Abstract
Background
Relapsed and refractory acute myeloid leukemia (RR-AML) still poses major treatment concerns. Current treatments include high doses of cytarabine or fludarabine in combination with cytarabine and G-CSF (FLAG), but provide mixed results. Low-dose decitabine, a hypomethylating drug, in combination with aclarubicin and cytarabine (DAC) has shown safety and efficacy in the treatment of AML; however, clinical data are limited for the treatment of RR-AML.
Methods
In this study, we retrospectively compared the response and safety of DAC vs FLAG for RR-AML patients.
Results
For the 35 patients with RR-AML enrolled in this study, the overall response rates reached 100% and 55.6% in the DAC group and FLAG group, respectively (P=0.002). Complete response rates after DAC and FLAG treatment were 64.7% and 33.3%, respectively (P=0.002). Median overall survival (95% CI) of the DAC treatment group was significantly higher than for the FLAG group (median not achieved vs 16.8 months, P=0.021).
Conclusion
DAC treatment was also more effective in those patients with poor prognosis, suggesting that DAC resulted in a better outcome for RR-AML treatment. In conclusion, in our study, DAC therapy provided more safety and effectiveness and lower toxicity in the treatment of RR-AML compared to FLAG therapy.
Keywords: AML, refractory diseases, relapsed diseases, decitabine, chemotherapy
Introduction
Leukemia is a clonal disease of hematopoietic stem cells, and it is a malignant tumor that seriously threatens human lives.1 Acute myeloid leukemia (AML) is a common type of leukemia that occurs in both children and adults. It is characterized by a rapidly exploding population of aberrant white blood cells that accumulate and lead to decreased production of normal blood cells. Currently, treatments for AML include standard chemotherapy with anthracycline plus cytarabine, which has resulted in unfavorable survival rates.2–4 Significant numbers of patients cannot be remitted during initial treatment or have relapsed, so-called relapsed and refractory AML (RR-AML) patients. It is these difficult cases on which the emphasis of AML research is currently focused.
Since the determination of the optimal chemotherapy regimen still requires further evaluation, many novel therapies have been attempted to treat RR-AML.5 In a previous report, a regimen of fludarabine in combination with cytara-bine and G-CSF (FLAG) was used with some success to manage RR-AML,6 but the hematological toxicity and the high costs of the treatment constrain its clinical application. An alternative approach for the treatment of RR-AML is decitabine (5-aza-2′-deoxycytidine), a DNA methyl-transferase inhibitor.7,8 Based on the results from multiple clinical trials, decitabine has been approved by the United States food and drug administration as a frontline treatment of elderly patients with myelodysplastic syndrome, due to their increased survival after remission and high tolerability toxicity profile.9–11 It has also been approved by the European Medicines Agency for the treatment of elderly patients with untreated AML since a study has shown its safety and effectiveness.12 The clinical effectiveness of decitabine as a transition drug for allogeneic stem cell transplantation has been studied;8,13,14 and a first report of a combination of low-dose decitabine with aclacinomycin and cytarabine (DAC) for treatment of adult refractory AML patients provided a substantially positive clinical outcome.15 In adult patients, especially the elderly, the efficacy and safety of a regimen of DAC plus G-CSF has been extensively proven for newly diagnosed AML and thus holds promise as a treatment for RR-AML.16 However, clinical data are still limited to verify the value of DAC plus G-CSF as the primary treatment choice for RR-AML, and as superior to standard chemotherapy.
In our study, we retrospectively compared the treatment outcome of DAC vs FLAG in the management of RR-AML, in order to find a more effective and safe treatment strategy for RR-AML patients. We report here valuable clinical evidence for improving the prognosis of RR-AML patients.
