Abstract
Focused ultrasound is now capable of noninvasively penetrating the intact human skull and delivering energy to specific areas of the brain with millimeter accuracy. The ultrasound energy is supplied in high-intensities to create brain lesions or at low-intensities to produce reversible physiological interventions. Conducting Acoustic Emission Detection (AED) and Electroencephalography (EEG) during transcranial focused ultrasound may lead to several new brain treatment and research applications. This study investigates the feasibility of using a novel scalp senor for acquiring concurrent AED and EEG during clinical transcranial ultrasound. A piezoelectric disk is embedded in plastic cup EEG electrode to form the sensor. The sensor is coupled to the head via an adhesive/conductive gel-dot. Components of the sensor prototype are tested for AED and EEG signal quality in a bench top investigation with a functional ex vivo skull phantom.
Keywords: Transcranial Ultrasound, Acoustic Emission Detection, Electroencephalography, Scalp sensor
Introduction:
Transcranial Focused Ultrasound (tFUS) has been investigated since the 1950s [1]. Not until recently has the technology been approved for clinical use to treat movement disorders [2]. Several other tFUS applications are under clinical investigation for functional neurosurgery, drug deliver, neuromodulation, and to noninvasively probe the brain to further understand its workings. Electrophysiological recordings are useful during these procedures to investigate the influence of tFUS on brain neural activity. Functional effects have been observed during high-intensity tFUS thermal ablation [2] and may be a valuable modality for guiding other ablation procedures. Neuromodulation has been observed in humans using pulsed low-intensity tFUS [3], [4] where EEG was a key component for recording the suppression of evoked potentials. Monitoring acoustic emissions from transmitting ultrasound through the head is important during these investigations to verify the ultrasound is having the intended bioeffect on the tissue and not causing any identifiable adverse events. The goal of this study is to investigate the feasibility of using a novel scalp sensor for use in tFUS applications to simultaneously acquire AED and EEG.
tFUS devices can range from a conical single-element piezoelectric crystal using manual steering [5], [6] to a hemispherical phased array helmet-style transducer using electronic beam steering to position a focused ultrasound beam on an intended target [7]. A water or gel pathway must be used to transmit ultrasound from the emitting transducer surface to the scalp. Clinically approved hemispherical phased array transducers require the head to be partially submerged in a water bath [8]. The arrangement limits the exposure of the scalp whereas single-element piezoelectric requires minimal coupling to the head using a water bag or gel pad allowing room for placement of scalp EEG electrodes on the head [3], [9],[10]. Real-time monitoring of these devices all use a variety of imaging modalities and sensors to quantify treatment progression and monitor safety. Some include Magnetic Resonance Imaging (MRI), Ultrasound Imaging (UI), EEG, Global Cavitation Detection (GCD), and Passive Acoustic Mapping (PAM) [11]. The type of monitoring depends on the intensity of the tFUS sonication and aim of the application. For ablation procedures in functional neurosurgery, GCD is used to assure there is no unintended tissue damage due to tissue cavitation in the brain during ultrasound transmission [2]. High-intensity tFUS brain tissue ablation procedures use real-time MRI to produce thermal images for guiding the creation of thermal lesions. PAM is used as an investigational method to localize and quantity the progress of microbubble enhanced low-intensity tFUS drug delivery through the Blood Brain Barrier (BBB) [12]. The PAM method uses receiving piezoelectric sensors to record microbubble oscillations as they pass through the focal spot of an ultrasound beam yielding spatial information about the BBB opening location. Other studies have used pulsed low-intensity tFUS sonication for neuromodulation to study suppression of EEG recorded evoked potentials [3], [10].
EEG may be a promising method to identify the closing of the BBB during tFUS drug delivery applications alongside PAM. A difference in electric potential between the brain parenchyma and blood can be measured at the scalp when the BBB is changes from opened to closed within the millivolt range [13]. Current PAM studies have only shown the ability measure BBB opening but not the closing of the BBB after the treatment [14]. A recent study reports that direct current EEG shifts correlate with the opening and sealing of the BBB during intra-arterial infusion [15]. The current standard for monitoring BBB drug delivery with tFUS is using gadolinium contrast agents [16]. Though, new alternatives for monitoring BBB activity during tFUS drug delivery are warranted since repetitive use of gadolinium may increase the risk of having a toxic effect on some patients [17] if used several times over a multi-therapy course of treatment. Brain mapping with tFUS single-element transducers are being investigated using neuromodulation [5] but there was no AED being used during the tFUS since the ultrasound exposures yielded a mechanical index below the Food and Drug Administration standard limit for diagnostic ultrasound imaging. Transmitting ultrasound powers with a mechanical index above 1.9 may result in soft tissue cavitation and cause tissue damage. However, a past investigation attempted to treat brain blood clots by sending low intensity tFUS through the temporal window continuously for up to more than an hour where AED was not used and resulted in 2 fatalities. A later study used a retrospective computational model to recreate the device and FUS parameters used in the clot-busting study. The model concluded that the device created standing wave regions yielding underestimated ultrasound intensities within the tFUS beam path causing subarachnoid space hemorrhaging and led to the adverse effects [18]. AED would have allowed real-time analysis of the tFUS exposure. Standing wave information may have been identifiable in the time or frequency domains of the AED signals and improved the safety monitoring while using the device. In this study, we demonstrate that both EEG and AED signals can be recorded simultaneously using a prototype scalp sensor during tFUS.
Methods:
Scalp Sensor Design:
The scalp sensor is designed to simultaneously acquire electric fields and detect transcranial acoustic emissions. To examine the intended feasibility of the sensor, multiple benchtop testing methods are used to test the sensor as if it was being used during a human tFUS application. The experiments include testing the senor for interference between the EEG and AED data acquisition systems during operation, confirm there is no signal noise in the EEG and AED acquisition signals from the tFUS sonication, and verify a transcranial acoustic emission signal can be acquired from within the skull in response to a tFUS sonication event.
