Abstract
We compared the effects of 8 weeks of high intensity, aerobic interval training (HIT) and isoinertial resistance training (IRT) on: (i)  O2 kinetics during heavy (HiEx) intensity exercise and; (ii) work economy during moderate (ModEx) intensity exercise in 12 healthy elderly men (69.3 ± 4.2 years). Breath-by-breath
O2 kinetics during heavy (HiEx) intensity exercise and; (ii) work economy during moderate (ModEx) intensity exercise in 12 healthy elderly men (69.3 ± 4.2 years). Breath-by-breath  O2 and muscle deoxygenation ([HHb] by means of NIRS) were measured in HiEx and ModEx at identical workloads before and after trainings. In HiEx,
O2 and muscle deoxygenation ([HHb] by means of NIRS) were measured in HiEx and ModEx at identical workloads before and after trainings. In HiEx,  O2 and HHb responses were modeled as tri-exponential and mono-exponential increasing functions, respectively. A two-way ANOVA for repeated measures analysis was made; Effect size (η2) was also evaluated. After HIT the amplitude and the time delay of the slow component of O2 uptake (
O2 and HHb responses were modeled as tri-exponential and mono-exponential increasing functions, respectively. A two-way ANOVA for repeated measures analysis was made; Effect size (η2) was also evaluated. After HIT the amplitude and the time delay of the slow component of O2 uptake ( O2sc) during HiEx were smaller (−32%; P = 0.045) and longer (+19.5%; P = 0.001), respectively. At Post IRT: (i) during ModEx, gain was lower (−5%; P = 0.050); (ii) during HiEx, τ2 (+14.4%; P = 0.050), d3 (+8.6%; P = 0.050), and τ3 (+17.2%; P = 0.050) were longer than at Pre IRT. After HIT, the decrease of the
O2sc) during HiEx were smaller (−32%; P = 0.045) and longer (+19.5%; P = 0.001), respectively. At Post IRT: (i) during ModEx, gain was lower (−5%; P = 0.050); (ii) during HiEx, τ2 (+14.4%; P = 0.050), d3 (+8.6%; P = 0.050), and τ3 (+17.2%; P = 0.050) were longer than at Pre IRT. After HIT, the decrease of the  O2sc amplitude was likely induced by the beneficial effects of training on a more responsive O2 delivery and consumption cascade leading to a better muscle metabolic stability. IRT training was able to increase exercise economy during ModEx and to reduce the amplitude and delay the onset of
O2sc amplitude was likely induced by the beneficial effects of training on a more responsive O2 delivery and consumption cascade leading to a better muscle metabolic stability. IRT training was able to increase exercise economy during ModEx and to reduce the amplitude and delay the onset of  O2sc during HiEx. These effects should be due to the reduction and the delayed recruitment of Type II muscle fibers. The better exercise economy and the delayed appearance of
O2sc during HiEx. These effects should be due to the reduction and the delayed recruitment of Type II muscle fibers. The better exercise economy and the delayed appearance of  O2sc induced by IRT suggests that strength training might be included in endurance training programs to improve exercise economy and resistance to fatigue in this population of old subjects.
O2sc induced by IRT suggests that strength training might be included in endurance training programs to improve exercise economy and resistance to fatigue in this population of old subjects.
Keywords: high intensity interval training, isoinertial strength training, heavy intensity exercise, near-infrared spectroscopy, oxygen uptake kinetics, elderly, muscle strength, slow component
Introduction
The kinetics of alveolar O2 uptake ( O2A) upon the onset of constant work rate (CWR) exercise of moderate intensity (ModEx) is usually described by a double exponential model (Poole and Jones, 2012). The first, rapid component – phase I – is characterized by a short time constant and it is caused by the prompt increase of cardiac output at the beginning of exercise. The second component – phase II – is considered to be a reliable proxy of muscular O2 uptake and is characterized by a time constant of about 20 s in young, healthy, and trained subjects (Poole and Jones, 2012).
O2A) upon the onset of constant work rate (CWR) exercise of moderate intensity (ModEx) is usually described by a double exponential model (Poole and Jones, 2012). The first, rapid component – phase I – is characterized by a short time constant and it is caused by the prompt increase of cardiac output at the beginning of exercise. The second component – phase II – is considered to be a reliable proxy of muscular O2 uptake and is characterized by a time constant of about 20 s in young, healthy, and trained subjects (Poole and Jones, 2012).
During heavy intensity exercise (HiEx), i.e., above the lactic threshold (LT), the attainment of the steady state oxygen consumption ( O2ss) is delayed due to the presence of a slow increase of
O2ss) is delayed due to the presence of a slow increase of  O2(
O2( O2sc) that starts about 150–200 s after the onset of exercise (Jones et al., 2011). Furthermore, if the exercise is performed in the very heavy domain (VHiEx), e.g., above the so called critical power,
O2sc) that starts about 150–200 s after the onset of exercise (Jones et al., 2011). Furthermore, if the exercise is performed in the very heavy domain (VHiEx), e.g., above the so called critical power,  O2ss cannot even be attained, since
O2ss cannot even be attained, since  O2 keeps increasing up to
O2 keeps increasing up to  O2max, a condition that heralds the interruption of exercise (Poole and Jones, 2012).
O2max, a condition that heralds the interruption of exercise (Poole and Jones, 2012).
From the performance standpoint,  O2sc is important, as it is related to increased susceptibility to fatigue:
O2sc is important, as it is related to increased susceptibility to fatigue:  O2sc amplitude, e.g., is linearly related to the time to fatigue in obese adolescents (Salvadego et al., 2010).
O2sc amplitude, e.g., is linearly related to the time to fatigue in obese adolescents (Salvadego et al., 2010).
There is compelling evidence that muscular mechanisms are largely responsible for  O2sc (Poole et al., 1991) and several data support the notion that the progressive recruitment of Type II muscle fibers during HiEx/VHiEx exercise is the main determinant of
O2sc (Poole et al., 1991) and several data support the notion that the progressive recruitment of Type II muscle fibers during HiEx/VHiEx exercise is the main determinant of  O2sc (Poole and Jones, 2012). Type II fibers are characterized by a higher ATP cost of force production (Stienen et al., 1996) and by higher O2 consumption for ATP synthesis (Willis and Jackman, 1994) than Type I fibers and it has been also demonstrated that
O2sc (Poole and Jones, 2012). Type II fibers are characterized by a higher ATP cost of force production (Stienen et al., 1996) and by higher O2 consumption for ATP synthesis (Willis and Jackman, 1994) than Type I fibers and it has been also demonstrated that  O2sc is more evident in humans with a higher percentage of Type II fibers (Barstow et al., 1996). Recent findings, however, have somehow challenged this view suggesting that the progressive recruitment of the less economic Type II fibers is not strictly necessary to induce
O2sc is more evident in humans with a higher percentage of Type II fibers (Barstow et al., 1996). Recent findings, however, have somehow challenged this view suggesting that the progressive recruitment of the less economic Type II fibers is not strictly necessary to induce  O2sc. Conversely,
O2sc. Conversely,  O2sc may be caused by events occurring inside the recruited fibers (Zoladz et al., 2008).
O2sc may be caused by events occurring inside the recruited fibers (Zoladz et al., 2008).
In addition, it has been shown that  O2sc can be modulated by manipulations of O2 delivery (Poole and Jones, 2012). Therefore, decreased O2 availability may affect the
O2sc can be modulated by manipulations of O2 delivery (Poole and Jones, 2012). Therefore, decreased O2 availability may affect the  O2sc of individuals in whom local O2 delivery during exercise is impaired (e.g., healthy aging) and a clear mismatch between O2 delivery and consumption is present (Murias et al., 2010a,b).
O2sc of individuals in whom local O2 delivery during exercise is impaired (e.g., healthy aging) and a clear mismatch between O2 delivery and consumption is present (Murias et al., 2010a,b).
The effects of physical training have been explored to disclose the mechanisms underpinning  O2sc (Jones et al., 2007). Endurance training improves the so-called metabolic stability, leading to a lower decrease in phosphocreatine concentration [PCr] and a diminished intramuscular acidosis during HiEx in connection with a less evident
O2sc (Jones et al., 2007). Endurance training improves the so-called metabolic stability, leading to a lower decrease in phosphocreatine concentration [PCr] and a diminished intramuscular acidosis during HiEx in connection with a less evident  O2sc (Poole and Jones, 2012). Since low levels of intramuscular [PCr] and of pH characterize HiEx/VHEx exercise (Jones et al., 2008, 2011), these results seem to suggest that the slow decrease in [PCr] and increase of [H+] occurring at these exercise intensities (Jones et al., 2008) are the main mechanistic determinants of
O2sc (Poole and Jones, 2012). Since low levels of intramuscular [PCr] and of pH characterize HiEx/VHEx exercise (Jones et al., 2008, 2011), these results seem to suggest that the slow decrease in [PCr] and increase of [H+] occurring at these exercise intensities (Jones et al., 2008) are the main mechanistic determinants of  O2sc. In addition, endurance training improves metabolic hyperemic response and optimizes the matching between local O2 delivery and utilization, especially in individuals with suboptimal vascular response, such as elderly subjects (Murias et al., 2010a,b). Therefore, the correlation between the indexes that describe amelioration of local peripheral perfusion and the attenuation of the amplitude of
O2sc. In addition, endurance training improves metabolic hyperemic response and optimizes the matching between local O2 delivery and utilization, especially in individuals with suboptimal vascular response, such as elderly subjects (Murias et al., 2010a,b). Therefore, the correlation between the indexes that describe amelioration of local peripheral perfusion and the attenuation of the amplitude of  O2sc might suggest a potential mechanistic link between O2 delivery and
O2sc might suggest a potential mechanistic link between O2 delivery and  O2sc.
O2sc.
