Abstract
In osteoarthritis research, imaging plays an important role in clinical trials and epidemiological observational studies. In this narrative review article, we will describe recent developments in imaging of osteoarthritis in the research arena, mainly focusing on literature evidence published within the past 3 years (2014–2017). We will primarily focus on MRI including advanced imaging techniques that are not currently commonly used in routine clinical practice, although radiography, ultrasound and nuclear medicine (radiotracer) imaging will also be discussed. Research efforts to uncover the disease process of OA as well as to discover a disease modifying OA drug continue. MRI continues to play a large role in these endeavors, while compositional MRI techniques will increasingly become important due to their ability to assess “premorphologic” biochemical changes of articular cartilage and other tissues in and around joints. Radiography remain the primary imaging modality for defining inclusion/exclusion criteria as well as an outcome measure in OA clinical trials, despite known limitations for visualization of OA features. Compositional MRI techniques show promise for predicting structural and clinical outcomes in OA research. Ultrasound can be a useful adjunct to radiography and MRI particularly for evaluation of hand OA. Newer imaging techniques such as hybrid PET/MRI may have a potential but require further research and validation.
Introduction
Three main imaging modalities utilized for osteoarthritis (OA) imaging are conventional radiography, MRI and ultrasound. In routine clinical practice, radiography is the first choice imaging modality for diagnosis of OA.1 In the research setting, more advanced imaging modalities play an important role in clinical trials and epidemiological observational studies. Of note, a series of recommendations pertaining the use of imaging in OA clinical trials were published by the OA Research Society International.2–4 In this narrative review article, we will describe recent developments in imaging of OA in the research arena, mainly focusing on literature evidence published within the past 3e years (2014–2017).
Literature search methodology
The searches in the electronic databases (PubMed, MEDLINE) were performed using the keywords “OA” and “magnetic resonance imaging”. Additional keywords were used to narrow the search including “radiography”, “ultrasound”, “nuclear medicine”, “PET”, “SPECT”, for articles published between January 2014 and September 2017 for the main searches. In particular, authors focused on papers describing human studies and written in English. This search strategy initially yielded 2857 abstracts, which were then screened for relevance. Additional older papers published before January 2014 were added when deemed essential for description and discussion on newer literature evidence which was based on available literature evidence or published scoring systems, classifications systems, or guidelines/recommendations. Initial literature search was performed by DH and screening for relevance was performed by all authors, based on the authors’ clinical and research expertise in the field of OA imaging. At the end, 97 papers were included in the current review.
Phenotypes of OA and role of imaging