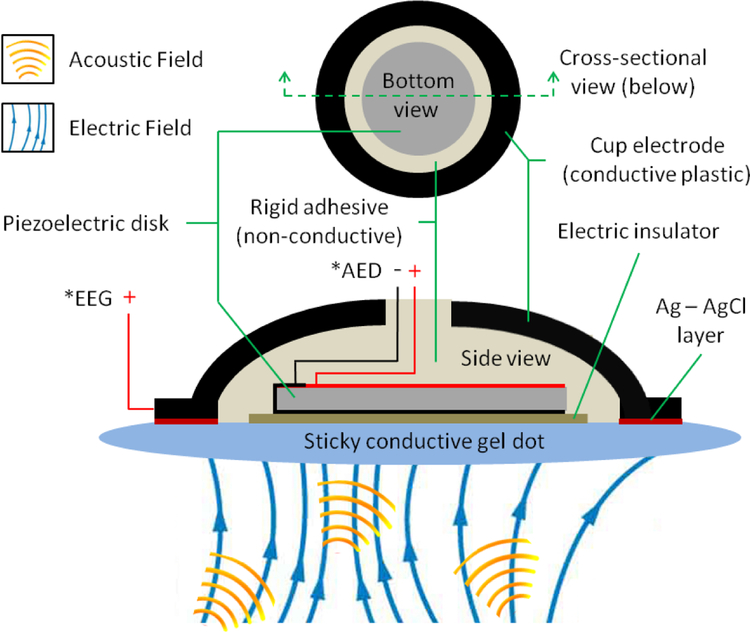
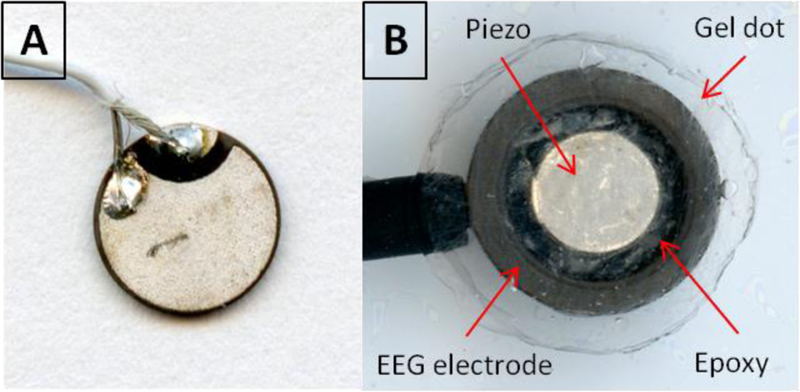
The scalp sensor contains a piezoelectric disk for AED and a conductive plastic cup electrode for EEG, (model: CPE/B, Ives EEG Solutions, Newburyport, MA) and is shown in a schematic in Fig. 1. A 10 mm diameter sticky conductive gel dot is used to couple the sensor to the scalp. The dot is electrically conductive and is a homogenous gel-like media, which allows a pathway for electric field oscillations and ultrasound transmissions to travel from the scalp to the sensor. Industrial glue dots (super high tack, Glue Dots International Inc., Germantown, WI) were chosen for the experiments in this study because their ultra-adhesive properties could adhere to the damp ex vivo skull. The piezoelectric used in the sensor is a 5 mm diameter, 0.4 mm thick disk (model #: SMD05T04R111WL, SM111, STEMiNC, Doral, FL) with a radial mode resident frequency of 450 kHz. Both sides of the piezo are electroplated where both positive and negative soldering points are on the piezo back side (ground) shown in Fig. 2.A. The core of 40 gauge coax is soldered to the back side and the shield wire to the piezo front side. Transparent double sided tape is placed on 3 mm thick transparent acrylic sheet for centering the piezo into the EEG cup electrode. The piezo front side is first stuck to the center of the tape and then the coax is slid through the receiving end of the electrode via a hole in the top of the electrode. This hole is a standard component of EEG electrodes and used for delivering conductive paste between the electrode and scalp during before conventional EEG procedures. Before the electrode is compressed onto the tape, the piezo position relative to the electrode can be seen through the underside of the transparent acrylic sheet for positioning by line of sight. A needle and syringe are used to deliver premixed parts A and B epoxy (2-Ton Clear Epoxy, ITW Deven Corp, Danvers, MA.) through a precut hole on the side of the electrode cup. Epoxy is injected until it purges through the electrode top hole and seen through the acrylic sheet to completely fill the gap between the electrode internal space and around the piezo. The double stick tape coupling prevents the epoxy from getting to the front end of the piezo and electrode. Once the epoxy solidifies after 24 hours, the sensor is removed from the tape. The epoxy was chosen since it is not an electrical conductor, which electrically isolates the piezo from the EEG electrode to eliminate electrical crosstalk between the AED and EEG sensing components. Fig. 2 shows a picture of the scalp sensor receiving side.
Fig. 1:
Schematic of the scalp sensor for AED and EEG data acquisition. Acoustic and electric fields can be obtained simultaneously though the same interface.
Fig. 2:
Picture of scalp sensor with the A) piezoelectric disk (backside showing) embedded in the B) scalp EEG electrode (electric insulator layer and Ag-AgCL layer removed).
Experimental Apparatus:
A bench top experimental apparatus using a phantom was constructed to mimic a human head undergoing therapeutic transcranial focused ultrasound. The goal was to emit an acoustic and electric field from the phantom for testing the sensor capability of acquiring AED and EEG, concurrently in the same senor. The phantom was designed to generate acoustic emissions within the cranium in response to external focused ultrasound stimulation, while simultaneously producing an alternating electric field source on the phantom exterior surface also being emitted from within the cranium.