Also, strength training, by decreasing the number of motor units (MUs) recruited at the same work rate (WR), may theoretically attenuate  O2sc, as a smaller number of less economic Type II fibers would be recruited at the same WR. However, this hypothesis has been somehow disproved in young adults in whom isometric strength training failed to abate the amplitude of
O2sc, as a smaller number of less economic Type II fibers would be recruited at the same WR. However, this hypothesis has been somehow disproved in young adults in whom isometric strength training failed to abate the amplitude of  O2sc (Zoladz et al., 2012). Yet, more effective strength training modalities applied to subjects with large muscular strength deficits may potentially elicit more evident and beneficial effects on
O2sc (Zoladz et al., 2012). Yet, more effective strength training modalities applied to subjects with large muscular strength deficits may potentially elicit more evident and beneficial effects on  O2sc via this mechanism.
O2sc via this mechanism.
Finally, it has also been suggested that strength training may improve mechanical efficiency during ModEx (Beattie et al., 2014). From the practical standpoint, a greater exercise economy associated with the attenuation of  O2sc induced by strength training may ameliorate exercise capability in subjects characterized by a low exercise capacity.
O2sc induced by strength training may ameliorate exercise capability in subjects characterized by a low exercise capacity.
Therefore, we studied in a group of healthy, moderately active elderly men the effect of high intensity interval training (HIT) and isoinertial strength training (IRT) on: (1)  O2 kinetics and muscular oxygenation of the exercising muscle by near-infrared spectroscopy (NIRS) during cycling HiEx performed at the same absolute WR before and after training; (2) work economy during ModEx at the same absolute WR. In addition, (3) Muscle cross sectional area (CSA) and muscle volume (Vol) of the quadriceps; and (4) muscular strength were assessed. We analyzed these data to determine the effects and relative mechanisms induced by HIT and IRT on the entity of
O2 kinetics and muscular oxygenation of the exercising muscle by near-infrared spectroscopy (NIRS) during cycling HiEx performed at the same absolute WR before and after training; (2) work economy during ModEx at the same absolute WR. In addition, (3) Muscle cross sectional area (CSA) and muscle volume (Vol) of the quadriceps; and (4) muscular strength were assessed. We analyzed these data to determine the effects and relative mechanisms induced by HIT and IRT on the entity of  O2sc.
O2sc.
Materials and Methods
Subjects
Twelve moderately active Caucasian men (mean ± SD; 69.3 ± 4.2 years, range, 65–75; 77.8 ± 10.4 kg; height 1.72 ± 0.05 m) volunteered to participate in the study. A medical examination, to determine exclusion criteria, and a cycle-ergometer stress test, to exclude abnormal responses to intense exercise, were preliminarily performed. The study protocol was approved by the institutional review board (approval on June 18th, 2013) and designed in accordance with ethical standards, the provisions of the Declaration of Helsinki and national and international guidelines. Written informed consent was obtained from each subject before the study.
Experimental Design
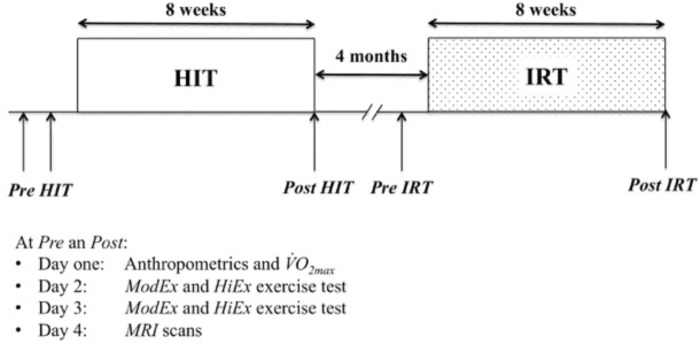
A two-factor within-subject design (A × B × S) (Keppel and Wickens, 2004) was used in which each subject (factor, S) received all the combinations that originated by crossing the two factors A and B. One fixed factor (A) was training modality (levels: HIT and IRT); the second fixed factor (B) was time (levels: Pre and Post training). The subjects were evaluated immediately before (Pre HIT) and immediately after 8 weeks of HIT (Post HIT). Then, after 4 months of recovery during which the subjects were asked to keep the same habitual lifestyle (Figure 1), the subjects were evaluated again before (Pre IRT) and immediately after 8 weeks of IRT (Post IRT). Before the first data collections, a familiarization session was conducted.
FIGURE 1.
Schematic representation of the experimental design.
MRI scans for measuring muscle CSA and Vol were obtained before and after HIT and IRT.
Tests were performed in the morning on four consecutive days: the main anthropometrical data and  O2 max were measured on day 1; CWR ModEx and HiEx exercise tests were performed on days 2 and 3; MRI scans were obtained on day 4.
O2 max were measured on day 1; CWR ModEx and HiEx exercise tests were performed on days 2 and 3; MRI scans were obtained on day 4.
Training Protocols
-
simple •
High intensity interval training (HIT). The subjects trained three times a week for 8 weeks. Training consisted of seven 2-min bouts of cycling (915 E, Monark, Varberg, Sweden) at about 85–95% of individual
 O2 max interspersed by 2 min of recovery at about 40% of
O2 max interspersed by 2 min of recovery at about 40% of  O2 max. Each series was preceded by 10 min of active warm-up.
O2 max. Each series was preceded by 10 min of active warm-up. -
simple •
Isoinertial resistance training (IRT). Resistance exercise was performed on a seated knee extension flywheel (4.2 kg) ergometer (YoYo Technology AB, Stockholm, Sweden) three times a week for 8 weeks. Each session consisted of four sets of seven maximal, coupled concentric extensions and eccentric flexions of the knee. The sets were interspersed by 3-min of rest and initiated immediately after performing two submaximal actions. Each exercise session was preceded by 10 min of active warm-up.
Anthropometry
Body weight (BW) and stature were measured with a Tanita electronic scale BWB-800 MA (Tanita, Arlington Heights, IL, United States) and a stadiometer (Holtain Ltd., Crymych, Pembs. United Kingdom).
Maximal Oxygen Uptake, Ventilatory Thresholds
All cycling tests were performed on an electromechanically braked cycle ergometer (Excalibur Sport, Lode, Netherlands) operated by a personal computer connected to a metabolic cart. Breath-by-breath gas exchanges were measured continuously at the mouth with a metabolic cart (Quark b2, Cosmed, Rome, Italy) that was calibrated following the manufacturer’s instructions before each experiment.
 O2 max and ventilatory thresholds were measured during a ramp test (Poole et al., 2008) and a supra maximal CWR test following the procedure illustrated by Bruseghini et al. (2015).
O2 max and ventilatory thresholds were measured during a ramp test (Poole et al., 2008) and a supra maximal CWR test following the procedure illustrated by Bruseghini et al. (2015).
Responses to Moderate Intensity and Heavy-Intensity Exercise
Responses to ModEx and HiEx exercise were evaluated at a WR corresponding to 90% of individual gas exchange threshold (GET) and to about 50% of the difference between GET and respiratory compensation point (RCP) determined at Pre HIT. The WR was calculated using the linear regression of  O2 vs. WR considering the lag of the
O2 vs. WR considering the lag of the  O2 increase with respect to that of the workload determined at the ramp test and it was maintained constant in all the sessions. After instrumentation and preparation, the subjects rested on the cycle ergometer for 3 min before starting to pedal for 3 min at 30 W; then, WR was increased to the preselected WR and maintained for 6 min. The procedure was repeated three times (twice for ModEx and once for HiEx) with 10 min of recovery between each test. The entire procedure was repeated the following day. Pedaling frequency was strictly maintained between 70 and 80 revolutions per minute by the aid of a visual pacemaker.
O2 increase with respect to that of the workload determined at the ramp test and it was maintained constant in all the sessions. After instrumentation and preparation, the subjects rested on the cycle ergometer for 3 min before starting to pedal for 3 min at 30 W; then, WR was increased to the preselected WR and maintained for 6 min. The procedure was repeated three times (twice for ModEx and once for HiEx) with 10 min of recovery between each test. The entire procedure was repeated the following day. Pedaling frequency was strictly maintained between 70 and 80 revolutions per minute by the aid of a visual pacemaker.
Muscle Oxygenation
Vastus lateralis muscle oxygenation during HiEx was evaluated by means of a frequency-domain-multidistance NIRS system (OxiplexTS, ISS, Champaign, IL, United States) that provided continuous measurement of absolute concentrations (μM) of oxyhemoglobin ([O2Hb]) and deoxyhemoglobin ([HHb]) (De Roia et al., 2012). The thickness of the skin and of the subcutaneous fat layer of the explored area was assessed by ultrasound (ACUSON P50 ultrasound system, Siemens, Erlangen, Germany) and it ranged from 6.4 to 11.8 mm, 8.1 mm ± 1.5. In addition, cutaneous landmarks were pen-marked on a transparent acetate sheet placed on the area of the probe so that it could be applied on the same site in the subsequent experimental sessions.
Muscular Strength and Morphology
Knee extension torque (Tk) of the dominant limb was evaluated with an isokinetic dynamometer (CMSi Cybex Humac Norm Dynamometer, Stoughton, MA, United States) during concentric contractions at 60° s−1 and 120° s−1 angular speeds. The subjects went through several practice trials and performed contractions while seated on the reclining chair of the dynamometer. The lower part of the leg was strapped to the end of the lever arm and the center of rotation of the knee was aligned with the axis of the dynamometer. Before the test, the subjects completed 10-min of warm-up exercise on a stationary bike. Three maximal trials were performed for each condition with 3 min of recovery between each trial. The highest Tk values (as peak values) were recorded for further analysis.
MRI scans were obtained after 1 h of supine rest to avoid the influence of posture-related fluid shifts on muscle size following the procedure illustrated by Bruseghini et al. (2015).
Data Analysis
Breath-by-breath
 O2 values were interpolated to 1 s intervals, time aligned with the onset of exercise transition, and treated by subtracting the
O2 values were interpolated to 1 s intervals, time aligned with the onset of exercise transition, and treated by subtracting the  O2 steady state value (average values of the last 30 s of trial) at 30 W. The data from the trials were then combined to obtain a single data file for each subject and condition.
