Abstract
Objective:
Helical tomotherapy (HT) is a new promising tool whose use remains to be studied. This work assesses its impact for local irradiation in terms of side effects, as well as tumour control in locally advanced (LABC) and metastatic breast cancer (MBC).
Methods:
We retrospectively reviewed data of 66 patients with LABC and MBC. Patients received standard fractionated radiotherapy by HT, with or without concurrent systemic treatment.
Results:
The median age was 60 years (28–77). The median follow-up of the population was 35.9 months (10.6–95.8). For 91% of patients, HT was concomitant with systemic treatments. Three patients experienced grade 3 skin toxicity and all had concurrent 5FU-vinorelbine. One patient who was receiving concurrent treatment with trastuzumab–pertuzumab had a decreased left ventricular ejection fraction by 14%. No late cardiac or lung toxicity was observed. A clinical benefit was observed in 75% of cases. At 2 months after HT, we observed tumour regression in 7/8 patients, as following: 1 complete, 4 partial responses, and 2 stable disease. The median survival for MBC group was 64.4 months (42.6–65.8) and 21.1 (6.1–36.1) months for LABC.
Conclusion:
This study suggests that the use of HT is well tolerated and feasible with a multimodal strategy that includes concurrent systemic treatments for patients with LABC and MBC.
Advances in knowledge:
The survival of LABC and MBC increases and new safe tools are needed to determine optimal strategies of treatment. To our knowledge, this is the first paper describing the use of HT for this population.
INTRODUCTION
Metastatic breast cancer (MBC) and locally advanced breast cancer (LABC) without response to a primary systemic treatment have a poor prognosis and treatment is usually palliative systemic treatment with improvement of the patient’s quality of life as the primary aim. Standard management is primary systemic treatment, surgery and radiotherapy.1 However, due to the development of more effective systemic therapies patients are surviving longer and this has led to new strategies to optimize local control.2 The aims of locoregional treatment are local control, to reduce the overall tumour burden and to contribute to the stabilization of the metastatic disease. Several retrospective studies suggest a decrease of mortality of 30 to 40% after locoregional treatment.3–6
Moreover, there are significant technological advancements in radiotherapy over the last 15 years. In particular, the integration of three-dimensional imaging into the planning process allows the reconstruction and visualization of the target volume inside the body. Helical tomotherapy (HT) is the evolution of these developments and integrates 3D imaging with intensity modulated radiotherapy (IMRT) and delivers radiation like a CT scanner with the radiation delivered as a modulated fan beam in a helical rotational manner as the patient moves through the scanner.7 HT improves the homogeneity of the dose distribution within the tumour volume and simultaneously aims to spare critical organs with reduced skin toxicity.8 The difficulties related to field junctioning in the treatment of larger complex volumes are overcome through delivery with continuous craniocaudal irradiation.9–12 Thus, HT can obtain a dosimetric gain in complex volumes with respect to the planning target coverage and sparing of organs at risk.13,14 This was shown in a study evaluating locoregional radiation in the setting of left breast cancer, where a significant reduction in the left lung and heart doses were demonstrated along with an improvement in the conformity index.15 The purpose of this work was to assess the feasibility and evaluate the toxicity of this promising tool for locally advanced and metastatic breast cancer.
Patients and methods
Patients
From 2008 to 2015, 66 females with advanced or metastatic breast cancer were treated with locoregional radiation therapy with HT at the Institut Curie.
All patients had histological confirmation of invasive cancer. Oestrogen and progesterone receptor (ER/PR) status and HER-2 status were reported in all patients. The initial patient staging assessment was classified using the seventh edition of the Tumour, Node and Metastasis classification.16 The history, physical examination, imaging results, and planning dosimetry were recorded prospectively. This project was approved by the Institut Curie Ethics Committee.
The initial characteristics of patients are shown in Table 1. Most females were slightly overweight with a median body mass index of 26 kg m−2 (range 16–66). The majority of the population was post-menopausal at the time of the diagnosis (n = 39; 65%). Five patients had a BRCA germ-cell mutation.
