Abstract
Darbepoetin alfa (Darbe) is a hyperglycosylated analogue of recombinant human erythropoietin (Epo). The aim of this study was to develop a population pharmacokinetic model for Darbe following intravenous (i.v.) and subcutaneous (s.c.) administration to infants. Data from 2 infant clinical studies (a single i.v. dose study following a 4 μg/kg dose of Darbe, and a single s.c. dose study following 1 μg/kg or 4 mg/kg dose of Darbe) were combined and analyzed simultaneously using nonlinear mixed-effect modeling approach. Darbe population pharmacokinetics was well described by a 2-compartment model with first-order elimination. The covariate analysis identified significant impact of gender on clearance and bodyweight on volume of distribution. The clearance of Darbe was estimated to be 0.050 L/h/kg in male infants and 0.031 L/h/kg in female infants. The predicted population mean value of Vp is 0.84 L/kg, which is associated with the subject’s bodyweight (p < 0.05). Following s.c. administration, the estimated absorption rate (i.e., ka) of Darbe was 0.062 L/h. The model provides a suitable starting point for the development of further pharmacokinetic-pharmacodynamic models in infants in a variety of disease settings. Because the covariate-pharmacokinetic parameter relationships were identified in only 22 infants, further investigation with larger sample size is warranted.
Keywords: pharmacokinetic/pharmacodynamic models, clinical pharmacokinetics, population pharmacokinetics, pharmacokinetics, clinical trials
Introduction
Darbepoetin alfa (Darbe; trade name Aranesp) is a hyper-glycosylated analogue of recombinant human erythropoietin (Epo) with 2 extra N-linked carbohydrate chains. The 2 chains are introduced into the primary Epo sequence using site-directed mutagenesis. Although Darbe stimulates erythropoiesis by the same mechanism as endogenous Epo, its increased carbohydrate content provides Darbe with lower clearance, longer half-life, and more sustained erythropoietic effects than Epo. This feature permits a reduction in the frequency of Darbe administration compared to Epo.1–3 Darbe is approved for the treatment of anemia in children and adults with chronic kidney disease and anemia in cancer patients as a result of chemotherapy. In addition to its approved indications, Darbe has been explored as a treatment option for anemia associated with other diseases, such as chronic heart failure and human immunodeficiency infection.4,5 Darbe effectively and safely maintains hemoglobin levels in these patient populations and can be administered at a monthly dosing interval. This is more convenient and less painful compared to more frequent dosing with Epo.4
Erythropoietin-stimulating agents (ESAs) such as Darbe also exert neuroprotective effects. This focus is an increasingly important area of active and intense research. In this regard, the Epo receptor has been found to be expressed throughout human brain.6,7Preclinical studies have demonstrated that Darbe can cross the blood-brain barrier.8 Results in an intracerebral hemorrhage rat model showed that weekly administered Darbe was effective in reducing neurologic impairment immediately after injury and conferred behavioral and histologic neuroprotection similar to that of daily Epo administration.9
The neuroprotective potential of ESAs such as Epo and Darbe may be particularly promising in infants, considering the high incidence of hypoxic-ischemic encephalopathy (HIE) in neonates and the high prevalence of cognitive delay in preterm infants.10,11 The potential application of Darbe in infants as either a neuroprotectant or an ESA requires an understanding of the Darbe dose-pharmacokinetic relationship, the inter-individual variability (IIV) in pharmacokinetics, and the covariates affecting pharmacokinetic variability; such information on quantitative understanding of Darbe in infants is very limited. Previously we conducted 2 single-dose clinical studies to evaluate the pharmacokinetics of Darbe in infants, one through intravenous (i.v.) administration and the other through subcutaneous (s.c.) administration.12,13 To better under stand Darbe dose-pharmacokinetic relationship and identify the covariates affecting pharmacokinetic variability, in the present study we developed a population pharmacokinetic model of Darbe in infants using a nonlinear mixed-effect modeling approach using the same pharmacokinetic data obtained in those 2 studies.12,13
Materials and Methods
Clinical Study
Darbe disposition was evaluated in infants following a single dose administered either s.c. or i.v. in 2 independent studies.
