Abstract
Background:
Term equivalent age (TEA) brain MRI identifies preterm infants at risk for adverse neurodevelopmental outcomes. But some infants may experience neurodevelopmental impairments even in the absence of neuroimaging abnormalities.
Objective:
Evaluate the association of TEA amplitude-integrated EEG (aEEG) measures with neurodevelopmental outcomes at 24–36 months corrected age.
Methods:
We performed aEEG recordings and brain MRI at TEA (mean post-menstrual age of 39 (± 2) weeks in a cohort of 60 preterm infants born at a mean gestational age of 26 (± 2) weeks. Forty-four infants underwent Bayley Scales of Infant Development, 3rd Edition (BSID-III) testing at 24–36 months corrected age. Developmental delay was defined by a score greater than one standard deviation below the mean (< 85) in any domain. An ROC curve was constructed and a value of SEF90 < 9.2, yielded the highest sensitivity and specificity for moderate/severe brain injury on MRI. The association between aEEG measures and neurodevelopmental outcomes was assessed using odds ratio, then adjusted for confounding variables using logistic regression.
Results:
Infants with developmental delay in any domain had significantly lower values of SEF90. Absent cyclicity was more prevalent in infants with cognitive and motor delay. Both left and right SEF90 < 9.2 were associated with motor delay (OR left: 4.7(1.2–18.3), p = 0.02, OR right: 7.9 (1.8–34.5), p < 0.01). Left SEF90 and right SEF90 were associated with cognitive delay and language delay respectively. Absent cyclicity was associated with motor and cognitive delay (OR for motor delay: 5.8 (1.3–25.1), p = 0.01; OR for cognitive delay: 16.8 (3.1–91.8), p < 0.01). These associations remained significant after correcting for social risk index score and confounding variables.
Conclusions:
aEEG may be used at TEA as a new tool for risk stratification of infants at higher risk of poor neurodevelopmental outcomes. Therefore, a larger study is needed to validate these results in premature infants at low and high risk of brain injury.
Keywords: Amplitude-integrated EEG, SEF90, Cyclicity, Neurodevelopmental outcome
1. Background
Preterm birth remains a significant problem in the United States, with a current rate of 11%. Multiple efforts are being directed towards decreasing the preterm birth incidence to a rate of 9.6% by 2020 [1]. There is a trend towards increase in survival of very preterm infants at the expense of increased long-term morbidities and disabilities [2]. Intraventricular hemorrhage (IVH) and white matter injury (WMI) continue to be important contributors of poor neurodevelopmental outcomes in this population. Primary care physicians and parents seek information on prediction of psychomotor and cognitive outcomes, both at discharge or early during follow up, for early institution of intervention services and helping this subset of population to achieve their best potential.
Magnetic resonance imaging (MRI) has emerged as an important tool for prediction of neurodevelopmental outcomes. White and gray matter abnormalities on MRI correlate well with childhood diagnoses of cerebral palsy and cognitive impairment with a reasonable specificity of 76–89% [3,4]. Despite this, a significant number of preterm infants experience neurodevelopmental impairments even in the absence of neuroimaging abnormalities, reflecting the importance of exploring other measures that may help with prediction of outcomes. In addition, MRI acquisition poses some practical issues such as transport of preterm infants out of the neonatal intensive care unit (NICU), the high cost of MRI, required personnel to perform and read the imaging studies.
An additional tool that accurately identifies brain injury and predicts neurodevelopmental outcomes at term equivalent age (TEA) would allow for better stratification of infants at risk of disability or developmental delay. Electroencephalography (EEG) and amplitude-integrated EEG (aEEG) are additional tools commonly used in the NICU to study the functional maturation of the preterm brain. They allow for objective measurements of brain function and may aid in accurate and reproducible assessment of the brain function.
The utility of amplitude-integrated EEG has been studied extensively in preterm infants. Measures from studies performed in the first days of life, such as continuity, cyclicity, and spectral edge frequency (SEF90), correlate with short- and long-term neurodevelopmental outcomes [5–7]. Early absence of cyclicity and lower SEF90 in the first week of life are predictive of intraventricular hemorrhage and white matter damage [7,8]. Other authors studied the predictive value of early aEEG (background pattern, cyclicity and presence of seizures) for neurodevelopmental outcomes at 3 years of age. Some of these studies did not take into account the various clinical confounders in the NICU, as well as the socio-economic status after discharge, both of which may affect outcomes [9]. A recent study showed that brain injury on MRI at TEA in premature infants is closely correlated with delay in brain functional maturation on term aEEG measures [10]. In addition, no prior studies have evaluated the role of aEEG at TEA for prognostication.