O2 steady state value (average values of the last 30 s of trial) at 30 W. The data from the trials were then combined to obtain a single data file for each subject and condition.
 O2 kinetics during HiEx exercise was modeled as a sum of three exponential increasing functions:
O2 kinetics during HiEx exercise was modeled as a sum of three exponential increasing functions:
| (1) |
where τ1, τ2, and τ3 are the time constants of the exponential increases during phase I, phase II, and phase III (the slow component), d1, d2, and d3 are the time delays and A1, A2, and A3 are the asymptotic amplitudes of the corresponding phases. U (t − d) is the unit step function defined as:
| (2) |
The value of the amplitude at the end of phase I, (A′1), which terminated at the start of phase II, was calculated as (2):
| (3) |
Note that the first addend of Eq. (1) is truncated as it reaches A′1 at t = d2, and doesn’t continue to rise toward its asymptotic value A1. The physiologically significant amplitude of the primary exponential (A′2) was defined as the sum of A′1 + A2 (Barstow et al., 1996). Because of the uncertain validity of the asymptotic value of A3, we used the value of the amplitude of the slow component at the end of the exercise (A′3) (Barstow et al., 1996). The change of O2 uptake from the  O2 steady state value at 30 W and the values of
O2 steady state value at 30 W and the values of  O2 at A′3 (Δ
O2 at A′3 (Δ O2EE) was given by A′2 + A′3. To compare the subjects working at different absolute workloads, the gain in the primary response (GPrim = A′2/ΔWR) and the gain in the total response at the end of HiEx exercise [GTot = (A′2 + A′3)/ΔWR] were calculated. The relative contribution of the slow component to the overall
O2EE) was given by A′2 + A′3. To compare the subjects working at different absolute workloads, the gain in the primary response (GPrim = A′2/ΔWR) and the gain in the total response at the end of HiEx exercise [GTot = (A′2 + A′3)/ΔWR] were calculated. The relative contribution of the slow component to the overall  O2 response was calculated as A′3/(A′2 + A′3).
O2 response was calculated as A′3/(A′2 + A′3).
The gain (G) during ModEx exercise was calculated as the ratio between net steady state  O2 and the corresponding net increase of WR (G = A′2/ΔWR).
O2 and the corresponding net increase of WR (G = A′2/ΔWR).
NIRS derived [HHb] response during HiEx was first interpolated to 1-s intervals, then time aligned with the onset of exercise transition and finally treated by subtracting the steady state value at 30 W. Then, the fitting window was constrained from the start of exercise to the onset of the slow component of [HHb] (Breese et al., 2013). Mean response time (MRT) was calculated as the sum of τ1 and d1. The primary [HHb] amplitude was divided by the phase II asymptotic amplitude A2 to yield the Δ[HHb]/Δ O2: it was considered as an index of the increase in fractional muscle O2 extraction required to sustain a given net increment in
O2: it was considered as an index of the increase in fractional muscle O2 extraction required to sustain a given net increment in  O2 during the primary phase (Murias et al., 2014). The net increase from the baseline of the values of [HHb] and of [O2Hb] after 120 s of exercise and at the end of the exercise were calculated over 30 s time windows, the first interval of time being centered on the 120th-second and second interval including the last 30 s of exercise.
O2 during the primary phase (Murias et al., 2014). The net increase from the baseline of the values of [HHb] and of [O2Hb] after 120 s of exercise and at the end of the exercise were calculated over 30 s time windows, the first interval of time being centered on the 120th-second and second interval including the last 30 s of exercise.
The net increases in [HHb] and in [O2Hb] were then added to obtain the net increase in total hemoglobin concentration ([Hbtot]) in the volume of tissue explored by the probe. [Hbtot] was only calculated at 120 s of exercise and at the end of exercise.
The parameters of the  O2 models were estimated by means of an iterative, weighted non-linear least-squares procedure (Marquardt, 1963) that was developed in G-Language (Lab-VIEW 7.0, National Instruments, Austin, TX, United States). Initial guesses of the parameters of the model were entered after visual inspection of the data. The 95% confidence intervals of the τ2 and τ3 of
O2 models were estimated by means of an iterative, weighted non-linear least-squares procedure (Marquardt, 1963) that was developed in G-Language (Lab-VIEW 7.0, National Instruments, Austin, TX, United States). Initial guesses of the parameters of the model were entered after visual inspection of the data. The 95% confidence intervals of the τ2 and τ3 of  O2 kinetics and of τ1 of HHb kinetics were generated by means of Monte Carlo simulation (Motulsky and Christopoulos, 2004) using commercial software for data analysis (GraphPad Prism version 6.00 for Macintosh, GraphPad Software, La Jolla, CA, United States). Amplitudes and time delays were constrained to the best-fit values and the time constants were allowed to vary.
O2 kinetics and of τ1 of HHb kinetics were generated by means of Monte Carlo simulation (Motulsky and Christopoulos, 2004) using commercial software for data analysis (GraphPad Prism version 6.00 for Macintosh, GraphPad Software, La Jolla, CA, United States). Amplitudes and time delays were constrained to the best-fit values and the time constants were allowed to vary.
MRI scans were transferred electronically from the scanner to a personal computer (Macintosh mac Book Pro, Apple, Cupertino, CA, United States) and analyzed with OsiriX (version 3.7.1 32 bit) by using manual planimetry to calculate CSA and Vol of the quadriceps of the dominant leg (Bruseghini et al., 2015). The same investigator carried out all measurements. The reliability of this measurement was assessed over five separate measurements of the CSA of three heads of the quadriceps muscle taken distally at 50% of the femur bone length; the average coefficient of variation of measuring the same image was 0.92% for total quadriceps femoris.
Statistical Analysis
All values in the text and the tables are presented as mean ± SD. Two-factor within-subject ANOVA analysis for repeated measures was carried out according to Keppel and Wickens (2004): (i) F values were calculated taking into account the possible violation of sphericity as suggested by Geisser and Greenhouse; (ii) single contrasts within subjects (time, Pre vs. Post) and between subjects (Training, HIT vs. IRT and interactions were computed; (iii) effect size was evaluated with partial squared correlation factor or η2, (, , , suffix are related to within, between, and interactions analysis) which expresses the ratio between explained variability and total variability in the population, but compensates for the size of the other treatment effect (either time or training); (iv) effect size (d) of the differences between the contrasted values was calculated. Calculations were carried out using an Excel spreadsheet (MO 2010, Microsoft Corp., Seattle, WA, United States) prepared for this purpose. Model 2 linear regressions between bivariate data were calculated according to the method of Deming (Motulsky and Christopoulos, 2004). Correlation between variables was computed using Spearman’s correlation coefficient.
Statistical analysis was made by a two-way ANOVA for repeated measures; Effect size was evaluated with partial squared correlation factor or η2. P was always set <0.05.
Results
The data concerning  O2 max, ventilatory threshold and muscular strength and mass have been already published in a paper that described the effects of HIT and IRT on several risk factors of cardiometabolic diseases and on the exercise capability in healthy elderly subjects (Bruseghini et al., 2015). The readers are kindly asked to refer to the indicated paper for further details. Here, only the essential results useful for supporting and discussing the hypothesis related to the present investigation will be summarized.
O2 max, ventilatory threshold and muscular strength and mass have been already published in a paper that described the effects of HIT and IRT on several risk factors of cardiometabolic diseases and on the exercise capability in healthy elderly subjects (Bruseghini et al., 2015). The readers are kindly asked to refer to the indicated paper for further details. Here, only the essential results useful for supporting and discussing the hypothesis related to the present investigation will be summarized.
Briefly, absolute  O2 max increased only after HIT (Pre HIT 2.34 ± 0.35 Post HIT 2.48 ± 0.38 L min−1
P = 0.015; d = 0.83; 95% CIDiff: 0.04 L min−1/0.22 L min−1), with no differences after IRT (Pre IRT 2.43 ± 0.43 Post IRT 2.44 ± 0.42 L min−1).
O2 max increased only after HIT (Pre HIT 2.34 ± 0.35 Post HIT 2.48 ± 0.38 L min−1
P = 0.015; d = 0.83; 95% CIDiff: 0.04 L min−1/0.22 L min−1), with no differences after IRT (Pre IRT 2.43 ± 0.43 Post IRT 2.44 ± 0.42 L min−1).  O2RCP, expressed as percent of
O2RCP, expressed as percent of  O2 max, was greater at Post HIT (P = 0.014; d = 0.85; 95% CIDiff: 2.1%/11.1%) and at Pre IRT (P = 0.007; d = 0.96; 95% CIDiff: 3.9%/16.2%) than at Pre HIT and it was greater at Post IRT than at Post HIT (P = 0.001; d = 1.24; 95% CIDiff: 1.4%/3.8%). Post hoc contrast analysis showed that CSA and Vol were increased after HIT: Vol, Pre HIT: 820 ± 199 cm3, post HIT: 866 ± 199 cm2; P = 0.002; d = 1.17; 95% CIDiff: 22.9 cm3/67.9 cm3) and after IRT Vol, Pre IRT: 813 ± 184 cm3, post IRT: 852 ± 188 cm2; P = 0.01; d = 0.90; 95% CIDiff: 13.9 cm3/64.6 cm3). Finally, maximal isokinetic torque was increased only after IRT: Tk
60° s−1, Pre HIT: 159.8 ± 24.5 N m, post HIT: 163.3 ± 22.2 N m; P = 0.360; d = 0.27; 95% CIDiff: −3.9 N m/10.9 N m; Tk
60° s−1, Pre IRT: 162.4 ± 25.8 N m, post IRT: 179.0 ± 31.1 N m; P = 0.001; d = 1.27; 95% CIDiff: 9.0 N m/24.1 N m.