Radiography is still the most commonly used imaging modality in any OA research despite known important limitations.5 The European League Against Rheumatism task force recently published recommendations regarding the use of imaging in the routine clinical management of patients with OA,1 essentially stating radiography is the primary imaging modality and additional imaging such as MRI and ultrasound should only be used in atypical cases or patients showing rapid progression of symptoms. It should be noted, however, that their recommendations are not applicable to OA research. It has been recognized that OA can be classified to various phenotypes.6, 7 While radiography alone may be sufficient for a simple phenotypical classification of foot OA (isolated first metatarsophalangeal joint OA and polyarticular OA),7 other more sophisticated classification needs more advanced imaging techniques.6 e.g. adequate assessment of “pain” phenotype6 or inflammatory phenotype8 require contrast-enhanced MRI and/or ultrasound imaging since synovial inflammation cannot be directly visualized by radiography. Other than synovitis, OA features that are correlated to pain such as bone marrow lesions (BMLs)9, 10 and meniscal abnormality11 also need MRI for evaluation. Ultrasound imaging enables real time, dynamic and multiplanar evaluation, and is particularly useful for assessment of inflammatory changes, i.e. synovial hypertrophy, synovitis and cortical erosions.8
Radiographic evaluation of OA
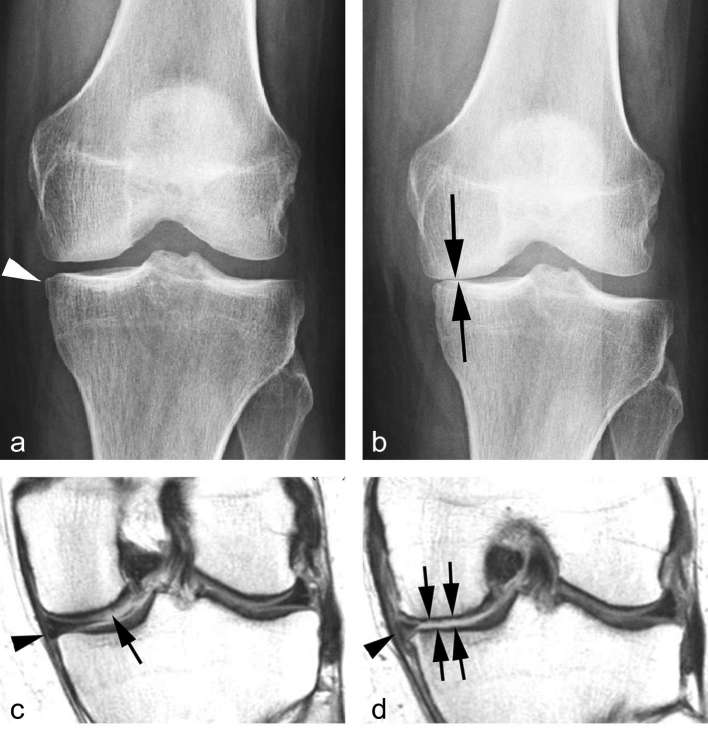
Inclusion and exclusion criteria for clinical trials of radiographic OA are commonly defined using Kellgren and Lawrence (KL) grading (Grade 0 = normal; Grade 1 = presence of equivocal osteophyte; Grade 2 = presence of definite osteophyte without joint space narrowing (Figure 1a); Grade 3 = presence of joint space narrowing; Grade 4 = complete loss of joint space, “bone on bone” appearance (Figure 1b).12 Limitations of radiography for imaging assessment of OA have been well documented. e.g. a large proportion of KL Grade 0 knees have OA features that can be detected by MRI.13 On the other hand, the opposite spectrum of the disease, KL Grade 4, is thought to be “end stage” radiographic OA. However, MRI can reveal that even KL Grade 4 knees can still progress further14 with respect MRI-detectable pathological features such as cartilage damage and BMLs. Another major limitation is that Grade 3 includes any severity of joint space narrowing (other than complete loss), and thus it is insensitive to change over time.5
Figure 1.
Progression of radiographic OA. (a) Baseline posterioranterior radiograph of the left knee shows small medial tibial osteophyte (arrow head) but no additional signs of osteoarthritis. No joint space narrowing is apparent. (b) Follow-up radiograph 2 years later shows marked progression of radiographic osteoarthritis with near bone-on-bone appearance of the medial tibiofemoral joint space (arrows). (c) Baseline coronal intermediate-weighted MRI shows discrete superficial focal cartilage defect at the weight-bearing medial femur (arrow). In addition there is a minimal meniscal extrusion medially (arrow head). (d) Follow-up MRI 2 years later shows diffuse weight-bearing tibial and femoral cartilage loss (arrows) and partial meniscal maceration with an increase in medial extrusion (arrow head). All three factors contribute to joint space narrowing on radiograph, i.e. cartilage loss, meniscal morphologic damage and meniscal extrusion. OA, osteoarthritis.
