Abstract
Objective:
In this paper, we describe trends in tobacco-related poison exposure calls (calls) involving young children in the US.
Methods:
Data were from the National Poison Data System between January 1, 2001 and October 31, 2016. We analyzed data on calls involving children younger than 5 years old. We describe trends in calls over time and call frequency by age, tobacco product type, level of care, and other characteristics of calls.
Results:
During 2001–2016, there were 123,876 calls involving young children. During the study period, calls increased for most product types; e-cigarette-related calls increased from 7 in 2010 to 2558 in 2015. In calls with information on level of care (92.2%), 278 children were admitted to an intensive care unit, 497 were admitted to a hospital noncritical care unit, and 19,834 were treated and released.
Conclusions:
Tobacco-related poison events commonly occur in the US and can have serious health consequences. More than 123,000 events among young children were reported during 2001–2016, but this likely represents a small portion of actual tobacco-related poison events due to underreporting. It is critical to continue to monitor tobacco-related poison events and develop strategies to prevent tobacco-related harm.
Keywords: tobacco, cigarette, cigar, smokeless, hookah, poison, children
Nicotine is a toxic substance whose ingestion can cause nausea, vomiting, and circulatory and respiratory effects.1,2 Severe nicotine poisoning can lead to convulsions, coma, respiratory depression, and cardiac arrests.1,2 Tobacco products containing nicotine are responsible for more than 10,000 telephone calls to poison control centers (PCCs) in the United States (US) each year; the majority of these calls involve accidental ingestion of tobacco products by young children.3 Connolly et al4 analyzed almost 14,000 cases of tobacco product ingestion by children younger than age 6 from 2006 to 2008 in the National Poison Data System (NPDS). Approximately 70% of these cases involved children younger than one year of age. The authors expressed particular concern about accidental ingestion of smokeless tobacco and the possibility of increased ingestion of dissolvable tobacco products due to their resemblance to candy.4 Appleton5 summarized American Association of Poison Control Centers (AAPCC) annual report data on tobacco-related poison exposure calls from 1983 to 2009 and noted a small number of fatal events involving tobacco products, but concluded that the frequency and severity of outcomes associated with accidental tobacco product ingestion was relatively low compared to other consumer products.
In addition to poison events associated with conventional tobacco products, poison events involving e-cigarettes are an increasing concern as use of these products increases. The US Centers for Disease Control and Prevention has reported that exposure calls to PCCs related to e-cigarettes increased from one per month in September 2010 to 215 per month in February 2014.6 Thus, e-cigarettes account for an increasing proportion of combined e-cigarette and conventional cigarette exposure calls, rising from 0.3% to 41.7% during the study period. Similar findings of rapid increase in exposure calls associated with e-cigarettes have been reported by others.7,8 Additionally, serious health effects associated with e-cigarettes involving children have also been reported,9,10 including death, burn, respiratory issues, and severe liver toxicity.
Despite these studies, information on the overall public health burden of tobacco-related poison events in the US remains limited, particularly as these events disproportionally affect young children and involve emerging tobacco products in the rapidly-changing tobacco use landscape. Cigarette smoking prevalence among US adults has declined from 20.9% in 2005 to a historic low of 15.1% in 2015.11 However, total consumption of non-cigarette combustible tobacco products such as cigars increased by 123% from 2000 to 2011.12 In addition, the prevalence of e-cigarette and hookah use has become increasingly common in recent years, especially among youth and young adults.13,14 With the increasingly diverse tobacco product use in the US, understanding acute health effects of these products is more important than ever. To address these issues and provide data for future assessment of regulatory policies, we analyzed data on tobacco-related poison exposure calls involving children younger than 5 years old,15 from NPDS between January 1, 2001 and October 31, 2016. We examined trends in the frequency of tobacco-related poison exposure calls over time and the frequency of calls by age, tobacco product type, route of exposure, healthcare facility level of care, and medical outcome. Data from our analyses may help inform regulatory policies aimed at reducing tobacco-related poison events among young children.
METHODS
Data Source
Data for this study were obtained from NPDS, a data repository of poison exposure calls to PCCs in the US. NPDS is owned and maintained by AAPCC. As of January 1, 2016, 55 regional PCCs serving the 50 states, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands reported poison event data to AAPCC. Healthcare professionals at these centers respond to telephone calls from the public regarding potential poison exposures. PCCs are available free of charge to the public, 24 hours a day, 365 days a year.
