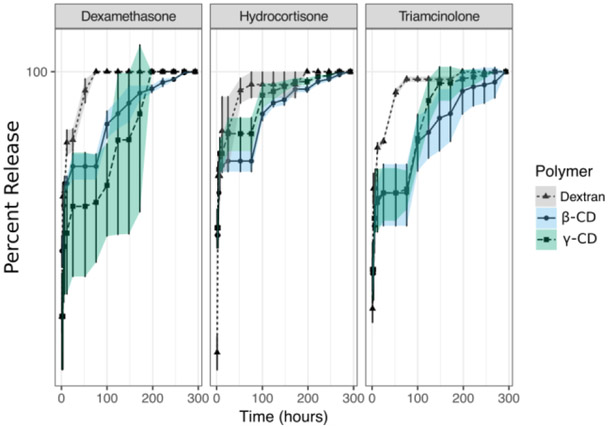
Release of corticosteroids from the insoluble polymer disk provide a rapid method to confirm the viability of using cyclodextrins as an affinity host to release corticosteroids. However, disks are not a clinically viable injectable delivery vehicle for the corticosteroids. Thus, we reformulated the polymer as an injectable fluid and repeated the release experiment. In the first experiment with this fluid formulation, we investigated the full panel of cyclodextrins against the three corticosteroids at room temperature. Figure 3 shows a clear difference in the release kinetics of all corticosteroids when the fluid cyclodextrins polymers are included at high concentrations. At low polymer concentrations the corticosteroids diffuse rapidly into solution from both the cyclodextrin and the dextran conditions. At low polymer concentrations, a classic burst-effect is observed, with most of the drug diffusing out of the formulation in less than two days after release is started, a behavior that was consistent across formulations whether affinity was present or not. Only when the concentration of affinity polymers is high (100mg/ml) a slower release is observed. This behavior was consistently observed for all cyclodextrin-corticosteroid conditions when polymer concentrations were increased but not when the affinity-free dextran control polymer concentrations were increased. Within the affinity containing conditions the cyclodextrin type influences the rate of release. In general, liquid polymers with a high concentration of α-CD released corticosteroids faster than liquid polymers of β-CD or γ-CD. There’s also differences in the shape of the release curves attributable to the type of corticosteroid. Hydrocortisone exhibited a clear linearity at high concentrations of cyclodextrins outlining the general trend that high concentrations of fluid affinity polymers slow the release of corticosteroids explained above. Triamcinolone exhibited drug partitioning, with drug remaining bound to the polymer at high concentrations of cyclodextrin liquid polymer as demonstrated by the lower amount of drug released at the end of the release assay for the high polymer concentrations of the affinity containing liquid polymer conditions and by absorbances consistently below the lower limit of detection of the assay (See supplementary information). For triamcinolone, this partition behavior is not observed for dextran at neither polymer concentration. Dexamethasone, demonstrates a burst release at low concentrations of polymers for the first days of release, a behavior that is consistent with the rest of the drugs. After ten days, the dexamethasone release profile became linear for all polymer types at both high and low concentrations of polymer. The dexamethasone released at the end of the assay also measured above the initial drug loaded into the system. This likely reflected a partition and degradation behavior between the polymer and the drug after the system reached equilibrium. This behavior was specific to dexamethasone and not observed for the other corticosteroids in this experiment and others (Supplementary Table 1 and Table 2).
Figure 3.
Average corticosteroid release from injectable liquid polymer at concentrations of 1mg/mL (Light shade circles) and 100mg/mL (Dark shade triangles) at 25°C. Release of corticosteroids shows a slower release profile at high polymer concentrations in an injectable fluid formulation of cyclodextrin polymers in a clinically relevant timeline of 1 week. High concentrations of liquid polymer shows strong binding to the affinity polymer with no measurable drug release. Release from a chemically similar polymer (dextran) showed burst and depletion in the first three days of release independent of polymer concentration.
Table 2.
