Abstract
Objective:
To study the influence of tumour diameter and anatomy on the success and complication rates of small renal mass (SRM, ≤4 cm) core biopsy.
Methods:
Retrospective analysis of SRMs that underwent ultrasound or CT-guided biopsy. Diagnostic and complication rates were compared according to tumour size (subcategorised as axial diameter ≤2 cm, >2 to– ≤3 cm, >3–≤4 cm) and anatomical disposition (exophytic/endophytic, centrality, polar location and anterior/posterior).
Results:
94 patients (54 male; age range 21.8–84.3 years) with 95 SRMs underwent biopsy. The first biopsy was diagnostic in 81/95 (85.3%). Seven patients underwent repeat biopsy (6/7 diagnostic), to give an overall diagnostic rate of 91.5%. The primary diagnostic rates in the ≤2, >2–≤3 , >3–≤4 cm groups were 21/25 (84%); 38/44 (86.4%) and 22/26 (84.6%) respectively and were similar (p = 1.00). Anterior and upper pole SRMs were more likely to fail initial biopsy (odds ratio 13.8, p < 0.01; and odds ratio 4.35, p = 0.04) respectively, but other anatomical factors were not relevant. Complications occurred in 14% (all conservatively managed perinephric haematomas; Clavien-Dindo Grade 1) and size or location were not relevant.
Conclusion:
Image-guided biopsy of SRMs has a high diagnostic rate irrespective of tumour size. Anterior and upper pole location had lower diagnostic rates. Biopsy should be considered for all patients with SRMs, if the result will impact on management and we list specific scenarios where an SRM biopsy may be helpful.
Advances in knowledge:
SRM size does not affect the likelihood of a diagnostic biopsy.
Introduction
The incidence of small renal masses (SRMs), defined as enhancing tumours ≤ 4 cm in diameter,1 has increased, likely due to increased detection with more widespread use of cross-sectional imaging.2 SRMs can be treated by nephrectomy, partial nephrectomy or thermal ablation.3 While these treatments are safe and effective, up to 30% of incidentally detected SRMs are benign.4 No imaging modality can reliably distinguish benign from malignant lesions and there is increasing interest in percutaneous biopsy of SRMs, to allow accurate diagnosis and direct future management.
Existing data have proven the safety and efficacy of image-guided biopsy of SRMs with a pooled primary diagnostic rate of 85.9% and a major complication rate of 2%,5 but to date, the effect of tumour size on the success of core biopsy is contradictory. Some studies suggest a higher failure rate with smaller renal masses6–10 but others have reported no difference.11–13 The effect of anatomical location has also not been investigated5 although some tumours, e.g. those in the upper renal pole or the anterior kidney, or endophytic masses, can be difficult to target under image guidance.14
We present our experience of image-guided percutaneous biopsy of SRMs over an 8 year period. The primary purpose was to study the effect of SRM size on the rate of diagnosis and safety. The secondary study intention was to investigate the effect of tumour location on diagnostic rate and safety.
Methods and materials
Study group and procedure
All patients who had undergone image-guided percutaneous biopsy of SRMs at our St George's Hospital, London between October 2008 to October 2016 were retrospectively identified by searching the Radiology Information System. Decisions to biopsy were made following discussion at the local renal cancer multidisciplinary team meeting. The decision to biopsy was based on imaging features suspicious for cancer (solid enhancing lesions and if cystic, with thick septations—with our without enhancement) and where the result would impact on management. SRM location did not affect the decision to biopsy, but may have altered the technique used (e.g. trajectory or imaging modality) and this was at the discretion of the radiologists performing the procedures, all of whom were experienced in percutaneous renal biopsy. Biopsy was carried out as a day case procedure, under local anaesthetic and with ultrasound or CT guidance. Core biopsies were only undertaken using a coaxial technique and the core needle size used and number of cores taken were at the discretion of the operator. After the biopsy, all patients were clinically observed for up to 4 h; and discharged if pain free, with stable pulse and blood pressure recordings.
This was a retrospective analysis of an established clinical service. Our hospital does not require formal institutional review board approval or informed consent from patients for retrospective service evaluation, using existing clinical data and without any change in patient care.
Data collection
Electronic records including the Picture Archiving and Communication System (PACS) were searched to create a database populated with patient demographics and the histopathological data from the SRM biopsies or subsequent surgical excisions. Histopathological results were categorised into malignant, benign or non-diagnostic, where a non-diagnostic result is defined as insufficient material, an inconclusive result or normal renal parenchyma.15 Where possible, biopsy histology was correlated with the surgical excision histology.