Table 1.
Initial characteristics of patients
| n | % | |
| Age [median(range)] | 60 (28–77) | |
| Body mass index [median(range)] | 26 (16–66) | |
| Menopause | 39 | 65 |
| Smoking habit | 19 | 28.7 |
| Diabetes | 4 | 6 |
| High blood pressure | 15 | 22.7 |
| Lung pathologies | 3 | 4.5 |
| Heart pathologies | 3 | 4.5 |
| Side | ||
| Right | 31 | 47 |
| Left | 27 | 41 |
| Bilateral | 8 | 12 |
| Histologic subtype | ||
| IDC | 57 | 86,3 |
| ILC | 3 | 4,6 |
| Mixed IDC + ILC | 5 | 7,6 |
| Mucinous | 1 | 1,5 |
| Histologic grade | ||
| I | 1 | 1,5 |
| II | 28 | 42,4 |
| III | 37 | 56,1 |
| Molecular subtype | ||
| HR+/HER2- | 38 | 57.6 |
| HR-/HER2+ | 10 | 15.1 |
| HR+/HER2+ | 7 | 10.6 |
| Triple negative | 11 | 16.7 |
| Disease stage at tomotherapy treatment | ||
| Locally advanced | 9 | 13.6 |
| Metastatic | 57 | 86.4 |
| Antracyclin regimen prior HT | 38 | 57.7 |
| Concomitant systemic treatment with HT | ||
| Hormonotherapy | 27 | 40.9 |
| FUN regimen | 9 | 13.6 |
| Trastuzumab and Pertuzumab | 11 | 16.6 |
| Trastuzumab alone | 2 | 3 |
| Cyclophosphamide | 6 | 9 |
| Capecitabine | 6 | 9 |
| Docetaxel | 1 | 1.5 |
| Surgery before HT irradiation | ||
| Before HT irradiation | 26 | 39.4 |
| After HT irradiation | 14 | 21.2 |
FUN regimen, 5 fluorouracil and vinorelbine regimen; HR, hormone receptor; HT, helical tomotherapy; IDC, invasive ductal carcinoma; IDL, invasive lobular carcinoma.
In view of the potential cardiac and pulmonary toxicities with HT, we carefully documented the patient’s comorbidities and smoking history at diagnosis. Almost 29% of them had a significant smoking history. Among the other cardiovascular risks, 6% of patients had diabetes and 23% a history of hypertension. Two patients had moderate mitral valve disease and one patient had documented Bouveret disease. When assessing for respiratory comorbidities, there was one patient with chronic obstructive pulmonary disease, one with multiorgan sarcoidosis, and one reported a history of myasthenia gravis.
In terms of clinical staging at diagnosis, 79% had distant metastatic disease. Clinical T-stage was T4 in 45%, T3 in 26% and T2 in 23%. Clinical lymph node involvement was found in 65 patients (98.5%) at diagnosis. Bilateral breast cancer represented 12% of patients in this study cohort, with 41% occurring in the left breast and 47% in the right breast. The radiological assessment of the tumour response consisted in RECIST guidelines assessment (v. 1.1)17 by mammograms at diagnosis, before and after radiotherapy for the locally advanced population. All patients’ files were discussed in the multidisciplinary breast radiation oncology meeting, after stabilization of disease with systemic therapy.
