Abstract
Influenza continues to pose a threat to public health by causing illness and mortality in humans. Discovering host factors that regulate influenza virus propagation is vital for the development of novel drugs. We have previously reported that sphingosine kinase (SphK) 1 promotes influenza A virus (IAV) replication in vitro. Here we demonstrate that the other isoform of SphK, SphK2 promotes the replication of influenza A virus (IAV) in cultured cells, and temporary inhibition of SphK1 or SphK2 enhances the host defense against influenza in mice. IAV infection led to an increased expression and phosphorylation of SphK2 in host cells. Furthermore, pharmacologic inhibition or siRNA-based knockdown of SphK2 attenuated IAV replication in vitro. Notably, oral administration of an SphK2-specific inhibitor substantially improved the viability of mice following IAV infection. In addition, the local instillation of an SphK1-specific inhibitor or an inhibitor that globally blocks SphK1 and SphK2 provided protection to IAV-infected mice. Collectively, our results indicate that both SphK1 and SphK2 function as proviral factors during IAV infection in vivo. Therefore, SphK1 and SphK2 represent potential host targets for therapeutics against influenza.
Keywords: influenza virus, sphingosine kinase 2, sphingosine kinase 1
1. Introduction
Influenza is a major threat to the human population worldwide. Seasonal influenza outbreaks occur annually, resulting in substantial morbidity and adverse economic effects (Iuliano et al., 2017). Moreover, pandemic influenza can cause elevated illness and mortality (Morens and Fauci, 2007). In 2009, pandemic influenza (H1N1) became prevalent globally, and the recurrent outbreaks of avian influenza are adding to concerns about the next potential influenza pandemic (Claas et al., 1998; Cowling et al., 2013; Fraser et al., 2009; Wang and Palese, 2009). Vaccines against influenza virus must be reformulated annually and have a lower efficacy than vaccines targeting other viruses mainly due to the frequent genetic mutations introduced into the IAV genome. Antiviral drugs that inhibit the function of viral proteins such as NA and M2 are available to treat disease caused by influenza virus infection. However, limitations exist for these therapies, as multiple viral strains were found to be resistant to the contemporary antiviral drugs (Cheng et al., 2010; Dharan et al., 2009; Marjuki et al., 2015; Poland et al., 2009). Therefore, it is important to discover new therapeutic targets that regulate influenza virus replication and are less susceptible to the high rate of genetic mutation of influenza virus.
Sphingosine kinase (SphK) has two isoforms, SphK1 and SphK2, which mediate the phosphorylation of sphingosine to form sphingosine 1-phosphate (S1P) (Oskouian and Saba, 2010; Spiegel and Milstien, 2011). These two isoforms are located in distinct subcellular compartments: SphK1 is positioned in the cytosol and at the plasma membrane, whereas SphK2 localizes primarily to the nucleus but can be found in the cytosol and endoplasmic reticulum under certain cellular conditions (Igarashi et al., 2003; Maceyka et al., 2005; Taha et al., 2006). SphK1 is known to promote cell survival and proliferation. In contrast, SphK2 was controversially reported to have anti-apoptotic properties (Min et al., 2007; Min et al., 2005; Pitson, 2011). SphK2, but not SphK1, mediates the phosphorylation of FTY720 (Don et al., 2007; Kharel et al., 2005; Zemann et al., 2006), which is an analogue of sphingosine and an immune modulatory drug used clinically for the treatment of multiple sclerosis (Brinkmann et al., 2010; O’Connor et al., 2009; Ziemssen et al., 2017). SphK1 was reported to be critical for TNF-α and NF-κB signaling during inflammatory responses, while both pro- and anti-inflammatory functions of SphK2 have been documented (Alvarez et al., 2010; Neubauer and Pitson, 2013; Pitson, 2011). SphK1 has been reported to regulate the replication of several viruses such as bovine viral diarrhea virus (BVDV), human cytomegalovirus (HCMV), influenza virus, and measles virus in vitro (Machesky et al., 2008; Seo et al., 2010; Vijayan et al., 2014; Yamane et al., 2009). Importantly, inhibition of SphK1 suppresses the activation of NF-κB, leading to decreased influenza viral RNA synthesis, and SphK1 inhibition interferes with CRM1/RanBP3-mediated nuclear export of the influenza viral ribonucleoprotein complex (Seo et al., 2010). However, the role of SphK2 during virus infections remains poorly understood. A few studies reported that SphK2 could regulate cellular gene expression during chikungunya virus (CHIKV) infection and maintain viral latency for Kaposi’s sarcoma-associated herpesvirus (KHSV) (Dai et al., 2014; Reid et al., 2015). Yet, the role of SphK2 in influenza virus propagation is unknown. Furthermore, the effect of specific inhibition of SphK1 and SphK2 during viral infection has not been tested in animal models.
In this study, we demonstrate that SphK2 is a proviral cellular factor that accelerates influenza A virus (IAV) replication and viral pathogenicity. SphK2 inhibition strongly suppressed IAV replication in vitro. Oral administration of an SphK2-specific inhibitor increased the survival rate of mice upon lethal IAV infection. Additionally, pharmacologic inhibition of SphK1 protected mice from IAV-induced mortality. Thus, targeting of the SphKs could be a novel strategy to manage influenza virus infection.