All patients had undergone a diagnostic post-intravenous contrast CT study within the 3 months prior to biopsy and these were re-evaluated on Picture Archiving and Communication System (by MS). The maximal axial tumour diameter was measured and the anatomy of the SRM was evaluated. Components of the R.E.N.A.L. Nephrometry score16 were used to classify the locational anatomy of the tumour. Briefly, the position of the SRM was categorised as in the upper renal pole, interpolar region or the lower renal pole and either anterior or posterior in relationship to the renal midline. Each renal mass was further categorised as either mainly or partly exophytic, or purely endophytic; and the closeness of the SRM to the renal sinus structures was graded.16 Immediate post-biopsy complications were classified according to the Clavien-Dindo criteria.17
Statistical analysis
For the purposes of this study, a diagnostic (or successful) biopsy was defined as biopsy analysis leading to a firm histopathological diagnosis of a malignant or benign cause of the SRM. For the primary study intention, the diagnostic biopsy rate was compared using three size thresholds (≤2, >2–≤3, and >3–≤4 cm). For secondary study purposes, the diagnostic biopsy rate was compared according to the individual locational variables as described above. Statistical analysis was performed using SPSS v. 23 (IBM Corp., Armonk, NY). Continuous values were compared using an appropriate t-test, Kruskal–Wallis or Mann–Whitney U test after testing for normality and the test used is stated in the text or relevant table. Fisher’s exact test or X2 tests were performed to compare categorical variables. A p-value <0.05 was considered statistically significant.
Results
Patient demographic data
A total of 99 SRM biopsies were identified, but 4 were discarded (three because the mass was found to be >4 cm in diameter on repeat measurement, and the last patient was already known to have renal lymphoma and the biopsy was undertaken to regrade the lymphoma). Thus, 95 SRM biopsies were performed on 94 patients (one patient had bilateral SRMs, and underwent biopsies on separate occasions). Mean patient age was 64.6 years (range 21.8–84.3) and mean lesion diameter was 2.6 cm (0.9–4). 64 and 39 cases were performed under CT and ultrasound guidance respectively (these figures include repeat biopsies—see “Overall biopsy accuracy”). Five patients had suspected N1 disease and two suspected M1 disease prior to biopsy. The detailed patient and lesion characteristics are listed in Table 1. 21 lesions, all renal cell carcinoma (RCC), underwent partial or total nephrectomy and 25 lesions underwent thermal ablation. The other SRMs are being maintained on imaging and clinical surveillance.
Table 1.
Patient (n = 94) and tumour characteristics of 95 renal masses ≤ 4 cm in diameter that underwent image-guided renal mass biopsy
| Gender | N (%) |
| Male | 54 (57.9) |
| Female | 40 (42.1) |
| Side | |
| Right | 44 (46.3) |
| Left | 51 (53.7) |
| Tumour location | |
| Upper pole | 27 (28.4) |
| Interpolar | 44 (46.3) |
| Lower pole | 24 (25.3) |
| Exophytic/endophytic | |
| ≥50% exophytic | 39 (41.1) |
| <50% exophytic | 30 (31.6) |
| Entirely endophytic | 26 (27.4) |
| Centrality | |
| ≥7 mm from collecting system/sinus | 36 (37.9) |
| >4 but ≤7 mm from collecting system/sinus | 18 (18.9) |
| ≤4 mm from collecting system/sinus | 41 (43.2) |
| Anterior/posterior | |
| Anterior | 28 (29.5) |
| Posterior | 54 (56.8) |
| Neither | 13 (13.7) |
Note: Patient characteristics are given for 94 individuals. The other variables are given for 95 renal masses (as one patient had two tumours that underwent biopsy on separate days).