Treatments
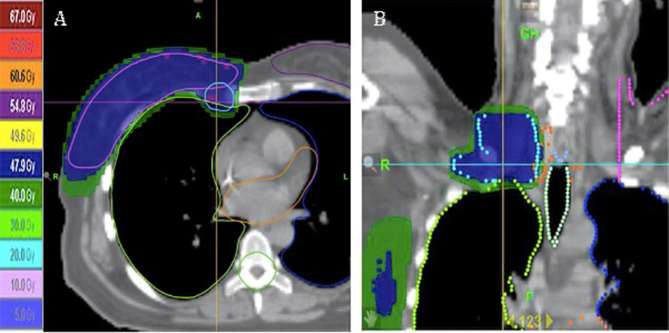
All females were treated with HT using normofractionation radiotherapy. The physician marked the breast, tumour or chest wall clinical target volume by clinical palpation and radio-opaque markers were applied for CT acquisition. The CT data was transferred to a treatment planning system (TomoTherapy Accuray system, CA) (Figure 1). The delineation of the breast, chest wall, regional nodal basins, surgical site and organs at risk were identified by the same physician according to locoregional status of every patient and also using published guidelines.18 The planning target volume (PTV) was created taking into account the clinical target volume with 5 mm margin. In the setting of post-mastectomy radiation, the target volume of the chest wall was delineated to represent the region under the excised breast, 3 mm below the skin. The irradiated volumes included the breast ± simultaneous integrated boost or chest wall, axillary lymph nodes, ± internal mammary (IMN), ± mediastinum as previously discussed.19–21 .
Figure 1.
(a) Sagittal view of dose distribution to breast and IMN. (b) Coronal view of dose distribution of HT treatment of supra and infra clavicular lymph nodes. HT, helical tomotherapy; IMN, internal mammary.
The doses were: breast 51.8 Gy; simultaneous integrated boost 63 Gy; chest wall 50 Gy; PTV lymph nodes 50.4 Gy; mediastinum 50 Gy. The Dmean of heart, ipsilateral lung and contralateral lung were respectively 8.9, 14.7 and 6.5 Gy. Dose constraints for cardiac volumes were used according to published literature related to cardiac complications.21, 22 The distribution of dose in three dimensions and the dose–volume histogram (DVH) were analysed and optimized. The beam energy used in all cases was 6 MV. The goal of plan optimization was to achieve a PTV coverage between 95 and 107%.
All toxicities were described using the Common Toxicity Criteria for Adverse Events v4 (CTCAE.v 3&4). The patient had weekly clinical review with the radiation oncologist and acute toxicity was recorded at each visit, along with treatment compliance and completion of treatment.
Systemic treatments were sometimes administered concurrently with HT in 91% of cases and sequentially in 8% (Table 1). The patients with a LABC were treated with an antracyclin and taxanes regimens prior to radiotherapy, whereas patients with a MBC were heavily treated before and after the radiotherapy. Hormonal therapy was the most commonly used concurrent systemic agent with HT (n = 27, 40.9%) followed by target therapy anti-HER2 (n = 13, 19.7%). The FUN regimen was the chemotherapy most often used concurrently (n = 9, 13.6%). It consisted of four cycles of 5-fluorouracil, 500 mg m-2 d-1, continuous infusion D1 to D5 and Vinorelbine, 25 mg m−2 D1 and D5, as previously reported.14, 23
Finally, anti-HER2 therapy—Trastuzumab or, more recently, the combination of Trastuzumab and Pertuzumab—was used concurrently with HT in 3 and 16.6% of cases respectively. Before irradiation, 57.7% of females received an antracyclin regimen known to have inherent cardiac toxicity.
We used the R statistical software (R-3.4.1, 2017, GNU GPL, Comprehensive R Archive Network, New Zealand) to define the survival from diagnosis to the end of the follow-up, the median survival at 3 and 5 years and the 95% confidence intervals.
Results
57 females with MBC and 9 females with LABC were irradiated by HT in our Institute between 2008 and 2015. Among them, eight patients with a LABC had a surgical procedure after local irradiation. The median age was 60 years (range 28–77). The median follow-up of the population was 35.9 months (range 10.6 to 95.8).
25 patients with an MBC had surgery before HT and 7 patients after HT. Three patients were irradiated before the surgical procedure because non response to primary systemic treatment. The sites treated with radiation are defined in the Table 2.
Table 2.