2. Materials and Methods
2.1. Virus and cells
Influenza A/WSN/33 (H1N1) virus was initially provided by Yoshihiro Kawaoka (University of Wisconsin-Madison). Influenza A/Puerto Rico/8/34 (H1N1) virus (PR8) was originally provided by Adolfo Garcia-Sastre (Mount Sinai School of Medicine). The pandemic influenza A/CA/04/09 (H1N1) virus was a gift from Wenjun Ma (Kansas State University) (Lee et al., 2017; Xia et al., 2018). The influenza A/Hong Kong/8/68 (H3N2) virus (ATCC VR-1679) and influenza B/Lee/40 virus (ATCC VR-1535) were purchased from ATCC. Viruses were amplified and titrated on Madin-Darby Canine Kidney (MDCK) cells as described (Neumann et al., 1999; Seo et al., 2010; Varble et al., 2014). For infection of cultured cells, cells were incubated with an indicated virus for 1 hour, and then washed with PBS. Following infection with A/WSN/33 (H1N1) cells were incubated with medium containing the fetal bovine serum (FBS), while cells that were infected with the other influenza viruses were incubated with FBS-free medium containing 0.3% BSA and TPCK-trypsin (1 µg/ml) for the indicated time. The supernatants containing infectious viruses were harvested for titration by plaque assay on MDCK cells. For the plaque assay, using serial dilutions of culture supernatants, viruses were adsorbed onto 4 × 105 MDCK cells/well in a 6-well plate for 1 hour, and then cells were incubated with 2 × EMEM (Gibco) mixed with an equal portion of 1% agarose (Seakem ME). Mice were infected by intranasal (i.n.) administration of influenza virus (Pritzl et al., 2015). The sources of human embryonic kidney (HEK) 293 cells, MDCK cells, and human lung epithelial A549 cells have been described (Min et al., 2007; Seo et al., 2013; Vijayan et al., 2014). Cells were cultured in a CO2 incubator at 37°C. HEK 293 cells and A549 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco) and MDCK cells were cultured in Minimum Essential Medium Eagle (MEM, Mediatech) (Seo et al., 2010; Seo et al., 2013; Vijayan et al., 2014; Xia et al., 2015). All media were supplemented with 10% FBS (Sigma-Aldrich) and Penicillin (100 U/ml)/streptomycin (100 µg/ml) (Invitrogen).
2.2. Mice
Wild type C57BL/6 mice were purchased from the Jackson Laboratory. Six to eight week old male or female mice were used in experiments (Pritzl et al., 2015). Mice were bred and maintained in a closed breeding facility according to institutional guidelines and animal protocols approved by the Animal Care and Use Committee of University of Missouri-Columbia.
2.3. Reagents and Antibodies
SphK2 inhibitor ABC294640 (Opaganib) (MedKoo) (Orr Gandy and Obeid, 2013), SphK1 inhibitor SK1-I ((2R,3S,4E)-N-methy1–5-(4’-pentylphenyl)-2-aminopent-4-ene-1,3-diol) (Tocris Bioscience) (Song et al., 2011), and SK inhibitors N,N-Dimethylsphingosine (DMS) (Cayman Chemical) and SKI-II (4-((4-(4-Chlorophenyl)-2-thiazolyl)amino)phenol) (Sigma-Aldrich) (Orr Gandy and Obeid, 2013) were purchased from the indicated manufacturers. Antibodies against human SphK2, influenza A viral NP, M1, and M2, and influenza B viral NP were purchased from Abcam; the antibody against influenza A viral NS1 was purchased from Santa Cruz; the antibodies against human SphK1 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were purchased from Cell Signaling Technology; the antibodies against human phospho-SphK2 and human phospho-SphK1 were purchased from ECM Biosciences.
2.4. Construct and Transfection
Doxycycline (DOX) inducible expression plasmid encoding SphK2 was generated by PCR from pVB201 (provided by Stephen Alexander, University of Missouri-Columbia) (Min et al., 2007) with primers 5’ -GGG GAC AAG TTT GTA CAA AAA AGC AGG CTT ACC ACC ATG GGG GGT TCT CAT CAT CAT- 3’ and 5’ -GGG GAC CAC TTT GTA CAA GAA AGC TGG GTG TCA GGC TTG TGG CTT TTG ACC TGC AGG- 3’. The amplified murine SphK2-encoding fragment was cloned into pINDUCER20 vector using BP and LR clonase kits (Invitrogen) according to the manufacturer’s instructions. The pINDUCER20 reagents were a gift from David Pintel at University of Missouri-Columbia (Adeyemi et al., 2014; Meerbrey et al., 2011). For transient expression, HEK293 cells (2 00D7 105/well) were seeded in a 24-well plate one day before transfection. Cells were then transfected with a plasmid encoding SphK2 (250 ng/well) using LipoD293 transfection reagent (SignaGen) and protocols recommended by the manufacturer. DOX (100 ng/ml, MP Biomedical) was added to the cell culture 24 hours post transfection to induce the transient expression of SphK2.
2.5. Western blot analysis
Western blotting was performed as described previously (Seo et al., 2010; Seo et al., 2013; Vijayan et al., 2014; Xia et al., 2015; Xia et al., 2018). Briefly, cells were lysed in 2× sample buffer containing β-mercaptoethanol and heated at 95°C for 10 min. Equ al amounts of protein samples were resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a nitrocellulose membrane (Bio-Rad). Membrane bound antibodies were detected using IRDye secondary antibodies (IRDye 800CW Goat anti-Mouse IgG and Goat anti-Rabbit IgG; LI-COR). The signals were imaged by Odyssey Fc (LI-COR), and data were analyzed using Image Studio V5.2 (LI-COR). Similar results were obtained from at least three independent experiments.
2.6. RNA interference
ON-TARGETplus Human SphK2 siRNA (si-SphK2) and universal scrambled negative control siRNA (SCR) were purchased from Dharmacon. All siRNAs were used at a final concentration of 20 nM to transfect A549 cells. The cells (2 × 105/well) were transfected with siRNA by reverse transfection using Lipofectamine RNAiMax reagent according to the manufacturer’s instructions. The transfected cells were then seeded in a 24-well plate. One day later, cells were infected with IAV and then harvested at one day post-infection (dpi). The knockdown of SphK2 was confirmed by Western blot analysis.
2.7. Statistical analysis
Data were analyzed and compared using a bidirectional, unpaired Student t test (Pritzl et al., 2015; Xia et al., 2018). Error bars represent means ± standard deviations (SD). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Differences in group survival were analyzed using Mantel-Cox Log-rank p test by using a GraphPad Prism 5 software.