Overall biopsy accuracy
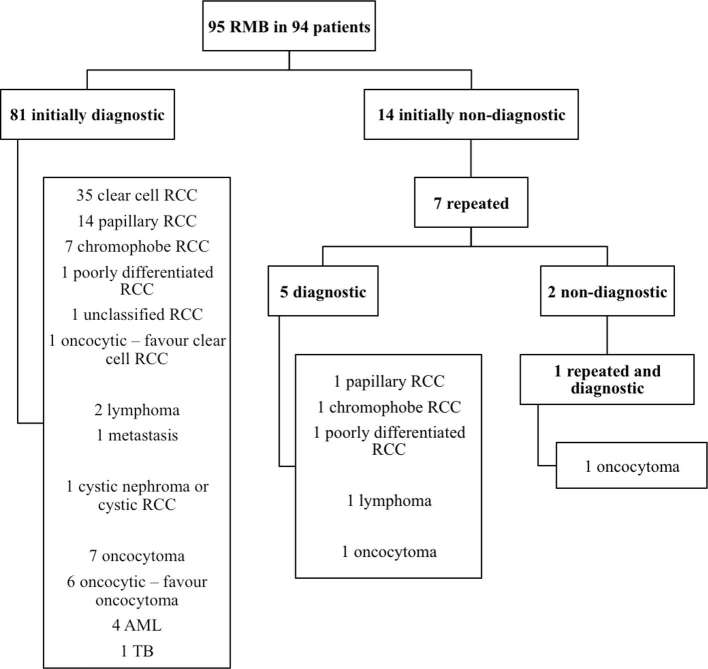
Initially, 81/95 (85.3%) SRM biopsies were diagnostic. Seven non-diagnostic biopsies were repeated (one SRM was rebiopsied twice) and a diagnosis was achieved in six (85.7%) of these. Therefore, a histological diagnosis was reached in 87/95 of all SRMs (91.5%). The results of the SRM biopsies are detailed in Figure 1 and a biopsy is illustrated in Figure 2. The proportion of benign vs malignant causes in the first and repeat biopsy cohort was 18/81 (22.2%) vs 2/7 (28.6%) respectively (p = 0.65, Fisher’s Exact Test). The seven tumours that did not undergo repeat biopsy are on surveillance and have not shown any radiological progression over a mean follow-up period of 27.6 months (range 8–60).
Figure 1.
SRM biopsy results and histopathological subtypes. AML, angiomyolipoma; RCC, renal cell carcinoma; RMB, renal mass biopsy; SRM, small renal mass; TB, tuberculosis.
Figure 2.
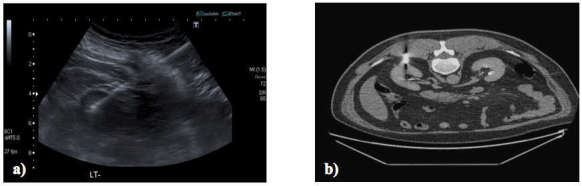
Renal mass biopsy of an upper pole lesion under ultrasound guidance (a) and a posteriorly located interpolar lesion biopsied under CT guidance (b). Upper pole lesions may require a steep trajectory and this may be easier under ultrasound guidance if the mass is clearly seen. Review of available imaging helps choose the appropriate guidance modality.
Biopsy accuracy vs size of renal mass
The mean tumour size in the patients with a diagnostic biopsy result at first attempt was not significantly different from those with unsuccessful biopsy: mean (95% confidence interval) tumour size was 2.6 cm (2.45–2.79) vs 2.5 cm (2.12–3.04) in the two groups; p = 0.85 (unpaired t-test); and the spread is illustrated in Figure 3. Initial biopsy success categorised according to the subgroups ≤ 2 cm diameter, > 2 to ≤3, > 3 to ≤ 4 cm was 21/25 (84%); 38/44 (86.4%) and 22/26 (84.6%) respectively and was also not significantly different (p = 1.00) (Table 2). The three subgroups were well-matched in terms of patient and tumour characteristics, except that SRMs were more centrally located in the > 3 to ≤ 4 cm group (Table 2), but this may reflect the larger tumour size in this subgroup. Centrality had no effect on biopsy success or overall complication rates (see below).
Figure 3.
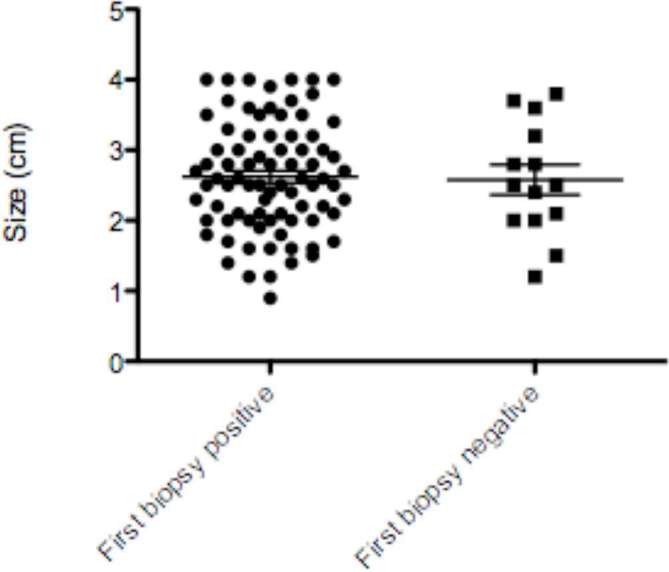
The distribution of tumour size in those with diagnostic (biopsy positive) and non-diagnostic (biopsy negative) biopsy of renal masses ≤ 4 cm in diameter [the errors bars refer to median (95% CI)]. CI, confidence interval.
