Summary
Mechanisms underlying α-synuclein (αSyn) mediated neurodegeneration are poorly understood. Intramuscular (IM) injection of αSyn fibrils in human A53T transgenic M83+/− mice produce a rapid model of α-synucleinopathy with highly predictable onset of motor impairment. Using varying doses of αSyn seeds, we show that αSyn-induced phenotype is largely dose-independent. We utilized the synchrony of this IM model to explore the temporal sequence of αSyn pathology, neurodegeneration and neuroinflammation. Longitudinal tracking showed that while motor neuron death and αSyn pathology occur within 2 months post IM, astrogliosis appears at a later timepoint, implying neuroinflammation is a consequence, rather than a trigger, in this prionoid model of synucleinopathy. Initiating at 3 months post IM, immune activation dominates the pathologic landscape in terminal IM-seeded M83+/− mice, as revealed by unbiased transcriptomic analyses. Our findings provide insights into the role of neuroinflammation in αSyn mediated proteostasis and neurodegeneration, which will be key in designing potential therapies.
Keywords: α-synuclein, inflammation, neurodegeneration, prionoid transmission, RNAseq
Introduction
Intracellular aggregates comprised of misfolded α-synuclein (αSyn) protein are neuropathological hallmarks in α-synucleinopathies, such as Parkinson’s disease (PD), multiple system atrophy (MSA) and dementia with Lewy bodies (DLB) (Uchihara & Giasson, 2016). Recent findings support the idea that αSyn pathology can be induced by conformational templating of soluble αSyn into aggregates which can spread by intra-synaptic transmission (Uchihara & Giasson, 2016). Rodent experiments and in vitro studies have provided insights into unique forms of αSyn conformers that facilitate the seeding and prionoid transmission process (Uchihara & Giasson, 2016). Although this unified hypothesis would explain both local molecular changes of αSyn as a molecular template and its stereotyped spread as a structural template, other cellular mechanisms, such as immune signaling, can be independently or synergistically involved in αSyn pathogenesis (Lim et al., 2016; Allen et al., 2015).
To characterize the molecular underpinnings of prionoid transmission of αSyn pathology, we have established an experimental paradigm by injecting αSyn fibrils in the hindlimb muscles (IM) of human A53T αSyn transgenic Line M83+/− mice that leads to induction of synucleinopathy along the neuraxis and a highly reproducible and rapid onset of paralysis phenotype (Sacino et al., 2014). Using this IM model, we have performed detailed temporal and spatial analysis of proteostasis onset, neurodegeneration, immune alterations and survival in mice injected with different amounts of preformed αSyn seeds. We find that altering the dose of IM-administered exogenous αSyn fibrils does not significantly alter time to paralysis. Though the overall prionoid transmission followed expected neuro-anatomic connectivity (muscle → spinal cord → brainstem → motor cortex), we observed both neuronal and glial αSyn inclusions simultaneously emerging at various points along the neuraxis, without following an obvious pattern of progressive retrograde propagation. Interestingly, immune activation closely follows, but does not precede, the emergence of αSyn pathology and motoneuron death in the spinal cord. Unbiased transcriptomic analyses reveals that end-stage IM seeded M83+/− mice are characterized by a robust inflammatory response, which is essentially similar to aged paralyzed M83+/+ mice, implicating immune pathways as a common mechanism associated with endstage α-synucleinopathy.
Materials and Methods
Mice.
All animal experimental procedures were performed according to University of Florida Institutional Animal Care and Use Committee regulatory policies. Mice were housed under stable conditions with a 12-hour light/dark cycle and access to food and water ad libitum. Transgenic mouse lines hemizygous (M83+/−) or homozygous (M83+/+) for human αSyn with the A53T mutation under control of the mouse prion protein promoter has been previously described (Giasson et al., 2002). M83+/− mice develop motor impairment leading to paralysis between 22 to 28 months of age with the concurrent accumulation of pathologic αSyn inclusions predominantly in the spinal cord, brainstem, midbrain, hypothalamus, thalamus and periaqueductal gray regions (Giasson et al., 2002). M83+/+ mice develop motor impairment progressing to paralysis and αSyn pathology between 8 to 16 months of age (Giasson et al., 2002).
αSyn Fibril preparation and IM injection.
Recombinant mouse αSyn was expressed in E. coli and purified using size exclusion and ion exchange chromatography as previously described (Sacino et al, 2014). Mouse αSyn protein (5 mg/ml in sterile PBS) was fibrillized by incubation at 37°C (Invitrogen) with continuous shaking for 5 days at 1050 rpm. αSyn fibril formation was validated with K114 fluorometry as previously described (Crystal et al., 2003). Mouse αSyn fibrils were diluted (1mg/ml in sterile PBS) and fragmented by water bath sonication at 40KHz for 1 hour at room temperature prior to injection as described before (Sorrentino et al., 2017).2 month old M83+/− mice were anesthetized with isoflurane (1 to 5%) inhalation, the back of the hind limb was shaved, and a 10-μl Hamilton syringe with a 27-gauge needle was inserted ~1 mm into the gastrocnemius muscle and injected with αSyn fibril or sterile PBS as previously described (Sacino et al., 2014) (Fig. S1, Movie S1). For dosage studies, three cohorts of 8 mice (equal males and females) each were injected in the left gastrocnemius muscle with 5μL of solution containing 2, 5, or 10 μg of mouse αSyn fibrils and control mice were injected with sterile PBS. Mice were euthanized at the onset of bilateral hindlimb paralysis (endstage). For time course studies, 32–35 mice each were bilaterally injected in a similar fashion with 10 μg of mouse αSyn fibril in 5μL of sterile PBS and euthanized at 1 month, 2 month, 3 month post injection or at endstage (~4 months post injection; n=8–9 mice per group).
Tissue processing, Immunohistochemistry and Immunofluorescence.