3. Results
3.1. IAV infection increases expression and activation of SphK2.
We have previously found that SphK1 regulates the replication process of IAV (Seo et al., 2010; Seo et al., 2013). In this study we sought to determine whether SphK2 is also involved in the regulation of influenza virus infection. We first analyzed SphK2 protein levels during IAV infection. Influenza A/WSN/33 virus (IAV H1N1) infection heightened the levels of SphK2 protein at 4 and 10 hpi (Fig. 1A). In order to test whether this increase is IAV type/subtype specific, A549 cells were infected with other influenza viruses such as 2009 pandemic influenza A/CA/04/09 (H1N1) virus (IAV pH1N1) (Fig. 1B), influenza A/Hong Kong/8/68 (H3N2) virus (IAV H3N2) (Fig. 1C), or influenza B/Lee/40 virus (IBV) (Fig. 1D). We observed similar up-regulation of SphK2 protein following infection by these viruses (Fig. 1A-D). Since the phosphorylation of SphK2 at Thr-578 represents its activation (Hait et al., 2007), the phosphorylation level of SphK2 was determined upon IAV infection. Infection of A549 cells with influenza viruses increased the amounts of phosphorylated SphK2 (pSphK2) (Fig. 1A-D). Interestingly, influenza viruses increased the levels of SphK2/pSphK2 more strongly at an early time point after infection than those of SphK1/pSphK1 (Fig. 1A-D) The modest increase of SphK1 and pSphK1 in A549 cells following IAV infection is consistent with the data previously reported (Seo et al., 2013). Also, no noticeable change was observed when different virus type/subtypes were tested. Together, these results indicate that IAV infection heightens the expression levels of SphK2 and activated SphK2 (phosphorylation of SphK2).
Fig. 1. IAV increases the levels of SphK2 and pSphK2.
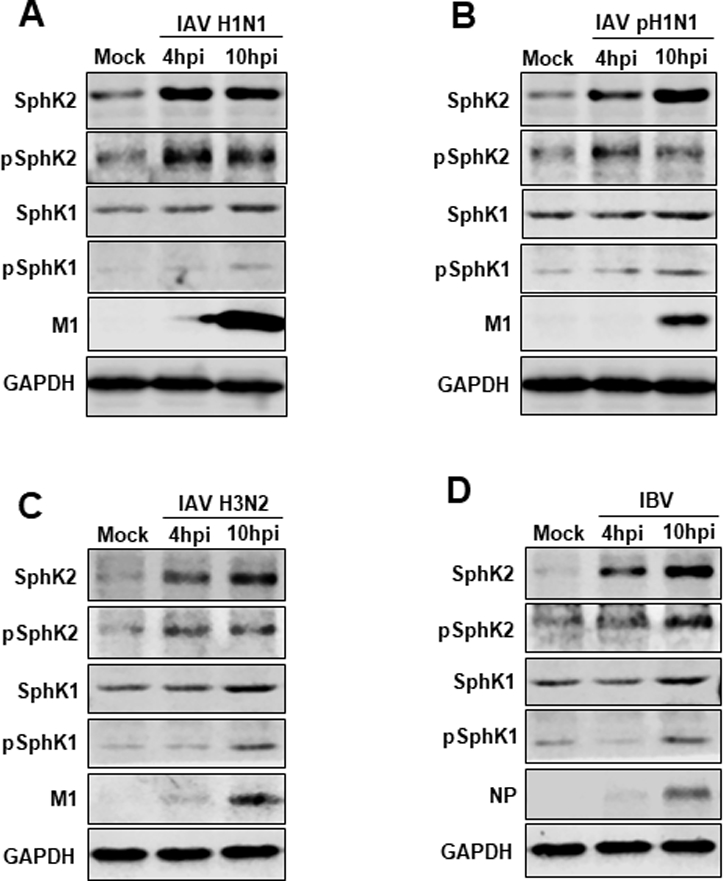
(A-D) A549 cells were infected with influenza A/WSN/33 (H1N1) virus (IAV H1N1) (A), pandemic influenza A/CA/04/09 (H1N1) virus (IAV pH1N1) (B), influenza A/Hong Kong/8/68 (H3N2) virus (IAV H3N2) (C), or influenza B/Lee/40 virus (IBV) at an MOI of 1. The levels of SphK2, pSphK2, SphK1, pSphK1, viral M1, viral NP, and GAPDH were analyzed by Western blotting at 4 hours post-infection (hpi) or 10 hpi. Data are representatives of 2 independent experiments.
3.2. SphK2 is crucial for efficient IAV replication in vitro.
Our observation that the expression and activation of SphK2 were increased by IAV infection led us to hypothesize that SphK2 is a cellular factor beneficial for influenza virus propagation. In order to test this idea, we employed several approaches to genetically control the level of SphK2 in the cells. Firstly, we transfected A549 cells with a Doxycycline (DOX) inducible plasmid encoding SphK2 (pInducer-SphK2) followed by infection with IAV in the presence or absence of DOX. Overexpression of SphK2 greatly increased the expression of viral proteins, NS1 and M1 at two different time points (4 hpi or 10 hpi) (Fig. 2A), suggesting that SphK2 is a proviral factor. Treatment of A549 cells with DOX alone did not affect virus replication (Fig. 2B). Secondly, we used a small interfering RNA (si-RNA) approach to down-regulate SphK2 expression. Knock-down of SphK2 suppressed the expression levels of viral proteins (NP, NS1, M1, and M2) when cells were infected with IAV at a multiplicity of infection (MOI) of 1 or 3 (Fig. 2C).
Fig. 2. SphK2 displays proviral activity and accelerates IAV replication.
