Abstract
Background
Postprandial hyperglycemia and glycemic fluctuations are significant cardiovascular disease risk factors for patients with type 2 diabetes. We investigated the effects of a single session of post-dinner moderate-intensity exercise on the postprandial glycemic response compared with a non-exercise condition in a study population of Chinese patients with type 2 diabetes.
Material/Methods
This randomized crossover self-controlled pilot study involved 29 patients with type 2 diabetes who participated in post-dinner exercise days using non-exercise days as a control. The interstitial glucose level was monitored using a continuous glucose monitoring system, with a standardized diet and medication. For the non-exercise control days, patients pursued normal daily activities but refrained from unusual strenuous physical activity. On the exercise days, participants walked on a treadmill for 20 minutes after dinner, with a heart rate reserve of 40%.
Results
Post-dinner moderate-intensity exercise reduced the 2-hour postprandial glucose spike, mean glucose level, and peak glucose level compared to the control condition. The cumulative glucose total area under the curve during 1-hour post-exercise was lower with exercise than under the control condition. The 12-hour standard deviation of blood glucose and the coefficient variation of glucose were significantly lower in the with exercise day compared to the control day, although the 12-hour mean amplitude of glycemic fluctuations did not reach statistical significance. No nocturnal hypoglycemia subsequently occurred on the exercise day.
Conclusions
A short session of moderate-intensity post-dinner exercise can improve postprandial hyperglycemia and glycemic excursions in Chinese patients with type 2 diabetes, with no potential hypoglycemia risk at a later period.
MeSH Keywords: Diabetes Mellitus, Type 2; Exercise; Hyperglycemia; Postprandial Period
Background
Postprandial hyperglycemia is associated with an increased risk of diabetes complications [1–4], particularly in patients with type 2 diabetes [5,6]. Therefore, better management of postprandial hyperglycemia is an important target in type 2 diabetes treatment. Despite the use of antihyperglycemic drugs and the consumption of a healthy diet, postprandial hyperglycemia and acute glucose swings remain the main characteristics of patients with type 2 diabetes [7,8]. Therefore, more treatment strategies to reduce the prevalence of postprandial hyperglycemia are required.
Structured exercise has long been considered a cornerstone for type 2 diabetes treatment [9]. Many studies have demonstrated that exercising during the postprandial period causes acute reductions in postprandial blood glucose [10–16]. However, post-dinner exercise has not always been differentially investigated, and most of the results have been derived from laboratory studies and no real-life conditions. In China, people are accustomed to exercising after dinner. To our knowledge, such effect of a bout of post-dinner exercise on glycemic levels in Chinese patients with type 2 diabetes has not been reported. However, exercise may lead to nocturnal hypoglycemia in children with type 1 diabetes [17,18] or a potential increase in a late-onset hypoglycemia risk for patients with type 2 diabetes [19]. For this reason, it was necessary to investigate whether a session of moderate-intensity exercise (MIE) after dinner could result in nocturnal hypoglycemia. In clinical practice, most nocturnal hypoglycemic episodes are missed. The continuous glucose monitoring system (CGMS) can record blood glucose for several days, which can be easily used to monitor exercise and activities of daily living, particularly during the night. A previous trial has shown that moderate-intensity walking after an evening meal may blunt postprandial glycemia, compared with pre-dinner exercise or no exercise in American individuals with type 2 diabetes, but CGMS was not performed [14].
Aside from regular exercise, more evidence [20,21] has suggested that non-exercise physical activities play an independent role in restoring or maintaining optimal glycemic control. Most previous laboratory studies have focused on the effects of post-meal exercise versus no exercise on blood glucose [10–16], and less attention has been paid to the comparison between exercise and daily activities. In this regard, a recent clinical trial [22] has shown that a single session of MIE had a greater impact on daily blood glucose homeostasis than repeated sessions of daily activities. However, this study had been restricted to exercise after breakfast and strictly defined daily activities. Moreover, another group [23] used normal daily activities as the control condition, however, the walking exercise protocols were performed during the lunchtime period. Therefore, the effects of exercise after dinner on glycemic control, using the CGMS, for type 2 diabetes in a real-life setting are unknown.