Human Subject Protections
The Institutional Review Board of Intermountain Health Care approved both studies; the parents of each participant in each study provided ongoing consent as part of the informed consent process. The initial consent was documented via signed informed consent document approved by the Institutional Review Board of Intermountain Health Care. The study population included a convenience sample of eligible preterm infants whose nonpopulation pharmacokinetic results have been previously reported.12,13 Eligibility criteria included a hemoglobin of ≤10.5 g/dL at the time of enrollment. Patients were deemed ineligible if the neonate was judged as being too ill to participate in a research study by the neonate’s attending neonatologist; factors considered by the neonatologist included requirement for mechanical ventilation with more than 50% inspired oxygen or a requirement for vasopressors to elevate the blood pressure. Patients were also deemed ineligible if the attending neonatologist judged they would likely require blood transfusion within 3 days of enrollment.
Study Designs
In the Darbe single s.c. dose study (referred to as “the first study” hereafter), pharmacokinetics was evaluated in 12 infants randomly divided into 2 groups: 6 infants received 1 μg/kg of Darbe and the other 6 received 4 mg/kg.12 Three subjects within each dose group had blood sampled before dose and at 6, 48, 72, and 96 h after dose; the other 3 subjects had blood sampled before dose and at 12, 60, 84, and 108 h after dosing.
In the second study, Darbe was administered as a single i.v. dose, pharmacokinetics was evaluated in 10 infants following a 4 μg/kg dose of Darbe that was administered over 4 h at a constant infusion rate.13 The lengthy infusion was done to avoid the possibility of the loss of drug in the urine. Blood was sampled before the dose and at 30 min and 4, 24, and 48 h after completion of the infusion.
A commercial Epo ELISA was used to quantify both Darbe and endogenous Epo (R&D Systems, Minneapolis, MN).12,13 Epo con centrations were expressed as mU/mL. The minimum detectable concentration was 0.6 mU/mL, with inter- and intra-assays within 5% (2.8% to 4.9%). In our modeling analysis, a conversion of 400 U Epo = 1 μg Darbe was used; this conversion was applied in the original reports13 and is consistent with manufacturer’s labeling.14
Population Pharmacokinetic Modeling
Population Model-Building Criteria
Population pharmacokinetic data from the single s.c. and i.v. doses of Darbe were analyzed simultaneously using the nonlinear mixed-effect modeling software NONMEM (version 7.2; Icon Development Solutions, Ellicott City, MD). Actual (i.e., not predetermined) sampling times were used in the analysis. Because ELISA detects both Darbe and endogenous Epo, the true Darbe concentration at each time point was calculated from the difference between measured Darbe concentration at each time point and the initial baseline Epo concentration for individual infants, referred to hereafter as “baseline corrected” data; hence, our tacit assumption was that the endogenous Epo concentration did not change significantly during the study.
Several criteria were used to evaluate the improvement in the model performance and to select the final model. The likelihood ratio test was used for comparing rival hierarchical models where a decrease in the NONMEM objective function (−2 log likelihood) of 3.84 points was necessary to consider the improvement in model performance statistically significant at α = 0.05. The Akaike information criterion was used for comparing rival nonhierarchical models. Other selection criteria included improved goodness-of-fit plots, successful estimation of the parameters precision, plausibility of the estimated parameters, and reduced variance of intersubject and residual errors. The most appropriate model was found to be a 2-compartment structural model parameterized in terms of bioavailability (F1, for s.c. data only), first-order absorption rate constant (ka, for s.c. data only), volume distribution of the central compartment (Vp), volume distribution of the peripheral compartment (Vt), distribution clearance (Q), and clearance (CL). The first-order conditional estimation method with the interaction (FOCEI) and a user-defined subroutine (ADVAN6) were used to estimate the typical population parameters, random IIV, and residual variability between observed and individually predicted plasma Darbe concentrations. Both FOCEI and ADVAN6 are built-in functions within NONMEM. All IIVs of the pharmacokinetic parameters of Darbe were estimated by an exponential model as follows:
| (1) |
where ηi is the proportional difference between the parameter estimate of the ith subject (Pi) and the typical population parameter (i.e., mean value estimate) value (TVP). ηi is assumed to be normally distributed with a mean of 0 and a variance of ω2.