O2 max, was greater at Post HIT (P = 0.014; d = 0.85; 95% CIDiff: 2.1%/11.1%) and at Pre IRT (P = 0.007; d = 0.96; 95% CIDiff: 3.9%/16.2%) than at Pre HIT and it was greater at Post IRT than at Post HIT (P = 0.001; d = 1.24; 95% CIDiff: 1.4%/3.8%). Post hoc contrast analysis showed that CSA and Vol were increased after HIT: Vol, Pre HIT: 820 ± 199 cm3, post HIT: 866 ± 199 cm2; P = 0.002; d = 1.17; 95% CIDiff: 22.9 cm3/67.9 cm3) and after IRT Vol, Pre IRT: 813 ± 184 cm3, post IRT: 852 ± 188 cm2; P = 0.01; d = 0.90; 95% CIDiff: 13.9 cm3/64.6 cm3). Finally, maximal isokinetic torque was increased only after IRT: Tk
60° s−1, Pre HIT: 159.8 ± 24.5 N m, post HIT: 163.3 ± 22.2 N m; P = 0.360; d = 0.27; 95% CIDiff: −3.9 N m/10.9 N m; Tk
60° s−1, Pre IRT: 162.4 ± 25.8 N m, post IRT: 179.0 ± 31.1 N m; P = 0.001; d = 1.27; 95% CIDiff: 9.0 N m/24.1 N m.
Response to ModEx
The average CWR was 72.5 ± 16.3 W in the ModEx condition, corresponding to 35–40% of  O2 max, i.e., <GET. G at Pre HIT and at Post HIT was not significantly different (12.1 mL min−1 W−1± 1.5 vs. 12.4 mL min−1 W−1± 1.0). Conversely, at Post IRT (12.0 mL min−1 W−1± 1.0) G turned out to be significantly smaller (P = 0.049; d = 0.63; 95% CIDiff: −0.05 mL min−1 W−1/−1.1 mL min−1 W−1) than at Pre IRT (12.6 mL min−1 W−1± 0.9).
O2 max, i.e., <GET. G at Pre HIT and at Post HIT was not significantly different (12.1 mL min−1 W−1± 1.5 vs. 12.4 mL min−1 W−1± 1.0). Conversely, at Post IRT (12.0 mL min−1 W−1± 1.0) G turned out to be significantly smaller (P = 0.049; d = 0.63; 95% CIDiff: −0.05 mL min−1 W−1/−1.1 mL min−1 W−1) than at Pre IRT (12.6 mL min−1 W−1± 0.9).
Response to HiEx
The average CWR was 144.3 ± 26.6 W in the HiEx condition and it corresponded approximately to 67–71% of  O2 max, i.e., >GET, but <RCP. The parameters describing the kinetics of
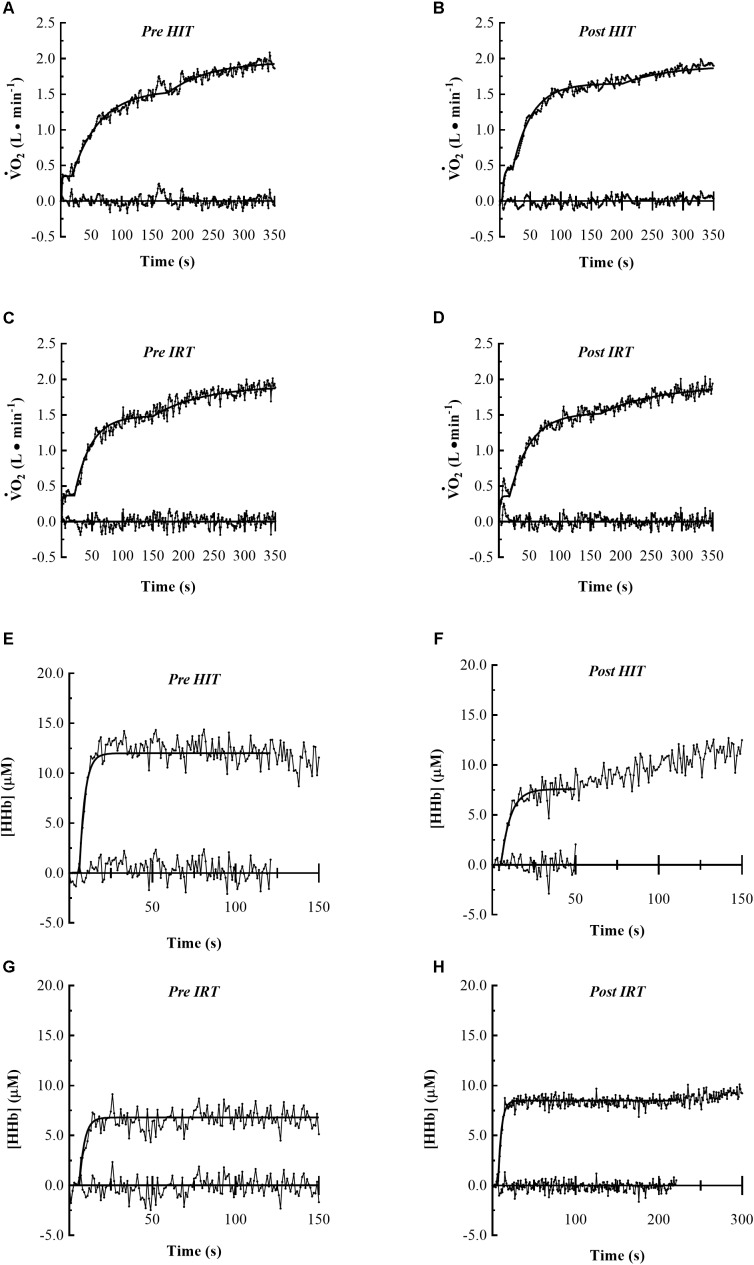
O2 max, i.e., >GET, but <RCP. The parameters describing the kinetics of  O2 and the NIRS signals obtained in the HiEx condition before and after HIT and IRT are presented in Tables 1, 2, respectively; Figures 2A–D demonstrates the kinetics of
O2 and the NIRS signals obtained in the HiEx condition before and after HIT and IRT are presented in Tables 1, 2, respectively; Figures 2A–D demonstrates the kinetics of  O2 at the onset of HiEx after HIT and IRT in a typical subject, respectively. Figures 2E–H shows the kinetics of [HHb] after HIT and IRT, respectively.
O2 at the onset of HiEx after HIT and IRT in a typical subject, respectively. Figures 2E–H shows the kinetics of [HHb] after HIT and IRT, respectively.
Table 1.
Mean values (SD) of the parameters describing  O2 kinetics at the onset of CWR exercise of heavy (HiEx) intensity.
O2 kinetics at the onset of CWR exercise of heavy (HiEx) intensity.
| Parameter | Training |
||||||
|---|---|---|---|---|---|---|---|
| HIT |
IRT |
||||||
| Pb; | Pw; | Pint; | Pre | Post | Pre | Post | |
| A’1 (L min−1) | 0.061; 0.271 | 0.098; 0.233 | 0.248; 0.123 | 0.37 (0.15) | 0.37 (0.20) | 0.23∗ (0.18) | 0.35‡ (0.10) |
| τ1 (s) | 0.006; 0.508 | 0.051; 0.305 | 0.535; 0.036 | 2.1 (1.5) | 3.9 (3.2) | 1.0∗ (0.7) | 2.0 (1.9) |
| d1 (s) | 0.024; 0.341 | 0.213; 0.136 | 0.264; 0.112 | 1.3 (1.5) | 2.3 (2.7) | 0.5 (1.1) | 0.5† (0.5) |
| A’2 (L min−1) | 0.101; 0.227 | 0.025; 0.381 | 0.751; 0.009 | 1.45 (0.27) | 1.53 (0.22) | 1.51 (0.22) | 1.58‡,(0.34) |
| τ2 (s) | 0.227; 0.131 | 0.410; 0.063 | 0.013; 0.444 | 27.7 (7.0) | 24.9 (4.3) | 25.7 (3.7) | 30.0‡,† (5.1) |
| 95% IC τ2 (s) | 26.1–29.4 | 23.9–25.9 | 24.2–27.1 | 27.9–30.9 | |||
| d2 (s) | 0.006; 0.508 | 0.249; 0.119 | 0.687; 0.015 | 17.7 (3.1) | 19.3 (8.7) | 12.1∗ (5.0) | 14.9 (3.8) |
| A’3 (L min−1) | 0.328; 0.088 | 0.019; 0.407 | 0.844; 0.004 | 0.19 (0.10) | 0.13∗ (0.07) | 0.21 (0.12) | 0.16 (0.10) |
| τ3 (s) | 0.003; 0.570 | 0.411; 0.062 | 0.127; 0.199 | 71.0 (8.5) | 69.6 (18.4) | 81.5 (16.7) | 92.0† (15.6) |
| 95% IC τ3 (s) | 70.3–71.8 | 69.4–71.2 | 78.6–81.7 | 90.0–94.7 | |||
| d3 (s) | 0.851; 0.003 | 0.000; 0.742 | 0.047; 0.313 | 165.8 (10.1) | 197.1∗ (13.6) | 172.2 (43.2) | 185.8‡ (42.6) |
Δ O2EE (L min−1) O2EE (L min−1) |
0.015; 0.433 | 0.398; 0.07 | 0.665; 0.018 | 1.62 (0.30) | 1.67 (0.27) | 1.72 (0.27) | 1.74 (0.34) |
| A’3/(A’2 + A’3) (%) | 0.329; 0.067 | 0.010; 0.471 | 0.706; 0.013 | 13.8 (7.5) | 8.5∗ (3.8) | 14.8 (9.5) | 10.8 (6.6) |
| GPrim (mL min−1ΔW) | 0.081; 0.251 | 0.040; 0.317 | 0.626; 0.012 | 10.1 (1.3) | 10.7 (0.5) | 10.5 (0.9) | 10.9 (0.8) |
| GTot (mL min−1/W) | 0.061; 0.292 | 0.637; 0.024 | 0.954; 0.000 | 11.5 (1.5) | 11.6 (0.6) | 12.3 (1.5) | 12.4 (1.5) |
The table reports also the mean of the 2.5% and 97.5% percentiles of the 95% confidence interval for τ2 and the P-values of the ANOVA analysis together with the values of the corresponding partial squared correlation factors. For the meaning of the symbols, please refer to the text. ∗Significantly different from Pre HIT; †significantly different from Post HIT; ‡significantly different from Pre IRT.