Final mass released at the end of the room temperature experiment
| Polymer Type |
Polymer Concentration (mg/ml) |
dexamethasone mass (μg) |
hydrocortisone mass (μg) |
triamcinolone mass (μg) |
|---|---|---|---|---|
| Alpha-CD | 1 | 3159.1 | 1117.7 | 261.3 |
| Beta-CD | 1 | 3545.6 | 813.6 | 469.3 |
| Gamma-CD | 1 | 4175.6 | 906.4 | 429.6 |
| Dextran | 1 | 2954.6 | 776.0 | 504.3 |
| Alpha-CD | 100 | 3357.6 | 722.9 | 81.9 |
| Beta-CD | 100 | 3238.1 | 359.6 | 0.0 |
| Gamma-CD | 100 | 2837.7 | 445.1 | 28.6 |
| Dextran | 100 | 3079.0 | 596.6 | 343.6 |
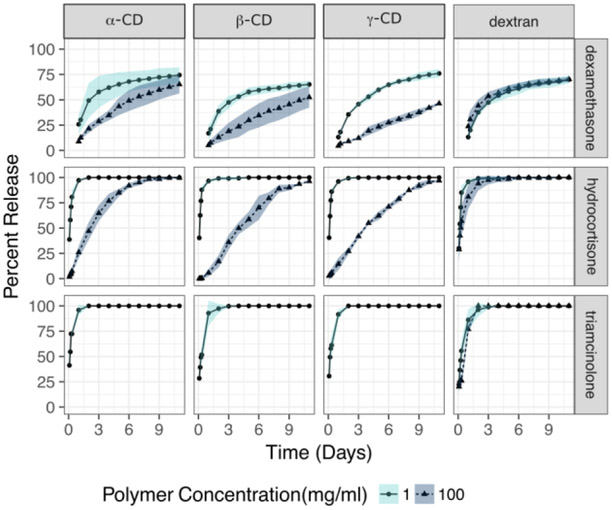
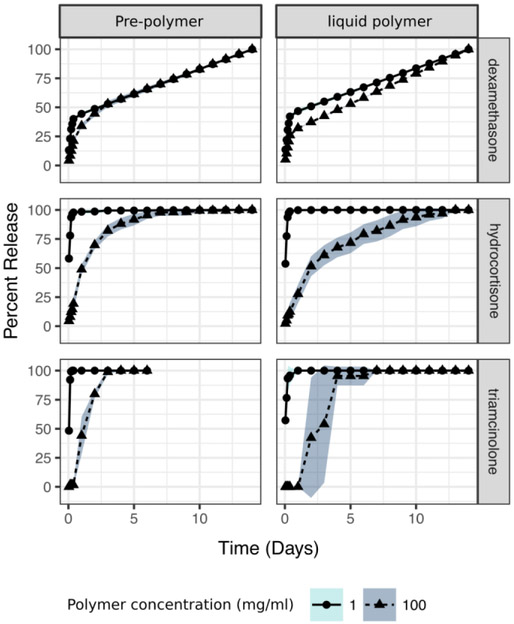
Non covalent affinity interactions such as those governing affinity release behavior are dependent on the temperature at which they occur. Predictions for cyclodextrin binding often assume room temperature conditions. Thus, experiments at this temperature are important for correlating affinity predictions with release. Clinically, release is bound to occur at higher temperatures. Thus, we studied the effects of release from the liquid polymer formulations at higher temperatures (Figure 4). We focused on γ-CD, as this polymer showed the greatest difference in release kinetics between high and low polymer concentrations. Here, we also compared the release profiles with the unreacted starting prepolymers to study the effects of increasing molecular polymer size. Like in Figure 3, we observed a slower release of all corticosteroids at higher concentrations of polymers for the crosslinked and uncrosslinked polymers. Like in the prior experiment, at high concentrations both the prepolymer and the liquid polymer showed a slower release of all corticosteroids. At high concentrations of affinity polymer, release was markedly slower for the crosslinked γ-CD fluid formulation when loaded with dexamethasone and hydrocortisone but not triamcinolone. At higher temperatures, the release was faster for dexamethasone for all γ-CD than at room temperature. At higher temperatures, triamcinolone overcame the partition effect observed at room temperature (Figure 3). If the reader compares Figure 3 and Figure 4 at low polymer concentrations, release of corticosteroids was faster for the low concentrations of γ-CD affinity polymers reflecting the faster diffusivity of the drugs out of the system as evidenced by the time needed to reach depletion.
Figure 4.
Corticosteroid release from starting polymer and γ-CD injectable liquid polymer (crosslinked) at concentrations of 1 mg/mL and 100 mg/mL at 37°C. At higher temperatures release is shortened in a drug dependent fashion. Importantly, a measurable delay in release effect is observed in the uncrosslinked state for all drugs. The effect is stronger for the crosslinked formulation. At higher temperatures triamcinolone overcomes the partition effect at this higher temperature.
Rheology
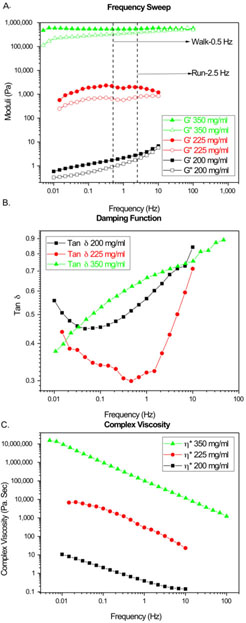
Flow behavior is important in the development of an injectable formulation for the characterization of the formulation, its injectability and long term behavior in the joints. Figure 5A, displayed the elastic and loss modulus of γ-CD liquid polymer formulations in water at concentrations of 350mg/mL, 225mg/mL and 200mg/mL. An increasing trend in both moduli with concentration was seen. Tests were also performed with the starting cyclodextrin prepolymer before crosslinking. The viscosities of these solutions at the sames concentrations as the liquid polymer formulation were low and fell beneath the sensitivity of the instrument with the chosen geometry. The damping function, a measure of viscoelasticity, shows similar shapes at the lower concentrations of polymer and a linear shape at the highest concentration (Figure 5B). Figure 5C displayed the complex viscosity of the polymer solutions over a range of frequencies. All curves showed the expected shear thinning even at high polymer fractions.