Target volumes irradiated by HT
| n | % | |
| Right breast | 19 | 28.8 |
| Left breast | 20 | 30.3 |
| Bilateral breast | 5 | 7.6 |
| Right side chest wall | 12 | 18.2 |
| Left side chest wall | 9 | 13.5 |
| Bilateral chest wall | 2 | 3 |
| Simultaneous integrated boost | 24 | 36.4 |
| Lymph nodes area | 62 | 94 |
| Internal mammary chain nodes | 34 | 51.5 |
| Mediastinum | 11 | 16.7 |
| Sternum | 6 | 9 |
The dose constraints for target volumes and organs at risk (OAR) consisted of minimal 95% coverage of 95% isodose and the dose constraints to OAR are given in Table 3.
Table 3.
Parameters for OAR during HT planning
| OAR | Priority | Blocking | Importance | Histogram dose–volume points |
| Controlateral lung | 1 | Directional | 1000 | 5%–7 Gy |
| 30%–3 Gy | ||||
| 50%–2 Gy | ||||
| Heart | 2 | Directional | 1000 | 15%–10 Gy |
| 5%–15 Gy | ||||
| Homolateral lung | 3 | Directional | 1000 | 50%–5 Gy |
| 15%–20 Gy | ||||
| 5%–30 Gy | ||||
| Controlateral breast | 4 | Directional | 1000 | 10%–3 Gy |
| Spinal cord | 5 | Directional | 300 | 30%–10 Gy |
| Liver | 6 | Directional | 300 | 20%–5 Gy |
OAR, organs at risk.
Most patients experienced Grade 1 and 2 skin toxicity: 35 (53%) and 14 (21%), respectively. Only three patients experienced Grade 3 skin toxicity and they required a treatment interruption to allow healing. They all received concurrent FUN regimen. There were 13 patients (19.7%) with Grade 1 and 4 patients presented with Grade 2 oesophageal toxicity. One patient received concurrent treatment with Trastuzumab and Pertuzumab had a decreased left ventricular ejection fraction by 14% compared to the initial value. She had no exposure to anthracycline. This female continued radiation treatment after cardiac assessment, stabilization of ejection fraction and after re-evaluation of therapy with respect to risks and benefits. There was no Grade 1 or 2 pulmonary fibrosis. Treatment interruptions were noted in eight patients. Three were due to Grade 3 radiodermatitis, two patients for a Grade 2 radiodermatitis, one for sepsis, one due to poor compliance and one for modification of the target volumes. There were no toxic deaths. The acute toxicity is shown in Table 4. No late toxicity was observed with a minimum follow up period of 10 months and a maximum follow up period of 8 years.
Table 4.
Acute toxicity
| Toxicity | n (%) |
| Skin | |
| Grade 1 | 35 (53) |
| Grade 2 | 14 (21) |
| Grade 3 | 3 (4.5) |
| Oesophageal | |
| Grade 1 | 13 (19.7) |
| Grade 2 | 4 (6) |
| Cardiac | |
| Grade 3 | 1 (1.5) |
| Respiratory | 0 |
At 2 months after locoregional irradiation, we reported tumour regression in seven of eight patients, as following: one complete remission, 4 partial responses, two patients were presented with stable disease. The radiological assessment was no feasible for one patient because of bulky disease in the breast but a clinical improvement was observed.
Among this population, five patients had a metastatic relapse on average 19.2 months (range 12.9–31.4) after the diagnosis.
Radiation therapy and the tumour response did impact the patients reported quality of life because the treatment of the symptoms associated with the locally advanced disease. This improvement was reported by the patients during their weekly clinics and documented by the radiation oncologist in the patients’ records. 19 of them had a complicated wound with inflammatory œdema, 1 with several areas of ulceration, and bleeding. One patient required blood transfusions because of haemorrhagic wounds. Of those 19 patients with tumour involving skin, HT led to significant clinical improvement in 75% of case. For MBC cases, the median survival was 64.4 months [95% CI (42.6–65.8)]. The patients with a LABC had a median survival of 21.1 months [95% CI (6.1–36.1)] (Figure 2).
Figure 2.
