Abstract
Surgical resection, when feasible, is the standard of care for hepatocellular carcinoma. However, many tumours are not resectable at the time of diagnosis. Recently, stereotactic body radiation therapy (SBRT) has emerged as a non-invasive local therapy for both non-resectable primary hepatic malignancies as well as hepatic metastases. Knowledge of the expected hepatic parenchymal appearance post treatment, as well as potential pitfalls and complications, is essential for accurate evaluation of treatment response. This pictorial review provides a fundamental description of the SBRT technique, outlines the expected cross-sectional imaging appearances of tumour response, and highlights potential pitfalls in interpretation. The expected liver parenchymal changes post-SBRT are also reviewed, along with some common radiation-induced complications.
INTRODUCTION
Surgical resection, when feasible, is the standard of care for hepatocellular carcinoma (HCC).1 However, many tumours are not resectable at the time of diagnosis due to a number of tumour or patient factors. Early stage unresectable disease is treated with either local ablative therapy, such as radiofrequency ablation, or hepatic transplantation. Local and regional therapies exist for intermediate stage disease to prevent further tumour progression.1 These therapies include chemoembolization, selective internal radiotherapy (SIRT) with Yttrium-90 microspheres, and recently, stereotactic body radiation therapy (SBRT).2
As SBRT emerges as an alternative therapy for HCC, it is essential that radiologists have a fundamental understanding of this technique. The expected imaging characteristics of treated tumours, as well as radiation-induced hepatic parenchymal changes, must be well understood in order to accurately assess treatment response. The aim of this pictorial review is to provide a fundamental description of the SBRT technique, outline the expected cross-sectional imaging appearances of tumour response, and highlight potential pitfalls in interpretation. The expected acute, subacute and chronic post-SBRT liver parenchymal changes are reviewed as well as some common radiation-induced complications.
SBRT—THE FUNDAMENTALS
Delivery of curative doses of radiation therapy (RT) has traditionally been limited by intolerance of the liver to radiation.3 Historically, treatment required large radiation fields, often encompassing the whole liver. Early studies deemed RT unsafe for whole liver treatment even at relatively low doses, below those required for adequate tumour control.3 Modern advances in treatment planning have reinvigorated interest in the use of RT for treatment of liver tumours, with new techniques allowing for high rates of tumour control with low risk of radiation-induced liver disease (RILD). SBRT delivers high doses of RT in a single treatment or small number of fractions using highly accurate radiation delivery systems with precise target definition (Figure 1).
Figure 1.
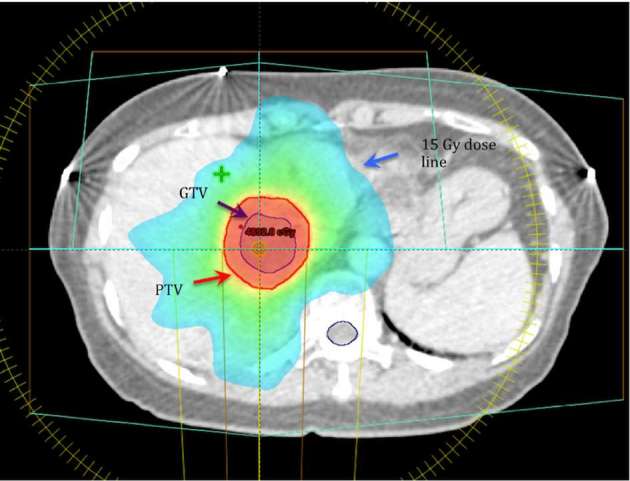
Radiation planning. In this example, prescribed dose is 45 Gy in five fractions. Mean liver dose is 14 Gy. GTV, gross tumour volume; PTV, planning target volume.