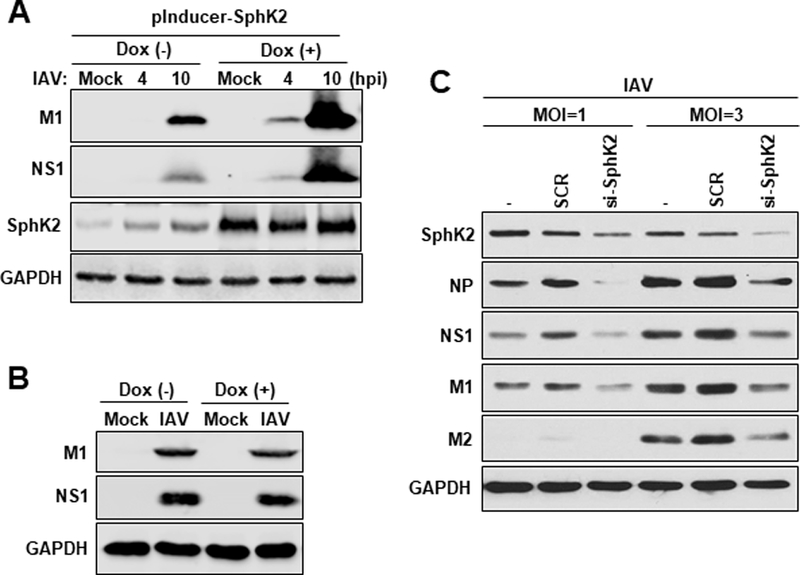
(A) A549 cells (2 × 105) were transfected with an inducible SphK2-encoding plasmid (pInducer-SphK2). 24 hours post-transfection, cells were infected with IAV at an MOI of 1 without (−) or with (+) the treatment of Dox (100 ng/mL). The levels of viral NS1, viral M1, SphK2, and GAPDH were analyzed at 4 hpi or 10 hpi by Western blotting. (B) A549 cells were infected with IAV at an MOI of 1 without (−) or with (+) Dox treatment (100 ng/mL). The levels of viral M1, viral NS1, SphK2, and GAPDH were analyzed by Western blotting at 10 hpi. (C) A549 cells were left untransfected (−), transfected with scrambled control siRNA (SCR) or siRNA targeting human SphK2 (si-SphK2). At 24 hours post-transfection, cells were infected with IAV at an MOI of 1 or 3. At 24 hpi, the levels of SK2, NP, NS1, M1, M2, and GAPDH were analyzed by Western blotting. All data are representatives of 2 or 3 independent experimental repetitions.
To further confirm the importance of SphK2 during IAV infection, we utilized an SphK2-specific inhibitor (ABC294640, referred to as ABC) (French et al., 2010; Orr Gandy and Obeid, 2013) to determine whether inhibition of SphK2 blocks IAV propagation in vitro. As shown in Fig. 3A, the expression levels of viral proteins (M2 and NS1) were markedly decreased by the treatment with SphK2 inhibitor. Furthermore, production of infectious virus particles from cells into the supernatant, which was measured by plaque assay, was significantly diminished (~10 fold) by SphK2 inhibitor treatment (Fig. 3B). We next determined the half maximal effective concentration (EC50), the effective concentration of ABC at which the titer of influenza virus produced from A549 cells was reduced by 50%. To this end, A549 cells were infected with IAV and treated with ABC at 6 different concentrations (Fig. 3C). The percentage of virus titer reduction of all concentrations were analyzed using GraphPad Prism 5 software to determine the EC50 (1.786 µM) (Haasbach et al., 2014). The decreased virus titer was not due to altered cell viability, since the inhibitor used at two highest concentrations (20 µM or 100 µM) did not display cytotoxicity in the experimental condition (Fig. 3D). Collectively, these results indicate that SphK2 functions as a proviral cellular factor, crucial for robust IAV replication.
Fig. 3. SphK2 inhibitor suppresses IAV replication in vitro.
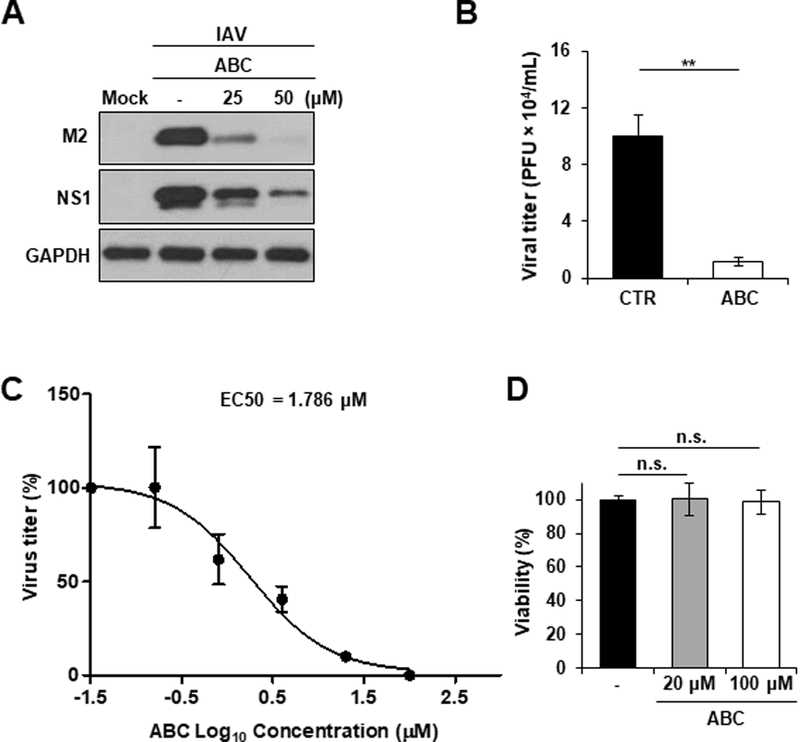
(A) A549 cells were infected with IAV at an MOI of 1. Cells were left untreated or treated with ABC294640 (ABC) at different concentrations as indicated. At 24 hpi, the levels of M2, NS1, and GAPDH were analyzed by Western blotting. The experiment was independently repeated twice. (B) A549 cells were treated with solvent control (CTR) or ABC (50 µM). At 1 hour post-treatment, cells were infected with IAV at an MOI of 0.5. The titer of infectious IAV in the supernatants of the culture was assessed by plaque assay on MDCK cells at 24 hpi (n = 3/group; **, p ≤ 0.01). (C) A549 cells were infected with IAV at an MOI of 0.001 for 1 hour. Infected cells were then treated with ABC at 6 different concentrations (0.032, 0.16, 0.8, 4, 20, or 100 µM) or left untreated. Virus titer in the supernatant was measured at 48 hpi by plaque assay on MDCK cells. No inhibition was seen when cells were treated with ABC at 0.032 µM (virus titer = 2.3 × 106 PFU/mL). The EC50 was determined with GraphPad Prism 5 software. The result represents the average of 3 replicative experiments. (D) A549 cells were treated with solvent (−) or ABC (20 µM or 100 µM) for 48 hours. Cellular viability was monitored by using a trypan blue exclusion assay. The total number of live cells in untreated group was set as 100%, and the relevant number of live cells in the ABC-treated groups are shown in percentages. The data represent means ± SD (n=3). n.s. = not significant.