The purpose of this study was to examine the effects of a single session of post-dinner MIE on the glycemic response to a standardized dinner in Chinese individuals with type 2 diabetes. It was hypothesized that 20 minutes (min) of post-dinner MIE would result in a lower peak postprandial glucose response than with normal daily activities as a control (CON) condition on a non-exercise day. We also tested the lasting effects of post-dinner exercise on the subsequent glycemic response.
Material and Methods
Participants
A total of 29 patients (7 females and 22 males) with uncomplicated type 2 diabetes treated with diet and/or a variety of oral glucose lowering medications volunteered for this study. Participants were excluded from the study if they had liver, renal, or cardiopulmonary disease or diseases contraindicating physical activity. The use of insulin, body weight instability (>3 kg/half a year), and regular exercise (> 150 min/week) were set as exclusion criteria.
This study was approved by the Ethics Committee of Nanjing First Hospital. All subjects gave informed consent prior to study participation. This study was registered with ChiCTR.org.cn, number ChiCTR-ONC-17010400 (http://www.chictr.org.cn/showproj.aspx?proj=17724).
Clinical and laboratory assessments
At the initial visit, data on height, weight, blood pressure, resting heart rate (HR), age, current clinical diagnoses, and medication usage were collected. The body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). Following a minimum 10 hours (h) of overnight fasting, all participants’ blood samples were collected. Plasma blood glucose levels were measured using the hexokinase method. Plasma total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid, and creatinine levels were measured with an auto-analyzer (Modular E170; Roche, Mannheim, Germany). HbA1c was determined by a DiaSTAT HbA1c analyzer (BIO-RAD Company; Hercules, CA, USA). Participants completed the aerobic capacity test using a treadmill. Cardiac function was monitored using a resting 12-lead electrocardiogram.
Study design
Participants performed trials consisting of 2 experimental periods in a crossover order: 1 non-exercise day as a control day and 1 post-dinner MIE day. Experiments were separated by at least 7 days and conducted in random order. All participants were asked to maintain their normal physical activity patterns throughout the study but to refrain from exhaustive physical labor and exercise training for 2 days before each experimental period. During the non-exercise day, participants were requested to refrain from participating in moderate or heavy exercise. During the post-dinner MIE day, exactly 30 min after the first bite, participants performed 20 min treadmill walking, and were requested to refrain from participating in moderate or heavy exercise at other times of the day. Participants rested during the 20-min exercise window on the non-exercise day. All participants were supervised on both study days in the department of endocrinology at Nanjing First Hospital.
A heart rate monitor was applied to monitor the exercise sessions. Heart rates during the exercise session were recorded. Exercise intensity was calculated using the heart rate reserve (HRR) method [24], which considers resting HR and estimated maximal HR (calculated as 220 minus the participant’s age), where the HRR is the difference between the estimated maximal HR (corrected for the age) and the resting HR. The intensity of exercise was set at 40% of the HRR by modulating treadmill speed [14], with all sessions completed on the treadmill. A 3-min warm-up and 2-min cool-down period were not included in the short session of exercise. The participants were asked to maintain the exercise intensity during the whole session. Timing of medication was standardized for each participant, meaning hypoglycemic agents were taken at the same time during both non-exercise and exercise conditions.
Continuous glucose monitoring
The day before each experimental session, the CGMS (Medtronic Incorporated, Northridge, CA, USA) sensor was placed on each participant’s abdomen between 16: 00 h and 17: 00 h, and it was removed on the day after the experimental day between 08: 00 h and 09: 00 h. The participants received a short training in the use of the device and collected blood glucose measurements 4 times daily, before the 3 main meals and before sleep. Nurses entered the 4 calibration data readings each day. Glucose concentration was continuously measured in the interstitial fluid of the subcutaneous tissue every 5 min. A total of 24 consecutive hours were considered for evaluation of glucose variations and responses to meals. The 24-h period was then divided into smaller time frames, namely, an exercise window, 1-h after exercise, 2-h postprandial periods after dinner, 3-h postprandial periods after dinner, night time (from midnight until 06: 00 h the following morning), and the 12-h timespan (17: 00 h–05: 00 h) for further analysis.