Materials and methods
Patients
This retrospective study was approved by the ethics committee of Harbin Medical University. All participants have signed the informed consent forms. We retrospectively reviewed a total of 35 consecutive patients with RR-AML who received either FLAG or DAC treatment regimen as induction therapy in the Institute of Hematology & Oncology of Heilongjiang Province from August 2006 to April 2014. These patients were diagnosed by the following the World Health Organization criteria: bone marrow (BM) or peripheral blood blasts ≥20%. Blood samples from patients with AML were used to detect the existence of fusion genes and mutations, while cytogenetic analysis was performed on BM samples. Refractory AML was diagnosed by the following criteria: 1) patients with AML for whom complete response (CR) could not be achieved after two courses of administration of a standard combined chemotherapy regimen (anthracycline plus cytarabine or homoharringtonine plus cytarabine); or 2) patients whose BM blasts did not decrease by more than 50% after one course of standard chemotherapy. Relapsed AML was defined as the reappearance of leukemic blasts in the peripheral blood, more than 5% blasts in the BM after CR was achieved (not attributable to other causes), or extramedullary relapse.
Treatment
All experiments were performed in accordance with relevant guidelines and regulations. When diagnosed with RR-AML, the patients were randomly assigned to FLAG or DAC therapy for one cycle. No previous transplantations had been performed before the therapy. Eighteen RR-AML patients received the standard FLAG regimen as reinduction remission treatment based on the Chinese guidelines for the diagnosis and treatment of RR-AML.17 The treatment regimen was as follows: intravenous injection of 30 mg/m2 fludarabine (Bayer, Leverkusen, Germany) on days 1–5; intravenous injection of 1–2 g/m2 cytarabine (Pfizer, New York, NY, USA) over 4 hours on days 2–6 after completion of the fludarabine injection; and intravenous injection of 200 µg/m2 G-CSF (Amoytop Bio, Xiamen, People’s Republic of China) on days 1–6. Alternatively, seventeen RR-AML patients were treated with low-dose decitabine (Chiatai Tianqing, Jiangsu, People’s Republic of China) combined with aclarubicin (Main Luck, Shenzhen, People’s Republic of China) and cytarabine (DAC regimen). The treatment regimen was carried out as follows: intravenous injection of 15 mg/m2 (low-dose) decitabine over 3 hours on days 1–3; and intravenous injection of 10 mg/m2 aclarubicin and 100 mg/m2 cytarabine over 4 hours on days 2–6 after completion of the decitabine injection. Depending on the BM blast number, with a cutoff of a 50% decrease18 on day 6, some patients had their treatment prolonged until day 8, with the same treatment as days 2–6.
Treatment response analysis
The parameters used to evaluate the treatment response include overall response (OR) and overall survival (OS). The OR can be divided into two parts: CR and partial response (PR). Treatment responses were defined according to standard BM morphological response criteria by the 2003 AML International Working Group.19 Chemotherapy-related myelosuppression was evaluated by the duration of agranulocytosis and the amount of blood transfused during the course of treatment.
Statistical analysis
Treatment response was assessed by descriptive χ2 analysis. Differences in the agranulocytosis period between two groups were analyzed by an independent samples t-test. The treatment effect on OS rate was analyzed by Kaplan–Meier curves. OS rate was defined as the number of months from initiation of treatment with FLAG or DAC therapy to death from any cause. All data analyses were generated using IBM SPSS statistics software (Version 20.0, IBM Corporation, Armonk, NY, USA). A P<0.05 was considered as statistically significant.