Semiquantitative radiographic assessment of OA can also be performed using another scoring system developed by OA Research Society International, published as an OARSI atlas.15 In this scoring system, knee OA can be assessed in regard to joint space narrowing which is graded as 0 (none), 1 (mild), 2 (moderate) and 3 (severe), and osteophytes which are graded as 0 (none), 1 (small), 2 (moderate) and 3 (large). Many epidemiological observational studies such as OA Initiative and the Multicenter OA Study use radiographic outcome to determine incidence and progression of OA based on semiquantitative assessment. Recent OAI-based studies showed that lower thigh muscle specific strength was a predictor of incident radiographic knee OA in females [odds ratio 1.47, 95% confidence interval (CI) (1.10–1.96)],16 and that meniscal surgery was associated with increased risk of radiographic joint space narrowing progression in persons without prior history of knee trauma [adjusted hazard ratio 1.27, 95% CI (1.00–1.63)].17
Another approach in radiographic imaging of OA is quantitative analysis of the joint space width, which can be measured manually or by using computer software. Recent studies using quantitative joint space width measurement have shown some interesting observations. In non-gout patients with knee OA, high serum urate levels predicted progression of joint space narrowing over 24 months [baseline serum uric acid levels distinguished progressors (joint space loss >0.2 mm) from non-progressors, p = 0.03 by multivariate analysis].18 Another study showed fixed-location joint space width measurement could better predict knee replacement surgery [odds ratio 1.57, 95% CI (1.23–2.01)] compared to minimum joint space width measurement [odds ratio 1.38, 95% CI (1.11–1.71)].19 The same study also showed radiographic joint space width measurement could predict future knee replacement surgery to similar accuracy compared to femoral cartilage thickness measurement on MRI (p = 0.001 by paired t-test and odds ratio 1.38).20 However, it has also been shown that, in healthy subjects, minimal joint space width reflected a combination of 3D quantitative cartilage and meniscal measures, particularly in females, and fixed-location joint space width was dominated by variance in cartilage thickness.21 Thus, the significant contribution of the meniscal position on minimal joint space width reinforces known concerns over validity of joint space width as an indirect measure of articular cartilage thickness (Figure 1c,d). To try to solve this issue, a novel approach to measure joint space width using 3D standing CT has been proposed and seems to have a potential for a tool to stratify participants in clinical trials, although more studies are needed to validate this methodology.22
Semiquantitative and quantitative radiographic analyses of OA are commonly used as inclusion/exclusion criteria as well as outcome measures in OA clinical trials such as vitamin D,19 chondroitin sulfate,23 glucosamine,24, 25 sprifermin,26 strontium ranelate,27 meniscal surgery15 and gene therapy,28 to name but a few.
It is worth noting that application of tomosynthesis in OA research has been published in the literature and offers better diagnostic performance for knee OA29 and hand OA30 features, using MRI and CT as a standard of reference.
Analytic approaches of MRI evaluation of OA
Broadly speaking, MRI analysis in OA research can be classified into semiquantitative and quantitative analyses. Additionally, physiological (compositional) imaging can be performed using advanced MRI techniques which are not routinely used in clinical practice at present. Whichever approach is chosen for a purpose of research, it is critically important to use appropriate MRI pulse sequences tailored for specific pathologic features to be evaluated. For assessment of focal cartilage defects and BMLs, fluid-sensitive fat-suppressed fast/turbo spin echo sequences (e.g. T2 weighted, proton density-weighted or intermediate-weighted) or short tau inversion recovery sequence should be used so as not to miss a small focal defect which may not be visible on gradient recalled echo type sequences (Figure 2).31–33