Figure 5.
A. Elastic (G’) and Viscous (G”) moduli of γ-CD injectable liquid polymer solution in water subjected to shear deformation B. Damping function as a function of frequency for all polymer solutions tested C. Complex Viscosity of γ-CD injectable liquid polymer solution in water subjected to shear deformation.
The authors would like to point out that there are skin effects due to evaporation observed during analysis for concentrations 225mg/mL and 350mg/mL. The dip in curve for 225mg/mL at 1Hz was the result of this effect.
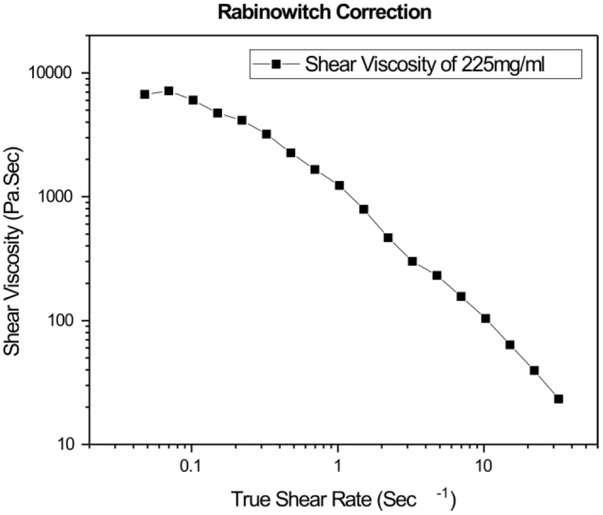
To determine how the system behaves when injected via syringe, shear rates of polymer flow have been deduced by imposing a Rabinowitsch correction from the apparent shear rates as shown in Figure 6, using the relevant equations in the supplement. To calculate the shear rates experienced by the polymer through the syringe needle, a power law fluid flow model has been fitted to account for shear thinning which takes into account the deviation from Newtonian behavior. Corresponding shear rates have been obtained by taking flow rates into consideration. It is known that current clinical setups for injecting viscosupplements employ a syringe with an internal diameter of 0.838mm for passing 2ml of fluid for a 10 seconds period. Our experiment involved similar parameters passing 1ml of fluid through a syringe with an internal diameter of 0.51mm over a period of 10 seconds. A relevant non- Newtonian flow and corresponding Rabinowitsch correction (Figure 6) was accounted for by calculating the shear rates at the wall. With the incorporation of the Rabinowitsch correction, we deduced that the current clinical setups would emulate a shear rate of 11254 sec−1 at the wall and our syringe experiment corresponds to imposed shear rate of 24966 sec −1 at the wall. The provided video demonstrated the feasibility of flow within the range of current standards and shows viscous resistance pooling. (Supplementary Video 1)
Figure 6.
Rabinowitsch correction is applied on apparent viscosity to account for the non-Newtonian behavior.
Cytotoxicity
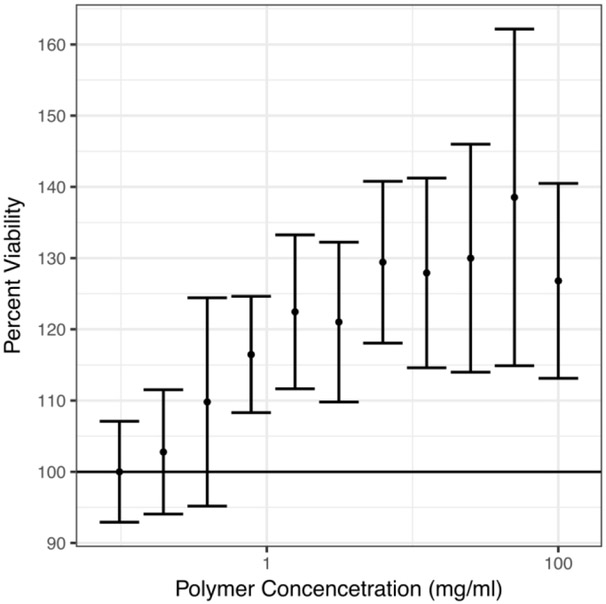
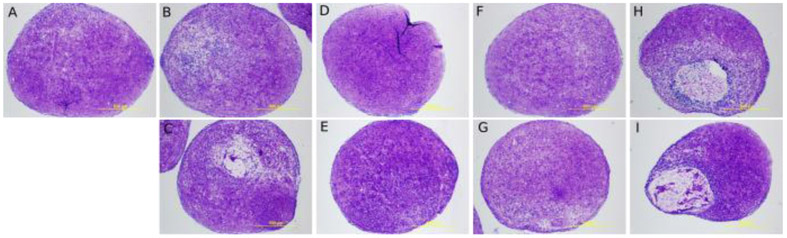
In order to know whether our polymers were overtly toxic, they were incubated with 3T3 cells and cell viability was determined by an MTS assay in the in half dilutions (100.00, 50.00, 25.00, 12.500, 6.25, 3.12, 1.56, 0.78, 0.39, 0.19, 0.10 mg/ml). The observed responses were standardized to the average of the minimum response. There was no measurable cytotoxicity at these concentrations. As polymer concentration increased the viability of the cells increased until 50mg/ml. (Figure 7) For clinical application, the polymer formulation must not harm the viability of human cells under long term exposures. To study long term exposure human mesenchymal stem cell aggregates were cultured with 1.0mg/mL of the injectable liquid polymer for 28 days in a chondrogenic differentiation assay. The pellets were stained with toluidine blue to check for morphology differences across conditions. The blue stain indicates the presence of proteoglycans.[27] As seen in Figure 8, there were no observed morphological differences in the gross appearance or diameter between carriers except for dextran, which suggest the affinity-based polymers were not cytotoxic to the cells.