Results
Patient characteristics
A total of 35 RR-AML patients treated at the Institute of Hematology & Oncology of Heilongjiang Province between August 2006 and April 2014 met the criteria for our research. For the 35 patients involved in this study, the median age was 28 years (range 6–67 years), with 20 (57%) being male and 15 (43%) female (Table 1). The result of the peripheral white blood cell count was from 0.23 to 297.9×109/L at the primary diagnosis. Among those patients, 20 patients had FLT-3 mutations, 13 had NPM1 mutations, and 33 had WT1 mutations. Fourteen patients had documented poor-risk cytogenetics, as defined by the National Comprehensive Cancer Network Guidelines For Acute Myeloid Leukemia, version 1. 2014, including complex (≥3 clonal chromosomal abnormalities), monosomal karyotype, -5, 5q-, -7, 7q-, 11q23-non t(9;11), inv(3), t(3;3), t(6;9), t(9;22), and normal cytogenetics with FLT3-ITD mutation. Twenty patients had hepatosplenomegaly before treatment. All patients had received standard chemotherapy of anthracycline and cytarabine as a frontline therapy without previous transplantation. When diagnosed with RR-AML, patients were given either standard FLAG or low-dose DAC treatment regimens as induction therapy as permitted by an open agreement. Eighteen patients who received the FLAG regimen had a median age of 20 years (range 10–63 years), and 17 patients who received DAC treatment had a median age of 30 years (range 6–67 years).
Table 1.
Characterization of patients with RR-AML in FLAG and DAC groups
| Characteristics | Overall | FLAG
|
DAC
|
||
|---|---|---|---|---|---|
| Number of patients | % | Number of patients | % | ||
| Patients | 35 | 18 | 51.4 | 17 | 48.6 |
| Classification | |||||
| Refractory | 23 | 12 | 83.3 | 11 | 64.7 |
| Relapsed | 12 | 6 | 16.7 | 6 | 35.3 |
| Age (years) | |||||
| <20 | 15 | 8 | 44.4 | 7 | 41.2 |
| 20–39 | 8 | 5 | 27.8 | 3 | 17.6 |
| 40–60 | 8 | 4 | 22.2 | 4 | 23.5 |
| >60 | 4 | 1 | 5.6 | 3 | 17.6 |
| Gender | |||||
| Male | 20 | 10 | 55.6 | 10 | 58.8 |
| Female | 15 | 8 | 44.4 | 7 | 41.2 |
| Initial WBC count (×109) | |||||
| <4 | 9 | 6 | 33.3 | 3 | 17.6 |
| 4–10 | 7 | 3 | 16.7 | 4 | 23.5 |
| >10 | 19 | 9 | 50.0 | 10 | 58.8 |
| Initial cytogenetics | |||||
| Normal | 14 | 8 | 44.4 | 6 | 35.3 |
| Abnomal | 21 | 10 | 55.6 | 11 | 64.7 |
| Fusion genes | |||||
| AML1/ETO | 5 | 4 | 22.2 | 1 | 5.9 |
| CBFB-MYH11 | 2 | 2 | 11.8 | ||
| ETO | 4 | 2 | 11.1 | 2 | 11.8 |
| EVI1 | 1 | 1 | 5.9 | ||
| HOX11 | 2 | 1 | 5.6 | 1 | 5.9 |
| P210 | 3 | 2 | 11.1 | 1 | 5.9 |
| PML-Rara | 1 | 1 | 5.9 | ||
| MLL-AF9 | 1 | 1 | 5.6 | ||
| Mutation | |||||
| WT1 | 33 | 17 | 94.4 | 16 | 94.1 |
| FLT3 | 20 | 10 | 55.6 | 10 | 58.8 |
| NPM1 | 13 | 5 | 27.8 | 8 | 47.1 |
| Subtypes | |||||
| M1 | 1 | 1 | 5.6 | ||
| M2 | 22 | 13 | 72.2 | 9 | 52.9 |
| M3 | 1 | 1 | 5.9 | ||
| M4 | 4 | 1 | 5.6 | 3 | 17.6 |
| M5 | 4 | 3 | 16.7 | 1 | 5.9 |
| Unknown | 3 | 3 | 17.6 | ||
| Hepatolienal characteristics | |||||
| Hepatomegaly | 2 | 1 | 5.6 | 1 | 5.9 |
| Splenomegaly | 13 | 5 | 27.8 | 8 | 47.1 |
| Hepatosplenomegaly | 5 | 2 | 11.1 | 3 | 17.6 |
| Others | 15 | 10 | 55.6 | 5 | 29.4 |
| Frontline therapy | |||||
