Abstract
Objective:
Hypofractionated radiotherapy in early stage breast cancer is an effective adjuvant treatment, but there is a lack of randomized data for patients with ductal carcinoma in situ (DCIS). The aim of this study is the evaluation of skin toxicity and cosmesis, and early clinical outcome of DCIS patients enrolled in an institutional Phase II trial of hypofractionated breast irradiation.
Methods:
137 DCIS patients were enrolled in the trial. All patients underwent volumetric modulated arc therapy (VMAT) to the whole breast with a total dose of 40.5 Gy in 15 fractions over 3 weeks, without tumour bed boost. Acute and late skin toxicities were recorded. Cosmetic outcomes were assessed as excellent/good or fair/poor. Early clinical outcome was reported.
Results:
Median age was 58 y.o. (range 30–86). The median follow-up time was 22 months (range 6–45). At the end of the radiotherapy, skin toxicity was grade G1 in 56% of the patients, G2 in 15%, no patients presented G3 toxicity. In the range of 3–9 months of follow-up, the skin toxicity was G1 in 28% of patients, no G2–G3 cases; cosmetic outcome was good/excellent in 95% of patients. In the follow-up interval of 9–24 months, the skin toxicity was G1 in 12% of patients, no G2-G3 toxicity; cosmetic outcome was good/excellent in 96% of patients. After an early evaluation of clinical outcomes, 5 patients (3.6%) presented an in-breast recurrence.
Conclusion:
Hypofractionated radiotherapy using VMAT is a viable option for DCIS. A longer follow-up is needed to assess clinical outcomes and late toxicity.
Advances in knowledge:
The use of hypofractionated VMAT is dosimetrically feasible for treating breast DCIS.
Introduction
Screening and advances in breast imaging led to a continuous increase of ductal carcinoma in situ (DCIS) diagnosis.1 Unfortunately, the management of this specific disease is still controversial regarding adjuvant therapy (radiation therapy and endocrine therapy) after the breast conserving surgery (BCS).
Four randomized trials have shown a decrease of the local recurrence (LR) using adjuvant radiotherapy (from 28 to 13% at 10 years) with conventional fractionation (50 Gy in 25 fractions),2–5 but there is no prospective trial data about the use of hypofractionated regimens in patients with DCIS.
Different authors reported analysis of small series, comparing standard radiotherapy with hypofractionated schedules,6–11 and all confirmed the equivalence in local control rates. Other investigators published toxicity, cosmetic and clinical outcomes of DCIS patients treated with hypofractionation,12–15 reporting encouraging results for the introduction of shorter schedules in the management of DCIS patients.
In this context, the role of the radiotherapy boost is another debated issue. A retrospective analysis of a mono-institutional experience published by the Florence group showed the negative prognostic impact of surgical margins <1 mm on LR rate, and the beneficial role of the radiation boost.16 A meta-analysis of observational studies17 confirmed a reduction of the risk for LR by adding the radiotherapy boost only in the presence of positive margins. Despite this results, a more recent retrospective analysis, using data from a large multi-institutional database, suggested that a radiotherapy boost for DCIS is associated with a small but statistically significant benefit in decreasing long-term LR, regardless the patient age and the endocrine therapy with tamoxifen.18 To better assess the use of the boost for DCIS patients, several prospective randomized trials are ongoing, but the results are expected in about 10 years.19
In the meantime, in the absence of robust evidence, the clinical management of DCIS patients varies, among different institutions, in the choice of the fractionation schemes, the use of an additional boost and the endocrine therapy.
We previously reported on our Phase II trial on early stage breast irradiation with hypofractionated simultaneous integrated boost (SIB) and volumetric modulated arc therapy (VMAT) technique.20 Since 2013, with an amendment to the protocol, also patients presenting DCIS were considered eligible for receiving a hypofractionated treatment without SIB. The motivation of using an advanced technique as VMAT for breast radiotherapy in place of the most consolidated conventional tangential beams is supported, particularly for increased fraction doses, by the possibility to lower the doses to critical structures (mainly heart and lung) and reducing the target dose inhomogeneity (dose spillage). Patients could in principle benefit from such a dose distribution improvement, in terms of possible improved toxicity profile.
In the present analysis, we reviewed the preliminary data for the DCIS subgroup, treated with hypofractionated VMAT according to our institutional protocol, in terms of cosmetic outcomes, toxicity and local control.
Methods and materials
From September 2013 to July 2016, 137 patients with DCIS after BCS received hypofractionated adjuvant radiotherapy with RapidArc technology (VMAT) at our institution. The study received the approval by the Ethical Review Committee, in compliance with the Helsinki declaration. Informed consent was obtained from all individual patients.
The results concerning acute and early late skin toxicity and cosmesis, as well as the local control, were reported and evaluated in this study.
For radiotherapy treatment, all patients were simulated in supine position, with both arms above the head. CT dataset was acquired with 3 mm thick adjacent slices. Respiratory gating was not in the protocol requirements. The VMAT technique can deliver almost no high dose levels to the heart, confining the use of respiratory gating, in particular with deep inspiration breath hold, only to particularly complex patient anatomies.