3.3. SphK2 inhibition protects mice from lethal IAV infection.
Based on our results indicating that inhibition of SphK2 suppresses IAV replication in vitro, we sought to determine whether SphK2 inhibitor treatment protects mice from lethal infection with mouse-adapted influenza virus. C57BL/6 (B6) mice were intranasally (i.n.) infected with IAV followed by oral administration of SphK2 inhibitor (ABC) for two consecutive days. The inhibitor (ABC) was proven to display high bioavailability in cancer animal models when utilized via oral delivery; the inhibitor is highly specific to SphK2, without altering SphK1 activity (French et al., 2010; Orr Gandy and Obeid, 2013). The survival rate of infected mice was monitored for 18 days (Fig. 4A). 90% of IAV-infected, solvent-treated control mice succumbed to infection by 14 dpi. However, 60% of SphK2 inhibitor-administered mice survived longer than the control group from day 9 and 50% of the inhibitor treated mice fully recovered from lethal challenge with IAV (Fig. 4B). Moreover, the weight loss of virus-infected mice (control vs. ABC) was monitored. As shown in Fig. 4C, significant differences in the kinetics of weight loss were observed. ABC-treated mice lost weight more slowly than control group from day 4 and began to regain weight from day 9 (Fig. 4C). Further, we determined the virus titer in the lungs of infected mice at 3 dpi. Treatment with SphK2 inhibitor significantly decreased virus titer in the lungs (Fig. 4D), suggesting that this inhibitor protects mice against lethal IAV infection by reducing virus replication in vivo. Therefore, these results suggest that inhibition of SphK2 could protect mice against lethal IAV infection.
Fig. 4. Orally administered SphK2-specific inhibitor decreases the fatality observed in IAV-infected mice.
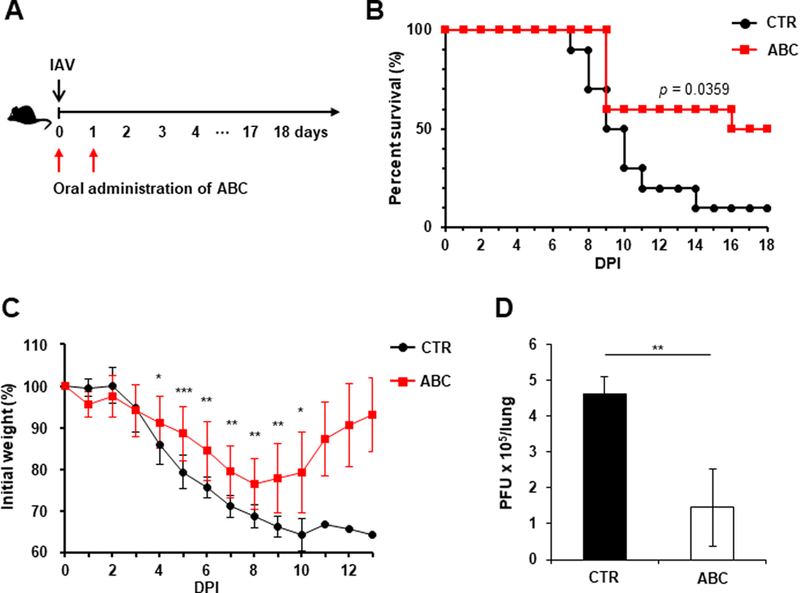
(A-B) C57BL/6 mice were infected with influenza A/PR/8/34 virus (IAV) intranasally (i.n.) at 1×103 PFU. Then, mice were orally administered solvent (PBS) control (CTR) (n=10) or ABC294640 (ABC; 75 mg/kg) daily for 2 days (day 0 and day 1; n=10). All groups were monitored daily for survival. The p value is shown (Log-rank test). (C) The body weights of the mice from (B) were measured daily from day 0 to day 13. The data represent means ± SD (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001). (D) C57BL/6 mice were infected with IAV intranasally at 1×103 PFU. The mice were then orally administered PBS control (CTR, n=4) or ABC (n=4) for 2 consecutive days starting from day 0. Lungs were collected at 3 dpi and viral titers were determined by plaque assay. The data represent means ± SD. **, p ≤ 0.01.
3.4. Inhibition of SphK1 protects mice against lethal IAV challenge.
Previously, we have shown that overexpressed SphK1 protein increases IAV replication, while downregulation or inhibition of SphK1 suppresses IAV replication in vitro (Seo et al., 2010; Seo et al., 2013). Therefore, we sought to investigate whether administration of SphK1 inhibitor could protect mice during lethal IAV challenge. To this end, mice were treated with an SphK1-specific inhibitor (SK1-I) (Paugh et al., 2008; Song et al., 2011) at 0.1 mg kg−1 administered i.n. following lethal IAV infection (Fig. 5A). The survival rate of IAV-infected mice was significantly increased by treatment with the SphK1 inhibitor (Fig. 5B). The result indicates that temporary inhibition of SphK1 provides protection to mice against influenza virus infection.
Fig. 5. Local administration of SphK1 inhibitor enhances the viability of IAV-infected mice.