Residual error in the population model was described by combined additive and exponential random effect model as follows:
| (2) |
where Cij is the measured plasma concentration of Darbe for ith individual at time j, is the corresponding model-predicted concentration in the same subject at the same time. ε1ij and ε2ij are the proportional and additive errors, respectively, and both parameters were assumed to be normally distributed with a mean of 0 and a variance of σ2.
The robustness of the final model was evaluated using bootstrap analysis. In this procedure, subjects were randomly sampled with replacement from the original data set to form 1000 new data sets. Each data set has the same number of participants as the original data set. The final model was then fit to the bootstrap data sets to obtain the mean, standard error, and CI of the final model parameter values as well as to evaluate the stability of the parameter estimates.
The adequacy of the final model was evaluated using the visual predictive check (VPC). The final model parameters were used when the concentration-time profiles were simulated.
Covariate Model Analysis
Covariate model building was conducted using NONMEM. Gender, bodyweight, blood hemoglobin concentration (Hb), and age were evaluated as covariates having a potential impact on CL and Vp of Darbe. The following model was used to describe the relationship between continuous covariates (e.g., age, bodyweight, and Hb) and a pharmacokinetic model parameter P (e.g., CL and Vp). Taking bodyweight as an example, this model would be:
| (3) |
where TVP is the model-predicted value for the pharmacokinetic parameter P given the covariate value for WGT. WGT represents weight, and θWGT represents the exponent of the relationship.
Reference values for the covariates evaluated included the following mean values for the study population of infants: bodyweight (WGTmean) = 1802 g, hemoglobin (HBmean) = 9.72 g/dL, and age (Agemean) 29.8 d.
The relationship between categorical covariates (i.e., gender in this example) and a pharmacokinetic parameter, P, was modelled as follows:
where TVP is the typical population value of a pharmacokinetic parameter P.
Covariate analysis was performed using the standard forward addition and backward elimination method. Forward addition was applied first to determine significant covariates. Only covariates that decreased the objective function value by more than 3.84 (i.e., p < 0.05) when compared to the base model were considered for the full covariate selection. Backward elimination was then applied to remove covariates from the model with an increase in the objective function value <6.63 corresponding to 1 df at p = 0.01. Only covariates that produced this magnitude of increase¼ were retained in the model.
Results
The disposition of Darbe was evaluated in 6 infants following a single s.c. dose of 1 μg/kg, 6 infants following a single s.c. dose of 4 μg/kg (study 1), and in 10 infants following 4-h i.v. infusion of 4 μg/kg dose (study 2). A summary of subject demographics for these 2 groups is presented in Table 1. The postnatal age of infants at the start of the study ranged from 31 to 64 days in study 1 and from 3 to 44 days in study 2.
Table 1.
Demographic Features of the Study Subjects in Study 1 and Study 2
| Variable | Study 1 (N = 12) | Study 2 (N = 10) |
|---|---|---|
| Mean (Range) | Mean (Range) | |
| Birthweight (g) | 1139 (820–1516) | 1481 (704–3025) |
| Gender | 2 Female | 5 Female |
| 10 Male | 5 Male | |
| Gestational age at delivery (wk) | 29.1 (27.0–30.5) | 30.6 (26.0–36.3) |
| Age at Darbe dose (d) | 43 (31–64) | 14 (3–44) |
| Weight at Darbe dose (g) | 1914 (1246–3260) | 1677 (922–3025) |
| Hemoglobin at Darbe dose (g/dL) | 9.7 (7.6–10.6) | 9.8 (8.0–10.7) |
The final structure model was a 2-compartment model with first-order elimination. For s.c. data, first-order absorption was included in the structure model. For i.v. data, zero-order input over 4 h was used. The base population model included IIV on CL, Vp, ka, and bioavailability (F). A combined (i.e., proportional + additive) error model was used to determine residual variability.