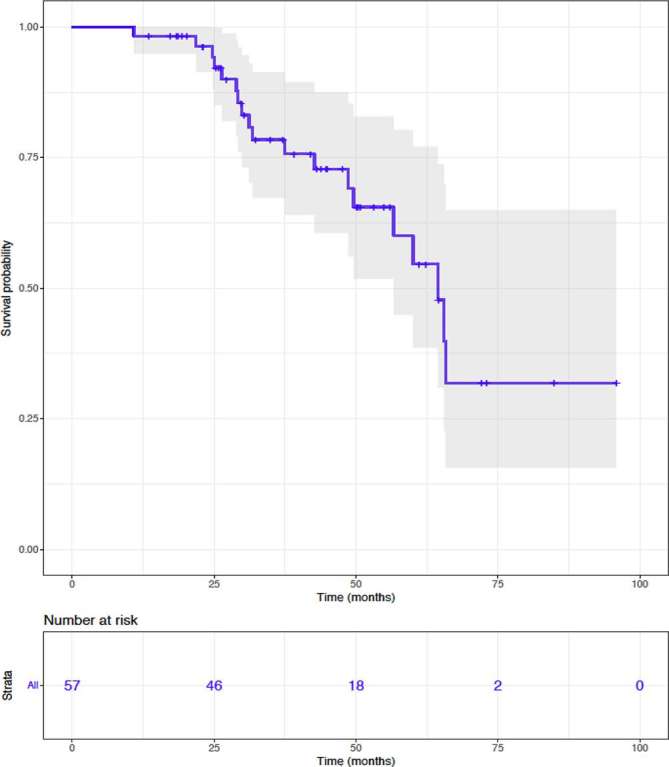
Kaplan–Meier estimates of overall survival for metastatic breast cancer population.
Discussion
This study presents the clinical results in the largest published cohort with longest follow up in patients with advanced and metastatic disease treated by HT. We found good local and regional control for patients with LABC. This cohort of patients had challenging local and regional volumes with 12% of patients presented with bilateral tumours, 41% had left sided cancers requiring cardiac avoidance, 26% had medial tumours and 21% had bulky disease in the breast. Nonetheless, the dosimetric constraints and protection of organs at risk were met. To our knowledge, this is the largest study which describes locoregional irradiation with this technology for females heavily pre and post-treated by systemic treatment.
Locoregional irradiation in association with different systemic treatments could be toxic. The combination of multiple treatments could cause cumulative unintended side effects. Previous reports have suggested hormonal therapy in the form of Tamoxifen with conventional radiotherapy correlates with pulmonary fibrosis.24, 25 Whereas, the combination of concurrent Trastuzumab and locoregional breast/chest wall radiotherapy did not show excess toxicities.26 There is limited and immature data regarding the triplet use of Trastuzumab, Pertuzumab and radiotherapy. Ajgal et al reported on the preliminary results in a study with 23 patients: 1 patient was diagnosed with pulmonary fibrosis and 1 patient with decreased left ventricular ejection fraction (more than 50% compared of the initial value).27 Other systemic regimens used were known to be potentially cardiotoxic with 58% in case of anthracycline-based chemotherapy and 35% in case of anti-HER2 therapy. The improvement of the efficacy with reduced toxicity represents an important part of this very sensitive population of patients because the necessity to improve the therapeutic ratio without discontinuation of the systemic treatment. In these series, we have not found any increased toxicity.