Mice were euthanized with CO2 inhalation and perfused using an intra-cardiac solution of ice-cold PBS containing heparin as per humane conditions. Brains and spinal cords were fixed in 70% ethanol containing 150 mM NaCl for 48 hours. Paraffin embedded tissue was immunostained using primary antibodies followed by Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) and visualized using 3,3′-diaminobenzidine (KPL, Gaithersburg, MD). The blocking and antibody diluent used was 5% FBS in 0.1M Tris (pH 7.6). Sections were counterstained with hematoxylin. Slides were digitally scanned using Aperio ScanScope CS instrument and images of representative areas of pathology were captured using the ImageScope software (Aperio, CA). Pathology quantitation was done using Pixel count algorithm (Aperio). For immunofluorescence analysis, sections were incubated overnight (4°C) in primary antibody and detected using secondary antibody conjugated to Alexa fluor 594nm or 488nm (Invitrogen). The blocking and antibody diluent used was 5% milk in 0.1M Tris (pH 7.6). Nonspecific fluorescence was quenched using 0.3% Sudan Black in 70% EtOH. DAPI counterstaining was performed followed by mounting with Fluoromount-G (SouthernBiotech). Slides were visualized using an Olympus BX51 microscope mounted with a DP71 Olympus digital camera. For both procedures, antigen retrieval was done in steam for 30 minutes (except for cd11b which required Dako Retrieval Solution, pH 6.0).
Antibodies.
LS4-2G12 (1:5000 IHC; 1:2000 IF) and 9C10 (1:10,000) are mouse monoclonal antibodies raised to pSer129 epitope of αSyn and total synuclein (residues 2–21) respectively (Dhillon et al., 2017). Other antibodies used include: rabbit anti-p62 (1:2000, ProteinTech), mouse anti-ubiquitin (1:1000, EMD Millipore), rabbit Iba-1 (1:1000, Wako), mouse Cd68 (1:200, Invitrogen), rabbit anti-GFAP (1:1000, Dako), and rabbit anti-cd11b (1:1000, AbCam).
Counting of Motor neurons.
The entire lumbar region of each mouse spinal cord was paraffin embedded and consecutively sectioned (7μm). Paraffin embedded sections were deparaffinized and incubated in 0.1% Luxol fast blue solution at 56°C overnight. After rinsing in distilled water, sections were differentiated in 0.05% lithium carbonate and 70% ethanol. Sections were counterstained with cresyl violet solution for 6 minutes before being differentiated in 95% ethanol and subsequently dehydrated. The number of motor neurons from every tenth section of the lumbar cord were then counted for each mouse assessed (n= 6–9/group).
RNA sequencing, GO analysis and TReNA.
Total RNA was extracted from flash-frozen spinal cords (RIN>6.5) and library was prepared with Illumina TrueSeq kits. Paired-end sequencing with 75 base pair reads were generated using Illumina Nextseq 500 sequencer at the University of Florida Interdisciplinary Center for Biotechnology Research. The read depth is approximately 100 million reads. Differentially expressed genes were identified using the R package edgeR, converting counts to cpm and dropping lowly expressed genes (Robinson et al., 2010). GO analysis was performed in R, using the package GO.db (http://www.bioconductor.org/packages/GO.db/). GO enrichment was assessed using the hypergeometric function in GOstats package. The conceptual framework for the transcriptional regulatory network using TReNA was described previously (Pearl etal., 2017). Briefly, DNase Hypersensitivity (DHS) fastq files from ENCODE for all available brain samples were downloaded and aligned using the SNAP method (Zaharia et al., 2011). Two alignments were performed using seed size 16 and 20 as the sequence data was typically > 50 bp in length. The peak calling algorithm F-seq was used to identify regions of open chromatin (Boyle et al., 2008). Footprinting algorithms for Wellington and HINT were generated using default parameters (Gusmao et al., 2016; Piper et al., 2013). For each individual gene model, footprints within the proximal promoter (±5 kb of the transcription start site) were considered as priors in assessing the relationship between the expression of the transcription factor and target gene. Using the R package trena (https://www.bioconductor.org/news/bioc_3_6_release/), which utilizes several LASSO regression techniques, Pearson and Spearman correlation, and random forest to prioritize a list of putative transcription factor regulators for each gene. Scores from all these approaches were scaled and projected into PCA space and their principle components added together to produce a single composite score (pcaMax). This approach was applied to the RNA-seq samples generated from the spinal cord samples. The cell type specificity of the transcription factors were plotted based on Zhang et al. (https://web.stanford.edu/group/barres_lab/brain_rnaseq.html; (Zhang et al., 2014)). Briefly, each transcription factor was allotted primary cell type assignment and secondary cell assignments based on whether the protein was exclusively expressed from one cell type or multiple cell types with variable expression levels. Final graphical and network analysis was done based on the primary cell type assignment. Transcription factors that were expressed from several cell types at comparable levels were labeled as ‘mixed’ lineage.
Statistics.
We used 1-way ANOVA for statistical comparisons (unless otherwise stated). For RNAseq data, we used multiple t test with Bonferroni’s correction and false discovery rate (FDR) of 0.05. Graphical representation of data was done using Prism 6 (GraphPad Software, La Jolla, CA) and final images were created using Photoshop CS4 (Adobe Systems).
Results
M83+/− mice overexpressing human A53T mutant αSyn develop αSyn inclusion pathology and motor impairment beyond 18 months of age whereas M83+/+ develop αSyn inclusion pathology and paralysis between 8–16 months of age (Giasson et al., 2002). IM administration of αSyn fibrils in Line M83 mice results in synchronized development of motor impairment progressing to rapid hindlimb paralysis with concomitant αSyn pathology along the entire neuraxis (Fig. S1; Movie S1) (Sacino et al., 2014). Utilizing this prionoid transmission model of αSyn (‘IM model’), we (a) explored whether αSyn transmission is a dose-dependent process, (b) assessed the spatio-temporal patterns of neurodegeneration, neuroinflammation and αSyn proteostasis along the path of transmission and finally (c) performed an unbiased transcriptomic study to characterize molecular signatures in the IM model and aged M83+/+ mice to explore whether the IM seeded model is physiologically and functionally equivalent to the transgenic M83+/+ mice.