| HAD | 32 | 18 | 100 | 14 | 82.4 |
| DA | 10 | 2 | 11.1 | 8 | 47.1 |
| MA | 14 | 7 | 38.9 | 7 | 41.2 |
| IA | 10 | 5 | 27.8 | 5 | 29.4 |
| CAG | 3 | 1 | 5.6 | 2 | 11.8 |
| ATO | 1 | 1 | 5.9 | ||
| FA | 1 | 1 | 5.9 | ||
| Remission duration after frontline therapy | |||||
| <3 months | 6 | 4 | 22.2 | 2 | 11.8 |
| 3–6 months | 5 | 1 | 5.6 | 4 | 23.5 |
| >6 months | 1 | 1 | 5.6 | ||
| Therapeutic response | |||||
| CR | 17 | 6 | 33.3 | 11 | 64.8 |
| PR | 10 | 4 | 22.2 | 6 | 35.3 |
| NR | 8 | 8 | 44.4 | 0 | 0 |
| Consolidated therapy | |||||
| Chemotherapy | |||||
| HAD | 4 | 2 | 11.1 | 2 | 11.8 |
| CAG | 1 | 1 | 5.6 | ||
| IA | 5 | 1 | 5.6 | 4 | 23.5 |
| DA | 2 | 2 | 11.8 | ||
| MA | 2 | 2 | 11.8 | ||
| MAE | 1 | 1 | 5.9 | ||
| alloSCT | |||||
| In remission | 9 | 6 | 33.3 | 3 | 17.6 |
| Outside remission | 2 | 2 | 11.1 | ||
| Treatment-related mortality | 1 | 1 | 5.6 | ||
Abbreviations: ATO, arsenic trioxide; CAG, low-dose cytarabine, aclarubicin and G-CSF; CR, complete remission; DA, daunorubicin and cytarabine; DAC, low-dose decitabine with aclacinomycin and cytarabine; FA, fludarabine and cytarabine; FLAG, fludarabine in combination with cytarabine and G-CSF; HAD, homoharringtonine, cytarabine and daunorubicin; IA, idarubicin and cytarabine; MA, mitoxantrone and cytarabine; MAE, mitoxantrone, cytarabine and etoposide; NR, no response; PR, partial response; RR-AML, relapsed/refractory acute myeloid leukemia; SCT, stem cell transplantation; WBC, white blood cell count.
The average age of patients in the two groups was not significantly different (P=0.384), 25.4 years in the FLAG therapy group and 32.2 years in the DAC therapy group. The initial diagnosis white blood cell data for the two groups was also not significantly different (P=0.453), 24.3 and 38.9 (×109/L), respectively. We also found no significant differences in the detailed genetics and molecular abnormalities of the patients in the two groups: poor-risk cytogenetics (39% vs 41%, P=1.000), WT1 mutations (94% vs 94%, P=1.000), FLT-3 mutations (56% vs 59%, P=1.000), and NPM1 mutations (28% vs 47%, P=0.238). Furthermore, 11% and 18% of patients in the FLAG and DAC groups, respectively, had hepatosplenomegaly at diagnosis (P=0.581).
Response rates for all patients or selected subgroups
In the FLAG group, 10 (55.6%) patients had an OR to the treatment, with 6 (33.3%) CR and 4 (22.2%) PR reported. In the DAC group, 17 (100%) patients showed an OR, with 11 (64.7%) patients with CR and 6 (35.3%) patients with PR. The difference in OR and CR rates between the two treatment groups were statistically significant (OR, P=0.002; CR, P=0.002) (Table 2).
Table 2.
Response of RR-AML patients with FLAG and DAC treatment
| Response | FLAG group (n=18) |
DAC group (n=17) |
Total (n=35) |
P-value |
|---|---|---|---|---|
| NR (%) | 8 (44.4) | 0 (0) | 8 (22.8) | – |
| OR (%) | 10 (55.6) | 17 (100) | 27 (77.1) | 0.002 |
| CR (%) | 6 (33.3) | 11 (64.7) | 17 (48.6) | 0.002 |
Abbreviations: CR, complete response; DAC, low-dose decitabine with aclacinomycin and cytarabine; FLAG, fludarabine in combination with cytarabine and G-CSF; NR, no response; OR, overall response; RR-AML, relapsed/refractory acute myeloid leukemia.