Therefore, the objective of this study is to evaluate the association of maturational measures as assessed by aEEG at TEA, with neurodevelopmental outcomes at 24–36 months corrected age in preterm infants born at < 30 weeks of gestational age (GA). We hypothesized that delayed maturation of aEEG measures such as cyclicity and SEF90 at TEA are associated with developmental delay at 24–36 months corrected age.
2. Methods
2.1. Study population
Infants in this study were selected from a cohort of babies recruited into a TEA neuroimaging study. To meet criteria for recruitment, infants had to meet the following criteria: 1-born at < 30 weeks GA, 2TEA MRI with moderate to severe injury, 3-TEA aEEG on same day as MRI. Infants were excluded if they were diagnosed with congenital infections or congenital brain malformations. The remaining 28/60 infants in the cohort were matched by gestational age at birth and enrolled from a control group of premature infants with none/mild injury on a TEA MRI as part of the same neuroimaging study.
Then, they were evaluated at 24–36 months corrected age using the Bayley Scales of Infant Development, 3rd edition. The study was conducted with approval from Washington University School of Medicine Human Research Protection Office.
Data regarding perinatal and neonatal factors were collected from each infant’s medical record including: gestational age at birth, birth weight, small for gestational age status, and clinical risk index for babies (CRIB-II) score [11]. Clinical variables included the use of oxygen therapy at 36 weeks post menstrual age (PMA), patent ductus arteriosus requiring treatment, postnatal steroids (hydrocortisone or dexamethasone), retinopathy of prematurity requiring surgery, surgical necrotizing enterocolitis, inotrope use (dopamine or dobutamine), sedative exposure (fentanyl, midazolam or morphine) at TEA (Table 1).
Table 1.
Clinical characteristics of the cohort.
| Variable | Cohort (N = 44) |
|---|---|
| Maternal characteristics | |
| Ethnicity | Caucasian: 23 (52.3%), Black: 21 (47.7%) |
| Maternal age | < 20 years: 7 (15.9%), > 20 years: 37 (84.1%) |
| Illicit drug use | None: 40 (91%), at least one: 4 (9%) |
| Marital status | Married: 17 (38.6%), Single: 27 (61.4%) |
| Insurance | Private: 20 (45.5%), Public: 24 (54.5%) |
| Birth weight in grams (mean ± sd) | 856 ( ± 280) |
| Gestational age (mean ± sd) | 26 ( ± 2) |
| PMA at study (mean ± sd) | 39 ( ± 2) |
| SGA status, n (%) | 6 (13.6%) |
| Antenatal steroids, n (%) | 29 (66%) |
| CRIB-II score (mean ± sd) | 11 ( ± 3) |
| NEC surgery, n (%) | 7 (15.9%) |
| PDA ligation, n (%) | 6 (13.6%) |
| ROP surgery, n (%) | 13 (29.5%) |
| Post-natal steroids, n (%) | 15 (34.1%) |
| Oxygen therapy at 36 wk., n (%) | 34 (77.3%) |
| Inotropes, n (%) | 21 (47.7%) |
| IVH grade 3–4, n (%) | 19 (43.2%) |
| Moderate/severe injury on MRI at TEA, n (%) | 25 (56.8%) |
| Motor delay, n (%) | 27 (61%) |
| Language delay, n (%) | 23 (52%) |
| Cognitive delay, n (%) | 24 (54%) |
PMA: Post-menstrual age, SGA: Small for gestational age, CRIB-II score: clinical risk index for babies score, NEC: necrotizing enterocolitis, PDA: Patent ductus arteriosus, ROP: retinopathy of prematurity, IVH: Intraventricular hemorrhage, diagnosed by cranial ultrasound.