Efficient induction of αSyn pathology and phenotype in IM model can occur at lower doses of αSyn fibrils
To study the efficiency of αSyn transmission, cohorts of M83+/− mice were unilaterally injected with increasing amounts of mouse αSyn fibrils (2 μg, 5 μg, or 10 μg) and the time to bilateral hindlimb paralysis was assessed (Fig. S2). All three doses had similar mean survival times of 142±28, 133±12, and 125±14 dpi for 2 μg, 5 μg or 10 μg dosage groups, respectively (p>0.05; χ2= 2.723, Log rank test) (Fig. S2). Notably, the 2 μg dose cohort had a broader range of terminal stage onset times (109–182 dpi) compared to the other cohorts which were more tightly clustered (117–145 dpi for 5 μg; 102–143 dpi for 10 μg).
We characterized the induced αSyn pathology in these mice injected with different doses of αSyn fibrils using antibodies against a) pSer129 αSyn, a canonical marker of pathological αSyn aggregates (LS4-2G12; Dhillon et al., 2017) (Fig. S3A), b) p62 (Fig. S3B) and c) N-terminus of αSyn (9C10; Dhillon et al., 2017) (Fig. S3C). We observed extensive pSer129 αSyn immunoreactivity (IR) in the neuritic processes and cell bodies in spinal cord, brainstem, midbrain and hypothalamus, with occasional IR in cerebellum, ventral thalamus, amygdala and motor cortex, similar to distribution and burden of pathological αSyn inclusions in end-stage aged M83+/+ mice (Fig. S3A). IR pattern for pathological αSyn was recapitulated using p62 antibody demonstrating the presence of inclusion pathology (Fig. S3B) and 9C10 IR further confirmed that these intracellular inclusions were composed of αSyn (Fig. S3C). We observed equivalent levels of increased astrocytosis (GFAP, Fig. S4A) and microgliosis (cd11b, Fig. S4B) in these mice. Co-immunofluorescence IR revealed that while astrocytic αSyn pathology was observed in both spinal cord and in pons of end-stage mice (Fig. S5A), cd11b+ microglia co-localizing with pSer129 αSyn was observed predominantly in the brainstem (Fig. S5B). A semi-quantitative histological scoring summarizes that most αSyn inclusion pathology in the spinal cord of end stage IM seeded M83+/− mice were present in astrocytes or neurons, similar to naïve paralyzed M83+/+ mice whereas microgliosis was mostly observed in the brainstem region of IM seeded M83+/− and naive M83+/+ mice (Table S1).
Temporal progression of neuropathological hallmarks in IM model
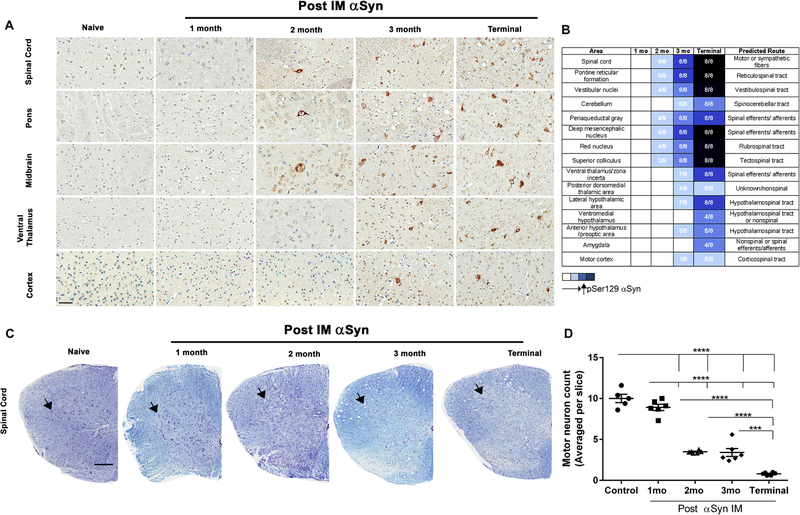
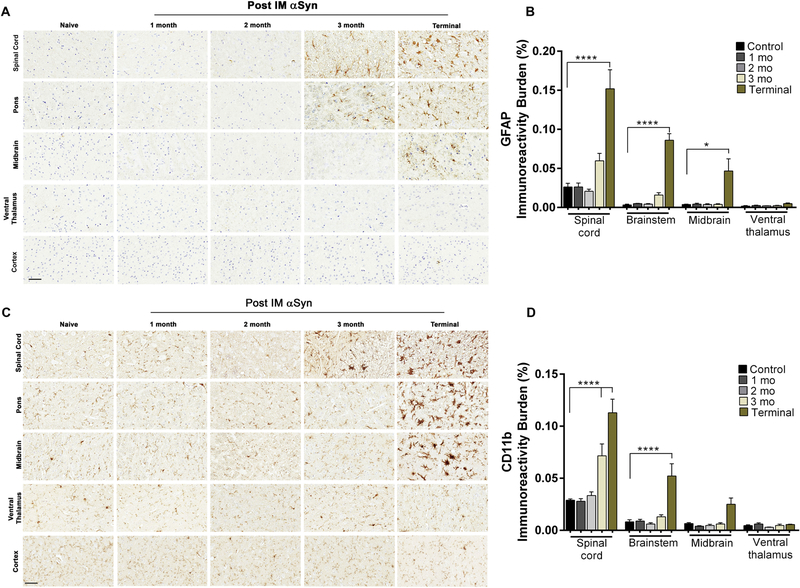
We next mapped the temporal progression of the αSyn pathology, spinal motor neuron death and immune activation in the neuraxis following bilateral IM administration of 10 μg αSyn fibrils. Given all three doses resulted in equivalent time to moribund and similar burden of αSyn pathology, these experiments were conducted using the highest effective dose to ensure complete penetrance of the phenotype. 