Data Collection and Measures
NPDS stores information on all poison events reported to PCCs. Since 1983, NPDS has accumulated more than 64 million human poison exposure case records, with more than 2 million records added annually.16 During each telephone call, information on caller location, exposure site, demographic characteristics of person experiencing poison exposure, products involved, route of exposure, clinical effect, healthcare facility level of care, and medical outcome are collected and recorded using a structured computer program. The information collected during each telephone call is then uploaded to NPDS automatically.16
NPDS contains poison event exposure information based on the generic and product codes for more than 419,000 products, including tobacco products. Generic codes are available for cigarettes, cigarette butts, cigars, chewing tobacco, dissolvable tobacco, snuff, e-cigarette devices, e-cigarette nicotine liquids (e-liquids), other types of tobacco (eg, products did not have a product code or did not belong to any established categories), and unknown types of tobacco. Generic codes for e-cigarette devices and liquids became available in September 2010. Poison events involving hookahs were identified using product codes for hookah and waterpipe. To identify all poison events involving dissolvable tobacco products accurately, which are relatively new and less frequently used than other products, we identified and reviewed events with product names known to be dissolvable tobacco products.17,18 These events were reclassified from other tobacco categories to the dissolvable tobacco category. Events with a product name known to be hookah (ie, narghile)19 were reclassified from other tobacco category to the hookah category. If a poison exposure event involves more than one substance or product, each substance is assigned a sequence number by PCC staff in order of its relative contribution to the observed clinical effects. The PCC staff makes this determination based on its clinical judgment and expertise. In this study, we restricted analyses to cases where a tobacco product was ranked as the number one substance. Poison events involving only one substance accounted for 99.4% of cases.
Tobacco products were classified according to generic codes. E-cigarettes and e-liquids were grouped together due to the evolving nature of these products, as early generations of e-cigarettes consisted of both a device and a liquid cartridge. Callers may not be able to differentiate between an e-cigarette device and e-liquid when reporting exposures. Healthcare facility level of care is categorized in NPDS as “treated/evaluated and released,” “admitted to critical care unit,” “admitted to noncritical care unit,” “admitted to psychiatric care facility,” “patient refused referral/did not arrive at healthcare facility,” “patient lost to follow-up/left the health-care facility against medical advice,” and “no health-care facility treatment received.” Route of exposure includes aspiration, bite/sting, dermal, ingestion, inhalation, ocular, otic, parenteral, rectal, vaginal, other, and unknown; more than one route can be reported for one event. Medical outcome is ascertained based on information available at the conclusion of a case with periodic follow-up until an outcome can be ascertained, when possible. Medical outcome is classified by NPDS as “no effect” (the patient did not develop any signs or symptoms as a result of the exposure), “minor effect” (the patient developed some minimal signs or symptoms), “moderate effect” (the patient developed signs or symptoms that were more pronounced than minor symptoms, but the symptoms were not life-threatening and caused no residual disability), “major effect” (the patient exhibited signs or symptoms that were life-threatening or resulted in significant residual disability), and “death.”
Data Analysis