Figure 7.
MTS assay of 3T3 cells exposed to serial dilutions of injectable γ-CD liquid polymer and normalized to the average of the lowest treatment concentration shows an increase in viability as polymer is increased.
Figure 8.
hMSC chondrocyte pellet differentiation assay where cells were exposed to 1mg/mL of injectable liquid polymer and stained with toluidine blue after 28 days of culture with the different crosslinked polymer formulations A. Carrier Control B,C. α-CD liquid polymer D,E. β-CD liquid polymer F,G. γ-CD liquid polymer H,I. Dextran crosslinked.
DISCUSSION
We developed an injectable liquid polymer that allows for sustained corticosteroid release, which is not currently possible by physically combining viscosupplements and corticosteroids. Other strategies to slow the release of drugs in the knee have relied on the chemical conjugation of drug to the viscosupplement or on the micronization of the drug to achieve sustained release. Instead of chemical conjugation or micronization, our system relied on the high density of cyclodextrin mediated affinity interactions within the polymer to slow the rate of corticosteroid transport, a behavior that should hold true for virtually any small molecule as long as the affinity interaction is strong enough. Combining the affinity polymer at high concentrations with three clinically relevant corticosteroids resulted in weeklong release profiles. To achieve a fluid state with sustained release, we crosslinked a cyclodextrin pre-polymer to its sol-gel transition point. After processing to remove solvents and unreacted crosslinker, the resulting polymer exhibited viscosities spanning the range of native synovial fluid and current clinically available viscosupplements in a concentration dependent manner. Importantly, the concentrations at which the polymer exhibits extended release and clinically relevant viscosities caused no measurable cytotoxicity.
To counter the quick drug release from viscosupplements, we increased the density and varied the strength of molecular interaction between the polymer carrier and the corticosteroids. Our lab and others have demonstrated that including a high density of affinity interactions in a polymer matrix results in substantial delays of a variety of clinically applicable drugs. To demonstrate this affinity release controlled behavior holds for corticosteroids, we released corticosteroids out of solid polymer disks made entirely out of one of the two most common cyclodextrin ring sizes (Figure 2). The disk polymers released measurable amounts of corticosteroids for 4 days, a timeframe not possible in the absence of an affinity component as shown by dextran controls in that same figure. To achieve an injectable version of our polymeric drug delivery system, we carried out the same polymerization we use to obtain disks but stopped the reaction at its sol-gel transition point (Table 1). The resulting polymer could be re-suspended up to 350mg/mL in water forming a viscous fluid with the capacity to flow through a 21-gauge needle, a common size used to inject viscosupplements into the knee. (Supplementary Video)
To achieve sustained release with this injectable formulation, we found the affinity interactions must be present at high concentrations. To demonstrate this behavior, we carried out a release experiment at different polymer concentrations while keeping the amount of loaded drug the same. High polymer concentrations of 100mg/mL exhibited a gradual sustained release of both hydrocortisone and triamcinolone for all cyclodextrin-containing polymers, but not for the dextran controls. Accordingly, low polymer concentrations of 1 mg/mL of liquid polymers resulted in a rapid release from all affinity containing polymer conditions for both drugs with release profiles, closely mimicking the high and low concentrations of the non-affinity dextran control. The difference in release behavior between the different cyclodextrin sizes further showed the effect of affinity strength on the corticosteroid release kinetics. For the high concentration condition, the linear portion of the β-CD and γ-CD release lasted 7.5 days as compared to the faster 5-day span seen from α-CD. The observations from these in vitro experiments go in accordance with the molecular docking strength of binding predictions where β-CD, and γ-CD are predicted to form stronger inclusion complexes than α-CD.