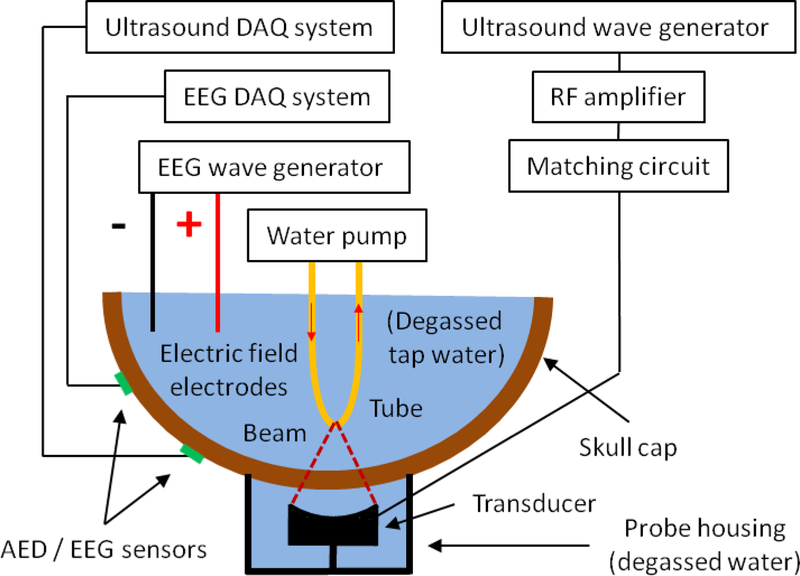
Fig. 3 shows a schematic of the experimental apparatus used to test the scalp sensor and Fig. 4 shows photos of the apparatus. The setup included an ex vivo adult human skull cap (previously fixed in formalin) positioned upside down on top of a bucket filled with degassed and deionizer water. A custom focused ultrasound transducer (fundamental frequency = 272 kHz, 5 cm diameter, focal length = 5 cm) was placed in the bucket and pointed upwards towards the skull. The center of the transducer beam focus was positioned approximately 2.5 cm beyond the inner surface of the skull. Water was filled to the brim of the bucket so the exterior surface of the skull was submerged in water directly above the transducer. The bucket housing the transducer sat in a secondary bucket for catching excess water spillage as the skull was positioned. A ring stand was used to rigidly hang two copper electric field antenna depth electrodes in the rear side of the skull cap but without touching the skull itself and is shown in Fig. 4.C. The antennas were wired to the core and shield wire of a coaxial cable, which was controlled by a function generator for producing a phantom EEG source signal. Next, the skull was filled with degassed and deionizer tap water. An oscillating electric field was created at the exterior surface of the skull when the EEG source function generator was turned on.
Fig. 3:
Experimental apparatus for testing the scalp senor with phantom AED and EEG signals sources.
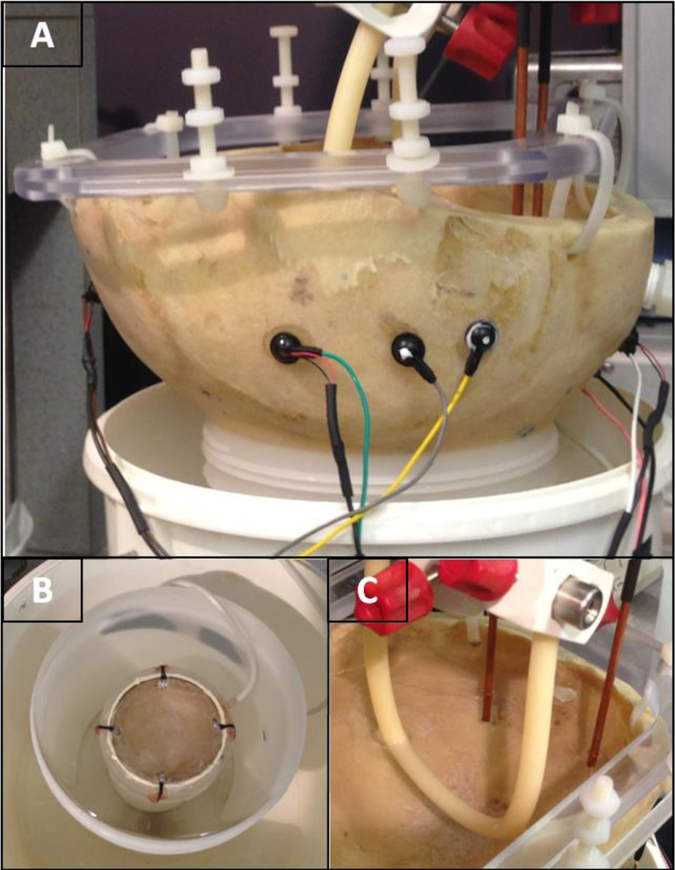
Fig. 4:
Picture of the AED/EEG scalp sensors placed on the A) phantom. B) Sonicating transducer (272 kHz) placed below the phantom. C) The rubber tube within the location of the focused ultrasound transducer beam focus and the location of the electric field antennae outside the beam focus.
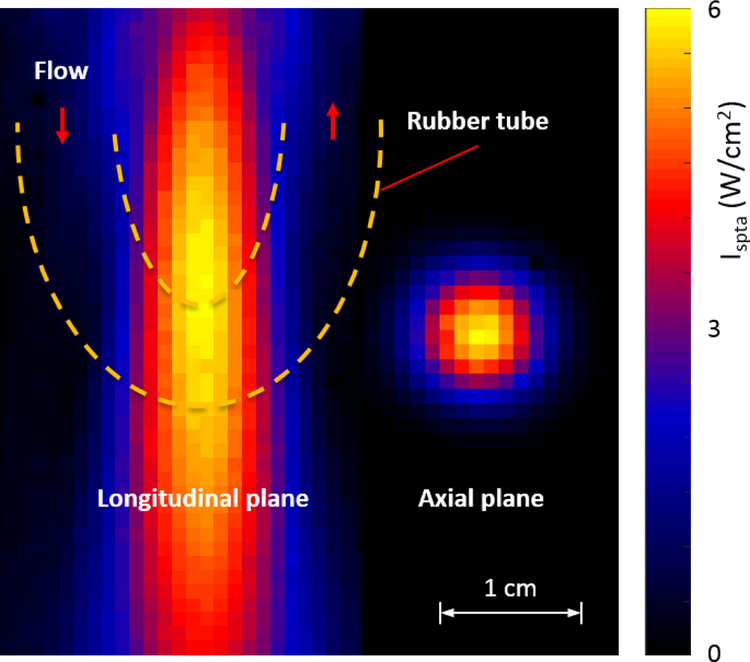
In addition, an automated 3D positioning system (MB603601J-S6, Velmex Inc.) and hydrophone (HNC-0400, Onda Corp.) was used to locate the approximate FUS beam focus position within the skull. Once the focus was found, a thin rubber hose (6 mm inner diameter and 1 mm wall thickness) loop was placed in the approximate location of the focus using the positing system. Fig. 5 shows the approximate location of the rubber hose in the beam focus. The hose loop ends were placed in a 300 mL glass beaker of degassed and deionizer water while a peristaltic pump (7144–05, Cole-Parmer Instrument Co.) circulated the water through the loop and as the beaker was placed on a magnetic stirrer (SP46615, Thermolyne Corp.). Approximately 10 g of talc powder (18654, M = 379.27 g/Mol, Sigma-Aldrich Corp.) was added to the beaker to create nucleation sites for cavitation. The stirrer kept the talc power evenly saturated in the water as it flowed through the tube at approximately 30 cm/s. Care was taken to make sure the vortex in the beaker created from the stirrer did not create any visible air bubbles. Talc-powder water was used to lower the threshold to create an acoustic emission signal containing frequency content which may be present during a tFUS procedure. The talc powered idea was taken from a past study investigating transdermal insulin delivery [19].