Table 2.
Patient and tumour characteristics of the 95 small renal masses subcategorised according to tumour diameter
| Diameter | ≤2 cm (n = 25) | > 2 to ≤ 3 cm (n = 44) | > 3 to ≤ 4 cm (n = 26) | p value |
| Diagnostic first biopsy/non-diagnostic first biopsy | 21/4 | 38/6 | 22/4 | 1.00b |
| Male/female | 14/11 | 25/19 | 16/10 | 0.91c |
| Mean age (95% CI) | 62.9 (57.2–68.6) | 66.5 (62.8–70.2) | 61.5 (55.0–68.0) | 0.40d |
| Right/left | 14/11 | 20/24 | 10/16 | 0.54c |
| Needle gauge (18G/20G)a | 17/5 | 35/8 | 20/5 | 0.94c |
| Image guidance (CT/ultrasound) | 19/6 | 30/14 | 13/13 | 0.13c |
| Tumour location | ||||
| Upper pole | 5 | 12 | 10 | 0.16c |
| Interpolar | 11 | 19 | 14 | |
| Lower pole | 9 | 13 | 2 | |
| Exophytic/endophytic | ||||
| ≥50% exophytic | 13 | 16 | 10 | 0.37c |
| <50% exophytic | 8 | 16 | 6 | |
| Endophytic | 4 | 12 | 10 | |
| Centrality | ||||
| ≥7 mm from collecting system/sinus | 17 | 11 | 8 | <0.01b |
| >4 but ≤7 mm from collecting system/sinus | 5 | 12 | 1 | |
| ≤4 mm from collecting system/sinus | 3 | 21 | 17 | |
| Anterior/posterior | ||||
| Anterior | 10 | 9 | 9 | 0.42b |
| Posterior | 13 | 28 | 13 | |
| Neither | 2 | 7 | 4 | |
CI, confidence interval.
Data on needle size were not available for all cases.
Fisher’s exact test.
X2 test.
Kruskal–Wallis test.
Biopsy accuracy vs anatomical factors
Biopsy success rates according to anatomical locations are listed in Table 3. Anteriorly located SRMs were significantly more likely to fail initial biopsy [odds ratio (OR) 13.8; p ≤ 0.01], despite no significant difference in whether CT or ultrasound guidance was used (20/28 anterior lesions were biopsied using CT guidance vs 42/67 midline/posterior lesions (OR for CT 1.49, p = 0.42; X2test). Upper pole masses were also more likely to fail initial biopsy compared to interpolar or lower pole lesions (OR 4.35, p = 0.04); but were more frequently biopsied under CT guidance (22/27 upper pole under CT vs 40/68 inter/lower pole, OR 3.08, p = 0.04; X2 test).
Table 3.
Patient and tumour characteristics in patients with a diagnostic first renal mass biopsy vs non-diagnostic biopsy
| First biopsy diagnostic (n = 81) | First biopsy non-diagnostic (n = 14) | p-value | |
| Male/female | 48/33 | 7/7 | 0.52c |
| Mean age (95% CI) | 63.5 (60.4–66.6) | 68.0 (60.7–75.3) | 0.32d |
| Right/left | 39/42 | 5/9 | 0.39c |
| Needle gauge (18G/20G)a | 64/13 | 8/5 | 0.13b |
| Image guidance (CT/ultrasound) | 51/30 | 11/3 | 0.37b |
| Tumour location | |||
| Upper pole | 19 | 8 | 0.04b |
| Interpolar | 39 | 5 | |
| Lower pole | 23 | 1 | |
| Exophytic/endophytic | |||
| ≥50% exophytic | 33 | 6 | 0.23b |
| <50% exophytic | 28 | 2 | |
| Endophytic | 20 | 6 | |
| Centrality | |||
| ≥7 mm from collecting system/sinus | 31 | 5 | 1.0c |
| >4 but ≤7 mm from collecting system/sinus | 15 | 3 | |
| ≤4 mm from collecting system/sinus | 35 | 6 | |
| Anterior/posterior | |||
| Anterior | 17 | 11 | <0.01b |
| Posterior | 52 | 2 | |
| Neither | 12 | 1 | |
CI, confidence interval.