Results from the covariate evaluation demonstrated that gender has a significant influence on clearance and that bodyweight has a significant impact on central compartment volume of distribution. Accordingly, both factors were included as covariates in the final population pharmacokinetic model. With inclusion of gender in clearance, the IIV of clearance decreased from 0.288 to 0.183. Similarly, incorporating bodyweight in Vp decreased the IIV of Vp from 0.506 to 0.228.
Table 2 summarizes the parameter estimates obtained from the final population pharmacokinetic model. The clearance of Darbe was estimated to be 0.050 L/h/kg in male infants, which is faster than the clearance in female infants (0.031 L/h/kg). The typical value (i.e., population mean) of Vp was 0.84 L/kg, and this value is dependent on subject bodyweight. The estimated peripheral volume of distribution Vt and distribution clearance, Q, were 0.268 L/kg and 0.027 L/h/kg, respectively. Following s.c. administration, the estimated absorption rate (i.e., ka) of Darbe is 0.062 L/h corresponding to an absorption half-life of 11.2 h.
Table 2.
Parameter Estimates From the Final Population Pharmacokinetic Model
| Parameter | Unit | Final Model Estimate | Mean Bootstrap Estimate | %RSE |
|---|---|---|---|---|
| F | NA | 0.99 | 0.99 | 3.0% |
| Ka | L/h | 0.062 | 0.061 | 21.0% |
| CL | L/h/kg | 0.050 (male) | 0.054 | 22.8% |
| 0.031 (female) | 0.033 | 66.1% | ||
| Vp | L/kg | 0.84 | 0.84 | 20.7% |
| vt | L/kg | 0.268 | 0.290 | 72.7% |
| Q | L/h/kg | 0.027 | 0.029 | 33.2% |
| ω2F | NA | 0.20 | 0.24 | 104% |
| ω2ka | NA | 0.0891 | 0.0863 | 210% |
| ω2CL | NA | 0.183 | 0.188 | 61.1% |
| ω2Vp | NA | 0.228 | 0.238 | 109% |
| (proportional residual error) | NA | 0.0643 | 0.107 | 98.2% |
| (additive residual error) | NA | 0.00839 | 0.00803 | 76.5% |
The shrinkage of ω2F ω2 ka, ω2CL, and ω2 Vp are 46.1%, 64.9%, 15.5%, and 24.6%, respectively. The shrinkage of and are 21.2% and 21.4%, respectively.
Abbreviations used: NA, not applicable; RSE, relative standard error.
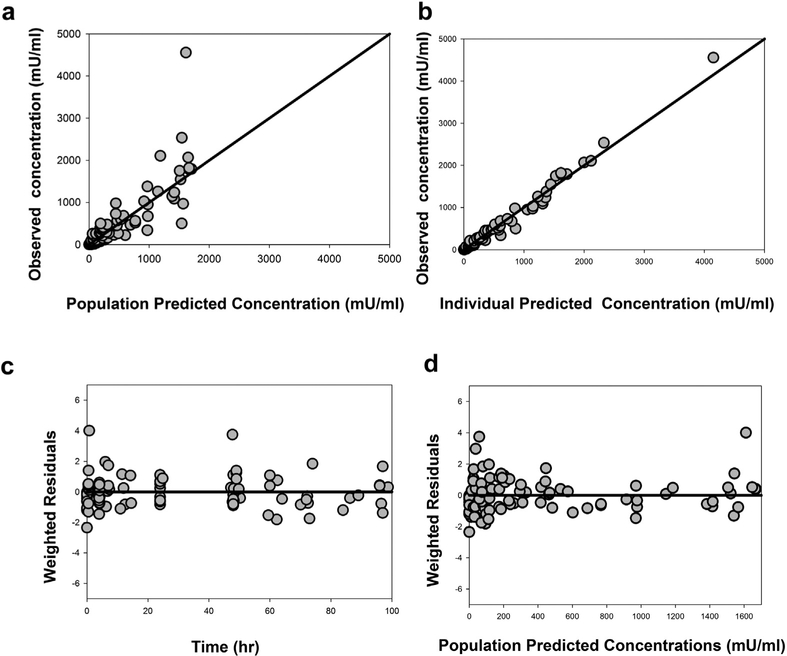
Figure 1 depicts the goodness-of-fit plots, including scatter plots of the observed versus population-predicted concentration data (Fig. 1a), observed versus individual-predicted concentrations (Fig. 1b), weighted residuals versus time (Fig. 1c), and population-predicted concentrations (Fig. 1d). As shown in Figures 1a and 1b, the population-and individual-predicted concentrations versus the observed concentrations were symmetrically distributed around the line of identity without bias, indicating that the final model describes Darbe pharmacokinetics adequately at both the population and individual levels. In addition, weighted residuals are distributed uniformly around the zero line when plotted either by time (Fig. 1c) or by population-predicted concentrations (Fig. 1d); these observations further suggest absence of significant systematic bias in the model fit.