2 month old M83+/− mice were injected in the hindlimb muscle with αSyn fibrils and cohorts were harvested at regular intervals of 1 month until onset of bilateral hindlimb paralysis. We observed the first instances of αSyn pathology at 2 months post IM in the intermediate and ventral regions of spinal cord, pontine reticular formation and deep midbrain structures (Fig. 1A–B). At 3 months post IM, the overall burden and distribution of αSyn underwent dramatic increase with robust pathology in the grey matter of spinal cord, pons, midbrain, with occasional IR in hypothalamus, ventral thalamus, cerebellum and cortex. At end-stage, pSer129-αSyn inclusion pathology was observed throughout spinal cord, midbrain and motor cortex, with additional αSyn IR observed in the white matter of spinal cord (Fig. 1A–B). Additional pathology that emerges at this stage are found in amygdala and posterior ventromedial hypothalamus. We did not observe any pSer129-αSyn IR in the dopaminergic (Thimmunopositive) neurons or astrocytes in the substantia nigra pars compacta region, though there was detectable levels of αSyn IR in this area (Fig. S6). Stereologic assessment of motor neurons showed that neurodegeneration initiates ~2 months post IM (Fig. 1C–D). By 2 months post IM, 65–70% of the motor neurons are lost proceeding to 90% loss at end-stage (Fig. 1D). Both 9C10 (Fig. S7A), p62 (Fig. S7B) and ubiquitin (Fig. S7C) IR patterns recapitulated pSer129 αSyn IR. We next characterized the temporal progression of immune alterations using different antibodies. At 2 months post IM, both astrogliosis (GFAP) and microgliosis (cd11b and Iba-1) levels in the spinal cord and brain were similar to baseline levels observed in naïve mice (Fig. 2A–D). We first detected astrogliosis at 3 months post IM in spinal cord and ventral part of the brain which progressed inexorably till terminal stage, when the dorsal regions, i.e. thalamus and ventral hypothalamus are also affected (Fig. 2A–B). We explored microglial activation using microglial markers cd11b (Fig. 2C–D), Iba-1 and Cd68 (Fig. S8). The pattern of cd11b and Iba-1 mirrored that of astrocytosis, initiating at 3 months post IM in the spinal cord and progressively increasing in the midbrain region in endstage mice (Fig. 2C–D, S8A). On the other hand, cd68 IR was largely unaltered in the spinal cord across the time course studied (Fig. S8B).
Figure 1. αSyn IM injection induces αSyn pathology and early motoneuron death in M83+/− mice.
Representative pSer129 αSyn IR (LS4-2G12 antibody) shows progressive induction of αSyn pathology in M83+/− mice bilaterally injected with 10 μg of αSyn fibrils (IM) at different timepoints (1 month, 2 months, 3 months post IM and end stage) (A). Relative abundance of LS4-2G12 IR in the neuraxis was used to predict transmission routes from the periphery to the CNS (B). Numbers indicate mice with existing pathology (darker shades of blue indicating increasing burden) or no pathology (white square). n=8–9/group. Massive motoneuron loss occurs as early as 2 months post IM, as indicated in representative images within the spinal cord stained with cresyl violet and Luxol fast Blue (C-D). Arrows indicate ventral horn area where motoneurons are found. Scale bar = 60 μm (a); 250 μm (b). n=5–6 mice/group. 1 way Anova, ****p<0.0001, ***p<0.001.
Figure 2. Progressive astrocytosis and microgliosis in the spinal cord and brain of M83+/− mice injected IM with αSyn fibrils.
Bilateral IM injection of αSyn fibrils in M83+/− mice leads to astrocytosis and microgliosis as assessed by anti GFAP (A-B) and anti cd11b (C-D) antibodies. Quantitative analysis in spinal cordand brain (except cortex that had baseline IR levels and not shown) showed gliosis is initiated 3 months post IM. Scale bar = 60 μm. n=8 mice/group.