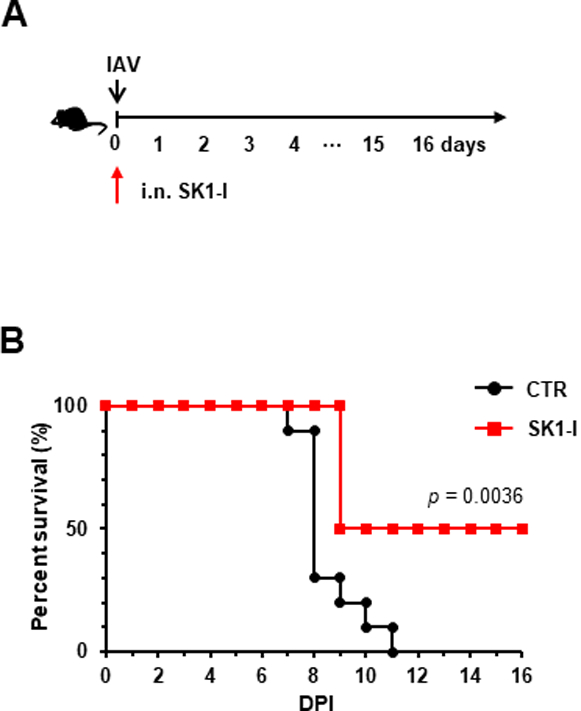
(A-B) C57BL/6 mice were infected with influenza A/PR/8/34 virus (IAV) i.n. at 5×103 PFU. Then, mice were treated i.n. with solvent (PBS) control (CTR) (n= 10) or SK1-I (0.1 mg kg-1, n=8). Mice survival was monitored daily. *, p = 0.0036 (Log-rank test).
3.5. Transient inhibition of both SphK1 and SphK2 rescues mice from lethal IAV infection.
Our results showed that inhibition of either SphK1 or SphK2 had a protective effect on IAV-infected mice. These results led us to test whether pan-SphK inhibitors, which can inhibit both SphK1 and SphK2, protect mice from lethal IAV infection. For this purpose, mice were infected with IAV and then treated with solvent control or a pan-SphK inhibitor such as DMS (Edsall et al., 1998; Orr Gandy and Obeid, 2013) (Fig. 6A and 6B) or SKI-II (French et al., 2003; Orr Gandy and Obeid, 2013) (Fig. 6A and 6C); these mice were monitored for mortality. DMS treatment substantially increased the survival rate of IAV-infected mice (Fig. 6A and 6B). Furthermore, mice that were administered DMS lost weight significantly less than solvent control-treated mice over time (data not shown). Similarly, SKI-II protected mice from lethal IAV infection (Fig. 6A and 6C). SKI-II also significantly reduced the virus titer in the lungs of infected mice (Fig. 6D). Thus, these results suggest that global inhibition of SphKs increases the host defense against pathogenic influenza in vivo.
Fig. 6. SphK inhibitors, DMS and SKI-II, decrease the mortality of IAV-infected mice.
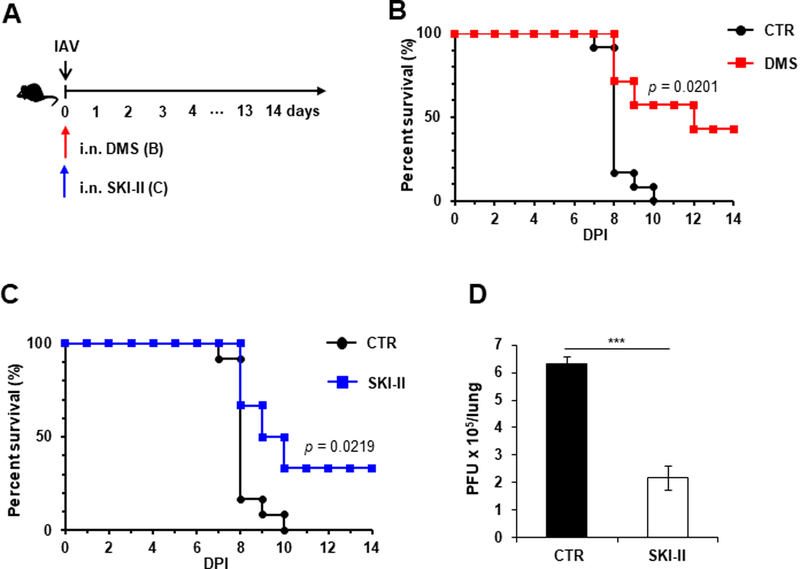
(A-C) C57BL/6 mice were infected with influenza A/WSN/33 virus (IAV) i.n. at 1×104 PFU. Then, mice were treated i.n. with solvent (DMSO; CTR; n=12), DMS (0.003 mg kg−1; n=7), or SKI-II (C) (0.012 mg kg−1; n=6) at day 0. Mice were monitored for their survival. p values are shown (Log-rank test). (D) C57BL/6 mice were infected with IAV at 1×104 PFU. The infected mice were then i.n. administered PBS (CTR, n=3) or SKI-II (0.012 mg kg−1; n=3) at day 0. Lungs were collected at 3 dpi and viral titers were determined by plaque assay. The data represent means ± SD. ***, p ≤ 0.001.
4. Discussion
In this study, we demonstrate a proviral role of SphK2 in IAV propagation in vitro and in vivo. Of note, mice administered with SphK1 or SphK2 inhibitors became more resistant to lethal IAV infection. Thus, SphK1 and SphK2 represent novel cellular targets for the management of influenza virus infection.
Although antiviral drugs that block the function of viral proteins have been developed to treat influenza virus infection, the emergence of viruses resistant to these drugs has been reported (Cheng et al., 2010; Dharan et al., 2009; Marjuki et al., 2015; Poland et al., 2009). Thus, it is of importance to identify novel antiviral targets and design broad-spectrum anti-influenza drugs to combat the infection. Direct targeting of viral components could result in viral escape mutants. Therefore, targeting a host cellular factor(s) such as SphK, which is hijacked for efficient viral replication, may reduce the chance of antiviral drug-resistant viruses emerging. A host factor-targeted approach could conceivably be used in combination with the viral factor-targeted drugs to maximize the drug’s efficacy against influenza.