Three volumes that are important to understand in SBRT planning are the gross tumour volume (GTV), clinical target volume (CTV), and planning target volume (PTV).4 The GTV is the actual tumour volume that can be demonstrated on imaging. An important role for the radiologist is to help the treating radiation oncologist define the GTV, so that an appropriate CTV and PTV can be determined. The CTV contains the demonstrable GTV, as well as a margin for subclinical disease spread. This subclinical margin is largely based on historical post-mortem series and biopsy, and is meant to encompass the maximum extent of invasion that could be seen in any patient for a given type of tumour.4 The PTV includes the CTV as well as an additional margin incorporating the surrounding normal liver to allow for uncertainties in planning or delivery, ensuring that the CTV receives the full prescription dose of RT.4 These uncertainties include variations in size, shape, and position of the CTV in relation to anatomic reference points (e.g. movement during respiration), as well as uncertainties in patient positioning and beam positioning. PTV is very much a geometric concept, and may extend beyond anatomical borders, or even outside of the patient.4 During treatment, the full prescription of RT dose is delivered to the PTV.
The unique delivery system of SBRT results in specific post-treatment changes in the treated tumour, as well as in the surrounding hepatic parenchyma.
EVALUATING RESPONSE
Responding tumours typically demonstrate reduced enhancement on CT and MRI, as well as gradual reduction in size, which is usually most evident at 3–4 months post treatment (Figure 2).5,6 Several established tools exist for systematically evaluating tumour response, since objective quantification of response can be difficult. The most widely used system in prospective studies is RECIST (response evaluation criteria in solid tumours).3 However, RECIST tends to underestimate response, as it does not discriminate between areas of enhancement and non-enhancement (necrosis). A modified version of RECIST has been described to take areas of non-enhancement into account (mRECIST), and is the preferred system at our institutions Vancouver General Hospital and BC Cancer Agency.7 The EASL (European Association for the Study of the Liver) system similarly accounts for regions of necrosis.1 A recent study by Price and colleagues comparing EASL with RECIST for evaluation of tumour response in patients who received SBRT for HCC concluded that percentage of necrosis is a more useful indicator of response than tumour size.8 At our institution, we image patients every 3 months for the first year following treatment, every 6 months for the second year, and yearly, thereafter. We have found this schedule suitable for identifying early complications or treatment failure, and for detecting recurrence.
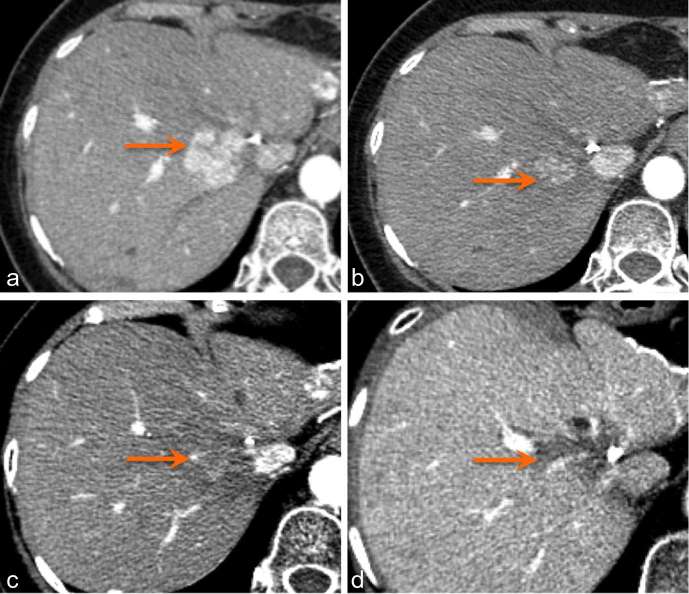
Figure 2.