Medication and food intake
Treatment with oral hypoglycemic agents was continued as normal throughout the entire experimental period. On each experimental period, participants were provided with a standardized diet, which was designed to ensure a total daily energy intake of 105 KJ/kg/day, consisting of 55% of energy from carbohydrate, 18% from protein, and 27% from fat. The foods were divided into 3 equal portions consumed by participants at 7: 00 h, 11: 30 h, and 17: 00 h. To ensure the matching of the diet between experimental days, participants were asked to consume the same diet provided by the hospital in the exercise day and control day.
Statistical analysis
The CGMS data were downloaded to a personal computer and converted into glycemic values for further analysis. The postprandial glucose total area under the curve (tAUC) for the standardized dinner was computed using the trapezoid method. Peak and mean glucose during the exercise session were also calculated. Peak post-dinner glucose values were obtained from the highest value recorded within 2 h or 3 h of dinner onset. Glucose spikes following dinner were calculated as the difference between the peak post-dinner glucose and pre-dinner glucose levels. The mean amplitude of glycemic excursions (MAGE) and other plasma glucose fluctuation parameters, such as the mean blood glucose (MBG) and the standard deviation of blood glucose (SDBG), were calculated. The coefficient variation (CV) of glucose was calculated by dividing the standard deviation (SD) with the mean of the corresponding glucose reading. MAGE was calculated by measuring the arithmetic mean of the differences between consecutive peaks and nadirs; measurement in the peak-to-nadir or nadir-to-peak direction was determined by the first qualifying excursion; only excursions of more than 1 SD of the mean glycemic values were considered [25].
All statistical analyses were conducted using SPSS 17.0 for Windows (Chicago, IL, USA). All data were checked for normality by the Kolmogorov-Smirnov and the Shapiro-Wilk tests. Variables with normal distribution were expressed as mean ±SD. Hypothesis testing was performed by determining the differences in the effects of the exercise and control conditions on the glycemic outcomes during the postprandial period after dinner, and the Student’s paired t-test was used to compare the indices of glycemic variability. Repeated measures analysis of variance (ANOVA) was used to assess differences between post-dinner exercise and the control condition at specific time points. The statistical tests were two-sided and a P-value of <0.05 was considered statistically significant.
Results
Participant characteristics
Participant characteristics and medications are presented in Table 1. Participants were approximately 50 years old, 76% were male, with a BMI of 24.8 kg/m2. The median duration of type 2 diabetes was 3 years and the participants had a mean HbA1c of 7.3% (56.2 mmol/mol) and a mean fasting plasma glucose of 7.2 mmol/L during screening. All participants were diagnosed with type 2 diabetes. Thirteen participants were being treated with antihypertensive medication and 9 participants were being treated with lipid-lowering medication. The participants reported excellent compliance with the standardized diet, and no conditions needed to be repeated or dropped from the analyses.
Table 1.
Participants characteristics.
| Characteristic | Values |
|---|---|
| Sex (M/F) | 22/7 |
| Age (years) | 51±11.2 |
| Type 2 diabetes diagnosis (years) | 5.7±6.0 (0.1–20) |
| Height (cm) | 167.8±7.4 |
| Weight (kg) | 69.9±10.6 |
| BMI (kg/m2) | 24.8±3.4 |
| Diastolic blood pressure (mmHg) | 78±8 |
| Systolic blood pressure (mmHg) | 125±13 |
| HbA1C (%) | 7.3±1.3 |
| HbA1c (mmol/mol) | 56.2±14.5 |
| Fasting glucose (mmol/L) | 7.2±1.3 |
| Blood uric acid (μmol/L) | 347.0±73.5 |
| Creatinine (μmol/L) | 72.2±15.2 |
| Cholesterol (mmol/L) | 4.5±0.9 |
| Triacylglycerol (mmol/L) | 1.9±1.5 |
| HDL-C (mmol/L) | 1.2±0.2 |
| LDL-C (mmol/L) | 2.2±0.6 |
| Glucose-lowering medication (n) | 14 |
| Metformin + Sulfonylurea | 4 |
| Metformin + Repaglinide | 1 |
| Metformin + Acarbose | 1 |
| Metformin + DPP4 inhibitor | 3 |
| Sulfonylurea + Thiazolidinedione | 1 |
| Sulfonylurea + Acarbose | 3 |
| Acarbose + Repaglinide | 1 |
Participants had an average resting HR of 70.9±7.9 beats per minute (bpm) (Table 2). Average exercise heart rates of the participants, measured during the final 5 minutes of the exercise session, were 111.0±7.9 bpm, which corresponded to 40.9±5.1% of the HRR, indicating the moderate intensity of their pace.