Figure 2.
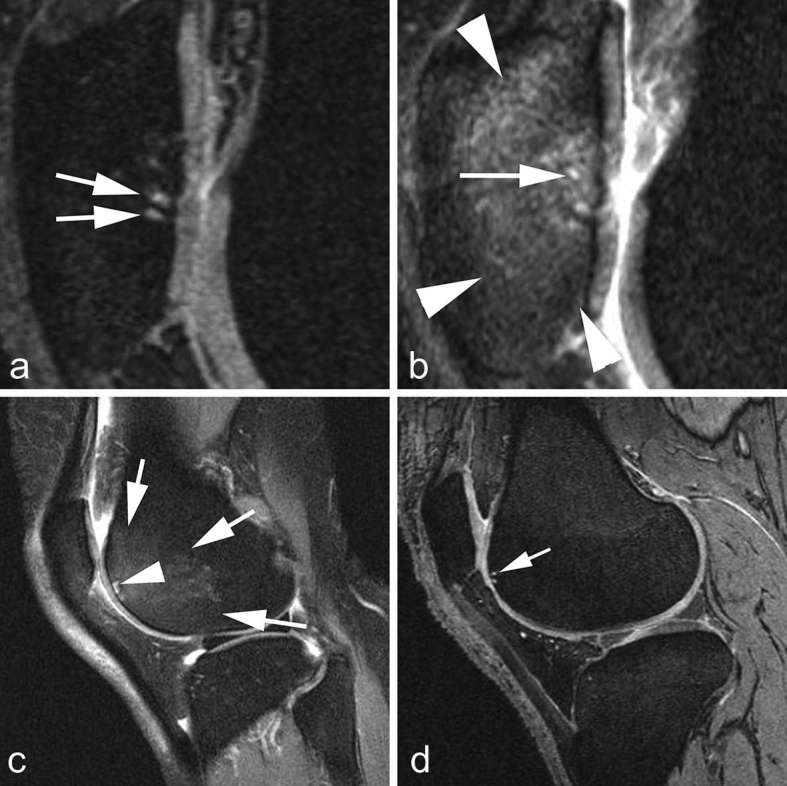
Depiction of BMLs using different pulse sequences on MRI. (a) Sagittal DESS MRI shows small cystic lesions in the subchondral patella (arrows). No diffuse BML is shown. (b) Corresponding sagittal intermediate-weighted fat-suppressed MRI shows small cystic lesions (arrow) but in addition there is a large diffuse ill-defined BML in the patella (arrow heads). Fast spin-echo sequences are much more sensitive for visualization of free water compared to gradient-echo sequences such as DESS. (c) Sagittal intermediate-weighted fat-suppressed MRI shows large BML in the anterior femoral trochlea and small subchondral cyst. (d) Corresponding DESS MRI only depicts the cystic lesion (arrow). BML, bone marrow lesion; DESS, dualecho at steady-state.
Semiquantitative analysis
Detailed description of each and every available MRI-based sem iquantitative scoring system is outside the scope of this review, but interested readers may find dedicated review articles in the literature for reference.34, 35 Briefly, these scoring systems divide the joint into more than one “subregions” and enable grading of OA features, such as cartilage damage, meniscal tear and extrusion, BMLs, osteophytes, synovitis and effusion, in each subregion and also as a whole joint. Existing semiquantitative scoring systems include, but not limited to, Whole Organ MRI Score (WORMS),36 MRI OA Knee Score (MOAKS),37 Hip OA MRI Score38 and outcome measures in rheumatology hand OA MRI scoring system.39 e.g. in MOAKS, the knee joint is subdivided into 15 subregions and BMLs can be graded as 0 = none, 1 = less than 33% of subregional volume, 2 = between 33 and 66% of subregional volume and 3 = greater than 66% of subregional volume.Over the last 3 years, several new scoring systems were developed and published. Anterior Cruciate Ligament Osteoarthritis Score allows reliable whole-organ scoring of acute ACL injury and longitudinal changes relevant to knee OA.40 ACLOAS is the only scoring system that has a specific focus on ACL injury in the context of knee OA. Assessed OA features include cartilage damage (Grade 0–7), traumatic articular surface damage and traumatic and degenerative subchondral BMLs (type of injury Grade 0–4; size of injury Grade 0–3), traumatic and degenerative BMLs (Grade 0–3), osteophytes (Grade 0–7), collateral ligaments (Grade 0–3), native ACL (Grade 0–3), ACL graft (Grade 0–3), posterior cruciate ligament (Grade 0–3), meniscal morphology (Grade 0–8), meniscal extrusion (Grade 0–2), joint effusion (Grade 0–3), Hoffa-synovitis (hyperintense signal changes within Hoffa’s fat pad as a surrogate marker of synovitis; Grade 0–3). In terms of reliability, the large majority of the measures showed substantial (0.61–0.80) or reached perfect (0.81–1.0) agreement, and were scored with overall percentage agreement above 80% for both intra- and inter-reader exercise.40 Scoring hip osteoarthritis with MRI (SHOMRI) enables a whole joint semiquantitative assessment of hip OA.41 SHOMRI divides the hip joint into 10 subregions and enables grading of articular cartilage loss (Grade 0–2), BMLs (Grade 0–3), and subchondral cysts (Grade 0–2). In addition, labral tears and ligamentum teres abnormalities are Graded 0–3, and the presence or absence of paralabral cysts, intra-articular bodies, and joint effusion are scored 0 or 1. SHOMRI allows semiquantitative scoring of the above features with excellent intra- and