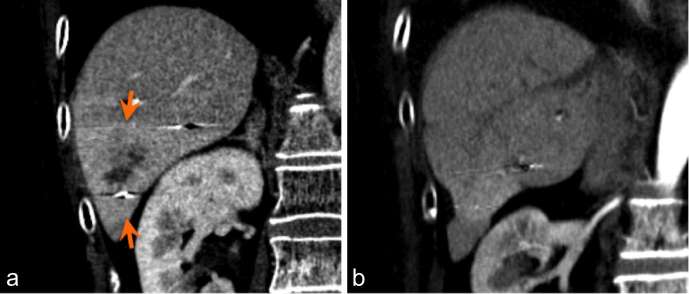
Arterial phase CT liver in a patient with HCC (arrow) demonstrates tumour appearance pre-treatment (a) and at 1 month (b), 4 months (c), and 2 years (d) post-SBRT. HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy.
While expected tumour response involves a decrease in overall tumour volume and in enhancing component, arterial hypervascularity in irradiated, non-tumorous parenchyma that surrounds the original tumour may lead to confusion (Figure 3). This finding is secondary to radiation-induced occlusive changes in portal venules, with a reactive increase in hepatic arterial flow.9 While conventional RT results in a sharply defined hyperenhancing parenchymal zone, the 3D conformal delivery of radiation in SBRT results in a much more irregular zone of hyperenhancement. Given the hypervascular nature of HCC, measuring tumour margins for evaluation of treatment response can be difficult. Park and colleagues demonstrated a high proportion of arterial hypervascularity in parenchyma surrounding original tumour, which occurred in 46% at 3 months post treatment.9 This phenomenon, termed “pseudoprogression”, may easily be misinterpreted as progression of disease (Figures 4 and 5). Several features are helpful in differentiating between true progression and pseudoprogression, including lack of wash out on delayed phase imaging and arterial hypervascularity that corresponds to the shape of the radiation field (Figures 6 and 7).9 In our experience, subsequent follow up imaging at 9 to 15 months often demonstrates reduced surrounding parenchymal hyperenhancement and increased necrosis, indicating a positive response.
Figure 3.
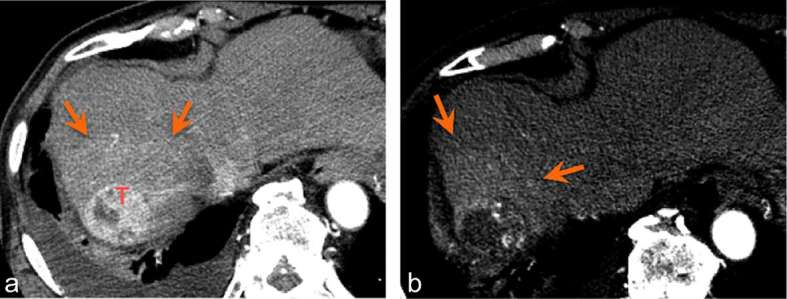
Arterial hypervascularity in irradiated hepatic parenchyma (arrows) surrounding the treated tumour (T). Arterial phase images 6 months (a) and 9 months (b) post-SBRT. SBRT, stereotactic body radiation therapy.
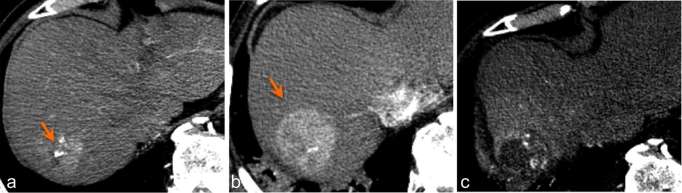
Figure 4.
Pseudoprogression. Arterially enhancing component appears to be much larger on the 3-month post-SBRT follow-up CT (b) compared to pre-treatment (a). 9-month follow-up CT (c) demonstrates tumour necrosis, indicating a good response. SBRT, stereotactic body radiation therapy.
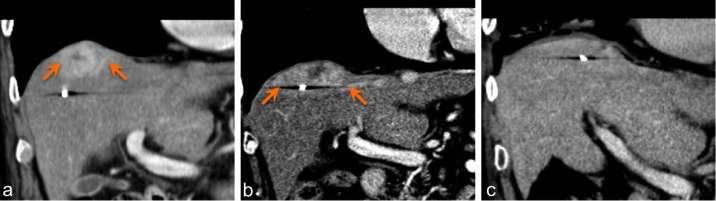
Figure 5.