Table 2.
Mean values (SD) of the parameters describing [HHb] kinetics at the onset of CWR exercise of heavy intensity (HiEx) together with the values of [HHb] and [Hbtot] after 120 s of exercise and at the end of exercise.
| Parameter | Training |
||||||
|---|---|---|---|---|---|---|---|
| HIT |
IRT |
||||||
| Pb; | Pw; | Pint; | Pre | Post | Pre | Post | |
| [Hbtot]120 (μM) | 0.052; 0.300 | 0.814; 0.005 | 0.772; 0.008 | 4.5 (3.4) | 3.8 (5.6) | 6.8 (6.3) | 7.0 (6.0) |
| [Hbtot]end (μM) | 0.016; 0.425 | 0.888; 0.002 | 0.917; 0.504 | 4.3 (2.3) | 4.3 (6.0) | 9.5∗ (7.5) | 9.1 (6.5) |
| [HHb]A1 (s) | 0.051; 0.307 | 0.246; 0.122 | 0.995; 0.000 | 8.3 (4.9) | 9.8 (6.8) | 12.2 (8.0) | 13.7 (9.9) |
| [HHb]d1 (s) | 0.572; 0.029 | 0.966; 0.000 | 0.199; 0.144 | 5.9 (1.0) | 6.7 (3.2) | 6.4 (1.4) | 5.7 (2.6) |
| [HHb]τ 1 (s) | 0.132; 0.188 | 0.731; 0.011 | 0.324; 0.091 | 4.5 (1.1) | 4.4 (0.9) | 3.9 (1.2) | 4.3 (1.0) |
| 95% IC [HHb]τ 1 (s) | 3.1–5.6 | 3.2–5.8 | 2.2–6.0 | 3.6–5.1 | |||
| [HHb]MRT (s) | 0.135; 0.191 | 0.821; 0.005 | 0.439; 0.055 | 10.4 (1.3) | 11.1 (3.2) | 10.3 (1.9) | 10.0 (3.3) |
| [HHb]120 (μM) | 0.091; 0.237 | 0.208; 0.140 | 0.627; 0.22 | 5.4 (3.2) | 7.3 (6.3) | 7.5 (4.6) | 8.5 (5.9) |
| [HHb]end (μM) | 0.042; 0.324 | 0.121; 0.204 | 0.590; 0.027 | 5.4 (3.0) | 6.4 (4.9) | 6.9 (4.1) | 9.0 (6.1) |
Δ[HHb]/Δ O2 (μM L−1 min−1) O2 (μM L−1 min−1) |
0.078; 0.255 | 0.425; 0.060 | 0.922; 0.001 | 5.6 (3.1) | 6.2 (3.7) | 8.0 (5.3) | 8.5 (6.2) |
Δ[HHb]/Δ O2 ratio is also reported. The table reports also the mean of the 2.5% and 97.5% percentiles of the 95% confidence interval for τ1 and the P-values of the ANOVA analysis together with the values of the corresponding partial squared correlation factors. For the meaning of the symbols, please refer to the text. ∗Significantly different from Pre HIT.
O2 ratio is also reported. The table reports also the mean of the 2.5% and 97.5% percentiles of the 95% confidence interval for τ1 and the P-values of the ANOVA analysis together with the values of the corresponding partial squared correlation factors. For the meaning of the symbols, please refer to the text. ∗Significantly different from Pre HIT.
FIGURE 2.
Pulmonary  O2 and muscle HHb kinetics of typical subjects at the onset of constant work rate exercise of heavy intensity are represented. The first four panels show the following
O2 and muscle HHb kinetics of typical subjects at the onset of constant work rate exercise of heavy intensity are represented. The first four panels show the following  O2 kinetics: Pre HIT
(A), Post HIT
(B), Pre IRT
(C) and Post IRT
(D). The last four panels show the muscle HHb kinetics: Pre HIT
(E), Post HIT
(F), Pre IRT
(G) and Post IRT
(H). Data are displayed on 1 s base and the residual plot is shown on x-axis.
O2 kinetics: Pre HIT
(A), Post HIT
(B), Pre IRT
(C) and Post IRT
(D). The last four panels show the muscle HHb kinetics: Pre HIT
(E), Post HIT
(F), Pre IRT
(G) and Post IRT
(H). Data are displayed on 1 s base and the residual plot is shown on x-axis.
The amplitude A′1 of phase I at Post IRT was significantly larger than at Pre IRT (P = 0.018; d = 0.81; 95% CIDiff: 0.03 L min−1/0.20 L min−1); the latter value was also significantly smaller than at Pre HIT (P = 0.029; d = 0.72; 95% CIDiff: 0.03 L min−1/0.24 L min−1). The time delay in phase I at Post IRT was significantly shorter than at Post HIT (P = 0.036; d = 0.69; 95% CIDiff: −0.3 s/−2.3 s) (Table 1).
A′2 was greater at Post IRT than at Pre IRT (P = 0.028; d = 0.73; 95% CIDiff: 0.01 L min−1/0.12 L min−1). The time constant of the primary phase of  O2 kinetics (τ2) during HiEx was significantly longer at Post IRT than before strength training (P = 0.010; d = 0.90; 95% CIDiff: 1.5 s/6.9 s) and after HIT (P = 0.010; d = 1.02; 95% CIDiff: 2.2 s/7.9 s). In addition, a significant interaction between training types and time at τ2 was noted (P = 0.010; d = 0.94; 95% CIDiff: 2.6 s/13.2 s). This further suggests that IRT was specifically able to induce the deceleration of the primary phase of
O2 kinetics (τ2) during HiEx was significantly longer at Post IRT than before strength training (P = 0.010; d = 0.90; 95% CIDiff: 1.5 s/6.9 s) and after HIT (P = 0.010; d = 1.02; 95% CIDiff: 2.2 s/7.9 s). In addition, a significant interaction between training types and time at τ2 was noted (P = 0.010; d = 0.94; 95% CIDiff: 2.6 s/13.2 s). This further suggests that IRT was specifically able to induce the deceleration of the primary phase of  O2 kinetics during HiEx. Finally, d2 at Pre IRT was shorter than before HIT (P = 0.004; d = 1.04; 95% CIDiff: −2.5 s/−8.8 s) (Table 1).
O2 kinetics during HiEx. Finally, d2 at Pre IRT was shorter than before HIT (P = 0.004; d = 1.04; 95% CIDiff: −2.5 s/−8.8 s) (Table 1).
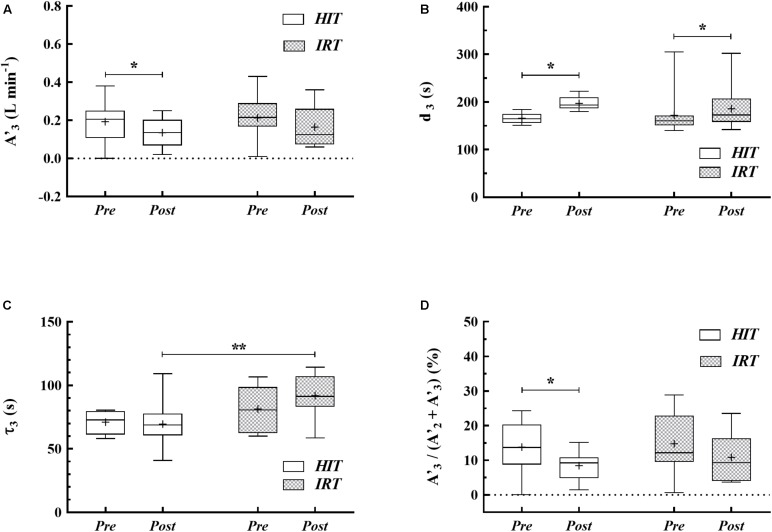
After HIT A′3 was significantly smaller (P = 0.045; d = 0.65; 95% CIDiff: −0.01 L min−1/−0.11 L min−1) and d3 was larger (P = 0.001; d = 1.55; 95% CIDiff: 19.6 s/43.1 s) than at Pre HIT (Table 1 and Figures 3A,B). The relative contribution of the slow component to the overall  O2 response [A′3/(A′2 + A′3)] (Figure 3D) was significantly smaller at Post HIT than at Pre HIT (P = 0.018; d = 0.80; 95% CIDiff: −1.4%/−9.2%). Also, IRT affected d3 (Figure 3B), as it was longer at Post IRT than at Pre IRT (P = 0.039; d = 0.67; 95% CIDiff: 2.0 s/25.3 s). In addition, a significant interaction between training types and time on d3 was observed (P = 0.022; d = 0.87; 95% CIDiff: 6.1 s/39.5 s), indicating that HIT induced a more marked effect than IRT on d3 (Figure 3B). Finally, τ3 was significantly greater at Post IRT than at Post HIT (P = 0.003; d = 1.107; 95% CIDiff: 10.7 s/34.1 s) (Figure 3C).
O2 response [A′3/(A′2 + A′3)] (Figure 3D) was significantly smaller at Post HIT than at Pre HIT (P = 0.018; d = 0.80; 95% CIDiff: −1.4%/−9.2%). Also, IRT affected d3 (Figure 3B), as it was longer at Post IRT than at Pre IRT (P = 0.039; d = 0.67; 95% CIDiff: 2.0 s/25.3 s). In addition, a significant interaction between training types and time on d3 was observed (P = 0.022; d = 0.87; 95% CIDiff: 6.1 s/39.5 s), indicating that HIT induced a more marked effect than IRT on d3 (Figure 3B). Finally, τ3 was significantly greater at Post IRT than at Post HIT (P = 0.003; d = 1.107; 95% CIDiff: 10.7 s/34.1 s) (Figure 3C).