The clinical target volume was the entire mammary gland. The planning target volume (PTV) was contoured by adding a 5 mm margin to the clinical target volume, limited to 4 mm within the skin surface, and excluded ribs and lung parenchyma. The treatment dose was prescribed as 40.5 Gy to the PTV in 15 fractions of 2.7 Gy over 3 weeks.21
The target plan objectives, for both dose coverage and homogeneity, were: D98% >95% (near-to-minimum dose, as dose received by at least 98% of the volume, greater than 95% of the prescribed dose), D2% <107% (near-to-maximum dose, as dose received by at most 2% of the volume, less than 107%). Concerning organs at risk (OAR)21: mean dose to ipsilateral lung <10 Gy, and the volume receiving more than 20 Gy should not exceed 10% (V20Gy <10%); related to heart: V40Gy <3% and V18Gy < 5%, mean dose <4 Gy (this last objective has been added during the period of the current data collection); minimize contralateral lung and breast irradiation.
Plans were optimized for RapidArc delivery, with two to four partial arcs in a range from the classical medial tangential beam (around ± 60 degree) to the almost posterior entrance (around ± 170 degree), through the PTV side; the collimator was rotated for the first two arcs to ± 20–30 degree, depending on the patient and target anatomy, the other two, when used, had collimator rotated to 90 degree; the field size was limited by the jaws to 15 cm in the leaf motion direction, and as long as needed to better cover the target in the other direction. Progressive resolution optimizer (PRO) algorithm was used to modulate multileaf collimator (MLC) shape and beam intensity during the gantry rotation. The strategy described by Nicolini et al22 for the skin flash was always adopted, using either the body expansion or the bolus. Dose calculations used the anisotropic analytical algorithm (AAA). Delivery was on 6 MV beams from Varian Clinac, Unique or TrueBeam (Varian Medical Systems, Palo Alto, CA), equipped with a multileaf collimator Millennium MLC-120.
A daily cone-beam CT acquisition (or 2D-2D matching for the patients treated on the Unique linear accelerator, not equipped with cone-beam CT) allowed the patient positioning verification before each treatment session.
Once a week, during the treatment, the patient clinical evaluation was assessed. Follow-up visits were scheduled at 1, 3 and 6 months after radiotherapy treatment, and then every 6 months for the first 2 years. Every 6 months haematologic examinations (i.e. complete blood count (CBC), liver and renal function, tumour marker Ca15.3), as well as breast ultrasound were scheduled; every 12 months a bilateral mammography was requested.
Acute toxicity was scored according to radiation therapy oncology group acute radiation morbidity scoring criteria, and late toxicity (from 6 month after radiotherapy) according to CTCAE (Common Terminology Criteria for Adverse Events) v. 4. The main endpoint for late skin toxicity was the hyperpigmentation; fibrosis and telangiectasia were also reported. Cosmetic outcomes were scored as excellent/good vs fair/poor, according to the Harvard scale.23 Skin toxicity was evaluated by two observers (a dedicated breast nurse and a radiation oncologist). Other toxicities were also evaluated: breast pain, (presence or absence, with no differentiation of pain intensity), presence of liponecrosis (through ultrasound examination); the lung toxicity was assessed with a thorax radiography every 12 months and was investigated in case of respiratory symptoms. Heart toxicity was evaluated only for symptomatic patients.
Dosimetric evaluation was based on dose-volume histogram analysis of targets and OAR. Reported data were mean doses, Vx (volume receiving more than x dose) and Dy (dose received by at least y volume).
Statistical analysis and data correlation was performed using the IBM SPSS software (Statistical Package for Social Science, v. 21.0). Standard descriptive statistics was used to describe the data. Univariate analysis, using ANOVA (analysis of variance) statistics for correlations, was performed to investigate the prognostic role of individual variables. Significance value was set to 0.05.
Results
The median follow-up of the 137 analysed patients was of 22 months (range 6–45 months).
Patient characteristics are summarized in Table 1. Two patients (one left and one right side) presented synchronous invasive carcinoma on the contralateral breast relative to the DCIS, and were treated simultaneously in 15 fractions on both mammary glands: as described in the Methods section for the DCIS, and with a simultaneous integrated boost up to 48 Gy (3.2 Gy/fraction) on the boost region for the invasive contralateral treatment.
Table 1.
Patient characteristics
| Number of patients | 137 | |
| Age (years old) | Median (range) | 58 (30, 86) |
| Breast laterality | Left | 75 (54.7%) |
| Right | 62 (45.3%) | |
| Performance status | 0 | 117 (85.4%) |
| 1 | 20 (14.6%) | |
| Menopausal | Yes | 91 (66.4%) |
| No | 46 (33.6%) | |
| Grading | G1 | 6 (4.4%) |
| G2 | 68 (49.6%) | |
| G3 | 56 (40.9%) | |
| Unknown | 7 (5.1%) | |
| Comedo subtype | Yes | 50 (36.5%) |
| No | 87 (63.5%) | |
| Multifocality | Yes | 35 (25.5%) |
| No | 102 (74.5%) | |
| Sentinel node biopsy | Yes | 87 (63.5%) |
| No | 50 (36.5%) | |
| Surgical margins | Negative | 118 (86.1%) |
| Close | 16 (11.6%) | |
| Positive | 3 (2.2%) | |
| pNsn | Negative | 82 (94%) |
| Positive | 5 (6%) | |
| Endocrine therapy | Yes | 15 (10.9%) |
| No | 122 (89.1%) |
pNsn, pathological sentinel node.