inter-reader reproducibility (Intraclass correlation coefficient >0.9).41 Knee Inflammation MRI Scoring System (KIMRISS) was developed to focus on potentially reversible MRI biomarkers of active knee OA, namely BMLs, with the use of an online interface. The scoring is performed with a template superimposed onto each sagittal slice of acquired MR images, and the reader would clicks segment that contain BML to score 1 for that segment. Femur and tibia are scored over 29 3 mm slices and the patellar is scored over ten 3 mm slices. Femoral template has 13 segments, tibial template has 10 segments and patellar template has 8 segments per slice, giving the overall maximum score per knee of 763 (290 for tibia, 377 for femur, and 96 for patella). The knee joint which enabled reliable scoring by both experienced (intraclass correlation coefficient 0.84 for baseline BML score and 0.82 for change in BML score) and inexperienced readers (intraclass correlation coefficient 0.89–0.98 for baseline BML score and 0.87–0.92 for change in BML score).42 The outcomemeasures in rheumatology MRI Task Force developed the thumb base OA MRI score (TOMS), based on the existing hand OA MRI scoring system to enable assessment of inflammatory and structural abnormalities in this specific hand OA subset.43 In this scoring system, the first carpometacarpal (CMC-1) and scaphotrapeziotrapezoid (STT) joints were specifically assessed for synovitis, subchondral bone defects (including erosions, cysts and bone attrition), osteophytes, cartilage and BMLs on a 0–3 scale. Subluxation was assess only in the CMC-1 as present/absent. Interreader reliability were good for all features (intraclass correlation coefficient 0.77–0.99 for CMC-1 and 0.74–0.96 for STT joint), with similar intrareader reliability.
Research studies deploying semiquantitative MRI scoring systems continue to play an important role for OA researchers to further their understanding of OA disease mechanism and to assist in their efforts to develop efficacious therapies for OA. Using data from the Foundation for the National Institute of Healthbiomarkers consortium project, studies showed 24-month MOAKS changes in cartilage thickness, cartilage surface area, effusion-synovitis, Hoffa-synovitis, and meniscal morphology were independently associated with OA progression, suggesting that these factors may serve as efficacy biomarkers in clinical trials of disease-modifying interventions for knee OA.44, 45
Meniscus is an important structure in the knee joint that is understood to play a role in the OA disease process and serves as a potential target for early detection of persons at risk of developing knee OA. In a study involving 407 middle aged obese females, baseline presence of meniscal extrusion was associated with incident radiographic knee OA [odds ratio 2.61, 95% CI (1.11–6.13)] and medial joint space narrowing [odds ratio 3.19, 95% CI (1.59–6.41)] after 30 months.46 Of note, meniscal root tear is considered a separate entity and is associated with greater pain than meniscal tears or maceration in knee OA (persons with meniscal root tears had adjusted mean WOMAC pain score of 45.2 with standard error of 2.7, while those without meniscal root tear had WOMAC pain score of 38.7 with standard error 1.2, p = 0.03 for difference).15 History of meniscal surgery can also affect knee OA incidence. In a study based on OAI data involving 355 knees, all 31 knees which had undergone partial menisectomy 165 knees with prevalent meniscal damage at baseline developed radiographic knee OA at 1 year follow-up [odds ratio 2.51, 95% CI (1.73–3.64)], demonstrating strong association between prior partial menisectomy and incident knee OA in 1 year.47