FIGURE 3.
Box-whiskers graphs of the amplitude (A′3) of the O2 uptake at the end of exercise (A), time delay (B) and slow phase time constant (C) of the  O2sc and relative contribution of A′3 to the overall
O2sc and relative contribution of A′3 to the overall  O2 response (D) at Pre HIT, Post HIT, Pre IRT, and Post IRT. Horizontal line marks the median of the data distribution, box extends from the 25th to the 75th percentiles; whiskers extend down to the 5th percentile and up to the 95th percentile; the cross represents the average. ∗P < 0.05; ∗∗P < 0.01.
O2 response (D) at Pre HIT, Post HIT, Pre IRT, and Post IRT. Horizontal line marks the median of the data distribution, box extends from the 25th to the 75th percentiles; whiskers extend down to the 5th percentile and up to the 95th percentile; the cross represents the average. ∗P < 0.05; ∗∗P < 0.01.
Training did not affect the parameters describing the increase in HHb at the onset of HiEx exercise (Table 2): no changes in A1, d1, τ1, and MRT were observed after either HIT or IRT as compared with the pre-training conditions.
Discussion
We investigated the effects of HIT and IRT on  O2 kinetics during CWR HiEx exercise performed at the same absolute WR before and after training in a group of healthy, moderately active elderly men.
O2 kinetics during CWR HiEx exercise performed at the same absolute WR before and after training in a group of healthy, moderately active elderly men.
Post-intervention assessment after 8 weeks of HIT mainly showed:
-
simple (i)
an increase in
 O2 max and an improvement of RCP;
O2 max and an improvement of RCP; -
simple (ii)
an increase in Vol and CSA of the quadriceps without a parallel increment of muscular strength;
-
simple (iii)
a decrease in the amplitude A′3 of the slow component of
 O2 kinetics assessed during HiEx together with a prolonged d3;
O2 kinetics assessed during HiEx together with a prolonged d3;
Post-intervention assessment after 8 weeks of IRT showed:
-
simple (i)
a decrease in the functional gain of the primary phase of
 O2 kinetics during ModEx;
O2 kinetics during ModEx; -
simple (ii)
an increase in muscular Vol and CSA in parallel with a significant increment in muscular strength;
-
simple (iii)
a deceleration in the primary component of
 O2 kinetics with an increased τ2 during HiEx;
O2 kinetics with an increased τ2 during HiEx; -
simple (iv)
a significant increase of d3 of
 O2sc In addition, the longer τ3 after IRT made the kinetics of
O2sc In addition, the longer τ3 after IRT made the kinetics of  O2sc significantly slower than the one after HIT.
O2sc significantly slower than the one after HIT.
Maximal Oxygen Uptake and Gas Exchange Thresholds
Several studies have demonstrated the efficacy of HIT in increasing  O2 max in different populations (Kohrt et al., 1991; Bruseghini et al., 2015). Our results are in line with the findings that 8–12 weeks of interval training can induce a significant increase in
O2 max in different populations (Kohrt et al., 1991; Bruseghini et al., 2015). Our results are in line with the findings that 8–12 weeks of interval training can induce a significant increase in  O2 max in elderly subjects (Lepretre et al., 2009). HIT induced also a significant improvement in RCP. A comparable trend of the positive effects of HIT was found at intensities corresponding to the ventilatory threshold in elderly subjects (Pogliaghi et al., 2006).
O2 max in elderly subjects (Lepretre et al., 2009). HIT induced also a significant improvement in RCP. A comparable trend of the positive effects of HIT was found at intensities corresponding to the ventilatory threshold in elderly subjects (Pogliaghi et al., 2006).
Muscle Morphology and Strength
The increases of CSA (plus 4.3% ± 3.6) and Vol (plus 5.6% ± 3.6) found after HIT are in agreement with previous findings (Sillanpää et al., 2008; Harber et al., 2012) that showed a significant increase (+6%) of the quadriceps muscle volume in elderly men after 12 weeks of aerobic training paralleled by the increase of CSA of myosin heavy chain type I (MHCI) fibers and a higher muscular thickness of vastus lateralis and intermedius after endurance training in older adults. IRT was followed by significant increases of CSA and Vol (plus 4.2% ± 4.4 and plus 4.9% ± 7.0). This confirms the results obtained in untrained elderly subjects in other occasions (Lee et al., 2008; Shunkert et al., 2008).
IRT was paralleled by a significant increase in the torque produced by the limb extensors. Therefore, we may also reasonably assume that, at Post IRT, our subjects were able to pedal at the same WR recruiting a smaller number of MUs. Indeed, from the individual WR and the isokinetic torque values we can calculate that the average torque maintained by the subject during HiEx exercise, when expressed as a percent of Tk, significantly decreased from Pre IRT to Post IRT: P = 0.008, CI of the difference 1.3/−0.5 N m for Tk 60° s−1; P = 0.012, CI of the difference 1.3/−0.25 N m for Tk 120° s−1.
Response to CWR of Moderate Intensity Exercise
The significant decrease in G after IRT reflected a decrease in the O2 cost of exercise and translated into a small, albeit significant, increase of 5% in work efficiency, η (Pre IRT η 22.9% vs. Post IRT η 24.0%; P = 0.041; 95% CIDiff: 0.1%/2.0%). This is consistent with previous findings of a significant decrease in the amplitude of the primary phase in cycling (Zoladz et al., 2012) after strength training. Accordingly, strength training results in an improved delta η (Bastiaans et al., 2001) and work η (Sunde et al., 2010) in cycling. The improvement in η found after IRT remains difficult to explain, though. One may surmise that, by increasing the absolute strength of the muscles involved in cycling, the subjects were pedaling against the same workload recruiting a smaller number of less efficient Type II fibers, wherefrom a smaller G and a larger η derived.
Response to CWR During Heavy-Intensity Exercise
Endurance training is followed by a substantial reduction of  O2sc (Casaburi et al., 1987; Womack et al., 1995). In addition, the contribution of
O2sc (Casaburi et al., 1987; Womack et al., 1995). In addition, the contribution of  O2sc to the overall
O2sc to the overall  O2 response has been related to the % of Type I fibers, which have been described to have a greater metabolic stability (Hochacka and McClelland, 1997). Therefore, an increase of the % of Type I fibers at Post HIT may have led to improved metabolic stability (Zoladz et al., 2006), attenuated the drop in intramuscular pH and [PCr] and decreased
O2 response has been related to the % of Type I fibers, which have been described to have a greater metabolic stability (Hochacka and McClelland, 1997). Therefore, an increase of the % of Type I fibers at Post HIT may have led to improved metabolic stability (Zoladz et al., 2006), attenuated the drop in intramuscular pH and [PCr] and decreased  O2sc (Jones et al., 2007).
O2sc (Jones et al., 2007).
In addition to these mechanisms directly linked to the plausible phenotypical shift in muscle fiber populations, also mechanisms intrinsic to each single fiber may be responsible for the observed decrease of  O2sc after HIT. A recent study (Zoladz et al., 2016) showed that endurance training in rats induced a temperature dependent enhancement of mitochondrial oxidative phosphorylation and a significant drop of mitochondrial uncoupling. Therefore, the decrease of O2 cost for oxidative ATP production in each recruited muscle fiber may have substantially potentiated the effect of endurance training on
O2sc after HIT. A recent study (Zoladz et al., 2016) showed that endurance training in rats induced a temperature dependent enhancement of mitochondrial oxidative phosphorylation and a significant drop of mitochondrial uncoupling. Therefore, the decrease of O2 cost for oxidative ATP production in each recruited muscle fiber may have substantially potentiated the effect of endurance training on  O2sc.
O2sc.
It has been also shown that  O2sc is modulated by manipulations of O2 delivery (Poole and Jones, 2012). HIT may improve O2 availability and induce a better matching between O2 delivery and utilization (Murias et al., 2010a,b). This may have a positive impact on
O2sc is modulated by manipulations of O2 delivery (Poole and Jones, 2012). HIT may improve O2 availability and induce a better matching between O2 delivery and utilization (Murias et al., 2010a,b). This may have a positive impact on  O2sc in the elderly in whom metabolic vasodilatation is impaired (Poole et al., 2003) and a mismatch of local O2 delivery to O2 muscular consumption is present (Murias et al., 2010a,b). However, the obtained results do not support this conclusion, as training did not modify any of the indexes that characterize HHb response during HiEx exercise. In particular, the primary time constant [HHb]τ1 was not affected: a constant τ1 would suggest a proportionally similar increase of the speeds of adjustment of local O2 delivery and muscular O2 uptake in the primary phase of
O2sc in the elderly in whom metabolic vasodilatation is impaired (Poole et al., 2003) and a mismatch of local O2 delivery to O2 muscular consumption is present (Murias et al., 2010a,b). However, the obtained results do not support this conclusion, as training did not modify any of the indexes that characterize HHb response during HiEx exercise. In particular, the primary time constant [HHb]τ1 was not affected: a constant τ1 would suggest a proportionally similar increase of the speeds of adjustment of local O2 delivery and muscular O2 uptake in the primary phase of  O2 kinetics during HiEx. This conclusion is somehow strengthened by the observation that [HHb]end was not modified in presence of a lower A′3 of O2 uptake response.
O2 kinetics during HiEx. This conclusion is somehow strengthened by the observation that [HHb]end was not modified in presence of a lower A′3 of O2 uptake response.
The primary phase of τ2 of  O2 kinetics during HiEx was decelerated after IRT. In analogy with ModEx, the primary component in HiEx exercise is thought to increase exponentially without other changes (Poole and Jones, 2012). However, because the statistical estimate of τ2 during HiEx is often based on a limited number of data, this unavoidable drawback may produce uncertain and unreliable values of τ2. Besides these methodological problems, specific physiological adaptations induced by IRT may have contributed to the increase of τ2. A constant [HHb]τ helps us infer that local O2 delivery response and muscular O2 utilization changed proportionally after training. Therefore, a substantial defect in O2 availability may be still present after IRT.