Fig. 5:
Hydrophone beam plot (1 mm resolution) of the FUS transducer used to excite the water in the rubber hose of the phantom. Scanning performed to create this image was conducted without the skull and in a water tank and at a lower acoustic power than used in AED experiments. The approximate size and location of the rubber hose exterior boundaries in relation to the beam focus is depicted with yellow dotted lines.
The custom transducer (272 kHz, 5 cm diameter, 5 cm focal length) was driven by an arbitrary waveform generator (33220A, Agilent Technologies), a 40 W linear RF amplifier (E&I 240L; Electronics & Innovation), and custom matching circuit. Two identical wave generators worked in tandem to transmit continuous wave or pulsed focused ultrasound into the phantom. The sonications consisted of either 272 kHz continuous wave exposures, or 1 ms pulses applied at a pulse repetition frequency of 500 Hz. The sonication duration was 0.5 s, and the time between sonications was 4 s. A third function generator acted as the EEG source to power the electric field antenna using frequencies at 6 or 12 Hz and at 200 or 1,000 mV. EEG was acquired with a clinical EEG system (EMU128 Xltek LTM Systems, Natus Neurology Inc.) using Natus software (Natus NeuroWorks 7.1.1, Xltek, a Division of Natus). AED cavitation testing was performed with a high-speed digitizer (NI PXIe-1082, National Instruments) linked to a Matlab interface for the cavitation experiments. For the experiments testing simultaneous AED and EEG, the acoustic signals were monitored using an oscilloscope (DPO 3034, Tektronix) during simultaneous AED and EEG testing. The sync output of the ultrasound wave generator was synchronized the FUS transmission with the EEG and AED systems.
Feasibility Testing:
Various tests were performed to evaluate the ability of the sensor to collect signals from an acoustic and electric field at the same time. All EEG channels were set to record relative to the common electrode in every experiment, which included 1) detecting cavitation frequency content in acoustic emissions recordings, 2) evaluating variability in EEG signal quality from embedding a piezo disk in an EEG cup electrode, and 3) investigating potential signal interference while simultaneously acquiring acoustic and electric fields.
-
1)
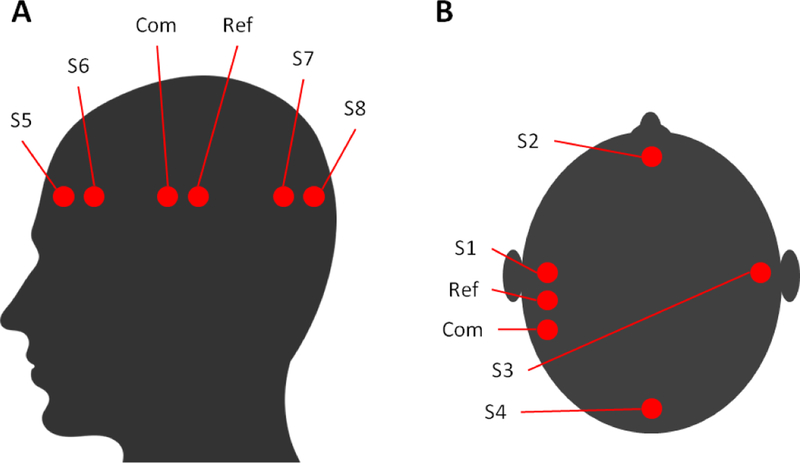
A sensor was placed at the S1 location shown in the Fig. 6.B. The S1 AED time signal was recorded during sonications directed at the rubber tube. Degassed and deionized water was constantly pumped through the rubber hose from the beaker while the sonication power was incrementally increased. The process was repeated three times and then the entire routine was also performed with the talc-powder water flowing through the hose. Recordings were obtained for each 10 ms per burst.
-
2)
Two sensors where positioned at S5 and S7 while two conventional scalp EEG electrodes not containing piezo disks were placed at S6 and S8. This arrangement is shown in Fig. 6.A along with the common and reference EEG electrode locations, which were both conventional EEG electrodes. S5, S6, S7, and S8 time signals where recorded with the EEG system. The EEG source wave generator amplitude was increased until a signal at approximately 30 μVpp was seen in the EEG acquisition. Once the background EEG at 6 Hz and 30 μVpp was observed in all four EEG channels, the frequency (6 Hz to 12 Hz) and amplitude (20 mVpp to 1 Vpp) was adjusted in the EEG source wave generator to examine the signals of the conventional electrodes vs. the sensors. The goal was to have S5 and S6 recording EEG from approximately the same location along with S7 and S8. Pulsed sonications where transmitted every 2 s to the rubber hose while the talc-powder water was flowing like in the cavitation experiments. Recordings were obtained for 9 seconds.
-
3)
Four sensors where placed at the S1, S2, S3, and S4 locations shown in Fig. 6.B along with two convectional EEG electrodes as the common and reference. The AED and EEG time signals of each sensor were recorded with the AED and EEG data acquisition systems, respectively. Continuous wave and pulsed wave sonications were used to induce cavitation in the talc-water hose while the EEG source wave generator was turned on. Recordings occurred for 10 s in each scenario.
Fig. 6:
Locations of sensors and electrodes during experiments and equvelnt locations where they would be placed on a human scalp.