Table 2.
Exercise subjects characteristics.
| Characteristic | Values |
|---|---|
| Resting HR (bpm) | 70.9±7.9 |
| Maximal HR (bpm) | 168.8±11.2 |
| HRR (bpm) | 98.0±11.7 |
| Target HR (bpm) | 109.9±5.3 |
| Exercise HR (bpm) | 111.0±7.9 |
Data are presented as means ±SD. HR – heart rate; bpm – beats per minute; HRR – heart rate reserve. Maximal HR is estimated as 220-age. HRR is the difference between the estimated maximal HR and the resting HR.
Data are presented as means ±SD (min–max). M – Male; F – Female; BMI – body mass index; HbA1C – glycated hemoglobin; HDL-C – high density lipoprotein cholesterol; LDL-C – low density lipoprotein cholesterol; DPP4 – dipeptidyl peptidase-4.
Glycemic control
The average CGM values (Figure 1A) and the CGM glucose change from baseline (Figure 1B) for the post-dinner MIE and control condition during the postprandial period after dinner are shown in Figure 1. There were no significant differences in the peak glucose (8.8±1.5 vs. 9.4±2.2 mmol/L, respectively; P=0.14) and mean glucose (8.5±1.5 vs. 8.9±2.2 mmol/L, respectively; P=0.22) levels during the time of the exercise session between the post-dinner MIE and the control condition, as well as in the tAUC (169.6±30.9 vs. 178.3±43.5 mmol/L×20 min, respectively; P=0.25) during the time of the exercise session.
Figure 1.
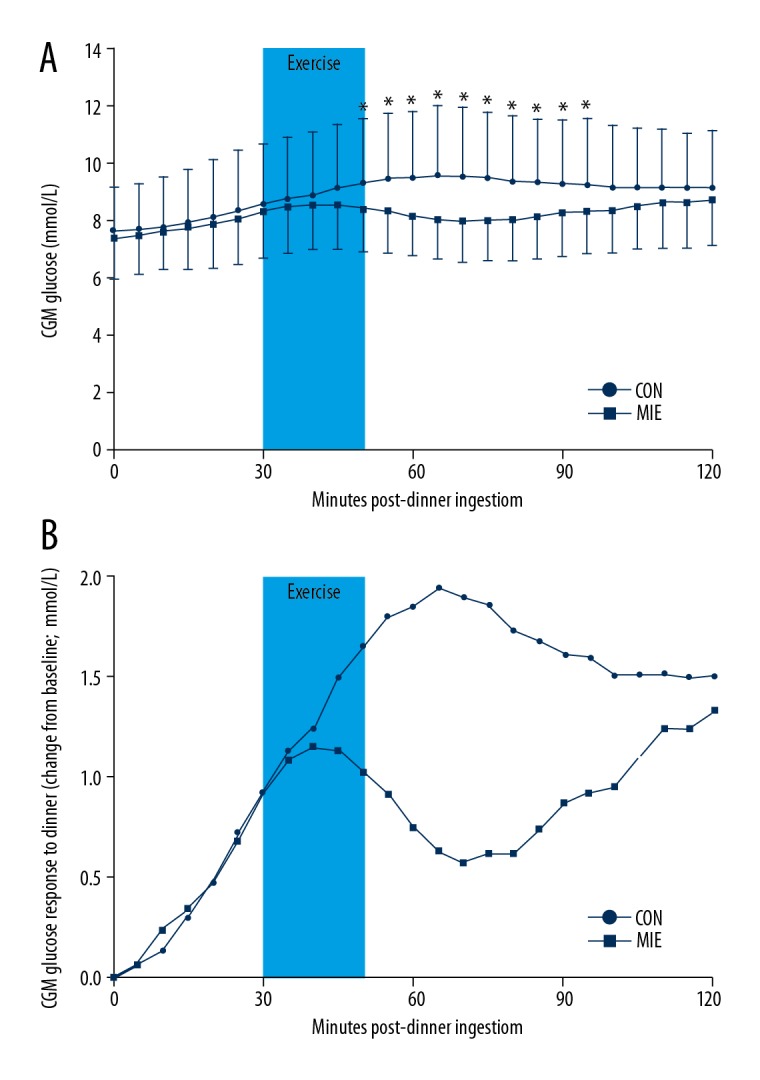