O2 kinetics during HiEx was decelerated after IRT. In analogy with ModEx, the primary component in HiEx exercise is thought to increase exponentially without other changes (Poole and Jones, 2012). However, because the statistical estimate of τ2 during HiEx is often based on a limited number of data, this unavoidable drawback may produce uncertain and unreliable values of τ2. Besides these methodological problems, specific physiological adaptations induced by IRT may have contributed to the increase of τ2. A constant [HHb]τ helps us infer that local O2 delivery response and muscular O2 utilization changed proportionally after training. Therefore, a substantial defect in O2 availability may be still present after IRT.
The changes induced by IRT on  O2sc are somehow ambiguous: the amplitude of
O2sc are somehow ambiguous: the amplitude of  O2sc, either in absolute or relative terms, turned out to be unaffected by IRT, but
O2sc, either in absolute or relative terms, turned out to be unaffected by IRT, but  O2sc appeared later and developed more slowly than at Post HIT.
O2sc appeared later and developed more slowly than at Post HIT.
The mechanism underpinning the delay in the appearance of  O2sc after HIT and IRT are of different origin. We first underline that IRT training was effective in increasing the strength of muscles involved in pedaling (see the section “Results”). Therefore, we can suggest that the pedaling subjects after IRT were utilizing a lower percentage of their maximal voluntary force at the same WR and that they were recruiting a smaller number of Type II MUs. Should this be true, the diminished recruitment of these MUs would result in a slower development of
O2sc after HIT and IRT are of different origin. We first underline that IRT training was effective in increasing the strength of muscles involved in pedaling (see the section “Results”). Therefore, we can suggest that the pedaling subjects after IRT were utilizing a lower percentage of their maximal voluntary force at the same WR and that they were recruiting a smaller number of Type II MUs. Should this be true, the diminished recruitment of these MUs would result in a slower development of  O2sc, as the utilized Type I muscle fibers are less liable to develop fatigue and their metabolic features make them less prone to cause
O2sc, as the utilized Type I muscle fibers are less liable to develop fatigue and their metabolic features make them less prone to cause  O2sc. However, this explanation does not clarify whether the main cause of
O2sc. However, this explanation does not clarify whether the main cause of  O2sc resides in intensive mechanisms, i.e., the progressive decay of the efficiency of the already recruited MUs, or, rather, it may be ascribed to an extensive process, i.e., the progressive recruitment of less efficient Type II fibers. It is worth noting, however, that the net decrease of d3 observed after IRT was positively correlated with the net increase of knee torque (P = 0.046, r = 0.60). Conversely, the two variables were not correlated in the case of HIT (P = 0.316, r = −0.32).
O2sc resides in intensive mechanisms, i.e., the progressive decay of the efficiency of the already recruited MUs, or, rather, it may be ascribed to an extensive process, i.e., the progressive recruitment of less efficient Type II fibers. It is worth noting, however, that the net decrease of d3 observed after IRT was positively correlated with the net increase of knee torque (P = 0.046, r = 0.60). Conversely, the two variables were not correlated in the case of HIT (P = 0.316, r = −0.32).
Also, after IRT, we were not able to find any significant changes of muscular oxygenation and of the indexes that describe amelioration of local peripheral perfusion. This might suggest that the impairment of local O2 delivery was not the main cause of  O2sc, at least in this specific population of subjects.
O2sc, at least in this specific population of subjects.
Points of Strength and Weakness of the Study
We compared for the first time the effects of HIT and of IRT on the dynamic response of pulmonary  O2 and muscular oxygenation during HiEx exercise in healthy, untrained elderly men.
O2 and muscular oxygenation during HiEx exercise in healthy, untrained elderly men.
However, a few methodological limitations should be mentioned. The experimental design was not counterbalanced for reasons of feasibility.
We did not evaluate the changes in muscle fiber expression during the two training interventions. Since the size of  O2sc has been positively related to the percentage of Type II fibers (Poole and Jones, 2012), a strong correlation between the observed changes in the slow component and the changes in the phenotypical expression of the trained muscles would have strengthened the hypothesis of a muscular origin of the slow component.
O2sc has been positively related to the percentage of Type II fibers (Poole and Jones, 2012), a strong correlation between the observed changes in the slow component and the changes in the phenotypical expression of the trained muscles would have strengthened the hypothesis of a muscular origin of the slow component.
We did not evaluate the possible changes in neuromuscular activation induced by the two training modalities during ModEx and HiEx. Comparison of the differences in recruitment patterns would have helped to strengthen or reject our working hypothesis on the role of Type II motor units involvement in the genesis of  O2sc after IRT.
O2sc after IRT.
HHb signal mainly reflects the fractional O2 extraction of the interrogated zone of the muscle resulting from the dynamic balance between muscular O2 uptake and local O2 delivery and HHb signal reflects changes in oxygenation mainly in the capillaries of the explored muscle volume (Grassi and Quaresima, 2016). However, the assessment of HHb obtained only from the surface of the vastus lateralis may be a substantial limitation to our analysis, since some spatial heterogeneity in terms of muscle oxygenation in an exercising muscle seems to exist (Koga et al., 2007). Nonetheless, using skin landmarks to accurately place the NIRS probe in the same site before all experiments minimized possible problems due to spatial inhomogeneity.
Finally, the study aimed to investigate the effects of training in a particular population of subjects, i.e., elderly healthy volunteers who may have larger strength deficits than young, active adults. Therefore, the meaning and the applicability of the results obtained in this study may be extended with some caution to other classes of subjects.
Conclusion
The amplitude of  O2sc during HiEx was substantially smaller after HIT than before, but its decrease was not correlated with an improvement in the O2 delivery-to-utilization ratio of the exercising muscles. This suggests that suboptimal local O2 delivery was not a possible factor contributing to
O2sc during HiEx was substantially smaller after HIT than before, but its decrease was not correlated with an improvement in the O2 delivery-to-utilization ratio of the exercising muscles. This suggests that suboptimal local O2 delivery was not a possible factor contributing to  O2sc in the elderly, whereas the improved metabolic stability induced by HIT was likely able to induce beneficial effect on
O2sc in the elderly, whereas the improved metabolic stability induced by HIT was likely able to induce beneficial effect on  O2sc.
O2sc.
IRT, by increasing muscle strength, resulted in a delayed appearance of  O2sc during HiEx because of a possible larger contribution of Type I fibers to a motor task of identical absolute intensity.
O2sc during HiEx because of a possible larger contribution of Type I fibers to a motor task of identical absolute intensity.
Perspectives
The results obtained in the present investigation may have practical applications. First, the association between a larger exercise economy and the delayed appearance of  O2sc found after IRT may be of interest, as it suggests that strength training should be included in the usual training programs of elderly people to improve exercise economy and resistance to fatigue (Beattie et al., 2014). Second, they prompt the investigators to better characterize the changes in neuromuscular activation and MUs recruitment induced by the two training modalities during HiEx performed at the same WR. This should be done in parallel with the invasive evaluation of the changes in muscle fiber expression induced by training interventions.
O2sc found after IRT may be of interest, as it suggests that strength training should be included in the usual training programs of elderly people to improve exercise economy and resistance to fatigue (Beattie et al., 2014). Second, they prompt the investigators to better characterize the changes in neuromuscular activation and MUs recruitment induced by the two training modalities during HiEx performed at the same WR. This should be done in parallel with the invasive evaluation of the changes in muscle fiber expression induced by training interventions.
Author Contributions
CC, PB, ET, FS, and EC planned the study. CC, ET, PB, EO, AP, RPM, SP, and EC collected and analyzed the data. CC, PB, and ET wrote the manuscript. CC, PB, ET, EC, and RPM revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many thanks to all the dedicated participants who made this study possible.
Footnotes
Funding. This study was supported by the European Space Agency (ESA) Contract # 4000102580 MAP Project “Astronaut Exercise Prescriptions Promoting Health and Fitness on Earth,” allocated to CC for the project “Cardiovascular and Skeletal Muscle Responses to Chronic Concurrent Exercise Using Flywheel Technology in Old Men.”
References
- Barstow T. J., Jones A. M., Nguyen P. H., Casaburi R. (1996). Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J. Appl. Physiol. 81 1642–1650. 10.1152/jappl.1996.81.4.1642 [DOI] [PubMed] [Google Scholar]
- Bastiaans J. J., Van Diemen A. B., Veneberg T., Jeukendrup A. E. (2001). The effects of replacing a portion of endurance training by explosive strength training on performance in trained cyclists. Eur. J. Appl. Physiol. 86 79–84. 10.1007/s004210100507 [DOI] [PubMed] [Google Scholar]
- Beattie K., Kenny I. C., Lyons M., Carson P. B. (2014). The effect of strength training on performance in endurance athletes. Sports Med. 44 845–865. 10.1007/s40279-014-0157-y [DOI] [PubMed] [Google Scholar]
- Breese B. C., McNarry M. A., Marwood S., Blackwell J. R., Bailey S. J., Jones A. M. (2013). Beetroot juice supplementation speeds VO2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305 R1441–R1450. 10.1152/ajpregu.00295.2013 [DOI] [PubMed] [Google Scholar]
- Bruseghini P., Calabria E., Tam E., Milanese C., Oliboni E., Pezzato A., et al. (2015). Effects of eight weeks of aerobic interval training and of isoinertial resistance training on risk factors of cardiometabolic diseases and exercise capacity in healthy elderly subjects. Oncotarget 6 16998–17015. 10.18632/oncotarget.4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Casaburi R., Storer T. W., Ben-Dov I., Wasserman K. (1987). Effect of endurance training on possible determinants of
 O2 during heavy exercise.