Enrolled patients were followed up for about 2 years. During 16.2 months of median follow-up (range from 4.5 to 30.1), 19 patients died of cancer (54.3%). Significantly fewer died in the DAC treatment group than in the FLAG group, 6 patients (35.3%) and 13 (72.2%), respectively (P=0.028). Among 17 RR-AML patients who were given DAC treatment, median OS (95% CI) was not achieved. We subsequently compared the OS of patients in the two groups and found the difference between the median OS of DAC therapy and FLAG therapy groups was statistically significant (not achieved vs 16.8 months, P=0.021) (Figure 1).
Figure 1.
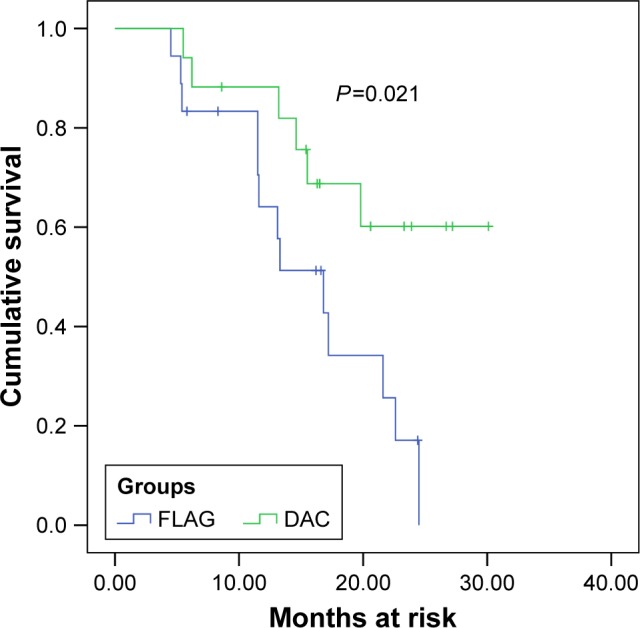
Kaplan–Meier plot of OS for FLAG and DAC regimen groups.
Notes: The blue line represents the FLAG group; the green line represents the DAC group.
Abbreviations: DAC, low-dose decitabine with aclacinomycin and cytarabine; FLAG, fludarabine in combination with cytarabine and G-CSF; OS, overall survival.
In addition, we compared the two treatment responses in RR-AML patients who had poor-risk cytogenetics and mutations status (Table 3), conditions that often correlate with poor prognosis. We found that RR-AML patients with poor-risk cytogenetics, WT1 or FLT-3 mutations, in the DAC treatment group achieved higher CR rates compared with those in the FLAG treatment group (P=0.040, 0.002 and 0.038, respectively), indicating that DAC treatment was also more effective in those RR-AML patients who had a poor prognosis. Considering that the difference is not significant between the CR rates of the two treatments in RR-AML patients with NPM1 mutations, which may be caused by the limited number of patients involved, we performed a power analysis (G*Power software, version 3.1.9.2, Franz Faul, Universität Kiel, Kiel, Germany), which showed that a total of 15 patients with NPM1 mutation needs to be recruited (data not shown).
Table 3.