The HT permitted to irradiate complex volumes with better sparing of the OAR. The use of HT for difficult anatomic volume irradiation illustrates how positioning of the patient, optimization of the treatment planning and delivery may reduce the probability of long-term adverse effects according to known dose–volume histogram parameters.26 The use of HT treatment planning compared to conventional techniques was evaluate in small cohort of 10 patients and seems superior for the coverage of planning treatment volumes with improved dose conformity and homogeneity.9,28–31 It also confirmed that lower doses were delivered to larger volumes of contralateral lung, contralateral breast and normal tissues. They concluded the risk benefit ratio was in favour of HT as opposed to 3D standard techniques, especially for complex volumes like funnel chest, tumour in the inner quadrant with an indication of irradiation of IMN and tumour bed, large inoperable breast tumours and post-implant breast construction.32 The study of Massabeau et al looked at 10 patients, who had implant breast reconstruction followed by irradiation with HT. It demonstrated that HT can achieve an improvement in target coverage and dose homogeneity whilst decreasing high dose to the heart and ipsilateral lung compared to standard 3D techniques.33 For locally advanced breast cancer, Chira et al published a study with five patients after failure of the treatment planning with 3D conformal techniques due to complex volumes. They showed it was possible with HT with acceptable and safe results.14 The benefit of HT as opposed to 3D-CRT would appear to be superior for complex target volumes like large inoperable cancers. More recently, a retrospective study of 29 patients with locally advanced breast cancer by Duma et al found acceptable acute toxicity for target volumes that surrounded a significant area of the thoracic region.34 Finally, Lamberth et al selected nine cases, where 3D conformal dosimetry was unacceptable and replanned them using HT. The potential indications were obesity, previous irradiation, pectus excavatum, and bilateral tumours. In each case, the use of HT improved at least one conformity index, and gave a more homogeneous dose to the target volume with better sparing of OAR.13 In our institute, HT utilization rates are increasing annually with 0.8% usage in 2009, rising to 9.4% in 2016 as the indications broaden along with clinical experience.35 At present, in view of our research, the current indications of HT are bilateral breast cancer with regional lymph nodes, deep IMN and in patients with particular anatomical shapes with known difficulty in obtaining adequate coverage with a classic field-in-field technique (especially funnel chest).35 Also, patients who have been heavily pretreated with systemic cardiotoxic agents or have comorbid heart and lung conditions are most likely to benefit from a locoregional therapy that spares OAR by reducing the overall burden of therapy. It seems logical to reduce the toxicity of radiotherapy in a context of multimodal treatment where quality of life and survival are both equally primary goals. At the same time, the use of HT increases the low doses to contralateral lung and breast as previously shown (10, 34), but in this population of patients with shorter survival, the risk of long-term complications is lower.
This study has some potential limits because it is a retrospective study analysing a small number of patients.
CONCLUSION
HT appears to be a helpful tool in the multimodal treatment of locally advanced and MBC. The acute toxicity profile is acceptable and with improved coverage and homogeneity of complex volumes. This study suggests an expanding indication for HT in the locoregional management of metastatic and locally advanced breast cancer.
Contributor Information
Laura Thery, Email: thery.laura@outlook.fr.
Alexandre Arsene-Henry, Email: alexandre.arsene-henry@curie.fr.
Susan Carroll, Email: susan.carroll@curie.fr.
Dominique Peurien, Email: dominique.peurien@curie.fr.
Louis Bazire, Email: louis.bazire@curie.fr.
Magalie Robilliard, Email: magalie.robilliard@curie.fr.
Alain Fourquet, Email: alain.fourquet@curie.fr.
Youlia M Kirova, Email: youlia.kirova@curie.fr.