The average CGM values (A) and the average postprandial CGM glucose change from baseline (B) for moderate-intensity exercise (MIE) condition and non-exercise as control (CON) condition during the postprandial dinner period. Time 0 represents the time of first bite. Post-dinner exercise began half an hour after first bite and lasted 20 minutes (light blue squares). * Significantly different compared with the control condition (P<0.05).
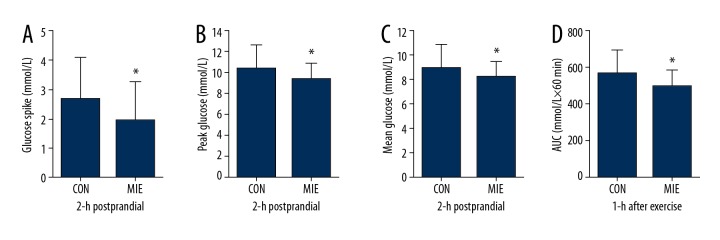
Significant glucose reductions in the 2-h postprandial glucose spike (1.9±1.3 vs. 2.7±1.4 mmol/L, respectively; P=0.04; Figure 2A), 2-h postprandial peak glucose (P=0.02; Figure 2B; Table 3), and 2-h postprandial mean glucose (P=0.04; Figure 2C; Table 3) levels were detected on the exercise day compared to those under the control condition. Significant differences were not observed in dinner 2-h postprandial glucose tAUC (Table 3). However, the glucose tAUC during 1-h after exercise in post-dinner MIE day was significantly lower than the non-exercise day (493.9±84.0 vs. 559.3±130.5 mmol/L×60 min, respectively; P=0.01; Figure 2D). Repeated measures ANOVA of the glucose at specific time points showed that differences between the exercise condition and control condition occurred from 50 min to 95 min within the postprandial period (P<0.05, Figure 1A).
Figure 2.
(A) 2-h postprandial glucose spike (difference between peak postprandial glucose and pre-dinner glucose), (B) 2-h postprandial peak glucose, (C) 2-h postprandial mean glucose, and (D) the glucose area under the curve (AUC) during 1-h after exercise. Data are estimated means ±SD (n=29 individuals). * Significantly lower compared with the control condition (P<0.05).
Table 3.
Continuous glucose monitor parameters.