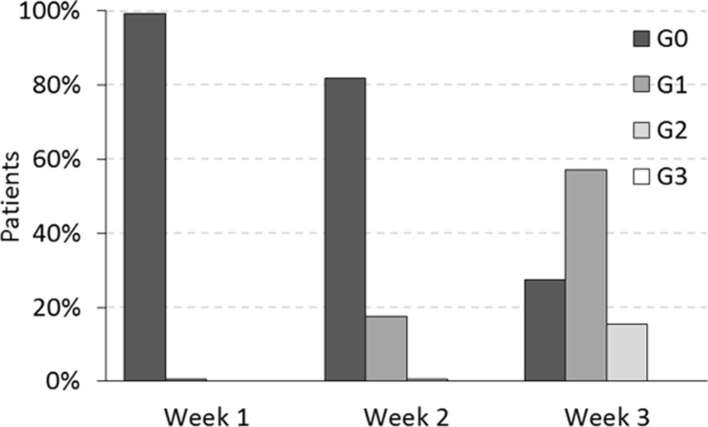
We recorded a relative prevalence of unfavourable prognostic factors: comedo subtype were reported in 36.5% of the patients and high nuclear grade in 40.9%. Endocrine therapy was administered only in the 10.9% of the entire cohort. Acute skin toxicities reported during the treatment course at 1, 2 and 3 weeks, are reported in Figure 1, where a maximum of Grade 2 (G2) acute toxicity was reported at the third week of treatment by 15% of patients. No grade higher than G2 was reported.
Figure 1.
Skin toxicity during the radiotherapy treatment.
As late skin toxicity, no patients presented fibrosis nor telangiectasia.
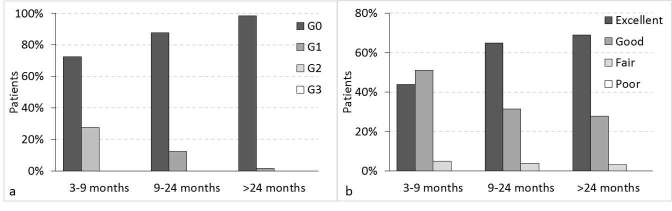
Skin toxicity and cosmetic results after the treatment are shown in Figure 2 for the temporal ranges of 3–9, 9–24 and >24 months after radiotherapy. No G2 or greater toxicity was reported. Only 2% of the patients reported G1 skin toxicity (the highest grading) as dermatitis, after 2 years from the treatment.
Figure 2.
(a) Skin toxicity during the follow-up; (b) cosmesis during the follow-up.
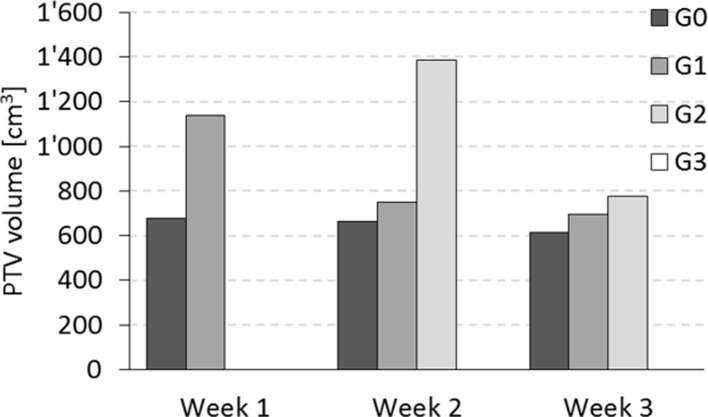
A highly significant correlation was shown between late skin toxicity (hyperpigmentation) and both the PTV volume (p = 0.007) and, more interestingly, the volume receiving at least 90% of the prescription dose (p < 0.001). The mean PTV volume of patients presenting late G0 and G1 was 651 ± 41 cm3 and 992 ± 148 cm3, respectively, as mean ± standard error of the mean. The body V90% for G0 and G1 groups were 789 ± 44 and 1469 ± 290 cm3. The same trend was found for acute toxicity during the treatment, with larger average volumes for higher toxicity level; however, those differences were not statistically significant in the current group of patients. Figure 3 shows the average PTV volumes for the different groups of patients. Similarly, the mean PTV volume for the patients showing excellent cosmesis was 592 ± 45, and 905 ± 83 cm3 for the patients with good cosmetic results (p = 0.002). The only four patients with fair cosmesis had a mean volume of 932 ± 226 cm3.
Figure 3.
Acute skin toxicity during the treatment related to the breast volume.