Five measures of the overall socio-economic status of each child’s family were collected from the mother’s medical record, including ethnicity, marital status, maternal illicit drug use, maternal age (early motherhood < 20 y of age), and insurance type as an indicator for family income. These five variables were combined to form a social risk index score [12,13].
2.2. aEEG recordings
Two sets of hydrogel electrodes (Natus Newborn Care, San Carlos, CA) were applied in the C3–P3 and C4–P4 positions for the newborn infant according to the International 10–20 system. Tracings were recorded using the BrainZ BRM3 monitor (Natus Newborn Care, San Carlos, CA) and analyzed offline using the software AnalyZe (Natus Newborn Care, San Carlos, CA). The median length of the aEEG recordings was 3 h (range: 1.5–5 h). Quantitative and qualitative analysis was performed. Spectral edge frequency (SEF90: defined as the frequency below which 90% of the spectral power is present) was computed using AnalyZe software.
Visual inspection of the tracings for presence or absence of cyclicity was performed according to Hellstrom-Westas [14]. The tracings were classified into the presence or absence of fully developed sleep-wake cycling defined as clear sinusoidal variations between discontinuous and more continuous background activity, with cycle duration ≥20 min. This qualitative analysis was computed by two independent, trained and blinded observers (N.E. and S.L.) and an interclass correlation coefficient was computed.
2.3. Neuroimaging
On the same day of the aEEG recording, infants underwent a non-sedated, non-contrast brain MRI following our institutional neonatal MRI guidelines [15]. Patients were fed and wrapped prior to placement in a MED-VAC vacuum immobilization bag (CFI Medical, Fenton, MI) that keeps babies from moving in the scanner. T1 and T2 images were obtained using Siemens TIM Trio 3.0 T MRI scanner (Siemens Medical Solutions, Erlangen, Germany).
An MRI scoring tool adapted from Kidokoro et al. [16] was used, which combines quantitative and qualitative measurements of brain abnormalities in preterm infants at term equivalent age. The quantitative scoring included: dilated lateral ventricles dimensions (score 0–3), biparietal diameter (corrected to the gestational age, scored 0–3), interhemispheric distance (score 0–3), deep gray matter (GM) volume (corrected to the gestational age, scored 0–3) and cerebellar volume (corrected to the gestational age and scored 0–3). Qualitative scoring included: white matter (WM) cystic lesions, WM focal signal abnormality, myelination delay, cortical GM signal abnormality, gyral maturation, deep GM signal abnormality and cerebellar signal abnormality. The variables were scored by an experienced reader (A.M.) blinded to the clinical information of the patients and the corresponding aEEG measures. The abnormality scoring system was divided into four grades: normal (0–3), mild [4–7], moderate [8–11] and severe (> 12) for the preterm brain at TEA.
2.4. Neurodevelopmental outcomes
Infants were followed up at age of 24–36 months corrected, and underwent testing using the Bayley Scales of Infant Development test, 3rd edition (BSID-III). BSID-III testing was performed by trained personnel blinded to the results of aEEG recordings. Infants were scored in three domains: language, motor and cognitive. Developmental delay was defined as a score greater than one standard deviation below a value of 100 (< 85) in any domain of the BSID-III.
2.5. Statistical analysis
Perinatal characteristics and electrophysiological measures were compared using independent samples t-test for continuous variables, and chi-square tests for categorical variables. Infants were initially divided into two groups (delay vs. no delay). SEF90 values and absent cyclicity were compared between the two cohorts. Then, an ROC curve was constructed to determine the value of SEF90 that yields the best sensitivity and specificity for moderate/severe brain injury on MRI.
The association between the aEEG measures and neurodevelopmental outcomes was assessed using odds ratio from χ2 analysis. Logistic regression was used to measure the same association after correcting for variables affecting development in preterm infants and social risk index score. These variables were accounted for in a multivariate regression model and included: gestational age, sex, antenatal steroids exposure, small for gestational age status, retinopathy of prematurity (ROP), oxygen requirement at 36 weeks GA, intraventricular hemorrhage (IVH) and postnatal steroids.
A p-value < 0.05 was considered significant. Results are displayed as odds ratios (OR) and 95% confidence intervals (CI).