| CON | MIE | P-value | |
|---|---|---|---|
| 2-h postprandial periods after dinner | |||
| MBG (mmol/L) | 8.9±2.0 | 8.2±1.3 | 0.04 |
| tAUC (mmol/L×120 min) | 1044.7±233.1 | 981.6±157.6 | 0.19 |
| Peak glucose (mmol/L) | 10.3±2.3 | 9.3±1.6 | 0.02 |
| Time to peak (min) | 75.3±28.3 | 68.3±42.4 | 0.41 |
| 3-h postprandial periods after dinner | |||
| MBG (mmol/L) | 8.9±1.9 | 8.3±1.3 | 0.08 |
| tAUC (mmol/L×180 min) | 1607.6±341.3 | 1501.1±238.5 | 0.08 |
| Peak glucose (mmol/L) | 10.6±2.3 | 9.6±1.6 | 0.007 |
| Time to peak (min) | 92.9±50.5 | 91.4±55.2 | 0.90 |
| Night time (0000 h–0600 h) | |||
| MBG (mmol/L) | 7.2±1.3 | 7.1±1.6 | 0.78 |
| tAUC (mmol/L×360 min) | 2575.1±479.2 | 2549.5±564.5 | 0.85 |
| Peak glucose (mmol/L) | 8.1±1.6 | 7.9±1.8 | 0.58 |
| Time to peak (min) | 159.7±128.0 | 177.1±146.5 | 0.55 |
| 12h after dinner (1700 h-0500 h) | |||
| MBG (mmol/L) | 7.9±1.5 | 7.7±1.5 | 0.55 |
| SDBG (mmol/L) | 1.2±0.5 | 1.0±0.4 | 0.009 |
| CV (%) | 15.6±7.1 | 12.8±6.4 | 0.009 |
| MAGE (mmol/L) | 3.1±1.6 | 2.6±1.9 | 0.14 |
Data are presented as means ±SD. CON – control; MIE – moderate-intensity exercise; MBG – mean blood glucose; tAUC – total area under the curve; SDBG – standard deviation of blood glucose; CV – coefficient of variation; MAGE – mean amplitude of glucose excursions.
Sustained glucose-lowering effects or hypoglycemia (<3.9 mmol/L) on subsequent nocturnal periods were not observed (Table 3). The 12-h SDBG (P=0.009) and the CV of glucose (P=0.009) were significantly lower in the exercise condition compared to the control day, although the 12-h MAGE (P=0.14) did not reach statistical significance (Table 3).
Discussion
The primary aim of the current study was to investigate a consistent glucose-lowering effect of post-dinner moderate-intensity exercise in inactive people with type 2 diabetes. The use of CGMS facilitated the tracking of glucose homeostasis over the course of the postprandial phase. Furthermore, as medication was kept stable, diets were standardized, and normal daily activities were monitored, the effects of exercise occurred independent of changes in medication, diet, and normal daily activities. We found that the 2-h postprandial glucose spike, peak glucose, and mean glucose were reduced with moderate-intensity post-dinner exercise. In addition, the glucose tAUC 1-h after exercise was reduced with exercise. In particular, post-dinner MIE resulted in lower glucose levels at the end of exercise than those at the same time point in the control condition, but the effect was limited to the 2-h after dinner.
Although the 12-h MAGE of CGM did not differ between experimental days, the 12-h SDBG and the CV of glucose did change with exercise. It appears that a short session of exercise after dinner can provide further improvement of glycemic fluctuations in the short term. Furthermore, the moderate-intensity exercise did not increase the hypoglycemic episodes as compared with the control condition in subsequent nocturnal periods.
As described, the blunting effect of postprandial exercise on blood glucose elevations has been well established [10–16]. A short session of moderate intensity exercise after breakfast decreases glucose levels in participants with type 2 diabetes, but the effect does not persist after lunch without additional exercise [10,11,16]. In the present study, the sustained glucose-lowering effects of 20 min MIE after dinner also did not extend to subsequent periods. Post-breakfast exercise significantly blunted the 2-h peak glucose and the 2-h glucose AUC, with the lowest peak postprandial glucose excursion observed with post-meal exercise and metformin combined [26]. However, according to Myette [27], adding an aerobic exercise session to regular daily metformin did not improve the average daily glucose concentrations and may increase the postprandial glucose levels over several meals. The findings of these studies appear contradictory. In our study, approximately half the patients took oral hypoglycemic agents and two-thirds of them took metformin, but the results showed that exercise could significantly improve postprandial blood glucose. This effect is also supported by Erickson [16], in a study where all the participants were treated with add-on glucose-lowering medication. Therefore, further research is required to confirm the effects.