FLAG and DAC treatment response in RR-AML patients who had poor-risk cytogenetics and mutation status
| Response | Mutations | FLAG group | DAC group | Total | P-value |
|---|---|---|---|---|---|
| NR (%) | Poor-risk cytogenetics | 4 (57.1) | 0 (0) | 4 | – |
| WT1 | 8 (47.1) | 0 (0) | 8 | – | |
| FLT-3 | 4 (40.0) | 0 (0) | 4 | – | |
| NPM1 | 3 (60.0) | 0 (0) | 3 | – | |
| OR (%) | Poor-risk cytogenetics | 3 (42.9) | 7 (100) | 10 | 0.018 |
| WT1 | 9 (52.9) | 16 (100) | 25 | 0.002 | |
| FLT-3 | 6 (60.0) | 10 (100) | 16 | 0.025 | |
| NPM1 | 2 (40.0) | 8 (100) | 10 | 0.013 | |
| CR (%) | Poor-risk cytogenetics | 1 (14.3) | 4 (57.1) | 5 | 0.040 |
| WT1 | 6 (35.3) | 11 (68.8) | 17 | 0.002 | |
| FLT-3 | 3 (30.0) | 5 (50.0) | 8 | 0.038 | |
| NPM1 | 2 (40.0) | 4 (50.0) | 6 | 0.058 |
Abbreviations: CR, complete response; DAC, low-dose decitabine with aclacinomycin and cytarabine; FLAG, fludarabine in combination with cytarabine and G-CSF; NR, no response; OR, overall response; RR-AML, relapsed/refractory acute myeloid leukemia.
Treatment-related myelosuppression
Treatment-related myelosuppression in the two treatment groups was comparable. After treatment with FLAG therapy, we found the duration of agranulocytosis to be 14.2±5.1 days (range, 0–21 days) and 12.6±7.4 days in the DAC therapy group (P=0.437). Since hemoglobin levels were below 60 g/L and platelet counts were <20×109/L, patients required red blood cell (RBC) and platelet transfusions to maintain their normal physiological functions. In the FLAG group, the mean RBC and platelet transfusion required was 10.0×109 cells/L (range, 0–14×109 cells/L) and 98.9×109 cells/L (range, 0–140×109 cells/L), respectively. The mean RBC and platelet transfusion in the DAC treatment group was 7.06×109 cells/L (range, 0–16×109 cells/L) and 72.9×109 cells/L (range, 0–200×109 cells/L), respectively. Statistical differences were not seen between the two groups (P=0.636 and P=0.844, respectively). Infection occurred with the resultant body temperature higher than 38°C in six and five patients, respectively, in the two groups. No deaths occurred due to chemotherapy-related toxicity.
Discussion
AML is a heterogeneous disease caused by a range of genetic defects.20 RR-AML patients often have a poor prognosis. An increasing number of studies have been performed on the effect of decitabine treatment in different patient groups, and most have achieved satisfactory clinical outcomes.9,12,21–23 Furthermore, relapsed AML patients, following autologous stem cell transplantation (auto-SCT) and allogeneic stem cell transplantation (allo-SCT), benefited from decitabine treatment.24,25 Although FLAG and DAC therapies have been studied for many years, very few were performed specifically to investigate the relative advantages of DAC therapy for RR-AML compared with standard FLAG chemotherapy.
Here, we retrospectively compared 35 patients with RR-AML treated with either FLAG or a regimen of DAC. We found that both OR and CR rates in the DAC group were significantly higher than those in the FLAG group. The CR rate in the DAC group was higher than previously reported,15 but in the FLAG group, the CR rate was lower than previously reported.26 Furthermore, we found better OS in the patients receiving DAC therapy during follow-up evaluations. DAC treatment was also more effective in those RR-AML patients who had a poor prognosis such as high-risk cytogenetics or WT1 or FLT-3 mutations. We had too few patients with NPM1 mutations to draw definite conclusions, so more eligible patients should be recruited for further investigation. Although therapy-related myelosuppression and the incidence of severe infections were similar with both treatments, we saw a trend that patients receiving DAC had relatively less toxicity and higher overall effectiveness. Thus, low-dose decitabine in combination with low-dose chemotherapy drugs may reduce the discomfort of patients during treatment. More importantly, we have demonstrated that RR-AML patients undergoing DAC therapy showed statistically better outcomes compared to those receiving the FLAG regimen. However, considering the small sample size in this study due to the rare occurrence of RR-AML, our result may underestimate the difference between treatment outcomes in different subgroups, classified by parameters such as age and status of genetic mutations, and should be further validated by additional studies involving more patients. Moreover, the genetic profiles of AML in children are known to differ from those in adults, including NPM1, FLT3-ITD27,28 which show significant influence in prognosis for AML. The treatment strategies should be further explored in various age groups of the RR-AML population.