Synovitis in OA can be assessed using MRI with or without intravenous gadolinium. MRI signal changes within Hoffa’s fat pad (“Hoffa synovitis”) and the presence of effusion (“effusion-synovitis”) are two indirect markers of synovitis in knee OA.36, 37,48 Hoffa synovitis is strongly associated with knee pain.49 However, effusion-synovitis may be preferred over Hoffa-synovitis as a surrogate marker when contrast-enhanced MRI (CEMRI) is not available, based on the finding that effusion-synovitis showing superior correlations (spearman correlation r = 0.41 for synovial thickness and 0.43 for synovial volume) compared to Hoffa synovitis (spearman correlation r = 0.32 for synovial thickness and 0.39 for synovial volume), using CEMRI-assessed synovial thickness as the reference.50 Ideally, synovitis should be assessed using the CEMRI which enables direct visualization of inflamed synovium and thus more accurate assessment of synovitis.51 Studies have shown synovitis detected by CEMRI is strongly associated with tibiofemoral radiographic OA and MRI-detected widespread cartilage damage.52 Based on CEMRI, increased severity of synovitis was shown to be strongly associated with increase in WOMAC pain score [odds ratio 1.82, 95% CI (0.05–3.58)]53 and increase in synovitis was shown to be associated with cartilage deterioration.54
Cartilage damage is one of central components of OA disease process. Partial-thickness and full-thickness focal cartilage defects seem to contribute equally to development of new cartilage damage in knee OA.55 For patellofemoral OA, knee sagittal dynamic joint stiffness may be a potentially modifiable risk factor for patellofemoral cartilage damage worsening over 2 years.56 In contrast, large cross-sectional areas of thigh extensors and vastus medialis muscle are associated with patellofemoral WORMS cartilage damage score increase over 48 months, suggesting maintenance of adequate extensor/flexor muscle balance for prevention of patellofemoral cartilage loss.57
Lastly, bone and bone marrow-related lesions are also important in the OA disease process. Marginal osteophytes were consistently associated with knee pain both cross-sectionally and longitudinally.58 BMLs are strong predictors of subsequent structural progression in knee OA including cartilage loss (Figure 3). Serum inflammatory markers were associated with BMLs in males and females with knee OA and predicted increased WORMS BML score in females, suggesting involvement of inflammation in BML pathogenesis in knee OA.59 In hand OA, BMLs are associated with pain, radiographic hand OA progression and incident joint tenderness.9, 60,61 Thus, BMLs can be a good target for OA clinical trials.
Figure 3.
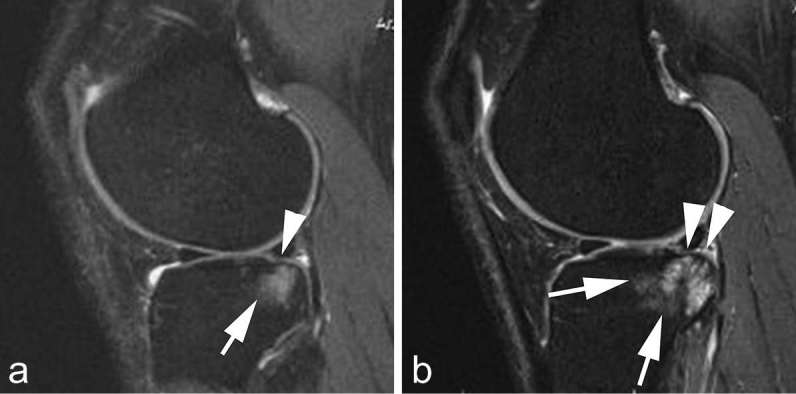
Structural predictors of progression. (a) Baseline sagittal intermediate-weighted fat-suppressed MRI shows minimal superficial focal cartilage defect at posterior lateral tibia (arrow head). In addition there is an associated large diffuse subchondral bone marrow lesion (arrow). (b) Follow-up MRI 2 years later shows progression of cartilage damage with now diffuse cartilage loss at the posterior tibia (arrow heads) and further progression of subchondral bone marrow lesion (arrows). Bone marrow lesions are strong predictors of subsequent structural progression including cartilage loss.
Quantitative analysis
Detailed review and overview of quantitative MRI research of OA has been published recently.62 Quantitative MRI analyses include, but not limited to, measurement size/thickness/shape/volume of cartilage (Figure 4), meniscus, effusion, synovitis, bone and BMLs. For quantitative MRI analysis of cartilage measures, the use of location-independent analysis has been proposed, given the spatial heterogeneity of cartilage loss in knee OA.63 A newly proposed Local-Area Cartilage Segmentation software method enables fast and responsive cartilage volume measurement on MRI.64
Figure 4.