J. Appl. Physiol.
62
199–207. 10.1152/jappl.1987.62.1.199
[DOI] [PubMed] [Google Scholar]
O2 during heavy exercise.
J. Appl. Physiol.
62
199–207. 10.1152/jappl.1987.62.1.199
[DOI] [PubMed] [Google Scholar] - De Roia G., Pogliaghi S., Adami A., Papadopoulou C., Capelli C. (2012). Effects of priming exercise on the speed of adjustment of muscle oxidative metabolism at the onset of moderate-intensity step transitions in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302 R1158–R1166. 10.1152/ajpregu.00269.2011 [DOI] [PubMed] [Google Scholar]
- Grassi B., Quaresima V. (2016). Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J. Biomed. Opt. 21:091313. 10.1117/1.JBO.21.9.091313 [DOI] [PubMed] [Google Scholar]
- Harber M. P., Konopka A. R., Undem M. K., Hinkley J. M., Minchev K., Kaminsky L. A., et al. (2012). Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 113 1495–1504. 10.1152/japplphysiol.00786.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochacka P. W., McClelland G. B. (1997). Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J. Exp. Biol. 200 381–386. [DOI] [PubMed] [Google Scholar]
-
Jones A. M., Grassi B., Christensen P. M., Krustrup P., Bangsbo J., Poole D. C. (2011). Slow component of
 O2 kinetics: mechanistic bases and practical applications.
Med. Sci. Sports Exerc.
43
2046–2062. 10.1249/MSS.0b013e31821fcfc1
[DOI] [PubMed] [Google Scholar]
O2 kinetics: mechanistic bases and practical applications.
Med. Sci. Sports Exerc.
43
2046–2062. 10.1249/MSS.0b013e31821fcfc1
[DOI] [PubMed] [Google Scholar] - Jones A. M., Wilkerson D. P., Berger N. J., Fulford J. (2007). Influence of endurance training on muscle [PCr] kinetics during high-intensity exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293 R392–R401. 10.1152/ajpregu.00056.2007 [DOI] [PubMed] [Google Scholar]
- Jones A. M., Wilkerson D. P., DiMenna F., Fulford J., Poole D. C. (2008). Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294 R585–R593. 10.1152/ajpregu.00731.2007 [DOI] [PubMed] [Google Scholar]
- Keppel G., Wickens T. D. (2004). Design and Analysis: A Researcher’s Handbook, 4th Edn, Upper Saddle River, NJ: Pearson Prentice Hall, 400–431. [Google Scholar]
- Koga S., Poole D. C., Ferreira L. F., Whipp B. J., Kondo N., Saitoh T., et al. (2007). Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J. Appl. Physiol. 103 2049–2056. 10.1152/japplphysiol.00627.2007 [DOI] [PubMed] [Google Scholar]
- Kohrt W., Malley M. T., Coggan A. R., Spina R. J., Ogawa T., Ehsani A. A., et al. (1991). Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J. Appl. Physiol. 71 2004–2011. 10.1152/jappl.1991.71.5.2004 [DOI] [PubMed] [Google Scholar]
- Lee C. M. Y., Huxley R. R., Wildman R. P., Woodward M. (2008). Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J. Clin. Epidemiol. 61 646–653. 10.1016/j.jclinepi.2007.08.012 [DOI] [PubMed] [Google Scholar]
- Lepretre P. M., Vogel T., Brechat P. H., Dufour S., Richard R. (2009). Impact of short-term aerobic interval training on maximal exercise in sedentary aged subjects. Int. J. Clin. Pract. 63 1472–1478. 10.1111/j.1742-1241.2009.02120.x [DOI] [PubMed] [Google Scholar]
- Marquardt D. W. (1963). An algorithm for least – squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 11 431–444. 10.1137/0111030 [DOI] [Google Scholar]
- Motulsky H. Y., Christopoulos A. (2004). Fitting Models to Biological Data Using Linear and Nonlinear Regression. New York, NY: Oxford University Press, 104–108. [Google Scholar]
-
Murias J. M., Kowalchuk J. M., Paterson D. H. (2010a). Speeding of
 O2 kinetics in response to endurance-training in older and young women.
Eur. J. Appl. Physiol.
111
235–243. 10.1007/s00421-010-1649-6
[DOI] [PubMed] [Google Scholar]
O2 kinetics in response to endurance-training in older and young women.
Eur. J. Appl. Physiol.
111
235–243. 10.1007/s00421-010-1649-6
[DOI] [PubMed] [Google Scholar] -
Murias J. M., Kowalchuk J. M., Paterson D. H. (2010b). Speeding of
 O2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization.
J. Appl. Physiol.
108
913–922. 10.1152/japplphysiol.01355.2009
[DOI] [PMC free article] [PubMed] [Google Scholar]
O2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization.
J. Appl. Physiol.
108
913–922. 10.1152/japplphysiol.01355.2009
[DOI] [PMC free article] [PubMed] [Google Scholar] - Murias J. M., Spencer M. D., Paterson D. H. (2014). The critical role of O2 provision in the dynamic adjustment of oxidative phosphorylation. Exerc. Sport Sci. Rev. 42 4–11. 10.1249/JES.0000000000000005 [DOI] [PubMed] [Google Scholar]
- Pogliaghi S., Terziotti P., Cevese A., Balestreri F., Schena F. (2006). Adaptations to endurance training in the healthy elderly: arm cranking versus leg cycling. Eur. J. Appl. Physiol. 97 723–731. 10.1007/s00421-006-0229-2 [DOI] [PubMed] [Google Scholar]
- Poole D. C., Jones A. M. (2012). Oxygen uptake kinetics. Compr. Physiol. 2 933–996. [DOI] [PubMed] [Google Scholar]
- Poole D. C., Schaffartzik W., Knight D. R., Derion T., Kennedy B., Guy H. J., et al. (1991). Contribution of excising legs to the slow component of oxygen uptake kinetics in humans. J. Appl. Physiol. 71 1245–1260. 10.1152/jappl.1991.71.4.1245 [DOI] [PubMed] [Google Scholar]
- Poole D. C., Wilkerson D. P., Jones A. M. (2008). Validity of criteria for establishing maximal O2 uptake during ramp exercise test. Eur. J. Appl. Physiol. 102 403–410. 10.1007/s00421-007-0596-3 [DOI] [PubMed] [Google Scholar]
- Poole J. G., Lawrenson L., Kim J., Brown C., Richardson R. S. (2003). Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am. J. Physiol. Heart Circ. Physiol. 284 H1251–H1259. 10.1152/ajpheart.00790.2002 [DOI] [PubMed] [Google Scholar]
- Salvadego D., Lazzer S., Busti C., Galli R., Agosti F., Lafortuna C., et al. (2010). Gas exchange kinetics in obese adolescents. Inferences on exercise tolerance and prescription. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299 R1298–R1305. 10.1152/ajpregu.00038.2010 [DOI] [PubMed] [Google Scholar]
- Shunkert H., Moebus S., Hanisch J., Steinhagen-Thiessen E., Hauner H., et al. (2008). The correlation between waist circumference and ESC cardiovascular risk score: data from the German metabolic and cardiovascular risk project (GEMCAS). Clin. Res. Cardiol. 97 827–835. 10.1007/s00392-008-0694-1 [DOI] [PubMed] [Google Scholar]
- Sillanpää E., Häkkinen A., Nyman K., Mattila M., Cheng S., Karavirta L., et al. (2008). Body composition and fitness during strength and/or endurance training in older men. Med. Sci. Sports Exerc. 40 950–958. 10.1249/MSS.0b013e318165c854 [DOI] [PubMed] [Google Scholar]
- Stienen G. J., Kiers J. L., Bottinelli R., Reggiani C. (1996). Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J. Physiol. 493 299–307. 10.1113/jphysiol.1996.sp021384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde A., Støren Ø., Bjerkaas M., Larsen M. H., Hoff J., Helgerud J. (2010). Maximal strength training improves cycling economy in competitive cyclists. J. Strength Cond. Res. 24 2157–2165. 10.1519/JSC.0b013e3181aeb16a [DOI] [PubMed] [Google Scholar]
- Willis W. T., Jackman M. R. (1994). Mitochondrial function during heavy exercise. Med. Sci. Sports Exerc. 26 1347–1353. 10.1249/00005768-199411000-00009 [DOI] [PubMed] [Google Scholar]
- Womack C. J., Davis S. E., Blumer J. L., Barrett E., Weltman A. L., Gaesser G. A. (1995). Slow component of O2 uptake during heavy exercise: adaptation to endurance training. J. Appl. Physiol. 79 838–845. 10.1152/jappl.1995.79.3.838 [DOI] [PubMed] [Google Scholar]
-
Zoladz J. A., Gladden L. B., Hogan M. C., Nieckarz Z., Grassi B. (2008). Progressive recruitment of muscle fibers is not necessary for the slow component of
 O2 kinetics.
J. Appl. Physiol.
105
575–580. 10.1152/japplphysiol.01129.2007
[DOI] [PubMed] [Google Scholar]
O2 kinetics.
J. Appl. Physiol.
105
575–580. 10.1152/japplphysiol.01129.2007
[DOI] [PubMed] [Google Scholar] - Zoladz J. A., Korzeniewski B., Grassi B. (2006). Training-induces acceleration of oxygen uptake kinetics in skeletal muscle: the underlying mechanisms. J. Physiol. Pharmacol. 10 67–84. [PubMed] [Google Scholar]
- Zoladz J. A., Koziel A., Woyda-Ploszczyca A., Chelichowski J., Jarmuszkiewicz W. (2016). Endurance training increases the efficiency. Pflugers Arch. 468 1709–1724. 10.1007/s00424-016-1867-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz J. A., Szkutnik Z., Majerczak J., Grandys M., Duda K., Grassi B. (2012). Isometric strength training lowers the O2 cost of cycling during moderate-intensity exercise. Eur. J. Appl. Physiol. 112 4151–4161. 10.1007/s00421-012-2405-x [DOI] [PubMed] [Google Scholar]