Crosslinking produced slower release rates without increasing the bulk viscosity. When release was carried with polymers not subjected to crosslinking, the release pattern followed that of the crosslinked version, high concentrations slowed the rate of release and low concentrations released as fast as dextran. However, this crosslinker free release was shorter than the crosslinked version of affinity polymers at high concentrations pointing to a crosslinking mediated effect in the rate of release. This experiment demonstrated that a high density of interactions by itself, without crosslinking, is enough to shift the shape of the release profile. We hypothesized that the further delay originated from local contributions the crosslinked cyclodextrin network. The crosslinked hard segments having less chain motility could increase the binding strength between the drugs and the cyclodextrin containing liquid polymer. A possible explanation was that increasing the crosslinking state of the polymer changes the diffusivity of the corticosteroids by altering the viscosity of the bulk solution. However, this scenario was ruled out because at the concentrations that we carried the drug release the polymers showed no measurable viscosity (Figure 5). There were differences in the release curve shape and variability between the disk and liquid polymer conditions which may have arisen from the fundamental geometrical differences between the two formulations such as the contributions diffusion path distance, swelling and microscopic pore structure of the disks.
We also found the drug to partition in the polymer network depending on the identity of the drug and the concentration of the polymer. This difference in total amount of drug released between hydrocortisone and triamcinolone reflected the higher solubility of hydrocortisone. Likely, the high density of crosslinked cyclodextrins provided a stable depot for drug that was more favorable than solution at higher concentrations of the crosslinked polymer. According to Eyring theory, affinity interactions are influenced by temperature.[28,29] To understand the effect of temperature, we carried out a release experiment at room temperature (25°C) and at body temperature (37°C). In accordance with the theory, we found that increasing the temperature shortens the linear portion of corticosteroid release in a drug dependent fashion. Dexamethasone reached the linear portion of the release in a much shorter time frame, whereas hydrocortisone timeframe remained similar at both temperatures. This observation is important, as corrections may need to be performed in predictive models, as many of the experimental determinations of affinity are carried out at 25°C.[30]
The rheological properties of the formulation are important for the injection of the formulation, its residence time within the synovial joint and for the proper function of the joint after polymer injection. Shear response of the polymer solution for the three concentrations tested showed an elastic moduli dominated behavior. Walking and running motions correspond to frequencies of 0.5 Hz and 2.5 Hz respectively.[31] The curves for both 200 mg/mL and 350 mg/mL endpoints showed too low and too high of a moduli respectively in the desired range. When a 225mg/mL concentration was tested the solution displayed moduli about an order higher than desired, as shown in Figure 5A. If matching the elastic moduli to synovial fluid is necessary, the liquid polymer concentrations needed should be between 200 and 225mg/ml to achieve the native synovial fluid rheological set point. Other hyaluronic acid derivatives and cyclodextrin derivatives have shown moduli in the range of 20-100Pa for frequencies between 0.5Hz and 2.5Hz.[23,31,32] Complex viscosity plots, shown in Figure 5B, displayed a three orders difference in magnitude between solutions. The wide range of viscosities demonstrated that our polymer solution may achieve the span of viscosities exhibited by native synovial fluid and up to that of the commercial viscosupplements. For the current viscosupplements there is a two orders of magnitude difference between the viscosity of non-crosslinked hyaluronic acid of a high molecular weight compared to a crosslinked hyaluronic acid as reported by Bhuanamantanondh.[31]
All experiments in Figure 5 were performed without any drug incorporation into the polymer. We hypothesized that the low weight fraction of therapeutic was incapable of inciting significant differences interactions with the polymer structure. When drug was added to the formulation we found the complex viscosity to behave similarly in the absence and presence of drug. The dynamic and loss moduli demonstrate lower response at lower shear frequencies in the presence of drug. At higher frequencies the dynamic and loss moduli behave in a similar fashion (Supplementary Figure S1). This may reflect different polymer drug interactions at the walking and running frequencies of the loaded polymer as shown below (Supplementary Figure S1). The polymer solutions tested show a gel-like behavior in the desired region of frequencies (0.5-2.5Hz) where the storage modulus was greater than the loss modulus. In addition, the crossover of moduli seemed to shift to higher frequencies as concentration increased. This effect may be attributed to the increase in entanglements with higher concentrations. Both behaviors have been reported earlier with similar polymeric systems.[23,31] Similarly, it can be seen that both moduli and complex viscosity increased by three orders in magnitude from 200mg/mL to 225mg/mL and another three orders from 225mg/mL to 350mg/mL. Although more experiments at different concentrations are needed to determine at which concentration this inflection takes place, we theorized that it happened between 200mg/mL and 225mg/mL. This crossover behavior has been demonstrated to a lesser extent with other cyclodextrin based solutions.