Our analyses focused on children under 5 years old, who are particularly at risk due to their natural curiosity and tendency to engage in oral exploration or imitation of adult behaviors.4,20 According to AAPCC, more than 80% of tobacco-related poison exposure calls involved young children.3 This is also the targeted age group (ie, under 5 years old) identified in the Poison Prevention Packaging Act (PPPA),21 which is the foundation of the Child Nicotine Poisoning Prevention Act (CNPPA) that became effective in July 2016.15
We extracted data from NPDS for this study in November 2016. We computed the frequency of tobacco-related poison exposure calls by year and type of tobacco product to monitor trends over time. We also computed the frequency and percentage of calls by age, healthcare facility level of care, and medical outcome. Statistical analysis was conducted using R version 3.0.2.22
RESULTS
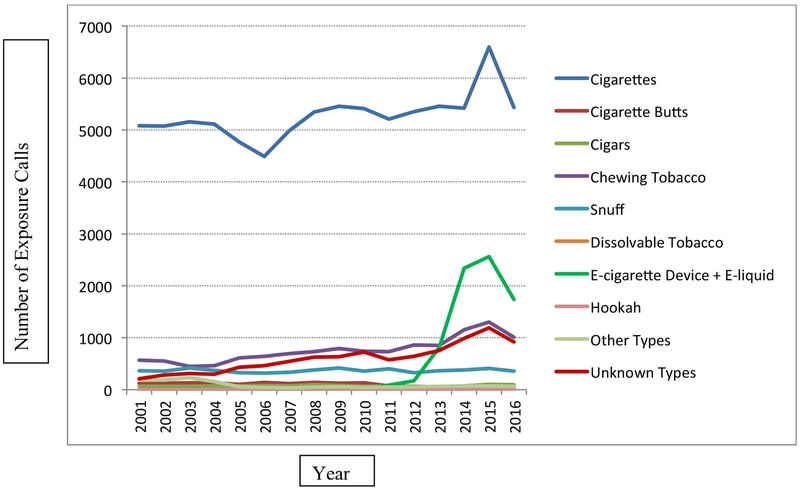
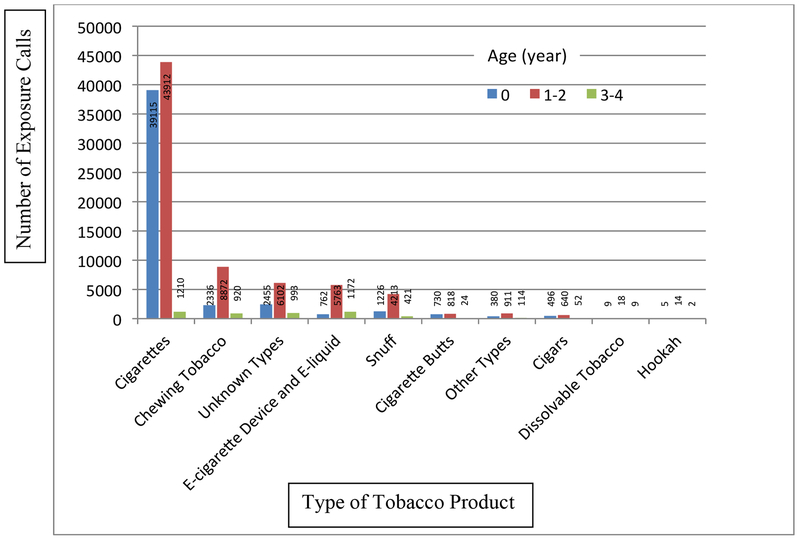
Overall, PCCs across the US received 123,876 tobacco-related exposure calls involving children younger than 5 years old between January 1, 2001 and October 31, 2016. The number of these calls increased from 6609 calls in 2001 to 12,295 calls in 2015 and 9658 calls in the first 10 months of 2016. Most calls involved conventional cigarettes, with around 5000 calls per year (Figure 1). The second leading cause of calls from 2001 to 2013 was chewing tobacco; calls more than doubled from 568 calls in 2001 to 1302 calls in 2015 and 1011 calls in the first 10 months of 2016. Calls involving e-cigarettes and e-liquid (N = 7707) increased dramatically from 7 calls in 2010 and 84 in 2011 to 2558 in 2015 and 1740 calls in the first 10 months of 2016, becoming the second leading cause of calls since 2014. There were 36 calls involving dissolvable tobacco products; the number of these calls decreased from 11 in 2009 to 7 in 2015 and 2 in the first 10 months of 2016. Hookah-related calls ranged from one in 2007 to 7 in 2015, with a total of 21 calls during the study period. Figure 2 illustrates that the overwhelming majority (96.0%) of tobacco-related calls involved children aged 2 years or younger. This pattern was observed for all tobacco products, ranging from 75.0% of calls for dissolvable tobacco products to 98.6% of calls for conventional cigarettes.
Figure 1.
Exposure Calls Involving Children Younger than 5 Years Old by Type of Tobacco Product and by Year, United States, January 1, 2001 – October 31, 2016
Figure 2.
Number of Tobacco-related Poison Exposure Calls Involving Children Younger than 5 Years Old by Age and Type of Tobacco Product, United States, January 1, 2001 – October 31, 2016
Table 1 shows that the majority of poison exposures occurred through ingestion for all products. Calls involving e-cigarette devices reported a relatively higher frequency of dermal exposure (10.9%) compared to other products. Calls involving e-liquid also reported a higher frequency of dermal exposure (12.1%) than other products. Calls involving hookah reported a much higher frequency of inhalation exposure (17.4%) than other products.
Table 1.
Route of Exposurea of Tobacco-related Poison Events among Children Younger than 5 Years Old by Type of Tobacco Product, United States, January 1, 2001 – October 31, 2016
| Type of Tobacco Product | Dermal | Ingestion | Inhalation | Ocular |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Cigarettes and Cigarette Butts | ||||
| Cigarettes | 850 (1.0) | 84178 (98.9) | 45 (0.05) | 81 (0.1) |
| Cigarette Butts | 35 (2.2) | 1569 (97.7) | 2 (0.1) | 0 (0.0) |
| Smokeless or Dissolvable Tobacco | ||||
| Chewing Tobacco | 287 (2.3) | 12052 (96.7) | 7 (0.06) | 116 (0.9) |
| Snuff | 168 (2.8) | 5801 (95.6) | 13 (0.2) | 84 (1.4) |
| Dissolvable Tobacco | 0 (0.0) | 36 (100.0) | 0 (0.0) | 0 (0.0) |
| Cigars | 10 (0.8) | 1187 (98.9) | 2 (0.2) | 1 (0.08) |
| E-Cigarette Device and E-Cigarette Liquid | ||||
| E-Cigarette Device | 698 (10.9) | 5334 (83.0) | 238 (3.7) | 154 (2.4) |
| E-Cigarette Liquid | 251 (12.1) | 1774 (85.9) | 5 (0.2) | 36 (1.7) |
| Hookah | 1 (4.3) | 17 (73.9) | 4 (17.4) | 1 (4.3) |
| Other Types | 37 (2.5) | 1371 (94.9) | 18 (1.2) | 18 (1.2) |
| Unknown Types | 352 (3.6) | 9338 (95.4) | 32 (0.3) | 62 (0.6) |
More than one route of exposure can be reported for one event.