Figure 1.
Goodness-of-fit plots for the final population pharmacokinetic model of Darbe. (a) observed versus population-predicted Darbe plasma concentrations; (b) observed versus individual-predicted Darbe plasma concentrations; (c) weighted residuals versus time; (d) weighted residuals versus population-predicted Darbe plasma concentrations. Solid lines represent the lines of identity in Figures 1a and 1b and the zero residuals in Figures 1c and 1d.
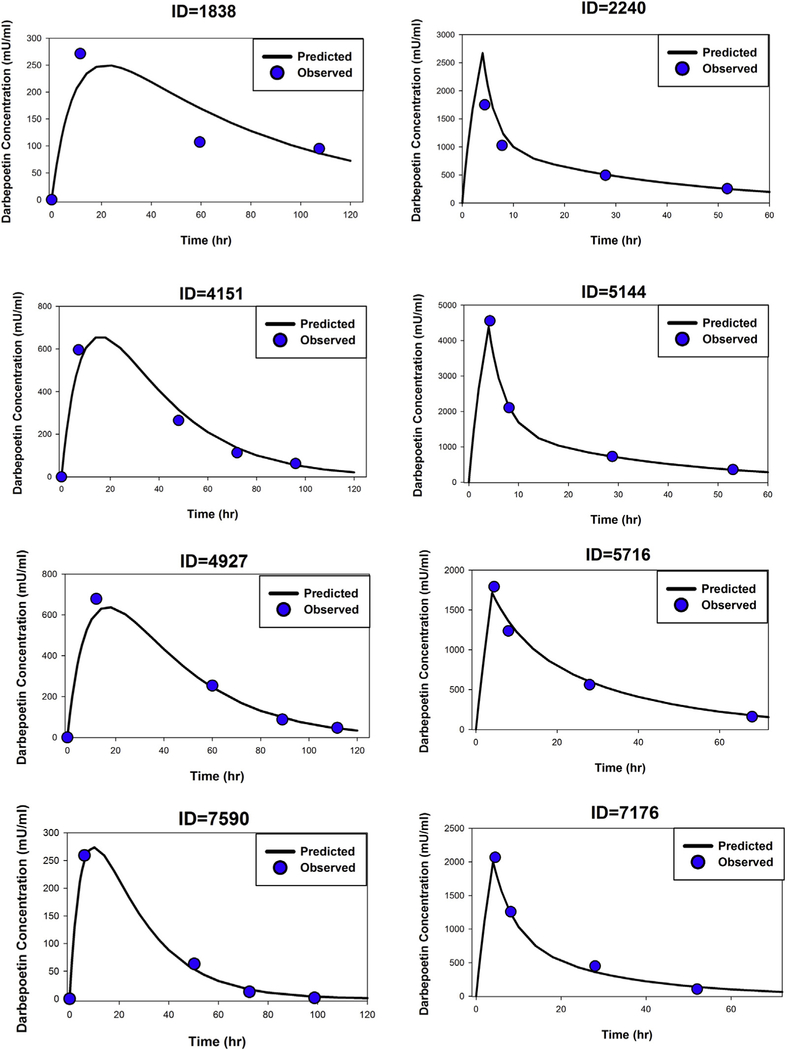
Figure 2 depicts the time course of observed versus individual-predicted plasma Darbe concentrations for 4 representative infants from each study. As judged by the goodness-of-fit plots, the proposed pharmacokinetic model captures the concentration-time profiles of Darbe well for both s.c. dose and i.v dose studies.