The findings in our study also concur with those of Colberg et al. [14], who found that 20 minutes of self-paced mild-to-moderate-intensity walking may be more effective at lowering the glycemic impact of the evening meal in type 2 diabetes compared with pre-meal exercise or no exercise, but they did not use CGMS to assess the blood glucose fluctuations. However, in the Hatamoto et al. study [28], the post-dinner peak glucose for 120 min was not significantly different between the postprandial exercise group and the no exercise group in young active healthy individuals. We speculate the reason for this difference resulted from young active healthy people being selected for the latter group and, possibly, exercise was more effective for postprandial hyperglycemia in patients with diabetes.
Physical activity may be associated with blood glucose fluctuations leading to hypoglycemia. Early hypoglycemia may occur immediately after exercise and delayed or nocturnal hypoglycemia may occur many hours after physical activity, even overnight, following periods of activity in the afternoon and evening [29]. The use of CGMS in our study facilitated the tracking of glucose homeostasis during periods of daily activity, particularly hypoglycemia during nocturnal periods. As stated previously in the Bacchi’s study [19], a single 60 min aerobic exercise session before dinner had the potential to increase the late-onset hypoglycemia risk in patients with type 2 diabetes. However, in the present study there were no sustained glucose-lowering effects or subsequent nocturnal hypoglycemia following the exercise day. This may be attributable to the short timeframe and the moderate-intensity of exercise in our study. So, we support short-term MIE after dinner in patients with type 2 diabetes.
Previous study findings have demonstrated that blunting of postprandial spikes in glucose improves inflammation, endothelial function and reduces carotid intima-media thickness [30,31]. The current study data indicated that short MIE after dinner improved postprandial hyperglycemia and may improve cardiovascular risk profiles in type 2 diabetes. Most previous studies had been performed in a sedentary environment, in strict laboratory conditions, as the control rather than involving daily activities. Although the interference from possible confounding events would be eliminated under strict laboratory conditions, the ecological effectiveness may have compromised [23]. It would be more easily evidenced that exercise can blunt postprandial hyperglycemia in normal daily activity condition than in a strict laboratory setting. The present study took a 20-min MIE, which was more feasible for sedentary individuals. Greater benefit can be achieved from simple exercise for most inactive patients with diabetes.
Our study does have some potential limitations. First, we estimated the exercise intensity based on the HRR and not on maximal oxygen uptake. However, assessment of exercise intensity using the heart rate is easier to implement in China. Second, the sample size was somewhat small, but it did not prevent us from investigating on the effects of different times, intensity, and duration of exercise on glycemia in the future. Third, we did not evaluate total daily activity, although participants were to refrain from unusual strenuous physical activity. Finally, measurements of insulin and additional hormonal responses would have helped interpret the findings more precisely.
Conclusions
We have demonstrated that a short session of moderate-intensity post-dinner exercise improves postprandial hyperglycemia and glycemic fluctuations in Chinese patients with type 2 diabetes, with no potential subsequent hypoglycemia risk. The data presented here could be promising for diabetes self-management education. In the future, it may be helpful to modulate exercise to improve cardiovascular complications in Chinese patients with type 2 diabetes.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the grants from Jiangsu Provincial Department of Science, Technology Project (BL2014010)
References
- 1.de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: The Hoorn Study. Diabetologia. 1999;42:926–31. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 2.Meigs JB, Nathan DM, D’Agostino RB, Sr, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: The Framingham Offspring Study. Diabetes Care. 2002;25:1845–50. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Hanefeld M, Leiter L, et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090–95. doi: 10.1001/archinte.164.19.2090. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–19. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 6.Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34:2237–43. doi: 10.2337/dc10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Praet SF, Manders RJ, Meex RC, et al. Glycaemic instability is an underestimated problem in Type II diabetes. Clin Sci (Lond) 2006;111:119–26. doi: 10.1042/CS20060041. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk JW, Manders RJ, Hartgens F, et al. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93:31–37. doi: 10.1016/j.diabres.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Colberg SR, Albright AL, Blissmer BJ, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42:2282–303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 10.Larsen JJ, Dela F, Kjaer M, Galbo H. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia. 1997;40:447–53. doi: 10.1007/s001250050699. [DOI] [PubMed] [Google Scholar]