Altered immune signaling dominates the pathological landscape in endstage αSyn mice
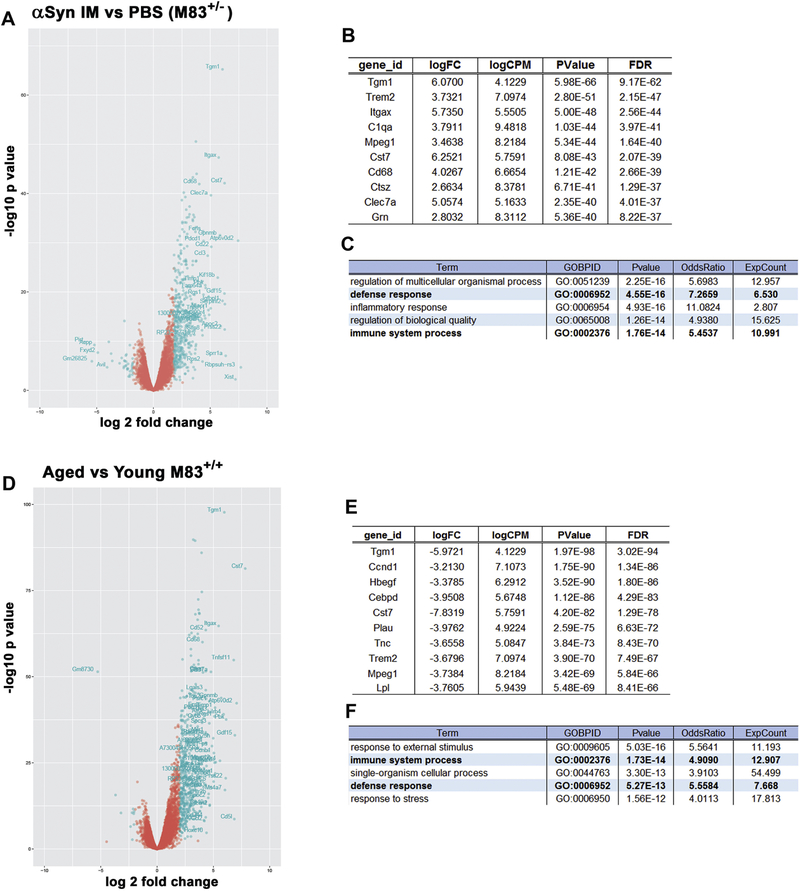
We carried out RNA sequencing of spinal cord to elucidate the pattern of differential expressed genes (DEG) underlying the pathologies in end stage IM model and further to understand how this mouse model compares with aged M83+/+ mice, both of which have similar burden of α-synucleinopathy and paralysis phenotype, albeit at different time scales. DEG analysis shows (1) 488 genes were altered in IM model compared to PBS injected healthy 6 month old M83+/− (p<0.05; fold change>2; FDR=0.05); (2) 510 genes were altered in end stage 12 month old naive M83+/+ mice compared to healthy 3 month old M83+/+ (p<0.01; fold change>2; FDR=0.05); (3) 308 genes were commonly upregulated in end stage IM model and naïve M83+/+ mice, and (4) 14 genes were altered in IM model compared to end stage 12 month old naive M83+/+ mice (p<0.05; fold change>2; FDR=0.05) (Fig. 3; Fig. S9; Tables S2–S3). In both these cohorts, microglia-specific genes were most highly altered (Tgm1, Trem2, Mpeg1 and Cst 7; p<10−40; FDR<10−37) (Fig. 3 A, B, D, E). This is reflected in the GO enrichment analysis which showed that immune response (GO:0002376) is upregulated in both cohorts (p<10−14; Bonferroni corrected) (Fig. 3 C, F). Additionally, the defense response (GO:0006952; p<10−13) exhibited by both these cohorts also signify engagement of the endogenous immune response that is triggered in response to tissue injury or infections (Fig. 3 C, F). Overall, these data sets show that upregulation of immune processes is a shared major response in end-stage IM model and aged M83+/+ mice.
Figure 3. Unbiased transcriptomic profiling of IM injected M83+/− and aged M83+/+ mice show robust immune activation.
RNA sequencing of spinal cords from age matched cohorts of endstage αSyn fibril (IM) injected M83+/− mice and PBS injected M83+/− mice (A-C) and 12 month paralyzed M83+/+ mice (‘aged’) and 3 month healthy M83+/+ (‘young’) mice (D-F) show massive upregulation of immune transcripts. DEG analysis showing the top 10 genes that are altered are mostly of immune origin (B, E). GO analyses confirms that immune pathways are commonly upregulated in M83+/− and M83+/+ mice (C, F, bold lettering). Multiple t test; p<0.05; fold change (log2)>2; FDR=0.05; n=2–4 mice/cohort.
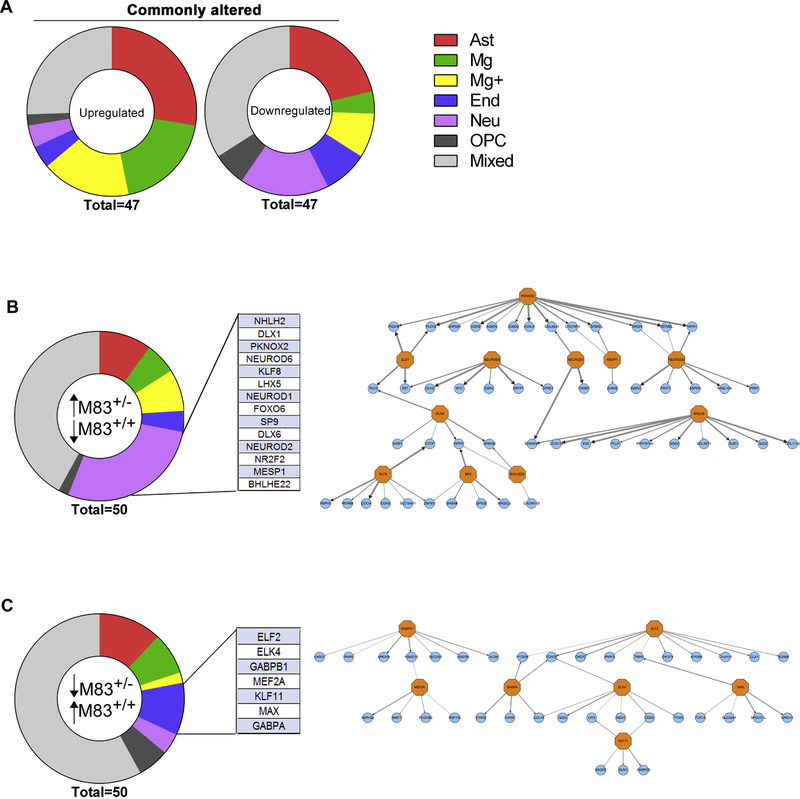
We next performed Transcriptional Regulatory Network Analysis (TReNA) to reconstruct transcriptional regulatory networks specific to IM model and aged M83+/+ mice. Combining datasets from brain-specific transcription factor (TF) binding sites with GTEx profile, we could infer a network of transcription factors (TRN) that are predictive of the altered DEG profiles in these two mouse models of synucleinopathy (Fig. 4; Tables S4–S8). We initially sorted the DEG according to fold change of individual genes between the two groups (IM model and aged M83+/+ mice normalized to their respective controls) (Table S4). We then used TReNA to rank-identify TFs that could explain the DEG changes in these mice (commonly altered TFs (upregulated: Table S5; downregulated: Table S6); and uniquely altered TFs (upregulated in IM model and downregulated in aged M83+/+: Table S7; downregulated in IM model and upregulated in aged M83+/+: Table S8). Following identification of these TFs, we used a reference RNAseq database to determine their cell-type enrichment (Zhang et al., 2014). Among the TFs predicted to be upregulated in both of these groups, at least 60% are enriched in immune cells - astrocytes (e.g., Srebf1, Rfx5 and Creb3l4) and microglia (e.g., Spi1, Irf5 and Runx1) (Fig. 4A; Table S5). This is consistent with the upregulation of immune pathways as observed in the GO enrichment profile (Fig. 3 C, F) as well as alterations in immune regulatory networks identified in neurodegenerative dementias in general (Zhao et al., 2016). Further, notable among the TFs that were downregulated in both the groups were of neuronal origin (e.g, NeuroD1, NeuroD2 and NeuroD6) and of OPC origin (e.g., Sox8, Sox6 and