Results:
Acoustic Emission Detection:
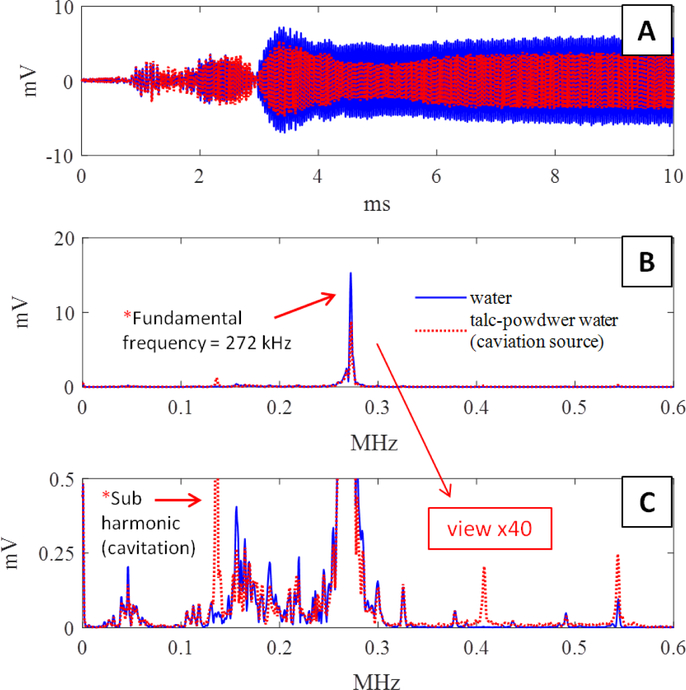
The goal of the cavitation experiments was to evaluate the capability of the sensor to detect transcranial cavitation activated from an external FUS transducer. Fig. 7 shows the AED results from sensor S1 during sonication of the rubber tube containing talc-powder water vs. degassed water. The sonication parameters were 272 kHz with 1 ms pulses at approximately 6.4 Watts acoustic power. Fig. 7.B & C contain the fundamental and sub-harmonic frequency peaks produced during the 272 kHz sonication. The spike indicated with an arrow labeled with “Sub harmonic (cavitation)” in Fig. 7.C shows a large increase in sub-harmonics during sonication of the talc-powder water, indicating the presence of cavitation.
Fig. 7:
AED results from sensor S1 during cavitation experiments. A) Time domain of acoustic emissions of the talc water (red) and degassed water (blue) flowing through the rubber tube during sonication. B) Frequency domain of the time series in (A). C) Magnified image of (B) showing the sub-harmonic peak. The example with cavitation is shown in red where; a large increase in sub-harmonics is evident during sonication in the talc water. Each data point is averaged after conducting the experiment 3 times.
Electroencephalography:
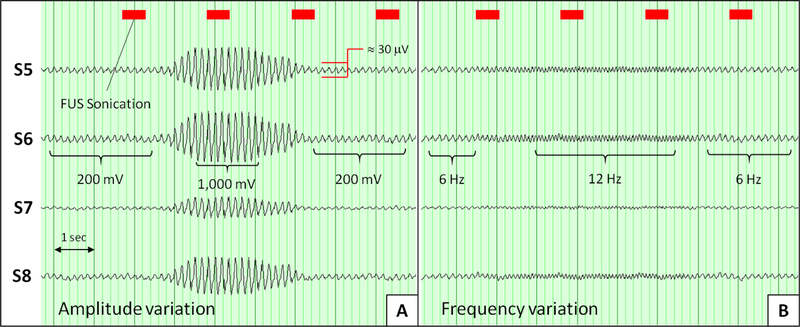
Recordings from conventional scalp EEG electrodes are compared to those made with the AED/EEG sensors are shown in Fig. 8. An EEG phantom source with a frequency of 6 Hz and an amplitude of 200 mVpp was used as the baseline EEG signal. The amplitude was increased from 200 mV to 1 Vpp for approximately 2 s and then decreased back to 200 mVpp. Recordings made from 2 conventional scalp electrodes and 2 AED/EEG sensors are shown in Fig 8.A. In Fig 8.B, the EEG is shown for each electrode position as the background source frequency was increased from 6 Hz to 12 Hz for approximately 2 s and then decreased back to 6 Hz.
Fig. 8:
EEG DAQ for simultaneous comparison of conventional scalp EEG electrodes vs. combined AED/EEG sensors. S6 and S8 are conventional scalp electrodes and S5 and S7 are the custom sensors. A) Amplitude variation test. B) Frequency variation test. No averaging was used. The FUS sonication timestamp is shown above the EEG signals.
Simultaneous acquisition of AED and EEG
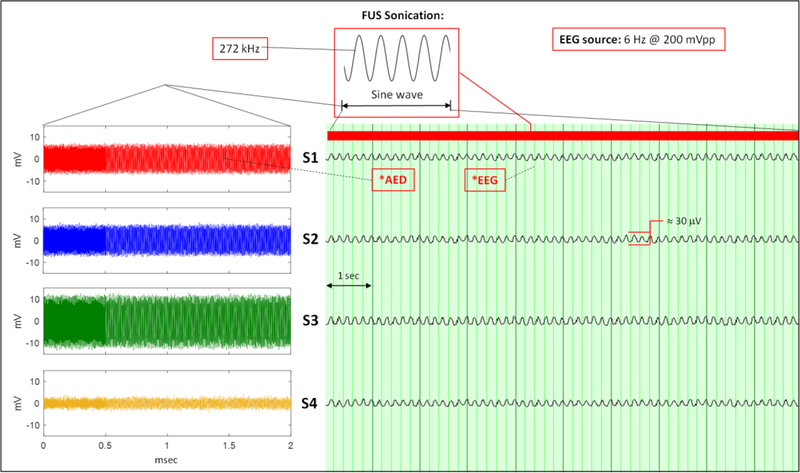
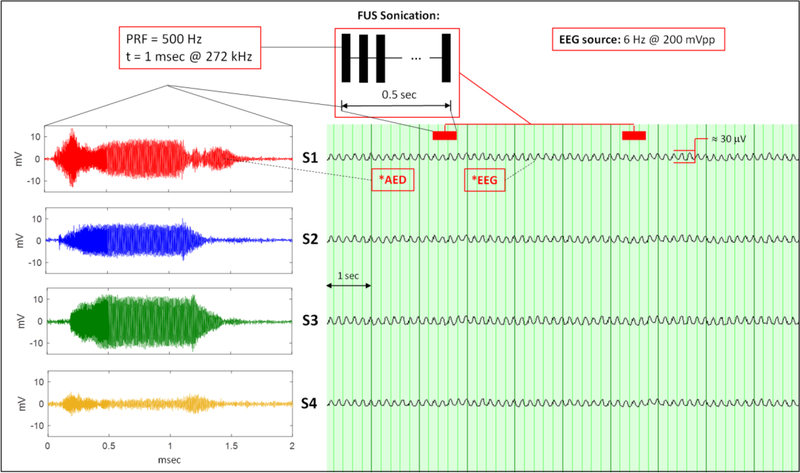
Four sensors at S1, S2, S3, and S4 acquired AED and EEG signals at the same time during continuous wave and pulsed wave sonication while the EEG source remained on continuously at 6 Hz and 200 mVpp. Fig. 9 shows the simultaneous recordings of AED and EEG during continuous wave sonication. Fig. 10 shows the same measurements during pulsed wave sonication.