3. Results
3.1. Study population
Our cohort included 60 infants born at < 30 weeks GA, 44 of whom returned for neurodevelopmental testing at age 24–36 months, corrected. Mean gestational age at birth for the cohort was 26 ( ± 2) weeks. aEEG recordings were performed at a mean post-menstrual age of 39 ( ± 2) weeks. Table 1 shows the characteristics of our study population. No significant differences in the demographic and clinical characteristics were noted in infants with developmental delay in any domain and those without delay except for the following variables: oxygen requirement at 36 weeks corrected GA, high grade IVH and moderate to severe injury on TEA MRI. Inter-rater reliability for qualitative scoring of aEEG was excellent with interclass correlation coefficient of 0.937 and p < 0.001. No significant differences were found between infants who followed at 24–36 months and those who did not in terms of infant’s clinical characteristics or family socio-economic background (Table 2).
Table 2.
Clinical and perinatal characteristics of the infants who followed up for BSID-III test and those who lost to follow up.
| Variable | Follow up (N = 44) | Lost to follow up (N = 16) | p-Value |
|---|---|---|---|
| Birth weight in grams (mean ± sd) | 856 ( ± 280) | 900 ( ± 237) | 0.5 |
| Gestational age (mean ± sd) | 26 ( ± 2) | 26 ( ± 2) | 0.8 |
| PMA at study (mean ± sd) | 39 ( ± 2) | 38.5 ( ± 2) | 0.4 |
| CRIB-II score (mean ± sd) | 11 ( ± 3) | 11 ( ± 3) | 0.8 |
| NEC surgery, n (%) | 7 (15.9%) | 3 (18.8%) | 0.7 |
| PDA ligation, n (%) | 6 (13.6%) | 2 (12.5%) | 0.9 |
| ROP surgery, n (%) | 13 (29.5%) | 3 (18.8%) | 0.5 |
| Post-natal steroids, n (%) | 15 (34.1%) | 2 (12.5%) | 0.1 |
| Oxygen therapy at 36 wk., n (%) | 34 (77.3%) | 14 (87.5%) | 0.5 |
| Inotropes, n (%) | 21 (47.7%) | 8 (50%) | 0.2 |
| IVH grade 3–4, n (%) | 19 (43.2%) | 7 (43.8%) | 0.9 |
| Moderate/severe injury on MRI at TEA, n (%) | 25 (56.8%) | 7 (43.8%) | 0.4 |
Motor delay was present in 61% of the children, while language and cognitive delay were prevalent in 52% and 54% of the children, respectively.
3.2. Neurodevelopmental outcomes at 24–36 months corrected age
Left and right SEF90 values were lower in infants with motor, language and cognitive delay. Absent cyclicity was more prevalent in infants with motor and cognitive delay (Table 3).
Table 3.
aEEG measures at TEA and developmental delay at 24–36 months corrected age.
| No motor delay | Motor Delay | p-Value | No language delay | Language delay | p-Value | No cognitive delay | Cognitive delay | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Left SEF90 | 9.5 ( ± 0.5) | 8.8 ( ± 1.1) | < 0.01* | 9.4 ( ± 0.7) | 8.6 ( ± 1.1) | 0.01* | 9.5 ( ± 0.6) | 8.6 ( ± 1.0) | < 0.01* |
| Right SEF90 | 9.5 ( ± 0.6) | 8.8 ( ± 0.8) | < 0.01* | 9.4 ( ± 0.6) | 8.8 ( ± 0.9) | 0.01* | 9.4 ( ± 0.7) | 8.9 ( ± 0.9) | 0.04* |
| Absent cyclicity | 3 (6.8%) | 15 (34.1%) | 0.01* | 7 (17.1%) | 9 (22.0%) | 0.44 | 2 (4.7%) | 15 (34.9%) | < 0.01* |
Denotes significance at p < 0.05. Left and right SEF90 values represent mean values ( ± standard deviation). Absent cyclicity and brain injury score values represent the number of patients and the corresponding percentages.