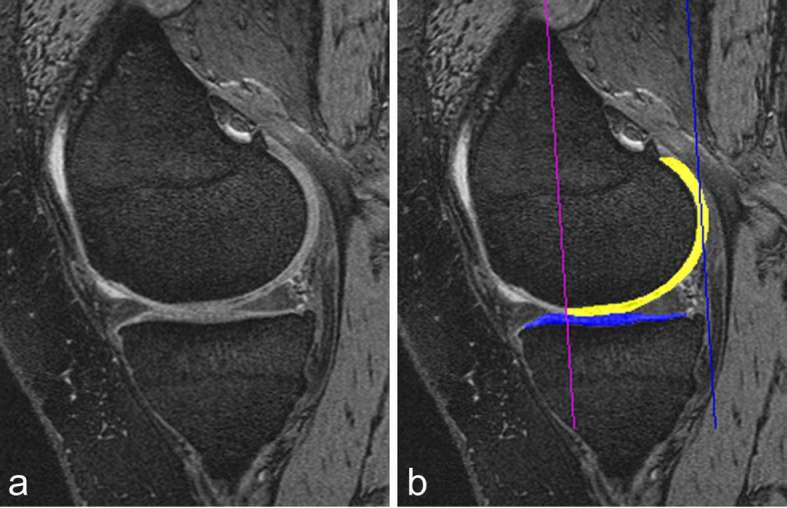
Cartilage quantification based on 3D-MRI. (a) Sagittal DESS MRI is commonly used for cartilage segmentation. Cartilage appears as a bright signal band between the subchondral bone and the intra-articular cavity. (b) The femoral segmentation is colour-coded in yellow and the tibial boundaries of cartilage are coded in blue. In addition the subregional demarcations are shown (two vertical lines). Image courtesy of Drs Wolfgang Wirth and Felix Eckstein, The Paracelsus Medical University, Salzburg, Austria. DESS, dualecho at steady-state.
Recent studies utilizing quantitative analyses have shown baseline lateral femoral cartilage volume is directly associated with medial JSN progression at 48-month follow-up,65 and bone curvature changes can predict efficacy of OA treatment on cartilage volume loss in a 2 year clinical trial of chondroitin sulfate.66
Although cartilage is the most commonly assessed structure in quantitative OA research, other articular and periarticular tissues can also be quantitatively analyzed. In females, but not males, low serum levels of endogenous estradiol, progesterone and testosterone were shown to be associated with increased knee effusion-synovitis volume.67 Authors of this study suggested that this finding might explain observed sex difference in OA. Stronger increase in 3D infrapatellar fat pad MRI signal and signal heterogeneity, but not IPFP volume, might be associated with radiographic/symptomatic progression of OA, compared to non-progressive OA or healthy knee.68
Quantitative analysis can also be applied to mensci in the knee. Quantitative measures of meniscal extrusion were shown to predict incident radiographic knee OA.69 A pilot study showed longitudinal change in quantitative 3D meniscus measurements, such as meniscal volume, area of tibial plateau coverage and extent of meniscal extrusion, may provide improved sensitivity to change compared with single slice analysis.70
One study highlighted the importance of choice of MRI pulse sequence for BML volume assessment. Changes in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was correlated with BML volume change on intermediate-weighted fat-suppressed sequence but not Dual Echo Steady State (DESS) sequence.31 Overall, BML quantification on intermediate-weighted fat-suppressed sequence offers better validity and statistical power than BML quantification on a 3D DESS sequence.