Gli1) (Fig. 4A; Table S6). Next we analyzed TFs that were upregulated in one group and downregulated in the other in order to understand whether TReNA could help explain the etiologies. We observed a unique set of neuronal-specific TFs that were enriched in the IM seeded M83+/− mice but downregulated in aged M83+/+ mice (Fig. 4B; Table S7). Further analysis revealed that this DEG is regulated by a common TF network (Fig. 4B) consisting of Dlx1, Dlx6 and Bhlhe22, that are critical for GABAergic neuron development (Feng et al., 2006, Li et al., 2012). The NeuroD family is involved in glutamatergic neurogenesis (Hevner et al., 2006). Nhlh2, mostly present in hypothalamic and spinal neurons, control cell-type determination and energy balance (Fox et al., 2007). Other TFs, Pknox2, Mesp1, Sp9 and Klf8, have unknown function in the CNS. Aged M83+/+ mice, on the other hand, had enriched endothelia-specific TF profile that was downregulated in the IM seeded M83+/− mice (Fig. 4C; Table S8). Of these TFs, the CNS function of Elf2 and Elk4 remain obscure, while Gabpa and Gabpb1 were shown to control CNS neovascularization (Jeong et al., 2006) and Klf11 is a PPARγ co-activator (Yin et al., 2013). Mef2a activates neurodevelopmental programs, regulating plasticity (Cole et al., 2012). Max is a major regulator of CNS gene expression (Hecker et al., 2017). Though these TFs are enriched in endothelial cells, many of them are also expressed in lower amounts in other cells, such as astrocytes and microglia. Overall, we surmise that some of the key differences between these two cohorts could be driven by involvement of different cell types as we could detect enrichment of cell type specific TFs associated with the respective DEGs.
Figure 4. Identification of cell-type specific TRNs in IM injected M83+/− and aged M83+/+ end-stage mice.
Distribution of cell type specific TFs that are commonly enriched (upregulated and downregulated) in αSyn fibril injected M83+/− mice and aged M83+/+ endstage mice (A). Cell type specificity of TFs has been imputed from http://web.stanford.edu/group/barres_lab/brain_rnaseq.html. TFs were either exclusively expressed from one cell type (‘primary’ cell type enrichment) or were present in multiple cell types, with highest (designated as ‘Primary’) and lower expression levels (designated as ‘Secondary’). Only the primary cell type enrichment is mapped here; for immune genes that are expressed equally from microglia, astrocytes and oligodendrocyte progenitor cells, these have been classified as Mg+. We further identified a neuronal-specific TRN and an endothelial cell TRN that are differentially enriched in endstage αSyn fibril (IM) injected M83+/− mice and aged M83+/+ mice respectively (B-C). Thickness of the arrows connecting the TFs in the network denotes rank (1 being the thickest) (B-C). Mixed=all cells; Neu=neuron; Ast=Astrocyte; Mg=Microglia; End=endothelia; OPC=oligodendrocyte progenitor cells.
Discussion
Prionoid transmission of αSyn is hypothesized to be a major driver of synucleinopathy (Uchihara & Giasson, 2015). However, the mechanisms of such prionoid transmission and the cellular networks that are majorly affected during this process remain largely unknown. Here, using a model of peripheral to CNS transmission of αSyn pathology, we show that (1) the transmission process is dose-independent as increasing the amount of exogenous αSyn seeds did not accelerate time to paralysis; (2) induction of αSyn proteostasis and motor neuron death occur early (2 months post IM) which is followed by induction of cd11b, Iba-1 and GFAP IR (3 months post IM), suggesting clear temporal delineation in proteostasis and immune signaling; (3) astrocytic αSyn pathology was highly evident in areas with αSyn inclusion pathology; (4) end-stage mice are characterized by inflammatory response, revealed by upregulation of microglia-specific DEG; and (5) TReNA shows that at endstage, though both IM seeded M83+/− mice and aged M83+/+ mice share overwhelmingly similar patterns of immune network disturbance, there are subtle differences in engagement of cell-type specific TRNs.