3.3. Association between aEEG measures and neurodevelopmental outcomes
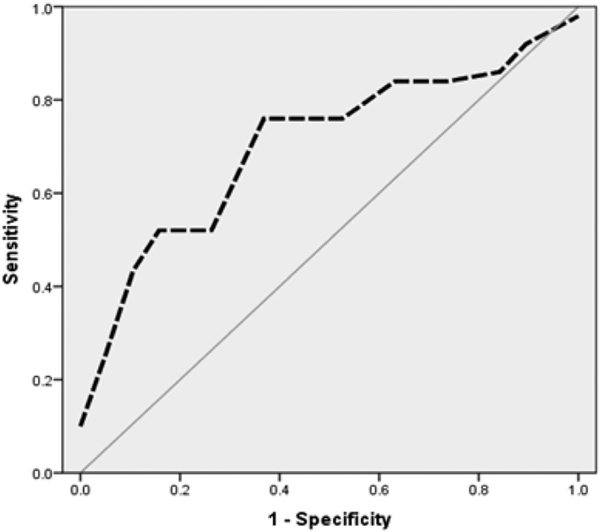
aEEG measures and MRI brain abnormality scores were closely correlated. Using linear regression analysis, left and right SEF90 showed an association with brain abnormality scores with lower values of SEF90 correlating with higher abnormality scores (F = 9.89, β = −0.38, p = 0.003 for left SEF90 and F = 11.26, β = −0.40, p = 0.001 for right SEF90). Using logistic regression, cyclicity also showed a strong negative relationship with brain abnormality scores on MRI (β = −0.10, p = 0.01). Therefore, we constructed an ROC curve to define the best value of SEF90 that yields the highest sensitivity and specificity for moderate/severe brain injury on MRI. A SEF90 < 9.2 correlated best with moderate to severe brain injury on MRI (AUC = 0.71, p = 0.01), as shown in Fig. 1.
Fig. 1.
ROC curve corresponding to SEF90 values for moderate/severe brain injury on MRI (AUC = 0.71, p = 0.01).
Then, we determined the odd ratios of developmental delay associated with absent cyclicity and SEF90 < 9.2. We found that SEF90 < 9.2 in either hemisphere is associated with motor delay, while absent cyclicity was associated with motor and cognitive delay. Table 4 describes the odd ratio of developmental delay associated with absent cyclicity and SEF90 < 9.2.
Table 4.
Odds ratio of developmental delay associated with SEF90 and absent cyclicity.
| Motor delay |
Language delay |
Cognitive delay |
|
|---|---|---|---|
| OR (95% CI), p-value | OR (05% CI), p-value | OR (05% CI), p-value | |
| Left SEF90 < 9.2 | 4.7 (1.2–18.3), p = 0.02* | 3.0 (0.8–10.7), p = 0.09 | 5.6 (1.5–21.2), p = 0.01* |
| Right SEF90 < 9.2 | 7.9 (1.8–34.5), p < 0.01* | 5.9 (1.5–23.1), p = 0.01* | 2.0 (0.5–6.9), p = 0.2 |
| Absent cyclicity | 5.8 (1.3–25.1), p = 0.01* | 1.6 (0.4–5.7), p = 0.4 | 16.8 (3.1–91.8), p < 0.01* |
| Absent cyclicity + SEF90 < 9.2 | 19.2 (3.9–94.1), p < 0.01* | 12.0 (2.2–65.5), p < 0.01* | 19.5 (3.5–108.5), p < 0.01* |
p < 0.05.
3.4. Adjusted odds ratio
In a multivariate regression model, we adjusted for confounding variables that can affect neurodevelopmental outcomes in preterm infants. These variables included gestational age, sex, antenatal steroids exposure, small for gestational age status, oxygen use at 36 weeks GA, ROP, IVH, post-natal steroids and the social risk index score. After adjustment, SEF90 < 9.2 in either hemisphere maintained its association with motor and cognitive delay (OR for motor delay = 6.25 (95% CI = 1.2–33), OR for cognitive delay = 7.6 (95% CI = 1.4–43)) but not for language delay (OR = 4.4, 95% CI = 0.7–26). Absent cyclicity also showed the same association with motor and cognitive delay (OR for motor delay = 6.2 (95% CI = 1.1–35), and OR for cognitive delay = 17.8 (95% CI = 2.7–115) and this association remained nonsignificant for language delay (OR for language delay =0.5, 95% CI = 0.1–3.5).