Data on needle gauge size were not available for all cases.
Fisher’s exact test.
X2 test.
Mann–Whitney U test.
Endophytic location or proximity to the renal sinus/collecting system did not influence biopsy success rates (Table 3). Needle gauge or the modalities used for biopsy guidance were also not important (Table 3). Overall R.E.N.A.L. nephrometry score also did not influence the diagnostic rate (mean R.E.N.A.L score was 6.79 in initially diagnostic biopsies vs 7.0 in non-diagnostic biopsies, p = 0.74; Mann–Whitney U test).
Accuracy of histological grading and relationship to mass size
On surgical excision, 17/21 (81.0%) SRMs had the same final RCC subtype as on biopsy (one poorly differentiated tumour was reclassified to tubular/spindle, one clear cell to papillary, one papillary and one chromophobe to unclassified). Führman grades were provided in 50/62 (80.6%) biopsy-proven RCCs: 6 (12%) were Grade 1, 4 (8%) were Graded 1–2, 37 (74%) had Grade 2 histology, 1 (2%) was Grade 2–3 and the finally 2 (4%) were Graded 3. 17 SRMs had Führman grades reported on both the biopsy and surgical specimens, with a concordance rate of 10/17 (58.8%). Führman grade was upgraded in six (all Grade 2 to 3) and downgraded in one (Grade 2 to 1) to give sample-upgrading and downgrading rates of 35.3 and 5.9% respectively. The likelihood of histological or cellular regrading was independent of tumour size. The mean size in those histologically reclassified was 2.9 cm (range 2.1–3.5) vs 2.8 cm (1.7–4.0) [for no change vs reclassified respectively (p = 0.93; Mann–Whitney U test)]. Corresponding values for masses with Führman grade reassignment were 2.8 cm (range 2.0–4.0) vs 2.9 cm (1.7–4.0) (p = 1.00; Mann–Whitney U test).
Complications
Complications were seen in 14 (13.6%) patients, all of which were small perinephric haematomas that did not require corrective blood transfusion or endovascular/surgical intervention (Clavien-Dindo Grade 1). An example is illustrated in Figure 4. Repeat biopsy did not increase the complication rate (1 of 7 (14%) had a perinephric bleed in the repeat biopsy group). Renal mass size, anatomical location or needle gauge did not influence complication rates (p > 0.05 for all comparisons). We were not able to study the influence of number of cores taken on bleeding rates, as this information was not retrospectively available. More perinephric haematomas were noted after CT guided biopsy, but this may be because CT is more sensitive for picking up small post biopsy collections.
Figure 4.

SRM of a posteriorly located lesion under CT-guidance (a). Repeat CT slice performed after the biopsy showing a small perinephric haematoma (arrow) that did not require transfusion or intervention (b). SRM, small renal mass.
Discussion
Our study shows that percutaneous biopsy of SRMs is safe and accurate, with an overall a diagnostic biopsy rate of over 91% and no major complications (defined as Clavien-Dindo Grade ≥2). High accuracy and safety can be achieved even with SRMs <2 cm in diameter. Tumour location influenced both biopsy success rate and choice of modality. Anterior or upper pole SRMs were more likely to require a repeat biopsy attempt, but repeat biopsy was as successful as first biopsy and without an increased complication rate. Location influenced the choice of image guidance, as upper pole masses were more frequently selected for biopsy under CT guidance.
Our data fall in line with those studies showing no effect of size on the outcomes of SRM biopsy.11–13 Although others have identified better diagnostic rates amongst larger renal masses,6–10 we argue that our study with a high proportion of ≤2 cm tumours (>25%) provides persuasive evidence that size does not negatively impact on biopsy success.