[23] Experiments revealed that the coil overlap parameter played a role in this crossover point. This phenomenon happens at a certain concentration where individual coils of the polymer come close enough to entangle and simulate the behavior of larger polymer coils in solution.[32] These rheological studies are relevant for decision making at the synthesis step. For example, if the material is lacking elastic modulus, one can aim to build a polymer with higher molecular weight and vice versa. Likewise, if an earlier crossover between moduli is desired, exploring branching on the polymer backbone could be a possible route. Currently, our system displays a crossover frequency above that of healthy human synovial fluid; however, the shift of crossover to lower frequencies can be achieved by incorporating rheological modifiers, which will impart more viscoelastic behavior to a more gel-like behavior.[32]
We also investigated the behavior of the the damping function (tan δ), another measure of viscoelasticity, for our polymeric system. This function is a ratio of the loss modulus to the storage modulus of a material and quantifies how a given material absorbs and disperses energy and remains understudied in the viscosupplement formulation literature. Higher tan δ values correspond to a greater amount of energy dispersed rather than gained and vice versa. If equal to 1, there is an equal amount of energy that is gained and dispersed. Figure 5B, showed the tan δ values for the three concentrations tested. Damping function values were similar for the 200mg/mL and 350mg/mL concentrations and lower for 225mg/mL solution with very dissimilar shape between the lower and higher concentrations. Further experiments in vivo at different ranges of concentrations are needed to ascertain as to which damping function behavior is desired and the biological role of this analysis. At lower concentrations this behavior is U shaped and at higher concentrations the behavior is linear. We believe that this shift in the shape of the damping function likely reflects the intermolecular differences in interaction at the different initial viscosity region (200mg/ml of Polymer) and solubility ends of the polymer (350mg/ml of Polymer).
The shear thinning behavior following a Newtonian regime in its terminal region presented in Figure 6 corresponded to that reported for non-crosslinked high molecular weight hyaluronic acid and crosslinked hyaluronic acid. [31] This behavior was expected as polymer solutions are shear thinning because they align and decrease their entanglement density with imposed shear. We employed a mathematical correction to account for the deviation from Newtonian behavior in the syringe flow experiment. This Rabinowitsch correction was required because flow through a syringe is in essence capillary pipe flow in which non-Newtonian behavior should be incorporated if a power law behavior of the material is observed.
The polymers exhibited no overt signs of cytotoxicity in two different assays of short term and long term exposure. For the long term exposure assay the polymers were incubated for 28 days in a hMSC chondrocyte pellet differentiation assay. Human MSCs are promising candidate as a potential source of cartilage regeneration and have been used to validate materials in vitro. [33] This allows us to observe if synthesis substances such as DMSO or unreacted isocyanate were being slowly released from the polymer causing adverse biological effects. We find no evidence of the polymers causing morphological changes in the appearances or the diameters of the pellets were observed. Because the pellet experiments were carried at low doses of polymer, we carried a similar experiment with NIH 3T3 cells at higher concentrations. In this cell line, concentrations of polymers above those that showed drug release behavior exhibited no measurable cytotoxicity. Surprisingly, as the concentration of polymer was increased the viability of the cells increased. This process could happen because the cells are uptaking the cyclodextrin polymer or some other factor altering the ratios of nutrient in the media that is currently unknown. Using these new affinity polymers embedded within the matrix of the culture could represent a new tool in the culture of hMSCS to engineer and study the development of functional cartilage and other tissues of clinical interest as a function of the rate of drug release. The superior capacity of affinity polymers to tune release rates depending on the choice of cavity and polymer could be of particular use for its in vitro study and development of lab engineered tissues from hMSC and other differentiable cell sources.
Recently, viscosupplement use has been associated with a delayed need for a first total knee replacement.[34] We determined that viscosupplements of our polymer system are in close agreement with reported behavior of hyaluronic acid and cyclodextrin derivatives.[22,23] Our polymers may allow for the exploration of formulations with new rheological properties while sustaining release of relevant pharmacological agents or have the release triggered by an endogenous or exogenous stimuli.[35] Similar work has been done in the design of small conjugates for environmental water treatment and found preferential loading of active pharmaceutical ingredients from certain crosslinkers over others.[36,37] This preferential loading may be a factor of molecular recognition provided by the cyclodextrin to crosslinker combination or alternatively, by an alteration of the thermodynamic parameters that drive the complexation behavior. Avenues for incorporating structural changes within precursors, addition of modifiers and optimizing polymerization reaction conditions should be explored in an attempt to engineer novel viscoelastic behavior, residence time and improved pharmacokinetics for this new class of affinity-based viscosupplements.