Breast pain was present in 31% of the patients at the last follow up (median 22, range 6–45 months). Although not statistically significant (p = 0.31), the patients presenting breast pain after the radiotherapy treatment had an average volume treated to 90% of the prescribed dose (treated volume) of 910 ± 103 cm3, while it was 861 ± 68 cm3 for the patients not presenting breast pain, showing a more pronounced trend of presenting pain for large breast cases (breast volume is strictly correlated to the treated volume).
Liponecrosis was reported in 7, 14 and 31% of the analysed cases in the intervals of 2–9, 9–24 and >24 months. About one fourth of the patients presenting liponecrosis had also breast pain at the last follow up.
No patients developed respiratory symptoms nor heart toxicity until the last follow-up.
During the follow-up, nine patients (6.6%) presented loco-regional recurrence. New contralateral breast cancers arose in four cases (1 DCIS and 3 invasive carcinomas).
Five patients (3.6%) experienced an ipsilateral breast recurrence IBR (1 DCIS and 4 invasive carcinomas), as summarized in Table 2. In 3 of the 5 patients, recurrence occurred at the original site of DCIS and in the remaining 2 cases outside the original tumour bed. Three of the 5 patients were estrogen and/or progesterone receptor, ER and PgR, positive; however, none of them received endocrine therapy. One patient had close margin after BCS and another one had multifocal disease; two patients had comedo subtype and high nuclear grade; one patient presented multifocality, comedo subtype and high nuclear grade. The analysis of these unfavourable features confirmed a relative higher rate of comedo subtype and high nuclear grade (60%) in the subset of ipsilateral breast recurrence cases compared to the total of the patients. The LR management was approached as follows: one patient with DCIS recurrence underwent BCS; among the patients with invasive carcinoma recurrence, one underwent mastectomy, two had chemotherapy administered after mastectomy and one had metastases at the time of the recurrence diagnosis and received chemotherapy without surgery.
Table 2.
Characteristics of the ipsilateral breast recurrence patients
| Patient | Age (y.o.) | Meno-pausal | Grading | Comedo | Multifoc. | Surg. Marg. | ER/PgR (%) | Endocr. therapy | Time to LR (months) | Recurrence histology |
| 1 | 49 | No | G2 | No | No | Close | 80/60 | No | 44.4 | Invasive |
| 2 | 58 | Yes | G3 | Yes | Yes | Negative | 0/0 | No | 12.7 | DCIS |
| 3 | 64 | Yes | G3 | Yes | No | Negative | 25/10 | No | 13.4 | Invasive |
| 4 | 40 | No | G2 | No | Yes | Negative | 90/5 | No | 35.3 | Invasive |
| 5 | 58 | Yes | G3 | Yes | No | Negative | 0/0 | No | 21.5 | Invasive |
ER/PgR, estrogen/progesterone receptor; LR, local recurrence; DCIS, ductal carcinoma in situ.
Time to progression is shown with the Kaplan–Meier curve in Figure 4, where only the loco-regional recurrences have been included as events, while the distant metastases event was censored, being related to another cancer.
Figure 4.
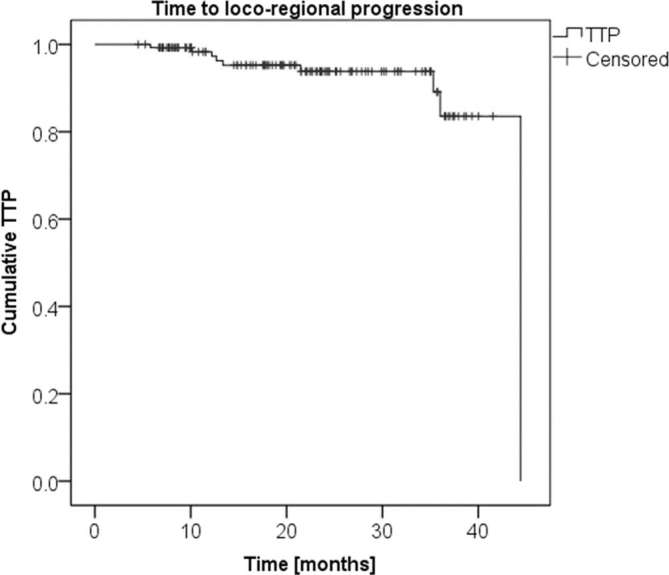
Kaplan–Meier curve of the time to loco-regional progression.
Concerning dosimetric results, in Table 3 a summary of some parameters related to the main OARs is reported. Of interest is the evolution of the dosimetric results with time. For example, the heart mean dose for left-sided breast cancer was requested to be lower than 4 Gy during the data collection (and treatment plan preparation). Plotting the mean heart dose sequentially according to the treatment date, a decreasing trend of mean heart dose is shown. In particular, the linear relationship of this data presented a mean heart dose of 4.8 Gy at the beginning of the data collection, and of 2.1 Gy at its end.
Table 3.