Fig. 9.
Continuous 272 kHz wave sonication during simultaneous acquisitions of AED and EEG. (Left: AED; right: EEG). The sonication timestamp is indicated above the EEG.
Fig. 10:
Pulsed wave sonication during simultaneous acquisitions of AED and EEG with Pulse Repetition Frequency (PRF) and 500 Hz and a pulse duration of 1 ms. (Left: AED; right: EEG). The sonication timestamp is indicated above the EEG.
Discussion:
The primary reasoning for designing the scalp senor is to acquire electrophysiological and acoustic emissions in the same point source location and in one system. A 10–20 EEG system is the standard grid used to place surface EEG electrode on the human head. Integrating the piezoelectric disks directly into the electrodes was chosen so the spatial identity of the acoustic emission source detector can potentially be defined by the 10–20 system in for future studies. EEG source localization methods often depend on the 10–20 system in order to spatial integrate EEG data with other brain imaging like MRI or CT so it may be beneficial to use the 10–20 system for acoustic emission sensor placement, as well. Before glue dots were used in experiments, hydrogel based TechDots (Ives EEG Solutions Inc., Newburyport, MA) designed to be used in human EEG applications as an alternative to EEG conductive glue were used in experiments. However, the Techdots are sensitive to water and would not stay attached to the wet skull specimen even though they are an adhesive/conductive gel. They are better suited for adhering to the dry scalp in an actual human procure.
Fabrication of the custom sensors was conducted in-house and included no design restraints regarding manufacturing procedures. It would be of interest to enhance the manufacturing process so the sensors could be produced in a mass production. The cost of materials to produce the sensors is within range of standard EEG scalp electrode but the effort it took to fabricate the sensors in prototype form used a time consuming and handmade manufacturing process. Design characteristics could also be improved. Small bubbles in the epoxy used to embed the disk in the electrode were present after the epoxy solidified. The process and type of epoxy used in the embedding process could be improved to minimize bubbles. Bubbles in the hardened epoxy did not show any visible effects in the AED signal during experiments. It is expected the bubbles in the epoxy oscillate in a frequency ranges several times larger in magnitude than the fundamental frequency and cavitation frequencies of below 1 MHz as the sensor acquires the acoustic emission fields. Another design improvement would be to add an alternative electric insulator layer over the disk in the sensor on the receiving side. A circular section of standard back electrical tape with a slightly larger diameter then the piezo was placed over the disk. This electrically isolated the receiving side of the disk from the EEG electrode and sticky conductive gel dot. During preliminary experiments, the sensor did not include the electrical tape layer. Electrical noise spikes occurred in the EEG signal at the same time the pulsed wave sonications were being transmitted while the EEG phantom source signal antenna was turned off. The electrical tape was then added to the sensor over the piezo disk and then the cross-talk was no longer observed. Since the piezoelectric disk produces an electric potential across its electrode surfaces in response to the acoustic pressure wave, the electric potential must have traveled into the EEG electrode causing the cross talk. An electrically-insulating coating could be used as an alternative to the electrical tape. Although the piezo disk selected has a resonant frequency of 450 kHz, the disk thickness could be adjusted to achieve different resonant frequencies for targeting specific AED applications.
The functional phantom constructed for testing the sensor could emit both oscillating electric fields and a cavitation signal from within an ex vivo skull cap. These simultaneous emissions were crucial for evaluating the sensor performance under the limits of using experimental bench top and ex vivo tissue methods. Using an electric field antenna to produce phantom EEG signals [20] and sonicating talc-powder water in a rubber tube to create a cavitation emission signal [19] has been used successfully in prior investigations. The phantom integrated the two ideas so the electric and acoustic field could be present at the same time at the exterior skull surface. The goal of the EEG source signal was to have the electric field propagate from a location inside the skull where the brain would be located. Using a fully saturated skull specimen in water is essential to keep the specimen electrically conductive so the electric field can reach the EEG electrodes. One important characteristic of the phantom setup is that the acoustic emissions received in Fig. 7 by were emitted from inside the skull in response to direct-wave sound transmission from the FUS transducer. The AED signals may have contained guided-wave sound transmissions traveling through the skull, which originate from the FUS beam entering the exterior of the skull. However, analyzing guided-wave sound transmission was not the focus of this study but their time of flight and attenuation characteristics may be important to explore for use in other transcranial FUS applications.
The fundamental frequency of the sonicating transducer is observable in the frequency domain in Fig. 7.B. Although the fundamental frequency emission in and of itself may not be relevant to detecting cavitation, its amplitude could be used to gauge the acoustic intensity levels during clinical procedures. The acoustic emission fundamental frequency amplitude has been used in a sonothrombolysis trial using a transcranial ultrasound device. In that study, the most efficient transmitting piezoelectric disk within in a multi-element array was used for recording [21]. Eight piezo disks were coupled to each temple to target the Circle of Willis while only one disk transmits at a time while the disks on the other side of the head receive the acoustic emissions. The disk producing the largest amplitude out of the eight transmitting disks is used for the therapy. A full 10–20 grid montage of AED scalp sensors could be used in the same way to monitor the acoustic field in three dimensions during ultrasound transmission into the skull and may provide useful information for guiding a and monitoring transcranial ultrasound applications.