Conclusion
The prevalence and rise of osteoarthritis with the aging population demands improvements of current treatment methods to deliver extended relief. However, engineering the slow release of small molecules from highly hydrated polymers remains difficult. Rapid diffusion of corticosteroid drug out of delivery platforms and the short residence time of the devices within the joint itself only provide short-term relief. Prior works of including viscosupplements to increase the residence time have only partially achieved the goal as burst release still occurs while the delivery system itself remains in the joint longer. Our work demonstrated three important strides towards the development of an osteoarthritic treatment that incorporates the pain and inflammation reduction from corticosteroids with the residence time of viscosupplements for long-term relief. Our works showed that the moduli behavior of our liquid polymer solution at high concentrations matched that of natural synovial fluid and existing viscosupplements. Further, the formulation achieved the shear rates of flow through a syringe observed in current clinical practice. In conclusion, we project that there will be a higher residence time within the intra-articular joint in comparison to current practices as it mimics the natural properties of the fluid within the body.
Supplementary Material
Table 3.
Average final mass released at the end of the 37°C temperature experiment for Gamma Cyclodextrin
| Polymer Type | Polymer Concentration (mg/ml) |
dexamethasone mass (μg) |
hydrocortisone mass (μg) |
triamcinolone mass (μg) |
|---|---|---|---|---|
| Pre-polymer | 1 | 3303.2 | 816.1 | 502.9 |
| Viscous | 1 | 3454.1 | 1003.1 | 379.3 |
| Pre-polymer | 100 | 3322.2 | 784.7 | 233.9 |
| Viscous | 100 | 2707.1 | 614.9 | 64.4 |
Acknowledgements
This work was supported by an ENGAGE research grant from the National Center for Regenerative Medicine awarded to AD; ERD was supported by a National Institute of Health grant [T32-AR007505]. The polymer cytocompatibility work was performed at the CWRU Center for Multimodal Evaluation of Engineered Cartilage and funded by the NIH/NIBIB grant [P41EB021911].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gladstone S, Viscosupplementation for Osteoarthritis of the Knee, N. Engl. J. Med. 372 (2015) 2569. [DOI] [PubMed] [Google Scholar]
- [2].Wehling P, Evans C, Wehling J, Maixner W, Effectiveness of intra-articular therapies in osteoarthritis: a literature review, Ther. Adv. Musculoskelet. Dis. 9 (2017) 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Evans CH, Kraus VB, Setton LA, Progress in intra-articular therapy, Nat. Rev. Rheumatol 10 (2014) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mathieu P, Conrozier T, Vignon E, Rozand Y, Rinaudo M, Rheologic behavior of osteoarthritic synovial fluid after addition of hyaluronic acid: a pilot study, Clin. Orthop. Relat. Res. 467 (2009) 3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brown TJ, Laurent UB, Fraser JR, Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit, Exp. Physiol. 76 (1991) 125–134. [DOI] [PubMed] [Google Scholar]
- [6].Migliore A, Giovannangeli F, Granata M, Laganà B, Hylan g-f 20: review of its safety and efficacy in the management of joint pain in osteoarthritis, Clin. Med. Insights Arthritis Musculoskelet. Disord. 3 (2010) 55–68. [PMC free article] [PubMed] [Google Scholar]
- [7].de Campos GC, Rezende MU, Pailo AF, Frucchi R, Camargo OP, Adding triamcinolone improves viscosupplementation: a randomized clinical trial, Clin. Orthop. Relat. Res. 471 (2013) 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miyamoto K, Yasuda Y, Yoshioka K, Hyaluronic acid derivative and drug containing the same, 7879817, 2011. https://www.google.com/patents/US7879817 (accessed September 4, 2017).
- [9].Larsen C, Ostergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, Andersen PH, Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders, J. Pharm. Sci. 97 (2008) 4622–4654. [DOI] [PubMed] [Google Scholar]
- [10].Tamura T, Higuchi Y, Kitamura H, Murao N, Saitoh R, Morikawa T, Sato H, Novel hyaluronic acid--methotrexate conjugate suppresses joint inflammation in the rat knee: efficacy and safety evaluation in two rat arthritis models, Arthritis Res. Ther. 18 (2016) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Larson N, Ghandehari H, Polymeric conjugates for drug delivery, Chem. Mater. 24 (2012) 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cyphert EL, Wallat JD, Pokorski JK, von Recum HA, Erythromycin Modification That Improves Its Acidic Stability while Optimizing It for Local Drug Delivery, Antibiotics (Basel). 6 (2017). doi: 10.3390/antibiotics6020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gianolio DA, Philbrook M, Avila LZ, Young LE, Plate L, Santos MR, Bemasconi R, Liu H, Ahn S, Sun W, Jarrett PK, Miller RJ, Hyaluronan-tethered opioid depots: synthetic strategies and release kinetics in vitro and in vivo, Bioconjug. Chem. 19 (2008) 1767–1774. [DOI] [PubMed] [Google Scholar]