We observed robust αSyn pathology in multiple segments of the spinal cord of IM seeded M83+/− mice arising simultaneously, with prominent inclusions in ventral horn and intermediate zone. If the seeds were being transmitted strictly unidirectionally and in a non-stochastic manner through inter-neuronal connections, we would expect a gradation of pathology along the spinal tracts. On the contrary, since we observed concurrently emerging αSyn pathology at all spinal levels, this suggests that different tracts or neurons may have differential molecular thresholds for templated induction of αSyn pathogenesis or there may exist other cellular modes that help in dispersal of the seeds. Indeed, we observed that astrocytes (but not cd11b-positive microglia) colocalize with αSyn inclusions, indicating that these cells may also play a role in αSyn transmission. This is consistent with reports that classical prions (Victoria et al., 2016) and αSyn seeds (Rostami et al., 2016) can be transferred between neurons and astrocytes, probably via exosomes or tunneling nanotubes. Another factor that can explain why the initial seeds cause interspersed pathologies in the spinal cord is the fact that atypical prions often behave in stochastic manner during lesion formation (Makarava & Baskakov, 2013). As opposed to classical templating in prions (Prusiner, 2013), it is possible that several iterations of ‘deformed templating’ (Makarava & Baskakov, 2013) lead to the emergence of several self-replicating states in vivo and only some of these deformed conformers have a selective advantage in transmitting along the neuraxis depending on their relative neurotoxicity, transmissibility, the vulnerability of the neuro-glial milieu or other endogenous barriers. Such unconventional paradigms can result in multiple modes of transmission in addition to canonical axonal transport along anatomic connections.
Based on the fact that there was a clear time-dependent progressive increment of αSyn burden that progressively increased in the spinal cord and later appeared in the brain areas that are neuro-anatomically connected, we conclude primitive motor pathways such as the reticulospinal, vestibulospinal, rubrospinal, tectospinal and spinocerebellar pathways are involved in αSyn transmission from periphery to brain. However, our previous data on intracerebral αSyn seeding showed that induction of αSyn pathology in the brain can be independent of strict tractographic transmission (Sorrentino et al., 2017). While the exact reasons for such distinct observations in the peripheral vs the CNS transmission models remains unclear, it is possible that endogenous mechanisms such as conformational plasticity of αSyn, selective vulnerability of certain neuronal populations to these αSyn prion conformers or astroglia mediated dissemination of seeds can contribute to this differential distribution of seeded pathology.
Unbiased systems biology evaluation of end-stage IM model and aged M83+/+ mice show that both these cohorts share an overwhelmingly similar DEG signature, though the former experimental model is more rapid and synchronized than the latter in terms of phenotypic progression. Indeed, the similarities between these models are reflected by the RNA sequencing data showing that only 14 genes are differentially altered between the two groups (p<0.05; fold change>2; FDR=0.05). This implies that αSyn seeding results in synchronization of the pathogenesis pathways leading to a rapid and reproducible model of α-synucleinopathy with an extremely tight variance. Both of these αSyn models show an overabundance of immune signaling pathways at end stage, suggesting a pathogenic link between inflammation and αSyn proteostasis. Previous data in an overexpression model of αSyn suggests that there can be a spatial and temporal separation in microglial and astrocytic response to αSyn spreading (Rusconi et al., 2018). Further studies are warranted to fully investigate whether this differential response of innate immune response is recapitulated in our IM seeded model (model of αSyn spreading) vis a vis the M83+/+ mice (overexpression model of αSyn). Further, analysis of TFs also confirms that microglial gene expression is the major response in these end-stage mice, though the transcriptional landscape of aged M83+/+ mice also revealed an endothelial TRN. On the other hand, a neuronal specific TRN was specifically enriched in the IM model.
We observed that robust microgliosis follows the appearance of αSyn pathology and motor neuron death in the seeded transmission model. αSyn can act as a damage associated molecular pattern, activating the immune system, which may then further trigger cellular pathologies and cell death (Lim et al., 2016; Allen et al., 2015). αSyn can also induce generation of reactive oxygen species directly leading to microglial activation in mesencephalic cultures (Zhang et al., 2007). However, a clear pathogenetic link between αSyn and activated microglia is still unclear (Croisier et al, 2005). On the whole, reconciling our observations with the prevailing literature would lead to a scenario that immune cells can successfully engulf and degrade neuronal debris and αSyn seeds (Koller et al., 2017, Loria et al., 2017, Morales et al., 2017) until a threshold is reached when overloading of this homeostasis mechanism, as well as bystander phenomena such as oxidative stress, can lead to the failure of the immune surveillance system causing runaway inflammation.
Our RNA sequencing data support this premise that runaway inflammation is a pathologic feature of endstage IM model and aged M83+/+ model, mirroring transcriptome changes observed in endstage neurodegenerative diseases (Zhao et al., 2016). However, determination of the early transcriptomic changes in the IM seeded model (1 – 2 month post IM) will be necessary to fully delineate whether there are subtle and region-specific alterations in immune response in the spinal and brain, undetected by conventional IR techniques. Further, while our study shows robust cd11b and Iba-1 reactive microgliosis in response to αSyn, detailed characterization of other immune markers is necessary to illuminate the complex role of innate immunity in disease pathogenesis. These information, guided by region-specific analysis of pathological αSyn, can also inform us whether neuroinflammation is truly secondary to αSyn induced motoneuron death or, whether αSyn pathology selectively affects innate immunity and cell death, depending on the context and regional vulnerability.
Conclusion
In summary, we demonstrate that peripheral to CNS transmission of αSyn seeds follow a model reminiscent of atypical prions leading to rapid induction of neurodegeneration and that immune system dysfunction, αSyn proteostasis and motor neuron death follow distinct temporal profiles in this seeded model of α-synucleinopathy.
Supplementary Material
Highlights.
Aggregated α-synuclein (αSyn), inflammation and neurodegeneration characterize α-synucleinopathies
Peripheral to central transmission of αSyn resulting in paralysis is independent of αSyn dose.
Motoneuron death, αSyn pathology and neuroinflammation follow distinct temporal profiles.
Endstage αSyn pathology is characterized by an overwhelming immune response.
Acknowledgements.
This work was supported by NIH grant NS099738 (PC), NS089622 (BIG), U01AG046139 (TEG) and U54EB020406 (NP).