Among tobacco-related poison events for children younger than 5 years old, there were 8 events with route of exposure as aspiration with ingestion, 2 events for bite/sting, 23 events for other, 10 events for otic, 2 events for parenteral, and 74 events with unknown route.
Of all 71,331 (57.6%) exposure calls with information on medical outcome, one e-cigarette device call concerned a death. There were 51 (0.07%) calls for exposures resulting in major medical effects, with 22 (43.1%) of these calls involving conventional cigarettes. Another 1620 (2.3%) calls had moderate effects, with 827 (51.0%) of calls involving conventional cigarettes (Table 2). Calls without information on medical outcome (N = 52,545) were largely due to decisions by PCCs staff as to whether the cases merited collecting information on medical outcome. Of these calls, 39,447 (75.1%) calls were not followed because no more than minor effects were expected; 5579 (10.6%) calls were judged as potential toxic exposures but staff were unable to follow the cases; 5042 (9.6%) were judged as nontoxic exposures; 2007 (3.8%) calls were considered unrelated effects (ie, the exposure was probably not responsible for the call); and 470 (0.9%) calls were confirmed non-exposure.
Table 2.
Medical Outcome and Healthcare Facility Level of Care of Tobacco-related Poison Events Involving Children Younger than 5 Years Old by Type of Tobacco Product, United States, January 1, 2001 – October 31, 2016
| Type of Tobacco Product | Medical Outcome | Healthcare Facility Level of Care | ||||
|---|---|---|---|---|---|---|
| Major Effect | Moderate Effect | Minor Effect | Treated and Released | Admitted to Critical Care Unit | Admitted to Non-critical Care Unit | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Cigarettes and Cigarette Butts | ||||||
| Cigarettes | 22 (43.1) | 827 (51.0) | 15429 (60.8) | 10633 (53.6) | 150 (54.0) | 249 (50.1) |
| Cigarette Butts | 1 (2.0) | 13 (0.80) | 236 (0.9) | 155 (0.8) | 1 (0.4) | 4 (0.8) |
| Smokeless Tobacco | ||||||
| Chewing Tobacco | 4 (7.8) | 262 (16.2) | 3715 (14.6) | 2517 (12.7) | 25 (9.0) | 45 (9.1) |
| Snuff | 3 (5.9) | 156 (9.6) | 1855 (7.3) | 1258 (6.3) | 15 (5.4) | 28 (5.6) |
| Dissolvable Tobacco | 0 (0.0) | 2 (0.1) | 9 (0.0) | 7 (0.0) | 0 (0.0) | 0 (0.0) |
| Cigars | 0 (0.0) | 17 (1.0) | 189 (0.7) | 165 (0.8) | 3 (1.1) | 3 (0.6) |
| E-cigarettes | ||||||
| E-cigarette device | 8 (15.7) | 92 (5.7) | 1133 (4.5) | 1979 (10.0) | 33 (11..9) | 51 (10.3) |
| E-cigarette liquid | 1 (2.0) | 29 (1.8) | 439 (1.7) | 745 (3.8) | 9 (3.2) | 21 (4.2) |
| Hookah | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other Type | 1 (2.0) | 29 (1.8) | 330 (1.3) | 266 (1.3) | 3 (1.1) | 5 (1.0) |
| Unknown Type | 11 (21.6) | 193 (11.9) | 2025 (8.0) | 2109 (10.6) | 39 (14.0) | 91 (18.3) |
| Total | 51 (100.0) | 1620 (100.0) | 25360 (100.0) | 19834 (100.0) | 278 (100.0) | 497 (100.0) |
Overall, 20,613 (16.6%) tobacco-related exposure calls involved events that led to children receiving medical care. Of these, 19,834 (92.2%) children were treated or evaluated by healthcare professionals and then released; 497 (2.4%) were admitted to a non-critical care unit and 278 (1.3%) were admitted to a critical or intensive care unit, with more than half of cases in both groups attributable to conventional cigarettes (Table 2). Overall, the tobacco products most commonly involved in events requiring hospital admission to a critical or intensive care unit were cigarettes, followed by unknown types of tobacco products, e-cigarettes, chewing tobacco, snuff, other types of tobacco products, cigarette butts, and cigars. No hookah-related calls reported hospitalization.
Table 3 shows the top 10 most frequently reported clinical effects or symptoms for all events and for events associated with e-cigarettes or e-liquid, respectively. The most common symptoms include vomiting, nausea, coughing or choking, and drowsiness or lethargy for all products and for e-cigarettes.
Table 3.