- [14].Zhang Z, Wei X, Gao J, Zhao Y, Zhao Y, Guo L, Chen C, Duan Z, Li P, Wei L, Intra-Articular Injection of Cross-Linked Hyaluronic Acid-Dexamethasone Hydrogel Attenuates Osteoarthritis: An Experimental Study in a Rat Model of Osteoarthritis, Int. J. Mol. Sci. 17 (2016) 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rivera-Delgado E, Sadeghi Z, Wang NX, Kenyon J, Satyanarayan S, Kavran M, Flask C, Hijaz AZ, von Recum HA, Local release from affinity-based polymers increases urethral concentration of the stem cell chemokine CCL7 in rats, Biomed. Mater. 11 (2016) 025022. [DOI] [PubMed] [Google Scholar]
- [16].Sakiyama-Elbert SE, Incorporation of heparin into biomaterials, Acta Biomater. 10 (2014) 1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suzuki T, Ishida M, Fabian WM, Classical QSAR and comparative molecular field analyses of the host-guest interaction of organic molecules with cyclodextrins, J. Comput. Aided Mol. Des. 14 (2000)669–678. [DOI] [PubMed] [Google Scholar]
- [18].Rivera-Delgado E, Ward E, von Recum HA, Providing sustained transgene induction through affinity-based drug delivery, J. Biomed. Mater. Res. A. 104 (2016) 1135–1142. [DOI] [PubMed] [Google Scholar]
- [19].Thatiparti TR, von Recum HA, Cyclodextrin complexation for affinity-based antibiotic delivery, Macromol. Biosci. 10 (2010) 82–90. [DOI] [PubMed] [Google Scholar]
- [20].Vulic K, Pakulska MM, Sonthalia R, Ramachandran A, Shoichet MS, Mathematical model accurately predicts protein release from an affinity-based delivery system, J. Control. Release. 197 (2015) 69–77. [DOI] [PubMed] [Google Scholar]
- [21].Mealy JE, Rodell CB, Burdick JA, Sustained small molecule delivery from injectable hyaluronic acid hydrogels through host-guest mediated retention, J. Mater. Chem. B Mater. Biol. Med 3 (2015) 8010–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dahl LB, Dahl IM, Engström-Laurent A, Granath K, Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies, Ann. Rheum. Dis. 44 (1985) 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chariot A, Auzély-Velty R, Novel Hyaluronic Acid Based Supramolecular Assemblies Stabilized by Multivalent Specific Interactions: Rheological Behavior in Aqueous Solution, Macromolecules. 40 (2007) 9555–9563. [Google Scholar]
- [24].Dallakyan S, Olson AJ, Small-molecule library screening by docking with PyRx, Methods Mol. Biol. 1263 (2015)243–250. [DOI] [PubMed] [Google Scholar]
- [25].Fu AS, Thatiparti TR, Saidel GM, von Recum HA, Experimental studies and modeling of drug release from a tunable affinity-based drug delivery platform, Ann. Biomed. Eng. 39 (2011) 2466–2475. [DOI] [PubMed] [Google Scholar]
- [26].Trott O, Olson AJ, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem. 31 (2010) 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geyer G, Linss W, Toluidine blue staining of cartilage proteoglycan subunits, Acta Histochem. 61 (1978)127–134. [DOI] [PubMed] [Google Scholar]
- [28].Eyring H, The Activated Complex in Chemical Reactions, J. Chem. Phys. 3 (1935) 107–115. [Google Scholar]
- [29].Evans MG, Polanyi M, Some applications of the transition state method to the calculation of reaction velocities, especially in solution, Transactions of the Faraday Society. 31 (1935) 875. [Google Scholar]
- [30].Rekharsky MV, Inoue Y, Complexation Thermodynamics of Cyclodextrins, Chem. Rev. 98 (1998) 1875–1918. [DOI] [PubMed] [Google Scholar]
- [31].Bhuanantanondh P, 10.5405/jmbe.834, J. Med. Biol. Eng. 32 (2012) 12. doi: 10.5405/jmbe.834. [DOI] [Google Scholar]
- [32].Balazs EA, Therapeutic use of hyaluronan, Struct. Chem. 20 (2009) 341–349. [Google Scholar]
- [33].Kang M-L, Kim J-E, Im G-I, Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis, Acta Biomater. 39 (2016) 65–78. [DOI] [PubMed] [Google Scholar]
- [34].Waddell DD, Bricker DC, Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis, J. Manag. Care Pharm. 13 (2007) 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cyphert EL, Fu AS, von Recum HA, Featured Article: Chemotherapeutic delivery using pH-responsive, affinity-based release, Exp. Biol. Med. . 242 (2017) 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xiao P, Corvini PF-X, Dudal Y, Shahgaldian P, Design and high-throughput synthesis of cyclodextrin-based polyurethanes with enhanced molecular recognition properties, Polym. Chem. 4 (2013)942–946. [Google Scholar]
- [37].Xiao P, Corvini P, Dudal Y, Shahgaldian P, Design of Cyclodextrin-Based Photopolymers with Enhanced Molecular Recognition Properties: A Template-Free High-Throughput Approach, Macromolecules. 45 (2012) 5692–5697. [Google Scholar]
- [38].Barnes HA, A Handbook of Elementary Rheology, 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.