Dosimetric data
| Structure | Parameter | Mean ± SD |
| Lung, ipsilateral | Mean (Gy)] | 7.00 ± 0.90 |
| V20Gy (%) | 6.19 ± 1.90 | |
| Lung, contralateral | Mean (Gy) | 2.21 ± 0.65 |
| Heart (left-sided cancer only) | Mean (Gy) | 3.94 ± 1.21 |
| V18Gy (%) | 1.21 ± 1.08 | |
| Heart (right-sided cancer only) | Mean (Gy) | 2.73 ± 1.09 |
| Breast, contralateral | Mean (Gy) | 2.26 ± 0.46 |
| PTV | SD (Gy) | 1.12 ± 0.15 |
| V105% (%) | 3.0 ± 2.6 |
PTV, planning target volume; SD, standard deviation.
Discussion
Hypofractionation represents the new standard radiation treatment for patients with early stage invasive breast cancer. Based on the long-term results of large randomized trials,24–26 the recent NCCN (National Comprehensive Cancer Network) guidelines update recommends the short schedule as preferred option for these patients.27
Nevertheless, there is a lack of robust evidence about the use of hypofractionation in DCIS patients.
Different authors have investigated this issue during the last years, whose results have been summarized in Table 4.
Table 4.
Synoptic table of previous studies on DCIS
| Author, year | Number of pts | Median follow-up (months) | Total dose/fractions | Boost | Nuclear grade G3 (% of pts) | Comedo subtype (% of pts) | Endocrine therapy (% of pts) | Late skin toxicity | Cosmetic outcome (% of good/excellent) | Local recurrence(% of pts) |
| Ciervide et al 201112 | 145 | 60 | 42 Gy/1540.5 Gy/15 | No48 Gy/15 (SIB) | 46 | NR | 43 | G2: 8 and 7%b | 91 | 4.1 |
| Hathout et al 201313 | 440 | 52.8 | 42.5 Gy/16 | 10 Gy/4 (28%) | 28 | NR | 52 | NR | NR | 3.2 |
| Guenzi et al 201314 | 113 | 30.5 | 46 Gy/2039 Gy/13 | 1.2–3 Gy/5 a1.2–1 Gy/5,3,4 a | 19.5 | 16.9 | 9.7 | G2: 0%G2: 3% | 97.677.8 | 0.8 |
| Cante et al 201415 | 103 | 48 | 45 Gy/20 | 50 Gy/20 (SIB) | 25 | 18 | 14 | G2: 8%; G3: 1% | 87 | 0 |
| Current study | 137 | 22 | 40.5 Gy/15 | No | 40.9 | 36.5 | 10.9 | G2: 0% | 96 | 3.6 |
NR, not reported; SIB, simultaneous integrated boost; pts, patients.
aConcomitant boost.
bHighest G2 toxicities: fibrosis and telangiectasia.
Ciervide et al12 published 5-year outcome of 145 patients enrolled in two trials, the first [New York University (NYU) 01–51] with a whole breast total dose of 42 Gy delivered in 15 fractions and the second trial (NYU 05–181) with a total dose of 40.5 Gy in 15 fractions to the whole breast and an additional concomitant boost (a total dose of 48 Gy to the surgical cavity). They reported an optimal cosmetic outcome (91% good to excellent) and a LR rate of 4.1% (3 patients in each trial, with a time to LR of 10–34 and 42–79 months in the boost and non-boost trials, respectively).
Hathout et al13 analysed data from 440 patients treated with a hypofractionated scheme in 16 fractions for a total dose of 42.5 Gy. An additional sequential boost was administered to 125 patients (28%) at a median dose of 10 Gy in 4 fractions. With a median follow up of 4.4 years, they showed a LR rate of 3.2% with no significant impact of the presence of the boost; however, a trend to lower LR rate in the various subgroups was shown for patients receiving the boost. No data were reported about cosmetic outcome or toxicity.
Guenzi et al14 reviewed data from 113 patients treated with two different adjuvant radiotherapy schedules: 46 Gy delivered in 20 fractions four times a week for 5 weeks (41 patients) and 39 Gy in 13 fractions four times a week for 3.5 weeks (72 patients). Both schemes included a concomitant boost to the tumour bed according to the surgical margin status. After a median follow up of 30.5 months, they recorded a cosmetic outcome excellent or good in 97.6% of patients treated with the first scheme and in 77.3% of patients treated with the shorter schedule. No Grade 3–4 of toxicity were described. Only one patient developed a LR (0.8%).
Lastly, Cante et al15 published the analysis on a total of 103 DCIS patients with a median follow-up time of 4 years. They prescribed a total dose of 45 Gy to the whole breast and 50 Gy to the tumour bed in 20 fractions with concomitant boost technique. No LR were recorded. Cosmetic outcome was excellent or good in 87% of patients. G3 acute toxicity was reported in 2% of the patients and G3 late toxicity in 1% of the patients (telangiectasia).
The here reported experience, according to the cited studies, confirmed an optimal cosmetic outcome (excellent or good in 96% of patients) and toxicity profile (no G2 or greater was recorded after the end of radiation treatment) of the hypofractionated scheme delivered using VMAT technique. Lower cosmetic scores were reported in some subgroups of patients in the Guenzi and Cante studies. To notice is the different treatment technique used in those studies, being conformal therapy with the use of wedges, which could generate inferior target dose homogeneity. Higher late skin toxicity was reported by Ciervide, where the beam geometry consisted again in two fixed tangential beams (conformal therapy and intensity-modulated beams for the two different protocols).