Portal venous phase coronal CT in a patient with a lesion at the liver dome. Pre-treatment (a) and 3 months post treatment (b) images show increase in the region of enhancement (arrows), which could be mistaken for progression. Imaging 6 months post treatment (c) demonstrates resolution of enhancement, in keeping with treatment response. The increased enhancement seen at 3 months was pseudoprogression. Note that the fiducial marker appears to have migrated medially due to focal atrophy.
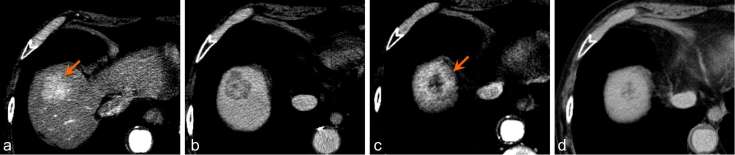
Figure 6.
Pre-treatment axial arterial phase (a) and delayed phase (b) CT of a Segment VII lesion (arrow) demonstrates tumour washout. Axial arterial phase CT 3 months post treatment (c) shows an enlarging area of arterial enhancement. Axial 3 min delayed phase 3 months post treatment (d) shows that this area does not demonstrate washout, suggesting treatment response and not progression.
Figure 7.
Segment VI HCC treated with SBRT. Coronal image3 months post treatment (a) show subtle hepatic parenchymal hyperenhancement corresponding to the radiation field (arrows). By 15 months (b), hepatic parenchymal arterial hyperenhancement has nearly resolved. HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy.
CT is the main imaging modality utilized in our institution to follow patients after SBRT. We do occasionally use MRI (1.5 T) for problem solving or when CT is contraindicated, usually in the setting of iodinated contrast allergy. Responding tumours demonstrate decreased enhancement on MRI, and subtraction pre-and post-contrast sequences are helpful to evaluate for subtle areas of hyper- or hypoenhancement (Figure 8). Signal changes may also be present, with decreased hyperintensity on T2 as well as on diffusion-weighted images (DWI) suggesting response (Figure 9c,d).10 Baseline tumour DWI signal may also be a useful predictor of tumour response, with absence of baseline DWI hyperintensity correlating with higher probability of complete response on subsequent MRI.10 MRI is also useful for evaluating parenchymal injury of the background liver following SBRT.6
Figure 8.
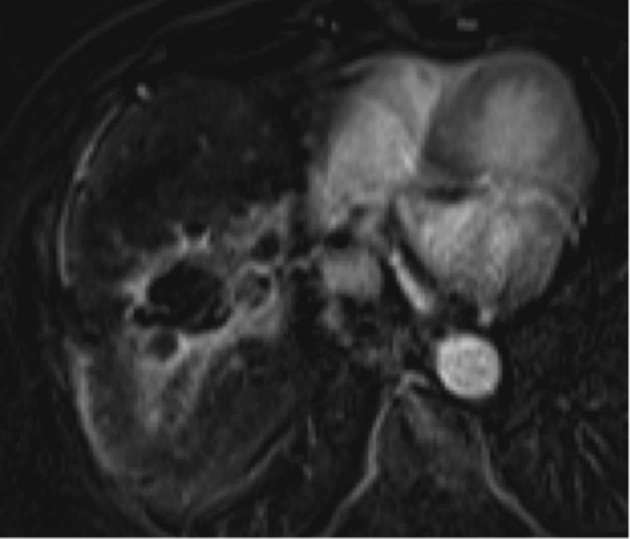
Subtracted (pre and post contrast) 3D GRE T1 weighted sequence with fat-saturation (VIBE) MRI in a patient treated with SBRT better delineates hyperenhancement surrounding a central region of non-enhancement. Contrast, gadoxetate disodium (Eovist®, Bayer Healthcare, Whippany, NJ).