To our knowledge, we demonstrate for the first time, the reduced diagnostic yield from anterior tumours that may be due to the technically challenging approach required for these SRMs. We have found that anterior SRMs can be mobile and can readily deviate away from the needle path. Upper polar location also reduced biopsy success also felt to be due to their challenging anatomy, as the adjacent liver or spleen can restrict percutaneous access. Whilst this finding has not been corroborated in other series,6,7,9–11 we suggest polar location should be considered during biopsy planning. A small upper pole renal mass in a relatively cephalad kidney may require a steeply angled trajectory to avoid the pleura and lungs. Although needle guidance in orthogonal planes is easier achieved using ultrasound (Figure 2a), some small upper pole masses are difficult to adequately visualise on ultrasound and are more confidently targeted under CT guidance. No patient required a transhepatic approach for a right upper pole SRM biopsy, but this has been described as safe and accurate.18 To our knowledge, there have been no cases of hepatic seeding post renal cancer biopsy, but this has been described after adrenal tumour biopsy.19 The other anatomical factors did not negatively influence biopsy accuracy, but we feel these should also be considered during biopsy planning. Endophytic location was a negative variable in a previous study9 and can be challenging on CT.14 Closeness of the tumour to the collecting system and central sinus structures did not affect the diagnostic or complication rate and should not preclude percutaneous biopsy.
Imaging modality used was also irrelevant, but there is a selection bias, as a given SRM may have been selected for ultrasound or CT guidance based on their prior imaging. Although more haematomas were noted after CT, it cannot be concluded that CT-guided biopsy is a more hazardous procedure because CT is known to better detect post-biopsy perinephric haematomas than ultrasound.20 Whilst the R.E.N.A.L nephrometry score can impact on surgical and ablative treatment decisions,21, 22 our data suggest that it does not help predict the success of SRM biopsy.
Nearly 15% of first biopsies were not diagnostic and other studies also report a 10–20% failure rate.7–13 The reasons for this near constant “miss” rate across studies has not been well investigated, but in one study, failure was more common in those with a skin to tumour distance of >13 cm, tumour size <4 cm, cystic or poorly enhancing tumours.23 Although our results show that size is unimportant, our data indirectly also suggest that tumour depth can limit biopsy rates. We have shown anterior location SRMs more often fail biopsy and anterior SRMs are also deeper tumours. Nevertheless, we advise repeat biopsy if feasible as the success rate is as good as first biopsy and is just as safe. The high success rate of repeat biopsy has been shown elsewhere.7 This implies that failed biopsy is due to technical and uncontrollable variables such as tumour heterogeneity, fragmentation on biopsy, respiratory movement interfering with needle targeting and the difficulty of renal histological analysis on small samples. In our view, repeat biopsy should be performed in a similar manner as the first biopsy, except perhaps the modality of needle guidance.
The histological concordance rate was strong (81.0%) in our data set but cellular upgrading was seen in 35.3% (all Führman Grade 2 to 3). This shortcoming of core renal mass biopsy has been previously highlighted9, 10 and is thought to be due to the heterogeneity of the nuclear grade of SRMs.24 We found that smaller tumour size did not negatively impact on histological or cellular concordance rates. Nonetheless, as Führman grade is a known prognostic factor for cancer specific survival, 25 there should be a low threshold for rebiopsy if the clinical or imaging follow up is discordant with a biopsy diagnosis of indolent or low-grade RCC.
Current European Association of Urology guidelines recommend partial nephrectomy without biopsy for all suspected T1a RCCs3 and in the past biopsy has been reserved for the elderly or infirm. Yet, 22.2% of the SRM biopsies in our study showed a benign disease and in a recent national nephrectomy audit, benign SRMs were more frequent in younger people with masses <3 cm diameter.26 Our data show SRM biopsy to be safe and not affected by lesion size and we would argue that where the result may impact on management, all SRMs should be considered for potential biopsy regardless of patients’ age or tumour size. Such a strategy may avoid unnecessary surgery and also avoid a lengthy, costly period of radiological surveillance, as those with benign tumours could be reassured and discharged from follow up. Table 4 list the clinical scenarios where an SRM biopsy may be helpful.
Table 4.
Clinical scenarios where an SRM biopsy may be helpful
| Prior to/during active surveillance, if the patient is a candidate for later active therapy | Where a patient is unsure whether to pursue definitive treatment |
| Prior to ablation | Patients with chronic kidney disease |
| Synchronous tumours to assess for genetic or syndromic aetiologies | Suspected metastases (particularly lymphoma) or an inflammatory mass where a diagnosis has not otherwise been achieved |
| Young patients with small lesions—may be benign | A central infiltrating lesion where a transitional cell carcinoma is possible on imaging and only if urine cytological and endoscopic assessment have been unsuccessful |
SRM, small renal mass.