REFERENCES
- 1.Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO-ESMO International consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 2017; 28: 3111–33. doi: 10.1093/annonc/mdx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 2002; 132: 620–7. doi: 10.1067/msy.2002.127544 [DOI] [PubMed] [Google Scholar]
- 4.Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol 2007; 14: 2187–94. doi: 10.1245/s10434-007-9438-0 [DOI] [PubMed] [Google Scholar]
- 5.AlJohani B, AlMalik O, Anwar E, Tulbah A, Alshabanah M, AlSyaed A, et al. . Impact of surgery on survival in stage iv breast cancer. Breast J 2016; 22: 678–82. doi: 10.1111/tbj.12662 [DOI] [PubMed] [Google Scholar]
- 6.Le Scodan R, Stevens D, Brain E, Floiras JL, Cohen-Solal C, De La Lande B, et al. . Breast cancer with synchronous metastases: survival impact of exclusive locoregional radiotherapy. J Clin Oncol 2009; 27: 1375–81. doi: 10.1200/JCO.2008.19.5396 [DOI] [PubMed] [Google Scholar]
- 7.Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, et al. . Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys 1993; 20: 1709–19. doi: 10.1118/1.596958 [DOI] [PubMed] [Google Scholar]
- 8.Fournier-Bidoz N, Kirova Y, Campana F, El Barouky J, Zefkili S, Dendale R, et al. . Technique alternatives for breast radiation oncology: conventional radiation therapy to tomotherapy. J Med Phys 2009; 34: 149–52. doi: 10.4103/0971-6203.54849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddu SM, Chaudhari S, Mamalui-Hunter M, Pechenaya OL, Pratt D, Mutic S, et al. . Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys 2009; 73: 1243–51. doi: 10.1016/j.ijrobp.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Hijal T, Fournier-Bidoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, et al. . Simultaneous integrated boost in breast conserving treatment of breast cancer: a dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol 2010; 94: 300–6. doi: 10.1016/j.radonc.2009.12.043 [DOI] [PubMed] [Google Scholar]
- 11.Schreiner LJ. MO-B-BRB-00: three dimensional dosimetry. Med Phys 2016; 43: 3695. doi: 10.1118/1.4957182 [DOI] [Google Scholar]
- 12.McCormick B, Hunt M. Intensity-modulated radiation therapy for breast: is it for everyone? Semin Radiat Oncol 2011; 21: 51–4. doi: 10.1016/j.semradonc.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 13.Lamberth F, Guilbert P, Gaillot-Petit N, Champagne C, Looten-Vieren L, Nguyen TD. Potential indications for helical tomotherapy in breast cancers. Cancer Radiother 2014; 18: 7–14. doi: 10.1016/j.canrad.2013.07.148 [DOI] [PubMed] [Google Scholar]
- 14.Chira C, Kirova YM, Liem X, Campana F, Peurien D, Amessis M, et al. . Helical tomotherapy for inoperable breast cancer: a new promising tool. Biomed Res Int 2013; 2013: 1–8. doi: 10.1155/2013/264306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coon AB, Dickler A, Kirk MC, Liao Y, Shah AP, Strauss JB, et al. . Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys 2010; 78: 104–10. doi: 10.1016/j.ijrobp.2009.07.1705 [DOI] [PubMed] [Google Scholar]
- 16.Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC cancer staging atlas: a companion to the seventh editions of the ajcc cancer staging manual and handbook. 2nd ed New York, NY: The British Institute of Radiology.; 2012. [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. . ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 2015; 114: 3–10. doi: 10.1016/j.radonc.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Monge R, Fernandes PS, Gupta N, Gahbauer R. Cross-sectional nodal atlas: a tool for the definition of clinical target volumes in three-dimensional radiation therapy planning. Radiology 1999; 211: 815–28. doi: 10.1148/radiology.211.3.r99jn40815 [DOI] [PubMed] [Google Scholar]
- 20.Dijkema IM, Hofman P, Raaijmakers CP, Lagendijk JJ, Battermann JJ, Hillen B. Loco-regional conformal radiotherapy of the breast: delineation of the regional lymph node clinical target volumes in treatment position. Radiother Oncol 2004; 71: 287–95. doi: 10.1016/j.radonc.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 21.Kirova YM, Servois V, Campana F, Dendale R, Bollet MA, Laki F, et al. . CT-scan based localization of the internal mammary chain and supra clavicular nodes for breast cancer radiation therapy planning. Radiother Oncol 2006; 79: 310–5. doi: 10.1016/j.radonc.2006.05.014 [DOI] [PubMed] [Google Scholar]