Figure 9.
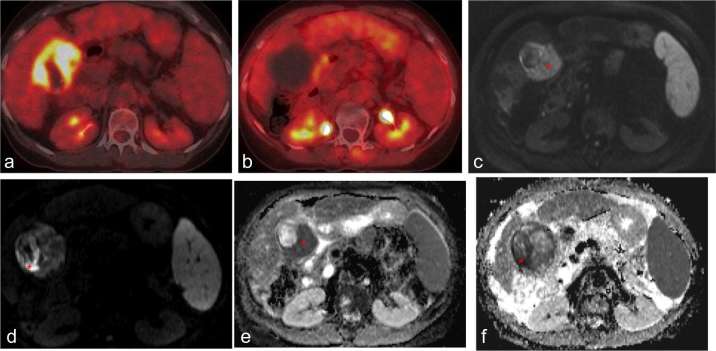
Axial 18F PET of HCC before (a) and 1 month after (b) treatment with SBRT demonstrate decreased uptake in treated tumour. Corresponding MR DWI (c, d) and ADC (e, f) sequences of the same tumour demonstrate restricted diffusion (red asterisk) in the tumour before treatment (c, e), with only minimal restriction remaining after treatment (d, f). ADC, apparent diffusion coefficient; DWI, diffusion-weighted image; HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy.
18F (fludeoxyglucose) PET is not routinely performed at our institution for evaluation of response after SBRT. Reduced uptake of 18F on PET may be demonstrated in responding FDG-avid tumours (Figure 9a,b). However, sensitivity of 18F PET for diagnosis of HCC is low (approximately 55%) compared to CT.11 Interestingly, a study by Huang and colleagues found 18F PET may be useful in predicting local HCC tumour control, with a baseline SUVmax below 3.2 predicting a better 4 year tumour control rate following SBRT.12
CT perfusion imaging has been shown to be a potential tool for response assessment for lung metastases treated with SBRT.13 To our knowledge, its utility has not been well-established in evaluation of post-treatment response for liver tumours, and remains an area of current investigation at our institution.
POST-TREATMENT HEPATIC CHANGES
Hepatic oedema is one of the earliest encountered changes of acute radiation injury that corresponds to the radiation field and is the result of decreased venous outflow due to injury and obliteration of venules and sinusoids. Hepatocytes in this region have decreased function, which results in relative low T1 signal intensity on MRI compared to the remaining liver parenchyma following the addition of hepatocyte-specific contrast agent.6 Periportal oedema is another commonly reported manifestation of acute radiation injury (Figure 10) which tends to appear and resolve quickly, lasting several days to weeks after treatment.6
Figure 10.
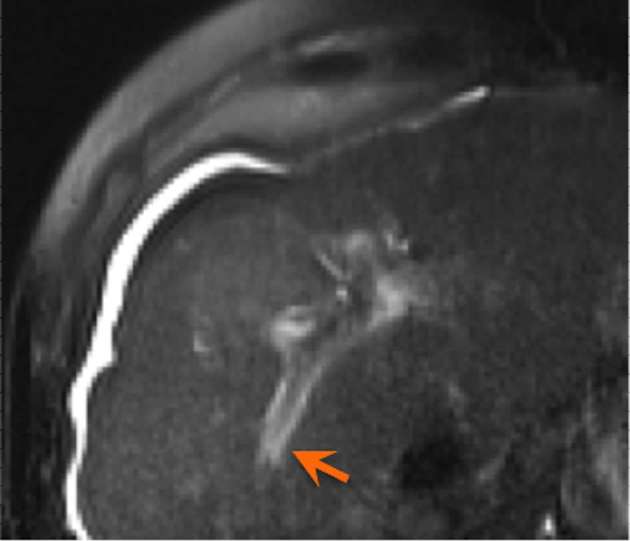
Axial T2 weighted sequence with fat suppression demonstrates periportal oedema following SBRT treatment of HCC. HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy.