This is a retrospective study and thus has its limitations, e.g. there is a potential of selection bias, as we did not collect data on those SRMs that were not biopsied. It is possible that radiologically typical or aggressive tumours were selected for surgery without biopsy confirmation. Conversely, those deemed at higher surgical risk, e.g. endophytic SRMs or older patients may have been selected for biopsy. The number with non-diagnostic biopsy was small and the possibility of Type 2 statistical error cannot be discounted. There is also the possibility of verification bias, as surgical correlation was feasible only in those with renal cell cancer on biopsy. The study period was relatively long because the study accrual rate increased over time, reflecting increasing confidence with the accuracy and safety of image guided biopsy. Our study was not designed to evaluate the influence of experience on the chances of success, and this effect cannot be excluded. Some workers have advocated a biopsy-all approach prior to surgery, arguing that this prevents unnecessary surgeries and is cost effective,27 whilst others have suggested that this approach may not be beneficial for good surgical candidates and advise a patient-centred approach.28 Our study was not designed to study the costs and clinical impact of SRM biopsy and a prospective, randomised trial would be required to answer this question.
In conclusion, our study shows that SRM size does not affect the likelihood of achieving a diagnostic biopsy. Biopsy of anterior and upper pole SRMs is more likely to be unsuccessful, in which case repeat biopsy has a high success rate. Biopsy of smaller SRMs does not lead to more complications and most bleeding is minor and does not require any intervention. Although SRM biopsy is accurate for histological classification, there is a potential for Führman misclassification, but this is not related to mass size. We confirm that a significant number of SRMs are benign and as the accuracy and safety of SRM biopsy is not related to mass size, we advise that biopsy should be considered for all patients presenting with SRMs if the result may impact on management and list potential scenarios where SRM biopsy may be helpful.
Contributor Information
Matthew J Seager, Email: matthew.seager1@nhs.net.
Uday Patel, Email: uday.patel@stgeorges.nhs.uk.
Christopher J Anderson, Email: chris.anderson@stgeorges.nhs.uk.
Michael Gonsalves, Email: michael.gonsalves@stgeorges.nhs.uk.
REFERENCES
- 1.Gill IS, Aron M, Gervais DA, Jewett MA. Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624–34. doi: 10.1056/NEJMcp0910041 [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98: 1331–4. doi: 10.1093/jnci/djj362 [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015; 67: 913–24. doi: 10.1016/j.eururo.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Tsivian M, Mouraviev V, Albala DM, Caso JR, Robertson CN, Madden JF, et al. Clinical predictors of renal mass pathological features. BJU Int 2011; 107: 735–40. doi: 10.1111/j.1464-410X.2010.09629.x [DOI] [PubMed] [Google Scholar]
- 5.Patel HD, Johnson MH, Pierorazio PM, Sozio SM, Sharma R, Iyoha E, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol 2016; 195: 1340–7. doi: 10.1016/j.juro.2015.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon HG, Seo SI, Jeong BC, Jeon SS, Lee HM, Choi HY, et al. Percutaneous kidney biopsy for a small renal mass: a critical appraisal of results. J Urol 2016; 195: 568–73. doi: 10.1016/j.juro.2015.09.073 [DOI] [PubMed] [Google Scholar]
- 7.Leveridge MJ, Finelli A, Kachura JR, Evans A, Chung H, Shiff DA, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 2011; 60: 578–84. doi: 10.1016/j.eururo.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 8.Menogue SR, O'Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Int 2013; 111: E146–E151. doi: 10.1111/j.1464-410X.2012.11384.x [DOI] [PubMed] [Google Scholar]
- 9.Richard PO, Jewett MA, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol 2015; 68: 1007–13. doi: 10.1016/j.eururo.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 10.Richard PO, Jewett MA, Tanguay S, Saarela O, Liu ZA, Pouliot F, et al. Safety, reliability and accuracy of small renal tumour biopsies: results from a multi-institution registry. BJU Int 2017; 119: 543–9. doi: 10.1111/bju.13630 [DOI] [PubMed] [Google Scholar]