Fig. 7.A shows the ability of the scalp sensor to detect cavitation from inside the skull. The amplitude at the 136 kHz sub-harmonic is distinctly visible with talc-powder water compared to water alone in the rubber tube. Although this experiment offers no information on spatial information regarding the location of the cavitation event, the information could be used to tune sonication power levels during drug delivery therapy. The ExAblate 4000 (InSightec Inc., Israel) uses GCD to monitor unwanted cavitation during ablation procedures [2]. High amplitude noise around sub or ultra-harmonics detected in the GCD signal is an indication of inertial cavitation. Inertial cavitation can cause tissue damage in or around an ablation target, which may result in blood bubbling or tissue liquefaction. These responses are considered an adverse effect in tFUS ablation procedures. The ExAblate 4000 immediately shuts off sonication if the cavitation is detected. Several studies have used single passive cavitation detectors for sensing cavitation or controlling sonication power [22]–[24]. Having multiple detectors placed directly on the scalp would both improve the SNR of these recordings. If one could determine the position of the detectors, it may possible reconstruct images showing the location of the emissions [12], [14], [25], [26]. Having the detectors on the scalp could improve the SNR in these images. Having flexibility to choose the placement of the detectors could also allow one to tailor the imaging field of view to each patient.
The EEG results in Fig. 8 indicate the scalp sensor is capable of acquiring EEG signal quality equivalent to EEG electrodes not containing piezoelectric disks. EEG is very reliant upon the location of the electrode recording [27]. However, the conventional electrode cannot be placed in the exact same position as the scalp sensor to compare them simultaneously in the same recording location. The conventional electrodes and scalp sensors were placed approximately 10 mm adjacent to one another and tested in temporal synchronization with the same EEG phantom source. Human scalp EEG frequencies during awake and resting state are in the Alpha (8 −13 Hz) range and typically has an amplitude between 10 to 100 μV. Neurological dysfunctions in the brain have specific signatures in EEG waveforms. For example, a seizure can manifest as an emergence of a rhythmic pattern of slow waves or a sudden increase in amplitude seen in a portion of EEG electrodes [28]. To impersonator these events, the EEG phantom signal parameters were set near the Alpha wave range and the amplitude was also varied to mimic a neuroglial event.
The EEG amplitude and frequency variation test in Fig. 8 were conducted to simulate different EEG waveforms for evaluating the sensors. There is some limitation in conducting this as a bench top experiment. Typically, the Low Pass Filter (LPF) and High Pass Filter (HPF) are set to 0.5 Hz and 60 Hz, respectively and a notch filter of 60 Hz is used to eliminate utility electric noise. In these experiments, the filters were set to LPF = 1 Hz and HPF = 6 Hz to eliminate the vibration noise caused by being placed on a table and general laboratory background noise. Common scalp EEG signal quality evaluation is usually based on the ability to identify artifact and observe events differing from the background signal in frequency and amplitude. The experiments conclude the scalp sensors can obtain these variations. Further evaluation is needed with human subjects to examine the scalp sensor EEG ability in a full 10–20 montage.
Therapeutic FUS is delivered in continuous or pulsed wave sonication to the brain. The acoustic intensity depends on the application where high-intensity FUS ablation mostly uses continuous wave and neuromodulation applications use low-intensity FUS use pulsed wave. The sonication parameters of 272 kHz, pulse repetition frequency = 500 Hz, pulse duration = 1 ms used in this study have been used in prior human applications [2], [5]. Both scenarios were examined to evaluate the sensors ability to acquire the acoustic and electric field at the same time without any interference between each other. Fig 9 and Fig. 10 demonstrate the ability of 4 sensors to record AED and EEG simultaneously during continuous and pulsed wave sonication, respectively. In both the continuous and pulsed wave cases, the 6 Hz EEG phantom source signal is observable without any identifiable interference from the acoustic field during sonication or vice versa. This is most noticeable in the pulsed wave sonication of Fig. 10 since the FUS transducer in turned off in between sonications. Talc-powered water was circulated in the rubber hose during all data experiments except for the data collected in Fig. 7 where water was circulated in the hose. The most significant difference to note between the EEG and AED acquisition is the sample rate of each system. The EEG and AED system sampled at 1 kHz and < 5 MHz, respectively. Given EEG source signals of interest are < 100 Hz and in this study the AED source signals were > 10,000 Hz, the electric field and acoustic field emissions are distinctly separate in nature and this simplifies the ability to acquire the two spectra simultaneously.
Conclusion
We have designed, constructed, and tested a combined sensor for simultaneous recording of EEG and acoustic emissions for use during tFUS therapies. Synchronizing the acquisition of these two modalities may be a promising tool for monitoring FUS ablation procedures, measuring the effect of ultrasound therapy in real-time for treating neurological disorders, or for monitoring electrophysiology during focused ultrasound neuromodulation. The AED/EEG scalp sensor shows promise for use in transcranial human applications within the limitations an experimental bench top evaluation.
Acknowledgments
The authors wish to acknowledge Thank you to Paul Dionne from Brigham and Women’s Hospital for his help with the EEG data acquisition system and discussions about EEG. This work was supported by the National Institutes of Health under grant P01CA174645 and R25CA089017.
Footnotes
Conflicts of Interest: JRI is head of R&D at Ives EEG Solutions design and manufacture of the conductive plastic EEG electrodes and the TechDots.