- 22.Kirova YM. Recent advances in breast cancer radiotherapy: evolution or revolution, or how to decrease cardiac toxicity? World J Radiol 2010; 2: 103–8. doi: 10.4329/wjr.v2.i3.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollet MA, Belin L, Reyal F, Campana F, Dendale R, Kirova YM, et al. . Preoperative radio-chemotherapy in early breast cancer patients: long-term results of a phase II trial. Radiother Oncol 2012; 102: 82–8. doi: 10.1016/j.radonc.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 24.Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 1996; 88: 918–22. doi: 10.1093/jnci/88.13.918 [DOI] [PubMed] [Google Scholar]
- 25.Koc M, Polat P, Suma S. Effects of tamoxifen on pulmonary fibrosis after cobalt-60 radiotherapy in breast cancer patients. Radiother Oncol 2002; 64: 171–5. doi: 10.1016/S0167-8140(02)00136-6 [DOI] [PubMed] [Google Scholar]
- 26.Jacob J, Belin L, Pierga JY, Gobillion A, Vincent-Salomon A, Dendale R, et al. . Concurrent administration of trastuzumab with locoregional breast radiotherapy: long-term results of a prospective study. Breast Cancer Res Treat 2014; 148: 345–53. doi: 10.1007/s10549-014-3166-5 [DOI] [PubMed] [Google Scholar]
- 27.Ajgal Z, de Percin S, Diéras V, Pierga JY, Campana F, Fourquet A, et al. . Combination of radiotherapy and double blockade HER2 with pertuzumab and trastuzumab for HER2-positive metastatic or locally recurrent unresectable and/or metastatic breast cancer: assessment of early toxicity. Cancer/Radiothérapie 2017; 21: 114–8. doi: 10.1016/j.canrad.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Schubert LK, Gondi V, Sengbusch E, Westerly DC, Soisson ET, Paliwal BR, et al. . Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011; 100: 241–6. doi: 10.1016/j.radonc.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 29.Caudell JJ, De Los Santos JF, Keene KS, Fiveash JB, Wang W, Carlisle JD, et al. . A dosimetric comparison of electronic compensation, conventional intensity modulated radiotherapy, and tomotherapy in patients with early-stage carcinoma of the left breast. Int J Radiat Oncol Biol Phys 2007; 68: 1505–11. doi: 10.1016/j.ijrobp.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 30.Qiu J, Liu Z, Yang B, Hou X, Zhang F. Low-dose-area-constrained helical TomoTherapy-based whole breast radiotherapy and dosimetric comparison with tangential field-in-field IMRT. Biomed Res Int 2013; 2013: 513708–6. doi: 10.1155/2013/513708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caudrelier JM, Morgan SC, Montgomery L, Lacelle M, Nyiri B, Macpherson M. Helical tomotherapy for locoregional irradiation including the internal mammary chain in left-sided breast cancer: dosimetric evaluation. Radiother Oncol 2009; 90: 99–105. doi: 10.1016/j.radonc.2008.09.028 [DOI] [PubMed] [Google Scholar]
- 32.Lauche O, Kirova YM. Helical tomotherapy in breast cancer treatment. Breast Cancer Manag 2014; 3: 441–9. doi: 10.2217/bmt.14.34 [DOI] [Google Scholar]
- 33.Massabeau C, Fournier-Bidoz N, Wakil G, Castro Pena P, Viard R, Zefkili S, et al. . Implant breast reconstruction followed by radiotherapy: can helical tomotherapy become a standard irradiation treatment? Med Dosim 2012; 37: 425–31. doi: 10.1016/j.meddos.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 34.Duma MN, Heinrich C, Schönknecht C, Chizzali B, Mayinger M, Devecka M, et al. . Helical TomoTherapy for locally advanced or recurrent breast cancer. Radiat Oncol 2017; 12. doi: 10.1186/s13014-016-0736-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arsene-Henry A, Fourquet A, Kirova YM. Evolution of radiation techniques in the treatment of breast cancer (BC) patients: from 3D conformal radiotherapy (3D CRT) to intensity-modulated RT (IMRT) using helical tomotherapy (HT). Radiother Oncol 2017; 124: 333–4. doi: 10.1016/j.radonc.2017.07.002 [DOI] [PubMed] [Google Scholar]