The current results are similar to those reported in our previous study on hypofractionated VMAT treatments for patients with invasive breast cancer: excellent or good cosmesis at 1 year in 96% of the patients, only one case of G3 skin toxicity after 1 month by the end of the treatment, and no patients with more than G1 toxicity after 1 year.20
The current study confirmed a prevalence of breast pain and a significant correlation of skin toxicity with patients having large breasts. This finding was also reported by Fiorentino et al28 in a study focused on radiotherapy treatment in elderly breast cancer patients; they presented a statistically significant correlation between G2 skin toxicity and the breast volume larger than 700 cm3 (p = 0.04). Regarding breast pain, Mak et al29 conducted a questionnaire-based study to characterize long-term breast pain in a population of early-stage breast cancer patients. They reported the volume of breast tissue treated to ≥105 or ≥110% of the prescribed dose using conformal techniques as predictive factor for pain. However, these values were not correlated to the absorbed dose, making difficult the comparison with different fractionation schemes. Nevertheless, the hot spots should clearly be avoided, enforcing the possible benefit in using the VMAT technique: only 3% of the target volume received more than 105% of the prescribed dose in the here presented VMAT patients. In our analysis, although not statistically significant (p = 0.31), the patients presenting breast pain had a larger volume treated to 90% of the prescribed dose (of 40.5 Gy in 15 fractions) compared to the other patients, parameter that is of course correlated with breast size.
Regarding dosimetric results, we observed an interesting progressive decrease of the mean OAR doses according to the treatment date to confirm a continuous improvement in VMAT planning, as described in the results for example with a reduction of the mean heart dose from an average of 4.8 Gy to the actual 2.1 Gy.
A possible concern of using the VMAT technique for breast cancer patient, especially for young females, is related to the second cancer induction risk, which in principle could be increased with this technique relative to the conformal tangential beam setting, due to the exposure of the normal tissues to low doses by the arc arrangement. For this reason, the use of VMAT has to be carefully evaluated in terms of achieved plan quality. Recently, two VMAT arc arrangements were compared with the tangential field-in-field conformal planning estimating the excess absolute risk of second cancer induction from the irradiation of the contralateral structures (breast and lung). The authors concluded that with a proper VMAT arc geometry, the excess absolute risk of contralateral breast cancer induction is 2.4 per 10,000 patients-year, to compare with 1.7 for the field-in-field conformal technique (8.5 for the other VMAT geometry); while for contralateral lung, the values are 1.6 and 1.5, respectively. Those values prove that the VMAT technique can be safely use in relation to second cancer induction, but only in the case where a high level of plan quality can be achieved, and has to be carefully considered for young patient treatment.30
In the current analysis, the LR rate was higher (3.6%) than those reported in the previous cited series, if we consider the shorter median follow-up time of 22 months (Ciervide and Hathout reported similar LR rate, but with a median follow-up of 60 and 53 months, respectively).
There are different possible reasons to explain this result. The first could be ascribed to the prescribed dose, with no additional radiotherapy boost. This factor however would concur with others, as the low percentage of patients (10.9%) having received endocrine therapy, as well as the patients characteristics, presenting a relative prevalence of unfavourable prognostic factors such as the comedo subtype, the multifocality and the high nuclear grade, surgical margin, age, that could have negatively influenced our clinical outcome. On the other hand, the small sample size is the major limitation of our study and it does not allow any definitive consideration on the relevant prognostic factors.
The wide heterogeneity of DCIS patients is probably the real key to understand the complex management of this disease. We should try to choose an ideal balance between efficacy and side effects, modulating adjuvant therapeutic strategies.31–33 Data from randomized trials have confirmed that tamoxifen use lead to a reduction of the incidence of recurrent ipsilateral DCIS and contralateral tumours, but having no effect on ipsilateral invasive disease.34 On the other side, preliminary data from observational studies have shown a benefit in decreasing of LR with the addition of the boost, but we have to wait for long-term results of the ongoing prospective randomized trials on this issue.19 Further investigations are then needed to identify in which DCIS patient the omission of adjuvant therapy is safe, or in which DCIS patients the additional radiotherapy boost or the endocrine therapy is recommended, especially considering compliance, toxicity, and the longer duration of this kind of treatment.
Conclusions
The 3-week hypofractionated schedule using VMAT technique is a well-tolerated radiation treatment for DCIS patients and it is associated with an optimal cosmetic and skin toxicity outcome. After a critical analysis of our LR rate, a subgroup of high-risk DCIS patients (patients with at least two unfavourable prognostic factors) should be identified, to intensify their treatment, for example with the additional radiotherapy boost. Considering the progressive increase in DCIS diagnoses, the definition of a risk-adapted therapeutic management becomes crucial to guide in the decision-making process.
Footnotes
Conflict of interest: L Cozzi acts as Scientific Advisor to Varian Medical Systems and is Clinical Research Scientist at Humanitas Cancer Center. All other co-authors declare that they have no conflict interests.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval: All procedures were in accordance with the ethical standards and the Helsinki declaration.