As previously mentioned, perfusional changes in irradiated hepatic parenchyma, specifically arterial hypervascularity on both CT and MRI, are a classic subacute finding post-SBRT (Figure 3). On MRI with gadoxetate disodium (Eovist®, Bayer Healthcare, Whippany, NJ), decreased uptake on the hepatobiliary phase may also be seen, corresponding to irradiated hepatic parenchyma (Figure 11). Transient biliary dilatation has been reported as a less common subacute finding after SBRT.6 Several cases have been noted at our institution following treatment, usually resolving over several months.
Figure 11.
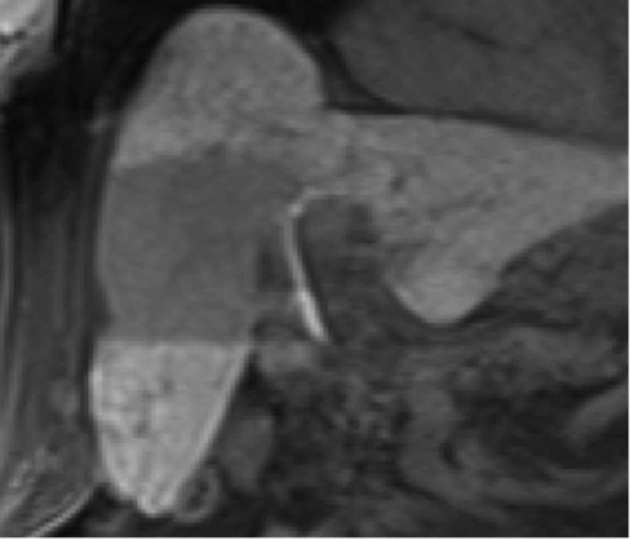
Coronal dynamic MRI following injection of gadoxetate disodium (Eovist®, Bayer Healthcare, Whippany, NJ) demonstrates a well-demarcated region of hypoenhancement on hepatobiliary phase corresponding to the location of the radiation field in this patient treated with SBRT for HCC. HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy.
Several months after RT, injured hepatocytes are gradually replaced by fibrosis. This is best detected on MRI as regions of low T1 and T2 signal corresponding to the radiation field. Depending on duration of onset, fibrosis may show moderate enhancement on arterial phase, with persistent progressive enhancement on delayed phase images.6 After several months, arterial enhancement decreases but there remains persistent progressive enhancement on delayed phase imaging for as long as 2 years.
There is usually a transient decrease in volume of the irradiated parenchyma in the first 2–3 months, with subsequent regeneration after 3 months.6 In some cases, volume loss and capsular retraction may persist for several years after therapy (Figure 12).
Figure 12.
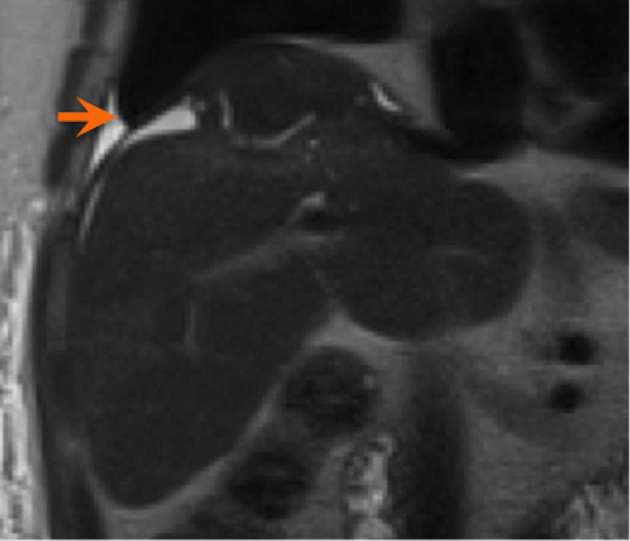
Coronal T2 weighted, fat saturated MRI images demonstrate capsular retraction.