- 11.Dave CN, Seifman B, Chennamsetty A, Frontera R, Faraj K, Nelson R, et al. Office-based ultrasound-guided renal core biopsy is safe and efficacious in the management of small renal masses. Urology 2017; 102: 26–30. doi: 10.1016/j.urology.2016.12.026 [DOI] [PubMed] [Google Scholar]
- 12.Salem S, Ponsky LE, Abouassaly R, Cherullo EE, Isariyawongse JP, Maclennan GT, et al. Image-guided biopsy of small renal masses in the era of ablative therapies. Int J Urol 2013; 20: 580–4. doi: 10.1111/iju.12010 [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Wolf JS, Wood DP, Higgins EJ, Hafez KS. Accuracy of percutaneous core biopsy in management of small renal masses. Urology 2009; 73: 586–90. doi: 10.1016/j.urology.2008.08.519 [DOI] [PubMed] [Google Scholar]
- 14.Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol 2014; 31: 20–6. doi: 10.1055/s-0033-1363839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivian M, Rampersaud EN, del Pilar Laguna Pes M, Joniau S, Leveillee RJ, Shingleton WB, et al. Small renal mass biopsy-how, what and when: report from an international consensus panel. BJU Int 2014; 113: 854–63. doi: 10.1111/bju.12470 [DOI] [PubMed] [Google Scholar]
- 16.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009; 182: 844–53. doi: 10.1016/j.juro.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SY, Park BK, Kim CK. Sonographically guided transhepatic core biopsies of right renal and adrenal masses: safety and short-term follow-up. J Ultrasound Med 2013; 32: 2013–21. doi: 10.7863/ultra.32.11.2013 [DOI] [PubMed] [Google Scholar]
- 19.Mody MK, Kazerooni EA, Korobkin M. Percutaneous CT-guided biopsy of adrenal masses: immediate and delayed complications. J Comput Assist Tomogr 1995; 19: 434–9. [DOI] [PubMed] [Google Scholar]
- 20.Ralls PW, Barakos JA, Kaptein EM, Friedman PE, Fouladian G, Boswell WD, et al. Renal biopsy-related hemorrhage: frequency and comparison of CT and sonography. J Comput Assist Tomogr 1987; 11: 1031–4. doi: 10.1097/00004728-198711000-00021 [DOI] [PubMed] [Google Scholar]
- 21.Canter D, Kutikov A, Manley B, Egleston B, Simhan J, Smaldone M, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology 2011; 78: 1089–94. doi: 10.1016/j.urology.2011.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naya Y, Kawauchi A, Oishi M, Ueda T, Fujihara A, Naito Y, et al. Comparison of diameter-axial-polar nephrometry and RENAL nephrometry score for treatment decision-making in patients with small renal mass. Int J Clin Oncol 2015; 20: 358–61. doi: 10.1007/s10147-014-0714-2 [DOI] [PubMed] [Google Scholar]
- 23.Prince J, Bultman E, Hinshaw L, Drewry A, Blute M, Best S, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol 2015; 193: 1899–904. doi: 10.1016/j.juro.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball MW, Bezerra SM, Gorin MA, Cowan M, Pavlovich CP, Pierorazio PM, et al. Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol 2015; 193: 36–40. doi: 10.1016/j.juro.2014.06.067 [DOI] [PubMed] [Google Scholar]
- 25.Rioux-Leclercq N, Karakiewicz PI, Trinh QD, Ficarra V, Cindolo L, de la Taille A, et al. Prognostic ability of simplified nuclear grading of renal cell carcinoma. Cancer 2007; 109: 868–74. doi: 10.1002/cncr.22463 [DOI] [PubMed] [Google Scholar]
- 26.Fernando A, Fowler S, O’Brien T, British Association of Urological Surgeons (BAUS). Nephron-sparing surgery across a nation - outcomes from the British association of urological surgeons 2012 national partial nephrectomy audit. BJU Int 2016; 117: 874–82. doi: 10.1111/bju.13353 [DOI] [PubMed] [Google Scholar]
- 27.Pandharipande PV, Gervais DA, Hartman RI, Harisinghani MG, Feldman AS, Mueller PR, et al. Renal mass biopsy to guide treatment decisions for small incidental renal tumors: a cost-effectiveness analysis. Radiology 2010; 256: 836–46. doi: 10.1148/radiol.10092013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel HD, Pierorazio PM. Kidney cancer: Undertreatment of small renal masses by overuse of biopsy. Nat Rev Urol 2016; 13: 701–3. doi: 10.1038/nrurol.2016.213 [DOI] [PubMed] [Google Scholar]