Figure 2.
Time courses of observed (symbols) and model-predicted (lines) Darbe plasma concentrations in 4 representative infants following single s.c. doses of Darbe (left panels) and in 4 infants following single i.v. dose of Darbe (right panels).
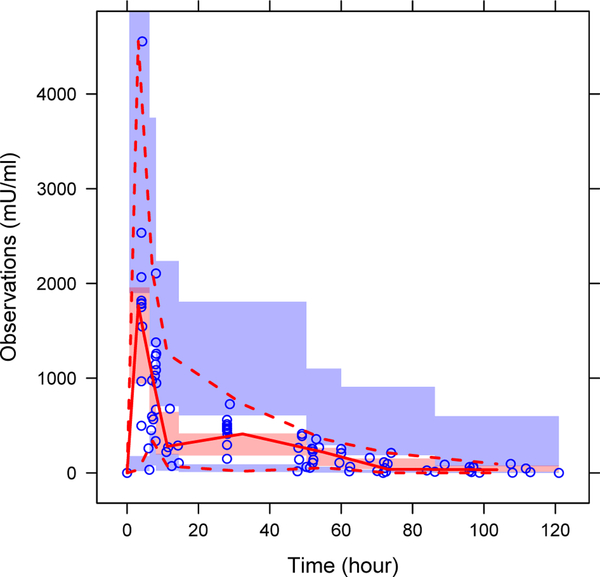
The results of the VPC of the final model are presented in Figure 3. As shown, the 5th, 50th, and 95th percentiles of the observations were within the 95% CI of the corresponding prediction percentiles in VPC plot, indicating that the final model is appropriate and sufficient.
Figure 3.
VPC of plasma Darbe data. The open circles represent the observed concentrations, the solid lines represent the median of the data, the dashed line represents the 5th and 95th percentiles for the data, and the shaded areas represent the 95% CI surrounding the 5th, 50th, and 95th of the prediction percentiles.
Discussion
In the present study, the model estimated mean terminal half-life of Darbe of 20.4 h in infants following i.v. administration agrees well with the half-life values observed in adults and hypothermic neonates.3,15 In adults, the mean terminal half-life of Darbe was 25.3 h following i.v. administration.3 In hypothermic neonates with HIE, the half-life after i.v. bolus was 23.6 h.15 However, the model-estimated half-life value of 20.4 h reported here is about twice the value we observed previously (t1/2 = 10.1 h)13; we speculate that the difference is attributable to use of a traditional standard 2-stage method (i.e., perform model fitting for each individual, then calculate descriptive summary statistics for each pharmacokinetic parameter) in the previous study. In contrast to the population pharmacokinetic approach applied currently, the standard 2-stage method requires a rich data set (i.e., frequent blood sampling) for optimum accuracy and often generates inaccurate estimates when the number of sample observations is limited. In the present study, each infant provided only 4 pharmacokinetic data points. Different from prior analysis, the current analysis used a population pharmacokinetic approach, which is applicable to sparse pharmacokinetic data set and allows for the identification of covariates that were used to reduce the variability in Darbe pharmacokinetic parameters.
In the present s.c. study, Darbe reached peak level at 15.2 ± 3.9 h following s.c. administration; this absorption process is faster than that reported in children and adults. In children, the time to reach peak concentration (i.e., Tmax) following s.c. administration was 36.2 h.16 In adults, Darbe demonstrated even slower absorption, reaching its peak at around 54.1 h after s.c. administration.3 The absorption rate constant of Darbe in infants following s.c. administration estimated using our model was 0.062 L/h, and the absorption half-life was 11.2 h. The absorption rate constant of Darbe (ka) in adults was reported to be 0.0212 L/h,17 corresponding to an absorption half-life of 32.7 h. The higher ka value and shorter absorption half-life in infants reflect a much faster absorption process than in adults, which is consistent with the observed data. The lymphatic system represents a major route for the absorption of protein drugs, including Epo.18,19 The lymphatic flow rate decreases with the increase in age.20 The age-dependent reduction in lymphatic flow rate may explain the decrease in absorption rate constant and longer time needed to reach the peak Darbe concentration with increases in age.