Contributor Information
Fiorenza De Rose, Email: fiorenza.de_rose@humanitas.it.
Antonella Fogliata, Email: Antonella.Fogliata@humanitas.it.
Davide Franceschini, Email: davide.franceschini@humanitas.it.
Cristina Iftode, Email: cristina.iftode@humanitas.it.
Rosalba Torrisi, Email: rosalba.torrisi@humanitas.it.
Giovanna Masci, Email: giovanna.masci@humanitas.it.
Andrea Sagona, Email: andrea.sagona@humanitas.it.
Corrado Tinterri, Email: corrado.tinterri@humanitas.it.
Alberto Testori, Email: alberto.testori@humanitas.it.
Wolfgang Gatzemeier, Email: wolfgang.gatzemeier@humanitas.it.
Bethania Fernandes, Email: bethania.fernandes@humanitas.it.
Daoud Rahal, Email: daoud.rahal@humanitas.it.
Luca Cozzi, Email: luca.cozzi@humanitas.it.
Armando Santoro, Email: armando.santoro@humanitas.it.
Marta Scorsetti, Email: marta.scorsetti@humanitas.it.
REFERENCES
- 1.Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010; 2010: 139–41. doi: 10.1093/jncimonographs/lgq027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet 2003; 362: 95–102. doi: 10.1016/S0140-6736(03)13859-7 [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998; 16: 441–52. doi: 10.1200/JCO.1998.16.2.441 [DOI] [PubMed] [Google Scholar]
- 4.Julien JP, Bijker N, Fentiman IS, Peterse JL, Delledonne V, Rouanet P, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet 2000; 355: 528–33. [DOI] [PubMed] [Google Scholar]
- 5.Donker M, Litière S, Werutsky G, Julien JP, Fentiman IS, Agresti R, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 2013; 31: 4054–9. doi: 10.1200/JCO.2013.49.5077 [DOI] [PubMed] [Google Scholar]
- 6.Williamson D, Dinniwell R, Fung S, Pintilie M, Done SJ, Fyles AW. Local control with conventional and hypofractionated adjuvant radiotherapy after breast-conserving surgery for ductal carcinoma in-situ. Radiother Oncol 2010; 95: 317–20. doi: 10.1016/j.radonc.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 7.Wong P, Lambert C, Agnihotram RV, David M, Duclos M, Freeman CR. Ductal carcinoma in situ-the influence of the radiotherapy boost on local control. Int J Radiat Oncol Biol Phys 2012; 82: e153–e158. doi: 10.1016/j.ijrobp.2011.03.045 [DOI] [PubMed] [Google Scholar]
- 8.Wai ES, Lesperance ML, Alexander CS, Truong PT, Culp M, Moccia P, et al. Effect of radiotherapy boost and hypofractionation on outcomes in ductal carcinoma in situ. Cancer 2011; 117: 54–62. doi: 10.1002/cncr.25344 [DOI] [PubMed] [Google Scholar]
- 9.Rakovitch E, Nofech-Mozes S, Narod SA, Hanna W, Thiruchelvam D, Saskin R, et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat 2013; 138: 581–90. doi: 10.1007/s10549-013-2455-8 [DOI] [PubMed] [Google Scholar]
- 10.Lalani N, Paszat L, Sutradhar R, Thiruchelvam D, Nofech-Mozes S, Hanna W, et al. Long-term outcomes of hypofractionation versus conventional radiation therapy after breast-conserving surgery for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys 2014; 90: 1017–24. doi: 10.1016/j.ijrobp.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 11.Isfahanian N, Al-Hajri T, Marginean H, Chang L, Caudrelier JM. Hypofractionation is an acceptable alternative to conventional fractionation in the treatment of postlumpectomy ductal carcinoma in situ with radiotherapy. Clin Breast Cancer 2017; 17: e77–e85. doi: 10.1016/j.clbc.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 12.Ciervide R, Dhage S, Guth A, Shapiro RL, Axelrod DM, Roses DF, et al. Five year outcome of 145 patients with ductal carcinoma in situ (DCIS) after accelerated breast radiotherapy. Int J Radiat Oncol Biol Phys 2012; 83: e159–e164. doi: 10.1016/j.ijrobp.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 13.Hathout L, Hijal T, Théberge V, Fortin B, Vulpe H, Hogue JC, et al. Hypofractionated radiation therapy for breast ductal carcinoma in situ. Int J Radiat Oncol Biol Phys 2013; 87: 1058–63. doi: 10.1016/j.ijrobp.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 14.Guenzi M, Giannelli F, Bosetti D, Blandino G, Milanese ML, Pupillo F, et al. Two different hypofractionated breast radiotherapy schedules for 113 patients with ductal carcinoma in situ: preliminary results. Anticancer Res 2013; 33: 3503–7. [PubMed] [Google Scholar]
- 15.Cante D, Franco P, Sciacero P, Girelli G, Marra AM, Pasquino M, et al. Hypofractionation and concomitant boost to deliver adjuvant whole-breast radiation in ductal carcinoma in situ (DCIS): a subgroup analysis of a prospective case series. Med Oncol 2014; 31: 838. doi: 10.1007/s12032-014-0838-2 [DOI] [PubMed] [Google Scholar]