Hypomethylating agent decitabine incorporates into DNA and forms irreversible covalent bonds with DNA methyltransferases, leading to their degradation with the resultant activation of genes involved in differentiation and apoptosis. In our study, the patients were given decitabine 1 day before treating with aclarubicin and cytarabine. Pretreatment with decitabine causes a state of hypomethylation in leukemia cells, increasing their sensitivity to chemotherapeutic drugs, aiding in their clearance. Aclarubicin is an anthracycline antibiotic which functions by inserting into DNA and interacting with topoisomerase I and II, thereby inhibiting DNA replication and DNA repair.29 In addition, all patients were given low-dose cytarabine four hours after decitabine for at least 6 hours. This continuous small amount of DNA synthesis inhibitor produced the lowest toxicity and greatest treatment effect. Our data show that our DAC regimen provided a good complete remission rate and no chemotherapy-related death. Thus, a significant advantage was demonstrated for DAC compared to FLAG treatment. More studies including more patients are warranted to verify our results.
Conclusion
We retrospectively compared the outcomes of FLAG and DAC treatment in patients with RR-AML. We found that DAC was an effective therapeutic regimen for the initial treatment of RR-AML, providing better outcomes than FLAG. DAC also showed a significantly better drug response than FLAG in RR-AML patients with WT1, FLT3, or NPM1 mutations. The DAC regimen may also provide a survival strategy for patients who do not achieve CR through classic FLAG chemotherapy.
Acknowledgments
This research was financially supported by the Heilongjiang Youth Science and Technology Fund (QC05C44).
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Juliusson G, Hough R. Leukemia. Prog Tumor Res. 2016;43:87–100. doi: 10.1159/000447076. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour EJ, Estey E, Kantarjian HM. Adult acute myeloid leukemia. Mayo Clin Proc. 2006;81(2):247–260. doi: 10.4065/81.2.247. [DOI] [PubMed] [Google Scholar]
- 4.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 5.Park H, Youk J, Kim I, et al. Comparison of cladribine- and fludarabine-based induction chemotherapy in relapsed or refractory acute myeloid leukaemia. Ann Hematol. 2016;95(11):1777–1786. doi: 10.1007/s00277-016-2774-z. [DOI] [PubMed] [Google Scholar]
- 6.Huhmann IM, Watzke HH, Geissler K, et al. FLAG (fludarabine, cytosine arabinoside, G-CSF) for refractory and relapsed acute myeloid leukemia. Ann Hematol. 1996;73(6):265–271. doi: 10.1007/s002770050239. [DOI] [PubMed] [Google Scholar]
- 7.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21(35):5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54(9):2003–2007. doi: 10.3109/10428194.2012.762093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 10.Ball B, Zeidan A, Gore SD, Prebet T. Hypomethylating agent combination strategies in myelodysplastic syndromes: hopes and shortcomings. Leuk Lymphoma. 2017;58(5):1022–1036. doi: 10.1080/10428194.2016.1228927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong VH, Komrokji RS, List AF. Update on the pharmacotherapy for myelodysplastic syndromes. Expert Opin Pharmacother. 2014;15(13):1811–1825. doi: 10.1517/14656566.2014.937705. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik P, Cashen AF. Decitabine in the treatment of acute myeloid leukemia in elderly patients. Cancer Manag Res. 2014;6:53–61. doi: 10.2147/CMAR.S40600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kröger N, Stübig T, Atanackovic D. Immune-modulating drugs and hypomethylating agents to prevent or treat relapse after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(2):168–172. doi: 10.1016/j.bbmt.