In the present study, the estimated terminal half-life of Darbe in infants following s.c. administration was 19.6 h, a value which is very close to the 20.4 h half-life obtained following i.v. administration. Contrary to what we observed in infants, the mean terminal half-life of Darbe in adults following s.c. administration (t1/2 =48.4 h) is longer than that after i.v. administration (t1/2 = 25.3 h).3 This phenomenon observed in adults is commonly referred to as flip-flop kinetics. This kinetic behavior occurs because the absorption process of Darbe is very slow in adults, such that absorption becomes the rate-limiting step of Darbe elimination. We did not observe this flip-flop phenomenon in infants. This is not surprising because the absorption process of Darbe is much faster in infants than that in adults and correspondingly may not represent a rate-limiting step of Darbe elimination. Indeed, our model estimates showed that the absorption half-life (i.e., 11.2 h) in infants is shorter than the elimination half-life (i.e., 19.6 h) following s.c. administration of Darbe.
Our analysis showed that Darbe pharmacokinetics in infants was well described by a 2-compartment model, which is consistent with the reports from the adult study. Agoram et al.17 performed population pharmacokinetic analysis to characterize Darbe disposition in healthy adults and reported that Darbe pharmacokinetics in adults was well described by a 2-compartment model, with clearance equal to 0.00234 L/h/kg and volume of distribution of central compartment equal to 0.085 L/kg. However, in contrast to the pharmacokinetic parameters in adults, our study revealed a clearance of Darbe in infants that is much more rapid (0.05 L/h/kg, in male infants, and 0.031 L/h/kg in female infants) and that the volume of distribution is much greater (0.84 L/kg). Our results are consistent with other infant studies; Roberts et al.15 evaluated population pharmacokinetics of Darbe in hypothermic neonates with HIE and reported a clearance equal to 0.015 L/h/kg and volume of distribution equal to 0.511 L/kg. Darbe is a synthetic analogue of Epo and is mainly eliminated by Epo receptors (EpoR). The bodyweight-normalized clearance of Epo is most rapid in sheep fetuses relative to newborn lambs and adult sheep and decreases progressively with maturation.21 The different expression of EpoR between newborn and adult may be associated with developmental differences in Epo and Darbe pharmacokinetics. The mechanism by which gender has a significant influence on Darbe clearance independent of its effect on bodyweight is currently uncertain, but interesting nonetheless. We speculate that this might be an Epo receptor effect as well.
Our current population pharmacokinetic model for Darbe has several limitations. First, the ELISA assay used quantifies both Darbe and endogenous Epo. In our model, Darbe concentrations in each individual were corrected by an individual-specific constant value. This may not be accurate because endogenous Epo has been reported to exhibit up to 2-fold diurnal variation in healthy subjects.22 However, the overall impact of potential variation of endogenous Epo is anticipated to be small based on the high exogenous Darbe doses used and correspondingly much higher Darbe concentrations at most study, most blood samples were collected during the elimination phase of Darbe. On account of this limitation, it is not possible to provide as precise estimates of parameters related to the absorption phase (e.g., absorption rate constant ka) or estimates of parameters influenced by absorption phase (such as bioavailability F).
In conclusion, we developed a population pharmacokinetic model of Darbe in infants based on a combination of single i.v and s.c administration data. The model can in the future be used to select the appropriate dose regimen of Darbe in infants. In addition, this population-based model provides an appropriate starting point for the development of pharmacokinetic-pharmacodynamic models in infants in varied disease settings. The covariate analysis identified significant impact of gender on clearance and of bodyweight on volume of distribution. Because these covariate-pharmacokinetic parameter relationships were identified in only 22 infants, further investigation with larger number of subjects is warranted.