Top 10 Most Frequently Reported Clinical Effects (ie, Symptoms) as a Result of Poison Exposure among Children Younger Than 5 Years Old for All Tobacco Products and for E-cigarettes or E-liquids, United States, January 1, 2001 – October 31, 2016
| All Events | Number of Reported Symptoms | Events Involving E-cigarettes or E-liquids | Number of Reported Symptoms |
|---|---|---|---|
| Vomiting | 27919 | Vomiting | 1218 |
| Nausea | 3214 | Cough or choke | 189 |
| Cough or choke | 2799 | Drowsiness or lethargy | 151 |
| Drowsiness or lethargy | 2226 | Nausea | 146 |
| Other | 1533 | Ocular irritation or pain | 139 |
| Agitated or irritable | 1440 | Tachycardia | 103 |
| Pallor | 1210 | Red eye or conjunctivitis | 96 |
| Tachycardia | 593 | Other | 85 |
| Diaphoresis | 488 | Oral irritation | 82 |
| Oral irritation | 473 | Agitated or irritable | 79 |
DISCUSSION
Between January 1, 2001 and October 31, 2016, PCCs across the US received more than 123,000 telephone calls due to tobacco product exposures among children under 5 years old. Tobacco-related poison events continue to be a significant public health problem, especially among the youngest children, with more than 650 incidents per month on average during the study period. Overall tobacco-related exposure calls increased over time for all products except cigarette butts and products classified as “other types.” According to AAPCC, 1.2% of all exposure calls involving children aged 5 years or younger to PCCs in 2015 were attributable to tobacco product exposures and the percentage has increased since 2013;3,16,23 electronic cigarettes were among the top 25 most frequently reported products for exposure calls in the same population in 2015.3 Moreover, electronic cigarettes were among the top 25 products with the greatest increase in exposure rate.3 Although tobacco-related poison events constitute a small percentage of exposure calls involving young children to PCCs, it is important to note that use of tobacco products within the US population is relatively high, with more than one-fourth (27.6%) of adults reporting current use of at least one type of tobacco product in 2013–2014.24 Given that exposure to tobacco can result in serious harm to young children, as reflected in NPDS data, tobacco-related poison events are a serious threat to public health. Our findings underscore the importance of monitoring poison events concerning all tobacco products, including emerging and re-emerging tobacco products.
Findings from this study are vastly different from the FDA’s reports on acute adverse experience (AE) associated with tobacco products.9,25 The FDA received fewer reports of AEs related to tobacco products than NPDS. This is likely due to several factors, including the different reasons for reporting to these 2 systems. Whereas both systems receive voluntary reports, NPDS houses data primarily from consumers seeking immediate professional advice on managing poison exposures whereas the FDA’s Safety Reporting Portal (SRP) for tobacco products is intended to gather information about tobacco product problems from citizens, health-care professionals, manufacturers, and researchers. Additionally, NPDS has been available for decades and public awareness of the system may be higher than the FDA’s SRP launched in 2014.
This study reveals that tobacco-related poison events can cause serious health effects that require intensive medical care in young children. Whereas more than half of the poison exposures to tobacco products occurred through ingestions, cases involving e-cigarette and e-liquid had higher rates of dermal exposures than other products; these cases also had higher rates of inhalation and or ocular exposures than other products except hookahs. In a study about e-cigarette-related poison events, Chatham-Stephens et al6 reported dermal exposure due to e-cigarette device leak, and eye irritation or pain due to mistakenly using e-liquids as eye drops. Lack of product standards, quality control, labeling, and appropriate packaging might be contributing factors in these cases. The actual number of tobacco-related poison events is likely to be much larger than what we observed due to potential underreporting. In a nationally representative sample survey, the Health Resources and Services Administration found that less than half of survey respondents (46%) were aware of PCCs, 26% had a poison control number posted in their home, and just 4% were able to provide AAPCC’s toll-free telephone number spontaneously.26 An Institute of Medicine (IOM) report found substantial underreporting for deaths associated with poison events.27–31 The IOM report also found that only about 20%–30% of poisoning cases treated in emergency rooms were reported to PCCs.32–34 This estimate is consistent with NPDS data showing that only 20% of poison exposure cases are reported to a PCC by staff at healthcare facilities.16 Although the exact reasons and determinants of using PCCs’ services are unknown, future studies assessing these factors may help PCCs identify deficiencies and develop programs to improve awareness and utilization of PCCs’ services that are available to everyone in the US. This study has presented additional insight into the nature of tobacco-related poison events in the rapidly-changing tobacco product market. Previously, Connolly et al4 expressed concern about the harm of accidental smokeless tobacco ingestion by young children, with particular concern about dissolvable tobacco products. Our study found that dissolvable tobacco products account for a small number of poison events, probably because of limited availability in the US35,36 and low rates of uptake and use of these products.37 E-cigarettes, in contrast, are responsible for a sizeable and growing number of poison events, indicating a serious public health issue. Our study also found a steady upward trend in exposure calls involving smokeless tobacco products, despite stable prevalence of smokeless tobacco use in recent years.38
Despite the significant decline in cigarette smoking prevalence in recent years,11,39 conventional cigarettes continue to be the leading cause of tobacco-related poison events among children aged 5 years or younger. Previous studies indicate that children’s accessibility to cigarettes is a major risk factor of poison events associated with conventional cigarettes.8,40 Developing targeted educational programs to increase the awareness of cigarette-related poison events among young children and associated harm may help prevent these events.