- 16.Meattini I, Livi L, Franceschini D, Saieva C, Meacci F, Marrazzo L, et al. Role of radiotherapy boost in women with ductal carcinoma in situ: a single-center experience in a series of 389 patients. Eur J Surg Oncol 2013; 39: 613–8. doi: 10.1016/j.ejso.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Nilsson C, Valachis A. The role of boost and hypofractionation as adjuvant radiotherapy in patients with DCIS: a meta-analysis of observational studies. Radiother Oncol 2015; 114: 50–5. doi: 10.1016/j.radonc.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Moran MS, Zhao Y, Ma S, Kirova Y, Fourquet A, Chen P, et al. Association of radiotherapy boost for ductal carcinoma in situ with local control after whole-breast radiotherapy. JAMA Oncol 2017; 3: 1060. doi: 10.1001/jamaoncol.2016.6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States National Library of Medicine. Radiation doses and fractionation schedules in non-low risk ductal carcinoma in situ (DCIS) of the breast. 2015. clinicaltrial.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT00470236 [27-8-2015]
- 20.De Rose F, Fogliata A, Franceschini D, Navarria P, Villa E, Iftode C, et al. Phase II trial of hypofractionated VMAT-based treatment for early stage breast cancer: 2-year toxicity and clinical results. Radiat Oncol 2016; 11: 120. doi: 10.1186/s13014-016-0701-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scorsetti M, Alongi F, Fogliata A, Pentimalli S, Navarria P, Lobefalo F, et al. Phase I-II study of hypofractionated simultaneous integrated boost using volumetric modulated arc therapy for adjuvant radiation therapy in breast cancer patients: a report of feasibility and early toxicity results in the first 50 treatments. Radiat Oncol 2012; 7: 145. doi: 10.1186/1748-717X-7-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolini G, Fogliata A, Clivio A, Vanetti E, Cozzi L. Planning strategies in volumetric modulated are therapy for breast. Med Phys 2011; 38: 4025–31. doi: 10.1118/1.3598442 [DOI] [PubMed] [Google Scholar]
- 23.van Dam FS, Aaranson NK, Engelsmen E. Various aspects of “quality of life” and the treatment of patients with breast cancer. Ned Tijdschr Geneeskd 1998; 132: 1323–6. [PubMed] [Google Scholar]
- 24.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010; 362: 513–20. doi: 10.1056/NEJMoa0906260 [DOI] [PubMed] [Google Scholar]
- 25.Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 2006; 7: 467–71. doi: 10.1016/S1470-2045(06)70699-4 [DOI] [PubMed] [Google Scholar]
- 26.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013; 14: 1086–94. doi: 10.1016/S1470-2045(13)70386-3 [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Cetwork. 2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf April 2017
- 28.Fiorentino A, Mazzola R, Giaj Levra N, Fersino S, Ricchetti F, Di Paola G, et al. Comorbidities and intensity-modulated radiotherapy with simultaneous integrated boost in elderly breast cancer patients. Aging Clin Exp Res 2017;: [Epubahead of print]. doi: 10.1007/s40520-017-0802-z [DOI] [PubMed] [Google Scholar]
- 29.Mak KS, Chen YH, Catalano PJ, Punglia RS, Wong JS, Truong L, et al. Dosimetric inhomogeneity predicts for long-term breast pain after breast-conserving therapy. Int J Radiat Oncol Biol Phys 2015; 93: 1087–95. doi: 10.1016/j.ijrobp.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 30.Fogliata A, De Rose F, Franceschini D, Stravato A, Seppälä J, Scorsetti M, et al. Critical Appraisal of the Risk of Secondary Cancer Induction From Breast Radiation Therapy With Volumetric Modulated Arc Therapy Relative to 3D Conformal Therapy. Int J Radiat Oncol Biol Phys 2017. [Epub ahead of print]. doi: 10.1016/j.ijrobp.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 31.Groen EJ, Elshof LE, Visser LL, Rutgers EJT, Winter-Warnars HAO, Lips EH, et al. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast 2017; 31: 274–83. doi: 10.1016/j.breast.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 32.Shah C, Wobb J, Manyam B, Kundu N, Arthur D, Wazer D, et al. Management of ductal carcinoma in situ of the breast: a review. JAMA Oncol 2016; 2: 1083–8. doi: 10.1001/jamaoncol.2016.0525 [DOI] [PubMed] [Google Scholar]
- 33.Lazzeroni M, Dunn BK, Pruneri G, Jereczek-Fossa BA, Orecchia R, Bonanni B, et al. Adjuvant therapy in patients with ductal carcinoma in situ of the breast: the pandora's box. Cancer Treat Rev 2017; 55: 1–9. doi: 10.1016/j.ctrv.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 34.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011; 12: 21–9. doi: 10.1016/S1470-2045(10)70266-7 [DOI] [PMC free article] [PubMed] [Google Scholar]