- 11.Larsen JJ, Dela F, Madsbad S, Galbo H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetic patients. Diabetologia. 1999;42:1282–92. doi: 10.1007/s001250051440. [DOI] [PubMed] [Google Scholar]
- 12.Hostmark AT, Ekeland GS, Beckstrom AC, Meen HD. Postprandial light physical activity blunts the blood glucose increase. Prev Med. 2006;42:369–71. doi: 10.1016/j.ypmed.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Derave W, Mertens A, Muls E, et al. Effects of post-absorptive and postprandial exercise on glucoregulation in metabolic syndrome. Obesity (Silver Spring) 2007;15:704–11. doi: 10.1038/oby.2007.548. [DOI] [PubMed] [Google Scholar]
- 14.Colberg SR, Zarrabi L, Bennington L, et al. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc. 2009;10:394–97. doi: 10.1016/j.jamda.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 15.DiPietro L, Gribok A, Stevens MS, et al. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care. 2013;36:3262–68. doi: 10.2337/dc13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson ML, Little JP, Gay JL, et al. Effects of postmeal exercise on postprandial glucose excursions in people with type 2 diabetes treated with add-on hypoglycemic agents. Diabetes Res Clin Pract. 2017;126:240–47. doi: 10.1016/j.diabres.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DM, Calhoun PM, Maahs DM, et al. Factors associated with nocturnal hypoglycemia in at-risk adolescents and young adults with type 1 diabetes. Diabetes, Technol Ther. 2015;17:385–91. doi: 10.1089/dia.2014.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsalikian E, Mauras N, Beck RW, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147:528–34. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacchi E, Negri C, Trombetta M, et al. Differences in the acute effects of aerobic and resistance exercise in subjects with type 2 diabetes: Results from the RAED2 Randomized Trial. PLoS One. 2012;7:e49937. doi: 10.1371/journal.pone.0049937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy GN, Dunstan DW, Salmon J, et al. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30:1384–89. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- 21.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–66. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 22.van Dijk JW, Venema M, van Mechelen W, et al. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care. 2013;36:3448–53. doi: 10.2337/dc12-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haxhi J, Leto G, di Palumbo AS, et al. Exercise at lunchtime: Effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur J Appl Physiol. 2016;116:573–82. doi: 10.1007/s00421-015-3317-3. [DOI] [PubMed] [Google Scholar]
- 24.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. 1988;5:303–11. doi: 10.2165/00007256-198805050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Mo Y, Zhou J, Li M, et al. Glycemic variability is associated with subclinical atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc Diabetol. 2013;12:15. doi: 10.1186/1475-2840-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson ML, Little JP, Gay JL, et al. Postmeal exercise blunts postprandial glucose excursions in people on metformin monotherapy. J Appl Physiol (1985) 2017;123:444–50. doi: 10.1152/japplphysiol.00213.2017. [DOI] [PubMed] [Google Scholar]
- 27.Myette-Cote E, Terada T, Boule NG. The effect of exercise with or without metformin on glucose profiles in type 2 diabetes: A pilot study. Can J Diabetes. 2016;40:173–77. doi: 10.1016/j.jcjd.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Hatamoto Y, Goya R, Yamada Y, et al. Effect of exercise timing on elevated postprandial glucose levels. J Appl Physiol (1985) 2017;123:278–84. doi: 10.1152/japplphysiol.00608.2016. [DOI] [PubMed] [Google Scholar]
- 29.Nadella S, Indyk JA, Kamboj MK. Management of diabetes mellitus in children and adolescents: engaging in physical activity. Transl Pediatr. 2017;6:215–24. doi: 10.21037/tp.2017.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho SS, Dhaliwal SS, Hills A, Pal S. Acute exercise improves postprandial cardiovascular risk factors in overweight and obese individuals. Atherosclerosis. 2011;214:178–84. doi: 10.1016/j.atherosclerosis.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Esposito K, Giugliano D, Nappo F, Marfella R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–19. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]