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Song LX, Xu L, Li X, et al. Clinical outcome of treatment with a combined regimen of decitabine and aclacinomycin/cytarabine for patients with refractory acute myeloid leukemia. Ann Hematol. 2012;91(12):1879–1886. doi: 10.1007/s00277-012-1550-y. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Chen Y, Zhu Y, et al. Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget. 2015;6(8):6448–6458. doi: 10.18632/oncotarget.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J, Chinese Society of Hematology, Chinese Medical Association Chinese guidelines for the diagnosis and treatment of relapsed and refractory acute myelogenous leukemia. Zhonghua Xue Ye Xue Za Zhi. 2011;32(12):887–888. [PubMed] [Google Scholar]
- 18.Zhu HH, Jiang H, Jiang B, et al. Cytarabine, aclarubicin and granulocyte colony-stimulating factor regimen represents an effective and safe salvage regimen for patients with acute myeloid leukemia refractory to first course of induction chemotherapy. Leuk Lymphoma. 2013;54(11):2452–2457. doi: 10.3109/10428194.2013.776679. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Estey E. Acute Myeloid Leukemia – Many Diseases, Many Treatments. N Engl J Med. 2016;375(21):2094–2095. doi: 10.1056/NEJMe1611424. [DOI] [PubMed] [Google Scholar]
- 21.Phillips CL, Davies SM, Mcmasters R, et al. Low dose decitabine in very high risk relapsed or refractory acute myeloid leukaemia in children and young adults. Br J Haematol. 2013;161(3):406–410. doi: 10.1111/bjh.12268. [DOI] [PubMed] [Google Scholar]
- 22.Issa JP, Garcia-Manero G, Huang X, et al. Results of phase 2 randomized study of low-dose decitabine with or without valproic acid in patients with myelodysplastic syndrome and acute myelogenous leukemia. Cancer. 2015;121(4):556–561. doi: 10.1002/cncr.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, He G, Depei W, et al. Outcome of dose-adjusted Decitabine regimen compared with FLAG regimen in the treatment of relapsed/refractory acute myeloid leukemia. Blood. 2013;122(21):5031. [Google Scholar]
- 24.Ustun C, Kalla A, Farrow S, Deremer DL, Jillella A. Decitabine as “bridge therapy” to a MUD transplant in relapsed AML postautologous stem cell transplantation. Am J Hematol. 2008;83(10):825–827. doi: 10.1002/ajh.21267. [DOI] [PubMed] [Google Scholar]
- 25.Ganguly S, Amin M, Divine C, Aljitawi OS, Abhyankar S, Mcguirk JP. Decitabine in patients with relapsed acute myeloid leukemia (AML) after allogeneic stem cell transplantation (allo-SCT) Ann Hematol. 2013;92(4):549–550. doi: 10.1007/s00277-012-1607-y. [DOI] [PubMed] [Google Scholar]
- 26.Lee SR, Yang DH, Ahn JS, et al. The clinical outcome of FLAG chemotherapy without idarubicin in patients with relapsed or refractory acute myeloid leukemia. J Korean Med Sci. 2009;24(3):498–503. doi: 10.3346/jkms.2009.24.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan PS, Ihsan R, Singh LC, Gupta DK, Mittal V, Kapur S. Mutation of NPM1 and FLT3 genes in acute myeloid leukemia and their association with clinical and immunophenotypic features. Dis Markers. 2013;35(5):581–588. doi: 10.1155/2013/582569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer. 2016;122(24):3821–3830. doi: 10.1002/cncr.30220. [DOI] [PubMed] [Google Scholar]
- 29.Hajji N, Mateos S, Pastor N, Domínguez I, Cortés F. Induction of genotoxic and cytotoxic damage by aclarubicin, a dual topoisomerase inhibitor. Mutat Res. 2005;583(1):26–35. doi: 10.1016/j.mrgentox.2005.01.012. [DOI] [PubMed] [Google Scholar]