4. Discussion
Our study shows that low values of SEF90 and absent cyclicity at term equivalent age are correlated with developmental delay at 24–36 months corrected age in preterm infants across all developmental domains, and these associations remained significant for motor and cognitive outcomes after adjusting for variables that can affect neurodevelopment in preterm infants. A value of SEF90 < 9.2 in either hemisphere was predictive of motor delay and cognitive delay (OR = 6.25, 95% CI (1.2–33) and OR = 7.6, 95% CI (1.4–43) respectively). Absent cyclicity was predictive as well of motor and cognitive delay (OR = 6.2, 95% CI (1.1–35) and OR = 17.8, 95% CI (2.7–115) respectively).
We found that motor, language and cognitive delays were present in 61%, 52% and 54% of infants in this cohort born at < 30 weeks GA. Our sample showed a similar proportion of disabilities, described in the literature in a cohort of preterm infants born at < 30 weeks GA [17,18].
No previous studies explored the predictive value of SEF90 at TEA in neurodevelopmental outcomes. A cutoff value of 9.2 was calculated after constructing an ROC curve correlating SEF90 values with MRI brain injury. An AUC value of 0.71 is a fair to good number, though not perfect for the best screening and diagnostic test. A SEF90 < 9.2 provides simultaneously the highest sensitivity and specificity for MRI brain injury in our cohort. Therefore, we assessed this value in prediction of neurodevelopmental outcomes.
SEF90 has been extensively studied as a marker of cerebral maturation in fetal lambs and newborn infants [19,20]. Bell et al. [19] showed that SEF90 measures increase with gestational age, indicative of maturation in neuronal connections. SEF90 has also been used for identification of white matter injury in preterm infants in the first few days of life [8]. Automated spectral analysis gained interest recently due to its calculated and objective quality, allowing for reproducible measurements of cerebral maturation in preterm infants in the first weeks of life [21–23]. Niemarkt et al. found an increase in the SEF90, corresponding to a decrease in delta power and an increase in beta power on EEG [22]. The normative values of SEF90 on aEEG were studied in a cohort of preterm infants by Vesoulis et al. [24] where it was found that SEF90 increases with age. The cohort included preterm infants, and normal SEF90 values in infants > 31 weeks GA ranged between 9.0 and 11.2. Our results showed that SEF90 < 9.2 at TEA predicts worse outcomes in preterm infants, especially for motor performance, even after adjustment for confounding variables that can affect neurodevelopmental outcomes in preterm infants. Our findings are in concordance with what has been described on conventional EEG studies in preterm infants. The physiologic maturation of EEG such as an increase in the frequency and decrease in the amplitude of delta activity was absent in preterm infants with cognitive impairment [25]. Richards et al. showed also that increased power in higher frequency range at term equivalent age correlated with normal outcome at five years of age [26].
Absent cyclicity on aEEG at TEA strongly correlated with motor and cognitive delay in the present cohort. This finding is somewhat expected due to the association of sleep-wake cycling with the maturity and development of the brain [27]. The presence or absence of sleepwake cycling in the first few days of life in preterm infants was shown as a predictor of brain injury [7,28]. These studies evaluated preterm infants early in life correlating absent cyclicity to short term outcomes such as IVH, and in some instances to long-term outcomes assessed at one year of age, but the sample size was small (12 infants only) in the affected infants [7]. Klebermass et al. evaluated the predictive value of aEEG within the first weeks of life in a cohort of 143 preterm infants born at < 30 weeks GA [9]. Absence of cyclicity in the first two weeks of life was associated with cerebral palsy with an OR of 33 (95% CI = 6.2–166), and severe impairment on Bayley developmental test at three years of age with an OR of 25 (95% CI = 5.2–111). Our findings found the same correlation of abnormal motor and cognitive outcome with absence of cyclicity at term equivalent age. In our study, we corrected for the medical and social factors that can affect adversely the neurodevelopmental outcomes in preterm infants, while this relationship was not taken into account in the aforementioned study.
The limitations of our study include the relatively small sample size which included 60 infants, with a 73% follow up rate. There were no significant differences between the infants who followed up at 24–36 months for developmental testing and those who did not follow up, reducing the selection bias in our study. The selected cohort does not represent the whole spectrum of premature infants born at < 30 weeks GA. Our cohort is on the sicker end of the spectrum with a high prevalence of ROP and IVH. Our odds ratios had associated wide confidence intervals, making the results less precise. We speculate that these results were affected by the small sample size. This is why a larger sample size is needed to reproduce and validate our results.