SphK1 and SphK2 share enzymatic activities to generate S1P from sphingosine (Cyster, 2005; Rosen and Goetzl, 2005; Takabe et al., 2008). However, the sphingosine analogue FTY720 is metabolized by SphK2, but not by SphK1, suggesting the presence of substrate specificity (Don et al., 2007; Kharel et al., 2005; Zemann et al., 2006). It has been reported that they could exhibit differential biologic activities (Alvarez et al., 2010; Lai et al., 2008a; Lai et al., 2008b; Pitson, 2011). However, both SK1 and SK2 were proven to functionally promote IAV replication. Our prior investigation indicates that SphK1 regulates multiple intracellular signaling pathways to control IAV replication (Seo et al., 2013). Currently, it is unknown how SphK2 controls IAV replication from its nuclear or other subcellular localization. Although both SphK1 and SphK2 enhance IAV replication, the molecular mechanisms by which these two enzymes regulate IAV infection may not be identical. Since SphK2 was reported to interact with histone deacetylases, HDAC1 and HDAC2, to regulate gene expressions in cancer cells (Hait et al., 2009), it is possible that activated SphK2 alters the cell’s gene expression profile to enhance IAV replication. This requires further investigation.
We previously reported that SphK1 displays proviral activity during IAV infection in vitro (Seo et al., 2013). As an extension of this observation, we demonstrated that administration of an SphK1 inhibitor increased the survival rate of lethally-infected mice with influenza virus in this study (Fig. 5). While intranasal administration of the SphK1 inhibitor increased survival rate of IAV-infected mice, intraperitoneal delivery of the inhibitor failed to protect mice (data not shown). Since influenza virus predominantly infects epithelial cells of the respiratory tract, locally (intranasally) instilled SphK1 inhibitor may directly act on virus-infected cells and be more effective than systemically (intraperitoneally) administered inhibitor. However, we could not exclude the possibility that systemic delivery of SphK1 inhibitor requires the optimization of treatment regimen, such as doses and frequency of the inhibitor treatment. We observed that a locally delivered high dose (0.1 mg kg−1) of SphK1 inhibitor exhibited greater protective effects on IAV-infected mice than the inhibitor used at low dose (0.01 mg kg−1) (data not shown). Thus, these results suggest that the anti-influenza viral efficacy of SphK1 inhibitor relies on the route of administration and concentration of the inhibitor. Since oral delivery is the most favored administration method for drug use from the practical standpoint, it would be interesting to reformulate the SphK1 inhibitor to an orally administrable drug for testing. In this regard, it is promising that orally instilled SphK2-specific inhibitor (ABC) substantially enhanced the viability of IAV-infected mice. Optimization of the delivery/doses/frequency and the modification/reformulation of these inhibitors to enhance their pharmacologic efficacy warrant future research.
Taken together, in this study, we demonstrate that SphK2 is a newly identified host factor critical for the rigorous replication of influenza virus, and transient inhibition of SphK1/SphK2 elevates host protection against pathogenic influenza in mice. In conjunction with continued development of new pharmacologic SphK-specific inhibitors, this host-targeted strategy could provide insights into future design of new therapeutics against influenza.
Highlights.
Sphingosine kinase (SK) 2 accelerates IAV replication in cultured cells.
IAV infection increases the expression and activation of SK2.
Oral administration of SK2 inhibitor improves the viability of mice following IAV infection. Transient inhibition of SK1 provides protection to IAV-infected mice.
Acknowledgments
We thank Yoshihiro Kawaoka (University of Wisconsin-Madison), Adolfo Garcia-Sastre (Mount Sinai School of Medicine), Wenjun Ma (Kansas State University), Stephen Alexander (University of Missouri-Columbia), and David Pintel (University of Missouri-Columbia) for their kind provision of research reagents as described in Materials and Methods. Also, we thank the animal facility at Medical Science Building at the University of Missouri-Columbia.
This work was supported by NIH/NIAID grant R21AI127404 (B.H.) and Chung-Ang University Research Grants (Y.S.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interests.
References
- Adeyemi RO, Fuller MS, Pintel DJ, 2014. Efficient parvovirus replication requires CRL4Cdt2-targeted depletion of p21 to prevent its inhibitory interaction with PCNA. PLoS Pathog 10, e1004055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S, 2010. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P, 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9, 883–897. [DOI] [PubMed] [Google Scholar]
- Cheng PK, To AP, Leung TW, Leung PC, Lee CW, Lim WW, 2010. Oseltamivir- and amantadine-resistant influenza virus A (H1N1). Emerg Infect Dis 16, 155–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG, 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351, 472–477. [DOI] [PubMed] [Google Scholar]
- Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z, Ni MY, Zhang Q, Ip DK, Yu J, Li Y, Wang L, Tu W, Meng L, Wu JT, Luo H, Li Q, Shu Y, Li Z, Feng Z, Yang W, Wang Y, Leung GM, Yu H, 2013. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 382, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 23, 127–159. [DOI] [PubMed] [Google Scholar]
- Dai L, Plaisance-Bonstaff K, Voelkel-Johnson C, Smith CD, Ogretmen B, Qin Z, Parsons C, 2014. Sphingosine kinase-2 maintains viral latency and survival for KSHV-infected endothelial cells. PLoS One 9, e102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, St George K, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM, Oseltamivir-Resistance Working G, 2009. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301, 1034–1041. [DOI] [PubMed] [Google Scholar]
- Don AS, Martinez-Lamenca C, Webb WR, Proia RL, Roberts E, Rosen H, 2007. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem 282, 15833–15842. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S, 1998. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 37, 12892–12898. [DOI] [PubMed] [Google Scholar]
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C, Collaboration WHORPA, 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324, 1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD, 2003. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res 63, 5962–5969. [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD, 2010. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther 333, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasbach E, Hartmayer C, Hettler A, Sarnecka A, Wulle U, Ehrhardt C, Ludwig S, Planz O, 2014. Antiviral activity of Ladania067, an extract from wild black currant leaves against influenza A virus in vitro and in vivo. Front Microbiol 5, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S, 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S, 2007. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem 282, 12058–12065. [DOI] [PubMed] [Google Scholar]
- Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S, 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem 278, 46832–46839. [DOI] [PubMed] [Google Scholar]
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS, Global Seasonal Influenza-associated Mortality Collaborator, N., 2017. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet [DOI] [PMC free article] [PubMed]
- Kharel Y, Lee S, Snyder AH, Sheasley-O’neill S L, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Pearson-White S, Lynch KR, 2005. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem 280, 36865–36872. [DOI] [PubMed] [Google Scholar]
- Lai WQ, Goh HH, Bao Z, Wong WS, Melendez AJ, Leung BP, 2008a. The role of sphingosine kinase in a murine model of allergic asthma. J Immunol 180, 4323–4329. [DOI] [PubMed] [Google Scholar]
- Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, McInnes IB, Melendez AJ, Leung BP, 2008b. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol 181, 8010–8017. [DOI] [PubMed] [Google Scholar]
- Lee J, Yu H, Li Y, Ma J, Lang Y, Duff M, Henningson J, Liu Q, Li Y, Nagy A, Bawa B, Li Z, Tong G, Richt JA, Ma W, 2017. Impacts of different expressions of PA-X protein on 2009 pandemic H1N1 virus replication, pathogenicity and host immune responses. Virology 504, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH Jr., Milstien S, Spiegel S, 2005. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem 280, 37118–37129. [DOI] [PubMed] [Google Scholar]
- Machesky NJ, Zhang G, Raghavan B, Zimmerman P, Kelly SL, Merrill AH Jr., Waldman WJ, Van Brocklyn JR, Trgovcich J, 2008. Human cytomegalovirus regulates bioactive sphingolipids. J Biol Chem 283, 26148–26160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjuki H, Mishin VP, Chesnokov AP, Jones J, De La Cruz JA, Sleeman K, Tamura D, Nguyen HT, Wu HS, Chang FY, Liu MT, Fry AM, Cox NJ, Villanueva JM, Davis CT, Gubareva LV, 2015. Characterization of drug-resistant influenza A(H7N9) variants isolated from an oseltamivir-treated patient in Taiwan. J Infect Dis 211, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE, Herschkowitz JI, Burrows AE, Ciccia A, Sun T, Schmitt EM, Bernardi RJ, Fu X, Bland CS, Cooper TA, Schiff R, Rosen JM, Westbrook TF, Elledge SJ, 2011. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci U S A 108, 3665–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S, 2007. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res 5, 801–812. [DOI] [PubMed] [Google Scholar]
- Min J, Traynor D, Stegner AL, Zhang L, Hanigan MH, Alexander H, Alexander S, 2005. Sphingosine kinase regulates the sensitivity of Dictyostelium discoideum cells to the anticancer drug cisplatin. Eukaryot Cell 4, 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Fauci AS, 2007. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis 195, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Neubauer HA, Pitson SM, 2013. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J 280, 5317–5336. [DOI] [PubMed] [Google Scholar]
- Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y, 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A 96, 9345–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L, Group FDS, 2009. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 72, 73–79. [DOI] [PubMed] [Google Scholar]
- Orr Gandy KA, Obeid LM, 2013. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta 1831, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B, Saba JD, 2010. Cancer treatment strategies targeting sphingolipid metabolism. Adv Exp Med Biol 688, 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S, 2008. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 112, 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM, 2011. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci 36, 97–107. [DOI] [PubMed] [Google Scholar]
- Poland GA, Jacobson RM, Ovsyannikova IG, 2009. Influenza virus resistance to antiviral agents: a plea for rational use. Clin Infect Dis 48, 1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzl CJ, Seo YJ, Xia C, Vijayan M, Stokes ZD, Hahm B, 2015. A ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J Immunol 194, 4339–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SP, Tritsch SR, Kota K, Chiang CY, Dong L, Kenny T, Brueggemann EE, Ward MD, Cazares LH, Bavari S, 2015. Sphingosine kinase 2 is a chikungunya virus host factor co-localized with the viral replication complex. Emerg Microbes Infect 4, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ, 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol 5, 560–570. [DOI] [PubMed] [Google Scholar]
- Seo YJ, Blake C, Alexander S, Hahm B, 2010. Sphingosine 1-phosphate-metabolizing enzymes control influenza virus propagation and viral cytopathogenicity. J Virol 84, 8124–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YJ, Pritzl CJ, Vijayan M, Bomb K, McClain ME, Alexander S, Hahm B, 2013. Sphingosine kinase 1 serves as a pro-viral factor by regulating viral RNA synthesis and nuclear export of viral ribonucleoprotein complex upon influenza virus infection. PLoS One 8, e75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Xiong H, Li J, Liao W, Wang L, Wu J, Li M, 2011. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non-small cell lung cancer. Clin Cancer Res 17, 1839–1849. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S, 2011. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha TA, Hannun YA, Obeid LM, 2006. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol 39, 113–131. [DOI] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S, 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varble A, Albrecht RA, Backes S, Crumiller M, Bouvier NM, Sachs D, Garcia-Sastre A, tenOever BR, 2014. Influenza A virus transmission bottlenecks are defined by infection route and recipient host. Cell Host Microbe 16, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan M, Seo YJ, Pritzl CJ, Squires SA, Alexander S, Hahm B, 2014. Sphingosine kinase 1 regulates measles virus replication. Virology 450–451, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Palese P, 2009. Unraveling the mystery of swine influenza virus. Cell 137, 983–985. [DOI] [PubMed] [Google Scholar]
- Xia C, Vijayan M, Pritzl CJ, Fuchs SY, McDermott AB, Hahm B, 2015. Hemagglutinin of Influenza A Virus Antagonizes Type I Interferon (IFN) Responses by Inducing Degradation of Type I IFN Receptor 1. J Virol 90, 2403–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Wolf JJ, Vijayan M, Studstill CJ, Ma W, Hahm B, 2018. Casein Kinase 1alpha Mediates the Degradation of Receptors for Type I and Type II Interferons Caused by Hemagglutinin of Influenza A Virus. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane D, Zahoor MA, Mohamed YM, Azab W, Kato K, Tohya Y, Akashi H, 2009. Inhibition of sphingosine kinase by bovine viral diarrhea virus NS3 is crucial for efficient viral replication and cytopathogenesis. J Biol Chem 284, 13648–13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A, 2006. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 107, 1454–1458. [DOI] [PubMed] [Google Scholar]
- Ziemssen T, Medin J, Couto CA, Mitchell CR, 2017. Multiple sclerosis in the real world: A systematic review of fingolimod as a case study. Autoimmun Rev 16, 355–376. [DOI] [PubMed] [Google Scholar]