Our study results indicate that these poison events continue to affect thousands of young Americans each year and remain a serious public health issue. Moreover, the youngest children are particularly at risk, with more than 80% of tobacco-related poison exposure calls involving children aged 5 years or younger.3 Nicotine has been identified as the toxic substance most commonly reported as causing symptoms such as nausea and vomiting in low doses, and more extensive neurological symptoms with higher doses among children who have consumed cigarettes or butts.41 The incidence of such harmful events could be reduced by eliminating young children’s access to tobacco products, given that previous research has shown that cigarettes and butts were most commonly ingested by young children in homes where smoking occurred in the presence of children and cigarettes and cigarette butts were accessible.40 The rapid increase in e-cigarette use and associated poison events in recent years raise additional public health concerns. National data indicate that current e-cigarette use has increased dramatically among US youth13 and adults.42 Currently, e-cigarette use in the US is still much lower than cigarette smoking, both in terms of prevalence in adults (3.7% vs 16.8% based on 2014 National Health Interview Survey data39,42) and sales (an estimated $2 billion vs $80 billion in 201343), but e-cigarettes now account for a disproportionate share of tobacco-related poison exposure calls. Further monitoring of this situation is warranted given the increasing prevalence of e-cigarette use and the growing number of associated poisoning events. On August 8, 2016, the FDA began the implementation of the Deeming Final Rule, which extends the Agency’s authority to all tobacco products, including e-cigarettes.44 Information from this study may inform future regulatory activities on these products.
This study has limitations. First, the number of tobacco-related poison exposure events is probably substantially underestimated, as NPDS is a passive surveillance system that relies on voluntary reporting. Second, these calls may not represent the actual spectrum of severity of outcome. Parents or caregivers may bring children with serious reactions or symptoms to the emergency department immediately or call 911 instead of calling poison control centers. Nationally representative data on tobacco-related injuries treated in hospital emergency departments may provide useful information that is complementary to this study. Third, we only included calls related to tobacco exposures as the primary substance identified by PCC staff as our goal was to assess poison exposure calls attributable to tobacco products. It is possible that tobacco could contribute to poison exposures where another substance was identified as the primary substance of the exposure by PCC staff. However, the potential underestimate of tobacco-related exposure events due to excluding cases involving more than one substance is minimal, approximately 0.6%. Fourth, some tobacco products may not be categorized appropriately, especially new and emerging tobacco products. For example, e-cigarettes may have been on the US market since 200744–46 and the first adverse event concerning an e-cigarette was reported to the FDA in 2008,25 but NPDS product codes for e-cigarettes were not available until 2010. In addition, the increased number of exposure calls involving unknown types of tobacco products might be attributable to new and emerging tobacco products. Finally, tobacco-related poison events and related information such as medical outcomes are available for only approximately half of the cases; medical outcome is often self-reported, which is subject to reporting bias. However, exposure calls to PCCs are answered by healthcare professionals and follow-up calls are made to obtain further information in nearly half of cases.16 The majority of cases without medical outcome information were those not followed as a result of clinical judgment.
IMPLICATIONS FOR TOBACCO REGULATION
Tobacco-related poison exposure is a public health concern. Conventional cigarettes continue to be the leading cause of tobacco-related exposure calls to PCCs in the US. Despite the substantial decline in cigarette consumption in the US over the past decade, the number of cigarette-related exposure calls has increased. In addition, e-cigarette-related calls have increased dramatically and exposure calls involving smokeless tobacco products are on the rise. Many of these events have serious health consequences. Approximately one in 6 children experiencing a tobacco-related poison event was taken to a healthcare facility, and one in 4 had minor to major medical effects. Findings from this study may inform targeted educational programs and potential regulatory actions aimed to prevent and reduce tobacco-related poison events. The recently implemented CNPPA requires nicotine exposure warnings and child-resistant packaging for liquid nicotine, nicotine-containing e-liquid(s), and other tobacco products.15 Under the May 2016 Deeming Final Rule,44 the FDA has the authority to require health warnings for product packages and advertisements, labeling, child-resistant packaging, and product standards for newly deemed products. As indicated in the Family Smoking Prevention and Tobacco Control Act of June 2009,47 the premarket review authority for new tobacco products also allows the FDA to assess the adequacy of the products child-resistant packaging as part of the assessment of whether allowing the product on the market is for the protection of public health. These measures may help reduce and prevent accidental exposure to excessive levels of nicotine. Findings from this study underscore the importance of continued monitoring of trends in tobacco-related poison exposure events over time, identifying populations at risk and emerging issues with tobacco products, and assessing factors influencing the occurrence of tobacco-related poison events.