COMPLICATIONS
The most frequently encountered complication of liver irradiation is RILD (radiation hepatitis). It is a syndrome of fatigue, right upper quadrant pain, ascites, anicteric hepatomegaly, and elevated transaminases. Due to strict dose–volume constraints and proper patient selection, most early SBRT studies have demonstrated minimal risk of RILD. A meta-analysis by Sawrie and colleagues of trials involving SBRT for HCC and liver metastases demonstrated a rate of RILD of 2.4%, with toxicity correlated with dose–volume constraints.14
Complications related to the location of the radiation field and the nearby organs and structures that are exposed are relatively common (Figure 13). Loss of appetite and nausea are encountered frequently, more severe when treated lesions are in close proximity to the stomach or duodenum.2 Radiation induced enteritis with symptoms of diarrhea is also common. Stomach and bowel ulceration and perforation has been encountered when the radiation field is close to stomach or bowel.2 Treatment of hepatic lesions in the superior aspect of the liver leads to increased risk of pulmonary complications, including radiation pneumonitis, and possible eventual pulmonary fibrosis.2
Figure 13.
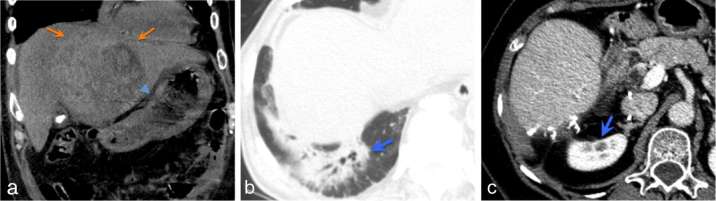
(a) Non-contrast (due to poor renal function) coronal CT demonstrates a large HCC (arrows) in close proximity to the stomach (arrowhead). Following treatment with SBRT, the patient developed endoscopic proven radiation gastritis. (b) Right lower lobe radiation pneumonitis in a patient who received SBRT for segment VII HCC. (c) Right renal cortical atrophy (blue arrow) following SBRT treatment of segment VI HCC. HCC, hepatocellular carcinoma; SBRT, stereotactic body radiation therapy.
CONCLUSIONS
While surgery remains the standard of care for resectable HCC, SBRT is becoming increasingly utilized as a safe and effective non-surgical treatment option for large or non-resectable disease. Since much of the current SBRT literature for HCC is aimed primarily at the radiation oncology community, our aim was to provide radiologists with an introduction to the SBRT technique and a comprehensive review of the post-treatment imaging appearances. Knowledge of expected post-treatment tumour appearance is essential for accurate evaluation of response, and we recommend using a response monitoring system that accounts for tumour necrosis (mRECIST or EASL) rather than size alone. Knowledge of potential pitfalls, such as pseudoprogression, is paramount to avoid mistaking post-radiation changes for progression of disease. While the highly focused nature of SBRT limits radiation dose to the surrounding normal liver, typical post-radiation changes are seen in the acute, subacute, and chronic setting, and it is important to be familiar with these expected findings at the various intervals of follow up. We have found that imaging every 3 months for the first year, every 6 months for the second year, and yearly thereafter, is a reasonable schedule to accurately evaluate response, assess for early complications or treatment failure, and detect recurrence.
Contributor Information
Trenton Kellock, Email: trentonkellock@gmail.com.
Teresa Liang, Email: teresaliang86@gmail.com.
Alison Harris, Email: Alison.Harris@vch.ca.
Devin Schellenberg, Email: dschellenberg@bccancer.bc.ca.
Roy Ma, Email: rma@bccancer.bc.ca.
Stephen Ho, Email: Stephen.Ho@vch.ca.
Wan Wan Yap, Email: wanwanyap@gmail.com.