This study demonstrates the utility of aEEG as a modality to assess cerebral maturation at term equivalent PMA. Delays in functional measures of aEEG are correlated with delays in development at 24–36 months corrected age in our cohort. These results indicate that brain injury at TEA can be detected by aEEG measures abnormalities and may be used at term equivalent age as a new tool for risk stratification of infants at higher risk of poor neurodevelopmental outcomes. A larger study is needed to validate these results in premature infants at low and high risk of brain injury.
Acknowledgements
We would like to thank Anthony Barton for his help in data collection, organization and processing.
Funding
This work was supported by the National Institute of Health (NICHD P30 HD062171, NCATS KL2 TR000450, NCATS UL1 TR000448, R01 HD057098).
Abbreviations:
- aEEG
Amplitude-integrated EEG
- SEF90
Spectral edge frequency
- BSID-III
Bayley Scales of Infant Development, 3rd Edition
- TEA
Term equivalent age
- MRI
Magnetic resonance imaging
- NICU
Neonatal Intensive care unit
- IVH
Intraventricular hemorrhage
- WMI
White matter injury
- GA
Gestational age
- PMA
Post-menstrual age
Footnotes
Conflicts of interest
None declared.
References
- [1].McCabe ER, Carrino GE, Russell RB, Howse JL, Fighting for the next generation: US prematurity in 2030, Pediatrics 134 (6) (2014) 1193–1199. [DOI] [PubMed] [Google Scholar]
- [2].Mukerji A, Shah V, Shah PS, Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis, Pediatrics 136 (6) (2015) 1132–1143. [DOI] [PubMed] [Google Scholar]
- [3].Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE, Neonatal MRI to predict neurodevelopmental outcomes in preterm infants, N. Engl. J. Med 355 (7) (2006) 685–694. [DOI] [PubMed] [Google Scholar]
- [4].Smyser CD, Kidokoro H, Inder TE, Magnetic resonance imaging of the brain at term equivalent age in extremely premature neonates: to scan or not to scan? J. Paediatr. Child Health 48 (9) (2012) 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bowen JR, Paradisis M, Shah D, Decreased aEEG continuity and baseline variability in the first 48 hours of life associated with poor short-term outcome in neonates born before 29 weeks gestation, Pediatr. Res 67 (5) (2010) 538–544. [DOI] [PubMed] [Google Scholar]
- [6].Hellstrom-Westas L, Klette H, Thorngren-Jerneck K, Rosen I, Early prediction of outcome with aEEG in preterm infants with large intraventricular hemorrhages, Neuropediatrics 32 (6) (2001) 319–324. [DOI] [PubMed] [Google Scholar]
- [7].Kidokoro H, Kubota T, Hayashi N, Hayakawa M, Takemoto K, Kato Y, et al. , Absent cyclicity on aEEG within the first 24 h is associated with brain damage in preterm infants, Neuropediatrics 41 (6) (2010) 241–245. [DOI] [PubMed] [Google Scholar]
- [8].Inder TE, Buckland L, Williams CE, Spencer C, Gunning MI, Darlow BA, et al. , Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants, Pediatrics 111 (1) (2003) 27–33. [DOI] [PubMed] [Google Scholar]
- [9].Klebermass K, Olischar M, Waldhoer T, Fuiko R, Pollak A, Weninger M, Amplitude-integrated EEG pattern predicts further outcome in preterm infants, Pediatr. Res 70 (1) (2011) 102–108. [DOI] [PubMed] [Google Scholar]
- [10].El Ters NM, Vesoulis ZA, Liao SM, Smyser CD, Mathur AM, Impact of brain injury on functional measures of amplitude-integrated EEG at term equivalent age in premature infants, J. Perinatol 37 (8) (2017) 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parry G, Tucker J, Tarnow-Mordi W, CRIB II: an update of the clinical risk index for babies score, Lancet 361 (9371) (2003) 1789–1791. [DOI] [PubMed] [Google Scholar]