Acknowledgments
The US Food and Drug Administration supported this work. This publication represents the views of the authors and does not necessarily represent the views of the US Food and Drug Administration.
Footnotes
Human Subjects Statement
The FDA’s Research Involving Human Subjects Committee approved this study
Conflict of Interest Statement
The authors have no competing interest to declare.
Contributor Information
Baoguang Wang, Office of Science, Center for Tobacco Products, US Food and Drug Administration, Silver Spring, MD..
Brian Rostron, Office of Science, Center for Tobacco Products, US Food and Drug Administration, Silver Spring, MD..
References
- 1.Gupta S, Gandhi A, Manikonda R. Accidental nicotine liquid ingestion: emerging paediatric problem. Arch Dis Child. 2014;99(12):1149. [DOI] [PubMed] [Google Scholar]
- 2.Salomon ME. Nicotine and tobacco preparations In Flomenbaum N, Goldfrank L, Hoffman R, et al. , eds. Goldfrank’s Toxicologic Emergencies. 8th ed. New York, NY: McGraw-Hill; 2006. [Google Scholar]
- 3.Mowry JB, Spyker DA, Brooks DE, et al. 2015 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol (Phila). 2016;54(10):924–1109. [DOI] [PubMed] [Google Scholar]
- 4.Connolly GN, Richter P, Aleguas A, Jr., et al. Unintentional child poisonings through ingestion of conventional and novel tobacco products. Pediatrics. 2010;125(5):896–899. [DOI] [PubMed] [Google Scholar]
- 5.Appleton S. Frequency and outcomes of accidental ingestion of tobacco products in young children. Regul Toxicol Pharmacol. 2011;61(2):210–214. [DOI] [PubMed] [Google Scholar]
- 6.Chatham-Stephens K, Law R, Taylor E, et al. Notes from the field: calls to poison centers for exposures to electronic cigarettes--United States, September 2010-February 2014. MMWR Morb Mortal. Wkly Rep. 2014;63(13):292–293. [PMC free article] [PubMed] [Google Scholar]
- 7.Vakkalanka JP, Hardison LS Jr., Holstege CP. Epidemiological trends in electronic cigarette exposures reported to U.S. Poison Centers. Clin Toxicol (Phila). 2014;52(5):542–548. [DOI] [PubMed] [Google Scholar]
- 8.Kamboj A, Spiller HA, Casavant MJ, et al. Pediatric exposure to e-cigarettes, nicotine, and tobacco products in the United States. Pediatrics. 2016;137(6). [DOI] [PubMed] [Google Scholar]
- 9.Durmowicz EL, Rudy SF, Chen IL. Electronic cigarettes: analysis of FDA adverse experience reports in non-users. Tob Control. 2016;25(2):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble MJ, Longstreet B, Hendrickson RG, Gerona R. Unintentional pediatric ingestion of electronic cigarette nicotine refill liquid necessitating intubation. Ann Emerg Med. 2017;69(1):94–97. [DOI] [PubMed] [Google Scholar]
- 11.Jamal A, King BA, Neff LJ, et al. Current cigarette smoking among adults – United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–1211. [DOI] [PubMed] [Google Scholar]
- 12.Tynan MAM T; Promoff G; Pechahek T Consumption of cigarettes and combustible tobacco – United States, 2000–2011. MMWR Morb Mortal Wkly Rep. 2012;61(30):565–569. [PubMed] [Google Scholar]
- 13.Arrazola RA, Singh T, Corey CG, et al. Tobacco use among middle and high school students – United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 14.Shearston JA, Park SH, Lee L, et al. Increasing hookah use among adolescent females in the US: analyses from the 2011–2014 National Youth Tobacco Survey (NYTS). Tob Prev Cessat. 2016;2(September):71. [Google Scholar]
- 15.Child Nicotine Poisoning Prevention Act, Pub. L., No. 114–6, 130 Stat 3, 2015. Available at: https://www.congress.gov/bill/114th-congress/senate-bill/142. Accessed August 6, 2017. [Google Scholar]
- 16.Mowry JB, Spyker DA, Brooks DE, et al. 2014 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol (Phila). 2015;53(10):962–1147. [DOI] [PubMed] [Google Scholar]
- 17.Lawler TS, Stanfill SB, Zhang L, et al. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem Toxicol. 2013;57:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stepanov I, Biener L, Yershova K, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel smokeless tobacco products: findings from round II of the new product watch. Nicotine Tob Res. 2014;16(8):1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maziak W, Taleb ZB, Bahelah R, et al. The global epidemiology of waterpipe smoking. Tob Control. 2015;24(Suppl 1):i3–i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CP, Blasco PA. Infant growth and development. Pediatr Rev. 1997;18(7):224–242. [DOI] [PubMed] [Google Scholar]
- 21.Congress US. Poison Prevention Packaging Act. 1970. Available at: https://www.cpsc.gov/s3fs-public/pdfs/blk_media_pppa.pdf. Accessed July 27, 2017.