REFERENCES
- 1. European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–43. [DOI] [PubMed] [Google Scholar]
- 2.Paravati AJ, Healy E, Murphy JD, Song W, Hattangadi-Gluth J. Stereotactic body radiation therapy for primary hepatic malignancies and metastases to liver: a technical and literature review. Transl Cancer Res 2013; 2: 507–20. [Google Scholar]
- 3.Tanguturi SK, Wo JY, Zhu AX, Dawson LA, Hong TS. Radiation therapy for liver tumors: ready for inclusion in guidelines? Oncologist 2014; 19: 868–79. doi: 10.1634/theoncologist.2014-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnet NG, Thomas SJ, Burton KE, Jefferies SJ. Defining the tumour and target volumes for radiotherapy. Cancer Imaging 2004; 4: 153–61. doi: 10.1102/1470-7330.2004.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook OR, Thornton E, Mendiratta-Lala M, Mahadevan A, Raptopoulos V, Brook A. CT Imaging findings after stereotactic radiotherapy for liver tuging Findings after Stereotactic Radiotherapy for Liver Tumors, CT imaging findings after stereotactic radiotherapy for liver tuImaging Findings after Stereotactic Radiotherapy for Liver Tumors. Gastroenterol Res Pract Gastroenterol Res Pract 2015; 2015: e126245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lall C, Bhargava P, Sandrasegaran K, Shanbhogue AK, Ramsinghani N, Koh YW, et al. . Three-dimensional conformal radiation therapy in the liver: MRI findings along a time continuum. J Comput Assist Tomogr 2015; 39: 356–64. doi: 10.1097/RCT.0000000000000219 [DOI] [PubMed] [Google Scholar]
- 7.Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcAssessment for Hepatocellular Carcinoma. Semin Liver Dis 2010; 30: 052–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 8.Price TR, Perkins SM, Sandrasegaran K, Henderson MA, Maluccio MA, Zook JE, et al. . Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer 2012; 118: 3191–8. doi: 10.1002/cncr.26404 [DOI] [PubMed] [Google Scholar]
- 9.Park MJ, Kim SY, Yoon SM, Kim JH, Park SH, Lee SS, et al. . Stereotactic body radiotherapy-induced arterial hypervascularity of non-tumorous hepatic parenchyma in patients with hepatocellular carcinoma: potential pitfalls in tumor response evaluation on multiphase computed tomography. PLoS One 2014; 9: e90327. doi: 10.1371/journal.pone.0090327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldrini G, Huertas A, Renard-Oldrini S, Taste-George H, Vogin G, Laurent V, et al. . Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS One 2017; 12: e0176118. doi: 10.1371/journal.pone.0176118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, et al. . Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol 2000; 32: 792–7. doi: 10.1016/S0168-8278(00)80248-2 [DOI] [PubMed] [Google Scholar]
- 12.Huang WY, Kao CH, Huang WS, Chen CM, Chang LP, Lee MS, et al. . 18F-FDG PET and combined 18F-FDG-contrast CT parameters as predictors of tumor control for hepatocellular carcinoma after stereotactic ablative radiotherapy. J Nucl Med 2013; 54: 1710–6. doi: 10.2967/jnumed.112.119370 [DOI] [PubMed] [Google Scholar]
- 13.Sawyer B, Pun E, Samuel M, Tay H, Kron T, Bressel M, et al. . CT perfusion imaging in response assessment of pulmonary metastases undergoing stereotactic ablative radiotherapy. J Med Imaging Radiat Oncol 2015; 59: 207–15. doi: 10.1111/1754-9485.12272 [DOI] [PubMed] [Google Scholar]
- 14.Sawrie SM, Fiveash JB, Caudell JJ. Stereotactic body radiation therapy for liver metastases and primary hepatocellular carcinoma: normal tissue tolerances and toxicity. Cancer Control 2010; 17: 111–9. doi: 10.1177/107327481001700206 [DOI] [PubMed] [Google Scholar]