Abbreviations used
- Darbe
darbepoetin alfa
- Epo
erythropoietin
- ESA
erythropoietin-stimulating agents
- HIE
hypoxic-ischemic encephalopathy
- Hb
hemoglobin
References
- 1.Macdougall IC. Optimizing the use of erythropoietic agents–pharmacokinetic and pharmacodynamic considerations. Nephrol Dial Transplant. 2002;17(Suppl 5):66–70. [DOI] [PubMed] [Google Scholar]
- 2.Macdougall IC. An overview of the efficacy and safety of novel erythropoiesis stimulating protein (NESP). Nephrol Dial Transplant. 2001;16(Suppl 3):14–21. [DOI] [PubMed] [Google Scholar]
- 3.Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10(11):2392–2395. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Sullivan JT, Ball S, et al. Once-monthly administration of darbepoetin alfa for the treatment of patients with chronic heart failure and anemia: a pharmacokinetic and pharmacodynamic investigation. J Cardiovasc Pharmacol. 2005;46(2):155–161. [DOI] [PubMed] [Google Scholar]
- 5.Tulpule A, Dharmapala D, Burian P, et al. Treatment of anemia with Aranesp (darbepoetin alfa) given once every two weeks in HIV seropositive patients. Blood. 2004;104:A3108. [Google Scholar]
- 6.Dame C, Bartmann P, Wolber E, Fahnenstich H, Hofmann D, Fandrey J. Erythropoietin gene expression in different areas of the developing human central nervous system. Brain Res Dev Brain Res. 2000;125(1–2):69–74. [DOI] [PubMed] [Google Scholar]
- 7.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43(1):40–49. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol. 2004;505(1–3):93–101. [DOI] [PubMed] [Google Scholar]
- 9.Grasso G, Graziano F, Sfacteria A, et al. Neuroprotective effect of erythropoietin and darbepoetin alfa after experimental intracerebral hemorrhage. Neurosurgery. 2009;65(4):763–769. discussion 769–770. [DOI] [PubMed] [Google Scholar]
- 10.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–338. [DOI] [PubMed] [Google Scholar]
- 11.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B, Network NN. Adverse neuro-developmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673–680. [DOI] [PubMed] [Google Scholar]
- 12.Warwood TL, Ohls RK, Wiedmeier SE, et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25(11):725–730. [DOI] [PubMed] [Google Scholar]
- 13.Warwood TL, Ohls RK, Lambert DK, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26(5):296–300. [DOI] [PubMed] [Google Scholar]
- 14.Highlights of prescribing information ARANESP. Thousand Oaks, CA: Amgen Inc.; 2016. Available at: http://pi.amgen.com/united_states/aranesp/ckd/aranesp_pi_hcp_english.pdf. Accessed February 22, 2017. [Google Scholar]
- 15.Roberts JK, Stockmann C, Ward RM, et al. Population pharmacokinetics of darbepoetin alfa in conjunction with hypothermia for the treatment of neonatal hypoxic-ischemic encephalopathy. Clin Pharm. 2015;54(12):1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner GR, Kale AS, Warady BA, et al. The pharmacokinetics of novel erythropoiesis stimulating protein (NESP) in pediatric patients with chronic renal failure (CRF) or end-stage renal disease. J Am Soc Nephrol. 2000;11:A1479. [Google Scholar]
- 17.Agoram B, Sutjandra L, Sullivan JT. Population pharmacokinetics of darbepoetin alfa in healthy subjects. Br J Clin Pharmacol. 2007;63(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310. [DOI] [PubMed] [Google Scholar]
- 19.Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–169. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier S, Ferland G, Tuchweber B. Lymphatic absorption of retinol in young, mature, and old rats: influence ofdietary restriction.FASEB J. 1996;10(9):1085–1090. [DOI] [PubMed] [Google Scholar]
- 21.Widness JA, Veng-Pedersen P, Modi NB, Schmidt RL, Chestnut DH. Developmental differences in erythropoietin pharmacokinetics: increased clearance and distribution in fetal and neonatal sheep. J Pharmacol Exp Ther. 1992;261(3):977–984. [PubMed] [Google Scholar]
- 22.Klausen T, Dela F, Hippe E, Galbo H. Diurnal variations of serum erythropoietin in trained and untrained subjects. Eur J Appl Physiol Occup Physiol. 1993;67(6): 545–548. [DOI] [PubMed] [Google Scholar]