- 22.R Development Core Team. R: A language and environment for statistical computing [computer program]. Version 3.0.2. Vienna: R Development Core Team; 2013. [Google Scholar]
- 23.Mowry JB, Spyker DA, Cantilena LR Jr, et al. 2013 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st annual report. Clin Toxicol (Phila). 2014;52(10):1032–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen IL. FDA summary of adverse events on electronic cigarettes. Nicotine Tob Res. 2013;15(2):615–616. [DOI] [PubMed] [Google Scholar]
- 26.Health Resources and Services Administration. Poison Help Campaign Fiscal Year 2012. Available at: http://poisonhelp.hrsa.gov/the-poison-help-line/campaignfiscalyear2012.pdf. Accessed July 27, 2017.
- 27.Institute of Medicine. Forging a Poison Prevention and Control System. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 28.Hoppe-Roberts JM, Lloyd LM, Chyka PA. Poisoning mortality in the United States: comparison of national mortality statistics and poison control center reports. Ann Emerg Med. 2000;35(5):440–448. [PubMed] [Google Scholar]
- 29.Linakis JG, Frederick KA. Poisoning deaths not reported to the regional poison control center. Ann Emerg Med. 1993;22(12):1822–1828. [DOI] [PubMed] [Google Scholar]
- 30.Soslow AR, Woolf AD. Reliability of data sources for poisoning deaths in Massachusetts. Am J Emerg Med. 1992;10(2):124–127. [DOI] [PubMed] [Google Scholar]
- 31.Blanc PD, Kearney TE, Olson KR. Underreporting of fatal cases to a regional poison control center. West J Med. 1995;162(6):505–509. [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyt BT, Rasmussen R, Giffin S, Smilkstein MJ. Poison center data accuracy: a comparison of rural hospital chart data with the TESS database. Toxic Exposure Surveillance System. Acad Emerg Med. 1999;6(8):851–855. [DOI] [PubMed] [Google Scholar]
- 33.Blanc PD, Jones MR, Olson KR. Surveillance of poisoning and drug overdose through hospital discharge coding, poison control center reporting, and the Drug Abuse Warning Network. Am J Emerg Med. 1993;11(1):14–19. [DOI] [PubMed] [Google Scholar]
- 34.Harchelroad F, Clark RF, Dean B, Krenzelok EP. Treated vs reported toxic exposures: discrepancies between a poison control center and a member hospital. Vet Hum Toxicol. 1990;32(2):156–159. [PubMed] [Google Scholar]
- 35.Southwell BG, Kim AE, Tessman GK, et al. The marketing of dissolvable tobacco: social science and public policy research needs. Am J Health Promot. 2012;26(6):331–332. [DOI] [PubMed] [Google Scholar]
- 36.Seidenberg AB, Rees VW, Connolly GN. R. J. Reynolds goes international with new dissolvable tobacco products. Tob Control. 2012;21(3):368–369. [DOI] [PubMed] [Google Scholar]
- 37.Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults – United States, 2012–2013. MMWR Morb Mortal. Wkly Rep. 2014;63(25):542–547. [PMC free article] [PubMed] [Google Scholar]
- 38.Agaku IT, Vardavas CI, Ayo-Yusuf OA, et al. Temporal trends in smokeless tobacco use among US middle and high school students, 2000–2011. JAMA. 2013;309(19):1992–1994. [DOI] [PubMed] [Google Scholar]
- 39.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults – United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 40.US Centers for Disease Control and Prevention. Ingestion of cigarettes and cigarette butts by children – Rhode Island, January 1994-July 1996. MMWR Morb Mortal Wkly Rep. 1997;46(6):125–128. [PubMed] [Google Scholar]
- 41.Novotny TE, Hardin SN, Hovda LR, et al. Tobacco and cigarette butt consumption in humans and animals. Tob Control. 2011;20(Suppl 1):i17–i20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1–8. [PubMed] [Google Scholar]
- 43.Robehmed N E-cigarette sales surpass $1 billion as big tobacco moves in. Forbes. 2013. Available at: https://www.forbes.com/forbes/welcome/?toURL=https://www.forbes.com/sites/natalierobehmed/2013/09/17/e-cigarette-sales-surpass-1-billion-as-big-tobacco-moves-in/&refURL=https://www.google.com/&referrer=https://www.google.com/. Accessed March 29, 2016.
- 44.Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act. Fed Regist. 2016;81(90):28973–9106. [PubMed] [Google Scholar]
- 45.McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17(10):1195–1202. [DOI] [PubMed] [Google Scholar]
- 46.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tob Control. 2013;22(1):19–23. [DOI] [PubMed] [Google Scholar]
- 47.Congress US. Family Smoking Prevention and Tobacco Control Act. Public Law 111–31. 2009. [Google Scholar]