Abbreviations
- αSyn
α-synuclein
- Ast
Astrocyte
- CD11b
cluster of differentiation molecule 11b
- CNS
Central Nervous System
- DEG
differentially expressed genes
- DHS
DNase Hypersensitivity
- DLB
dementia with Lewy bodies
- End
Endothelia
- FDR
False Discovery Rate
- GFAP
Glial fibrillary acidic protein
- IM
intramuscular
- IR
immunoreactivity
- Mg
Microglia
- MSA
multiple system atrophy
- Neu
Neuron
- OPC
Oligodendrocyte progenitor cells
- PBS
Phosphate buffered saline
- PD
Parkinson’s disease
- pSer129
phosphorylated at Ser129
- TReNA
Transcriptional Regulatory Network Analysis
- TF
transcription factor
- TRN
Transcriptional Regulatory Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest. The authors declare no potential conflict of interest.
References:
- Allen Reish HE, Standaert DG (2015) Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis 5:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AP, Guinney J, Crawford GE, Furey TS (2008) F-Seq: a feature density estimator for high-throughput sequence tags. Bioinformatics 24:2537–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CJ, et al. (2012) MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat Neurosci 15:1255–64 [DOI] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB (2005) Analysis of alpha-synuclein, dopamine and parkin pathways in neuropathologically confirmed parkinsonian nigra. Acta Neuropathol 113:253–63 [DOI] [PubMed] [Google Scholar]
- Crystal AS, et al. (2003) A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J Neurochem 86:1359–68 [DOI] [PubMed] [Google Scholar]
- Dhillon JS, Riffe C, Moore BD, Ran Y, Chakrabarty P, Golde TE, Giasson BI (2017) A novel panel of alpha-synuclein antibodies reveal distinctive staining profiles in synucleinopathies. PloS one 12: e0184731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L (2006) Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development 133: 4815–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DL, Vella KR, Good DJ (2007) Energy balance pathways converging on the Nhlh2 transcription factor. Front Biosci 12: 3983–93 [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM (2002) Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-ynuclein. Neuron 34: 521–33 [DOI] [PubMed] [Google Scholar]
- Gusmao EG, Allhoff M, Zenke M, Costa IG (2016) Analysis of computational footprinting methods for DNase sequencing experiments. Nat Methods 13:303–9 [DOI] [PubMed] [Google Scholar]
- Hecker N, Seemann SE, Silahtaroglu A, Ruzzo WL, Gorodkin J (2017) Associating transcription factors and conserved RNA structures with gene regulation in the human brain. Sci Rep 7: 5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C (2006) Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res 55: 223–33 [DOI] [PubMed] [Google Scholar]
- Jeong BC, Kim MY, Lee JH, Kee HJ, Kho DH, Han KE, Qian YR, Kim JK, Kim KK (2006) Brain-specific angiogenesis inhibitor 2 regulates VEGF through GABP that acts as a transcriptional repressor. FEBS Lett 580: 669–76 [DOI] [PubMed] [Google Scholar]
- Koller EJ, Brooks MM, Golde TE, Giasson BI, Chakrabarty P (2017) Inflammatory pre-conditioning restricts the seeded induction of alpha-synuclein pathology in wild type mice. Mol Neurodeg 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. (2012) Isolation of a novel rat neural progenitor clone that expresses Dlx family transcription factors and gives rise to functional GABAergic neurons in culture. Dev Neurobiol 72: 805–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Chun Y, Lee JS, Lee SJ. Neuroinflammation in Synucleinopathies (2016) Brain Pathol. 26:404–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria F, Vargas JY, Bousset L, Syan S, Salles A, Melki R, Zurzolo C (2017) alpha-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol 134: 789–808 [DOI] [PubMed] [Google Scholar]
- Makarava N, Baskakov IV (2013) The evolution of transmissible prions: the role of deformed templating. PLoS Pathog 9: e1003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I, Sanchez A, Rodriguez-Sabate C, Rodriguez M (2017) Striatal astrocytes engulf dopaminergic debris in Parkinson’s disease: A study in an animal model. PloS one 12: e0185989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JR, et al. (2017) Genome-scale transcriptional regulatory network models of psychiatric and neurodegenerative disorders. BioRxiv https://www.biorxiv.org/content/early/2017/09/19/190959 [DOI] [PubMed]
- Piper J, Elze MC, Cauchy P, Cockerill PN, Bonifer C, Ott S (2013) Wellington: a novel method for the accurate identification of digital genomic footprints from DNase-seq data. Nucl Acid Res 41:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB (2013) Biology and genetics of prions causing neurodegeneration. Ann Rev Genet 47: 601–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M, Bergström J, Roybon L, Erlandsson A (2017) Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes. J Neurosci 37:11835–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi R, Ulusoy A, Aboutalebi H, Di Monte DA (2018) Long-lasting pathological consequences of overexpression-induced α-synuclein spreading in the rat brain. Aging Cell 17:e12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino AN, et al. (2014) Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci USA 111: 10732–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino ZA, Brooks MMT, Hudson V 3rd, Rutherford NJ, Golde TE, Giasson BI, Chakrabarty P (2017) Intrastriatal injection of alpha-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Mol Neurodeg 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchihara T, Giasson BI (2016) Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131: 49–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria GS, Arkhipenko A, Zhu S, Syan S, Zurzolo C (2016) Astrocyte-to-neuron intercellular prion transfer is mediated by cell-cell contact. Sci Rep 6: 20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerman AL, et al. (2017) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol doi: 10.1007/s00401-017-1762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, et al. (2013) KLF11 mediates PPARgamma cerebrovascular protection in ischaemic stroke. Brain 136: 1274–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharia M, Bolosky WJ, Curtis K, Fox A Patterson D, Shenker S, Stoica I, Karp RM, Sittler T (2011) Faster and More Accurate Sequence Alignment with SNAP. arXiv arXiv:1111.5572 [Google Scholar]
- Zhang W, et al. (2007) Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 55:1178–88. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Forst CV, Sayegh CE, Wang IM, Yang X, Zhang B (2016) Molecular and genetic inflammation networks in major human diseases. Mol Biosyst 12: 2318–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.