References:
- [1].Fry WJ, Mosberg WH, Barnard JW, and Fry FJ, “Production of Focal Destructive Lesions in the Central Nervous System With Ultrasound,” J. Neurosurg, vol. 11, no. 5, pp. 471–478, 1954. [DOI] [PubMed] [Google Scholar]
- [2].Elias WJ, Lipsman N, Ondo WG, and Ghanouni P, “A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor,” N Engl J Med, al, vol. 375, pp. 730–739, 2016. [DOI] [PubMed] [Google Scholar]
- [3].Legon W et al. , “Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans,” Nat. Publ. Gr, vol. 17, no. 2, pp. 322–329, 2014. [DOI] [PubMed] [Google Scholar]
- [4].Lee W et al. , “Non-invasive transmission of sensorimotor information in humans using an EEG / focused ultrasound brain-to-brain interface,” pp. 1–20, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee W, Chung YA, Jung Y, Song IU, and Yoo SS, “Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound,” B M C Neurosci, vol. 17, no. 68, pp. 1–11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Legon W, Ai L, Bansal P, and Mueller JK, “Neuromodulation with single-element transcranial focused ultrasound in human thalamus,” Hum. Brain Mapp, vol. 39, no. 5, pp. 1995–2006, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McDannold N, Clement G, Hynynen K, Black P, and Jolesz F, “Transcranial MRI-guided focused ultrasound surgery of brain tumors: Initial findings in three patients,” Neurosurgery, vol. 66, no. 2, pp. 323–332, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eames MDC, Hananel A, Snell JW, Kassell NF, and Aubry J, “Trans-cranial focused ultrasound without hair shaving : feasibility study in an ex vivo cadaver model,” J. Ther. Ultrsound, vol. 1, no. 24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee W, Kim H, Jung Y, Song I, Chung YA, and Yoo S, “Image-Guided Transcranial Focused Ultrasound Stimulates Human Primary Somatosensory Cortex,” Sci. Rep, vol. 5, no. 8743, pp. 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee W, Kim H, Jung Y, Chung YA, Song I, and Lee J, “Transcranial focused ultrasound stimulation of human primary visual cortex,” Nat. Publ. Gr, pp. 1–12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leinenga G, Langton C, Nisbet R, and Götz J, “Ultrasound treatment of neurological diseases — current and emerging applications,” Nat. Rev. Neurol, vol. 12, no. 3, pp. 161–174, 2016. [DOI] [PubMed] [Google Scholar]
- [12].Crake C, Brinker ST, Coviello CM, Livingstone MS, and McDannold NJ, “A dual-mode hemispherical sparse array for three-dimensional passive acoustic mapping and skull localization within a clinical MRI guided focused ultrasound device,” Phys M ed Biol, vol. 16, no. 65008, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Voipio J, Tallgren P, Heinonen E, Vanhatalo S, and Kaila K, “Millivolt-Scale DC Shifts in the Human Scalp EEG: Evidence for a Nonneuronal Generator,” J. Neurophysiol, vol. 89, no. 4, pp. 2208–2214, 2002. [DOI] [PubMed] [Google Scholar]
- [14].Arvanitis CD, Clement GT, and McDannold N, “Transcranial Assessment and Visualization of Acoustic Cavitation: Modeling and Experimental Validation,” vol. 34, no. 6, pp. 1270–1281, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kiviniemi V, Korhonen V, Kortelainen J, Rytky S, and Siniluoto T, “Real-time monitoring of human blood-brain barrier disruption,” PLoS One, pp. 1–16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marty B et al. , “Dynamic study of blood-brain barrier closure after its disruption using ultrasound: A quantitative analysis,” J. Cereb. Blood Flow Metab, vol. 32, no. 10, pp. 1948–1958, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rogosnitzky M and Branch S, “Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms,” BioMetals, vol. 29, no. 3, pp. 365–376, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsivgoulis G and V Alexandrov A, “Ultrasound-Enhanced Thrombolysis in Acute Ischemic Stroke: Potential, Failures, and Safety ULTRASOUND IN ACUTE CEREBRAL,” vol. 4, pp. 420–427, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Feiszthuber H, Bhatnagar S, Gyöngy M, and Coussios CC, “Cavitation-enhanced delivery of insulin in agar and porcine models of human skin,” Phys. Med. Biol, vol. 60, no. 6, pp. 2421–2434, 2015. [DOI] [PubMed] [Google Scholar]
- [20].Collier TJ, Kynor DB, Bieszczad J, Audette WE, Kobylarz EJ, and Diamond SG, “Creation of a Human Head Phantom for Testing of Electroencephalography Equipment and Techniques,” IEEE Trans. Biomed. Eng, vol. 59, no. 9, pp. 2628–2634, 2012. [DOI] [PubMed] [Google Scholar]
- [21].Barlinn K et al. , “CLOTBUST-Hands Free Initial Safety Testing of a Novel Operator-Independent Ultrasound Device Device in Stroke-Free Volunteers,” Stroke, no. June, pp. 1641–1647, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun T et al. , “Closed-loop control of targeted ultrasound drug delivery across the blood-brain/tumor barriers in a rat glioma model,” Proc. Natl. Acad. Sci, vol. 114, no. 48, pp. E10281–E10290, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O’Reilly MA and Hynynen K, “Blood-Brain Barrier: Real-time Feedback-controlled Focused Ultrasound Disruption by Using an Acoustic Emissions–based Controller,” Radiology, vol. 263, no. 1, pp. 96–106, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marquet F, Tung YS, Teichert T, Ferrera VP, and Konofagou EE, “Noninvasive, transient and selective Blood-Brain barrier opening in Non-Human primates in vivo,” PLoS One, vol. 6, no. 7, pp. 1–7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Crake C et al. , “Enhancement and Passive Acoustic Mapping of Cavitation from Fluorescently Tagged Magnetic Resonance-Visible Magnetic Microbubbles In Vivo,” Ultrasound Med. Biol, vol. 42, no. 12, pp. 3022–3036, 2016. [DOI] [PubMed] [Google Scholar]
- [26].Deng L, O’Reilly MA, Jones RM, An R, and Hynynen K, “A multi-frequency sparse hemispherical ultrasound phased array for microbubble-mediated transcranial therapy and simultaneous cavitation mapping,” Phys. Med. Biol, vol. 61, no. 24, pp. 8476–8501, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tautan AM, Serdijn W, Mihajlovic V, Grundlehner B, and Penders J, “Framework for evaluating EEG signal quality of dry electrode recordings,” in 2013 IEEE Biomedical Circuits and Systems Conference, BioCAS 2013, 2013, pp. 186–189. [Google Scholar]
- [28].Cooper R, Osselton JW, and Shaw JC, EEG Technology, 2nd ed 1974. [Google Scholar]