- [12].Whitehead NS, The relationship of socioeconomic status to preterm contractions and preterm delivery, Matern. Child Health J 16 (8) (2012) 1645–1656. [DOI] [PubMed] [Google Scholar]
- [13].Foster-Cohen SH, Friesen MD, Champion PR, Woodward LJ, High prevalence/ low severity language delay in preschool children born very preterm, J. Dev. Behav. Pediatr 31 (8) (2010) 658–667. [DOI] [PubMed] [Google Scholar]
- [14].Hellström-Westas L, Rosén I, de Vries LS, Greisen G, Amplitude-integrated EEG classification and interpretation in preterm and term infants, NeoReviews 7 (2) (2006) e76–e87. [Google Scholar]
- [15].Mathur AM, Neil JJ, McKinstry RC, Inder TE, Transport, monitoring, and successful brain MR imaging in unsedated neonates, Pediatr. Radiol 38 (3) (2008) 260–264. [DOI] [PubMed] [Google Scholar]
- [16].Kidokoro H, Neil JJ, Inder TE, New MR imaging assessment tool to define brain abnormalities in very preterm infants at term, AJNR Am. J. Neuroradiol 34 (11) (2013) 2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Larroque B, Ancel PY, Marret S, Marchand L, Andre M, Arnaud C, et al. , Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study, Lancet 371 (9615) (2008) 813–820. [DOI] [PubMed] [Google Scholar]
- [18].Serenius F, Källén K, Blennow M, et al. , Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in sweden, JAMA 309 (17) (2013) 1810–1820. [DOI] [PubMed] [Google Scholar]
- [19].Bell AH, McClure BG, McCullagh PJ, McClelland RJ, Spectral edge frequency of the EEG in healthy neonates and variation with behavioural state, Biol. Neonate 60 (2) (1991) 69–74. [DOI] [PubMed] [Google Scholar]
- [20].Szeto HH, Spectral edge frequency as a simple quantitative measure of the maturation of electrocortical activity, Pediatr. Res 27 (3) (1990) 289–292. [DOI] [PubMed] [Google Scholar]
- [21].Victor S, Appleton RE, Beirne M, Marson AG, Weindling AM, Spectral analysis of electroencephalography in premature newborn infants: normal ranges, Pediatr. Res 57 (3) (2005) 336–341. [DOI] [PubMed] [Google Scholar]
- [22].Niemarkt HJ, Jennekens W, Pasman JW, Katgert T, Van Pul C, Gavilanes AW, et al. , Maturational changes in automated EEG spectral power analysis in preterm infants, Pediatr. Res 70 (5) (2011) 529–534. [DOI] [PubMed] [Google Scholar]
- [23].West CR, Harding JE, Williams CE, Gunning MI, Battin MR, Quantitative electroencephalographic patterns in normal preterm infants over the first week after birth, Early Hum. Dev 82 (1) (2006) 43–51. [DOI] [PubMed] [Google Scholar]
- [24].Vesoulis ZA, Paul RA, Mitchell TJ, Wong C, Inder TE, Mathur AM, Normative amplitude-integrated EEG measures in preterm infants, J. Perinatol. 35 (6) (2015) 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hayakawa F, Okumura A, Kato T, Kuno K, Watanabe K, Dysmature EEG pattern in EEGs of preterm infants with cognitive impairment: maturation arrest caused by prolonged mild CNS depression, Brain Dev. 19 (2) (1997) 122–125. [DOI] [PubMed] [Google Scholar]
- [26].Richards JE, Parmelee AH Jr., Beckwith L, Spectral analysis of infant EEG and behavioral outcome at age five, Electroencephalogr. Clin. Neurophysiol 64 (1) (1986) 1–11. [DOI] [PubMed] [Google Scholar]
- [27].Burdjalov VF, Baumgart S, Spitzer AR, Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates, Pediatrics 112 (4) (2003) 855–861. [DOI] [PubMed] [Google Scholar]
- [28].Natalucci G, Rousson V, Bucher HU, Bernet V, Hagmann C, Latal B, Delayed cyclic activity development on early amplitude-integrated EEG in the preterm infant with brain lesions, Neonatology 103 (2) (2013) 134–140. [DOI] [PubMed] [Google Scholar]