Compositional (physiological) MRI
Compositional MRI enables evaluation of the biochemical properties of joint tissues. Early, pre-morphologic changes that cannot be assessed on conventional MRI may be detected. Compositional MRI has been mostly applied for ultrastructural assessment of cartilage. Comprehensive review articles describing compositional MRI technique for cartilage imaging in OA are available in the literature.71, 72 However, the technique can be applied to other tissues such as meniscus73 and ligaments.74 The application of compositional MRI techniques including, but not limited to, T2/T2*/T1rho mapping, delayed gadolinium enhanced MRI of cartilage (dGEMRIC), sodium imaging, gagCEST imaging, and diffusion MRI, to cartilage and other articular and periarticular tissues continue to increase in the research setting.71, 72
T2 mapping is a well validated technique that is compatible with most MR systems (Figure 5). T2* mapping can be done with shorter acquisition time than T2 mapping thanks to 3D acquisition of data. Typically, pulse sequences based on spin-echos are required for T2 mapping while gradient echoes are required for field mapping. To overcome this limitation, a dual-pathway multi-echo steady state sequence and reconstruction algorithm have been developed to capture T2, T2* and field map information, enabling generation of T2 and field maps from the same acquired data rather than from separately.75 Another new approach – Extended Phase Graph (EPG) modeling – was developed to allow a simple linear approximation of the relationship between the two DESS signals, enabling accurate T2 estimation from one DESS scan.76 One should note, however, there can be potential discrepancies in T2 relaxation time quantification between different sequences and thus care should be taken when comparing results amongst different studies.77 As described earlier, compositional MRI techniques such as T2 mapping has a major advantage over conventional “morphologic” MRI in that they can be used for imaging of “pre-morphologic” stage of OA. e.g. T2 map signal variation can potentially predict symptomatic knee OA progression in asymptomatic individuals to serve as a possible early OA imaging marker.78 Also short-term longitudinal evaluation of T2 map and texture changes may provide early warning of cartilage at risk for progressive degeneration after ACL injury and reconstruction.79 Moreover, computer aided diagnosis of early cartilage degeneration in knee OA with T2 mapping has been attempted and seems to be technically feasible.80
Figure 5.
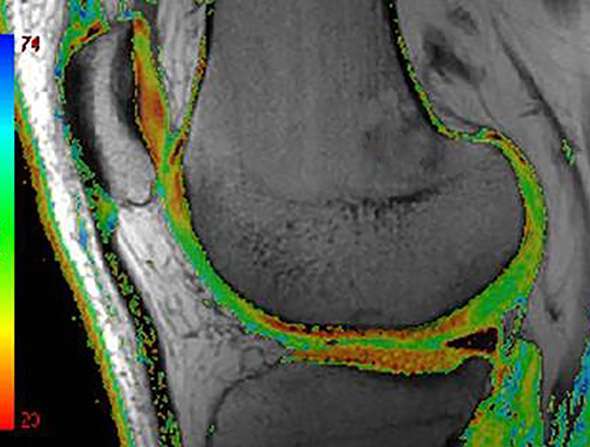
Compositional MRI. Compositional MRI is able to depict intratissue structural alterations that are not visible to the human eye. Colour-coded T2 map MRI shows areas of focal increase in T2 times as green areas. Several compositional techniques are applied with T2 relaxometrey the most commonly applied as it is available on most clinical MRI platforms.
T1rho mapping is sensitive to early cartilage degeneration and may complement T2/T2* mapping, but it requires special pulse sequences that are available at select few academic institutions and acquisition can be time consuming.81 Sodium imaging correlates directly with GAG content, but is limited by the need for specialized hardware, long examination times and low spatial resolution. Interestingly, machine learning seems to be a potentially applicable technique for classifying OA patients and controls using sodium MRI data.82 dGEMRIC can help accurately measure cartilage sulfated GAG content in vivo in patients with knee OA.83 This technique required intravenous injection of gadolinium contrast agent and examination time is prolonged by the need for time gap between injection and imaging. gagCEST is a relatively newer compositional MRI technique and research efforts to optimize image acquisition technique continue.84, 85 Technical feasibility of the diffusion-weighted stimulated echo-based sequence as a tool for early diagnosis and characterization of knee OA at 3T has been demonstrated.86
Compositional MRI techniques can potentially supplement conventional clinical MRI sequences to identify cartilage degeneration at an earlier stage than is possible now. Different techniques are complementary and offer information on different biochemical components of cartilage and other joint tissues. The applicability and responsiveness of these techniques in clinical trials need to be established in the near future.
Metabolic imaging with radiotracers and hybrid imaging
PET imaging with 18F-fluorodeoxyglucose (FDG) or 18F-fluoride (18F-) enable imaging of active metabolism and visualization of bone turn over changes seen in OA disease process. A pathologic feature of OA that is particularly relevant to PET imaging is synovitis, which shows increased metabolism (Figure 6). Detection of active synovitis in OA is clinically meaningful, since increased 18F-FDG uptake by knee synovium was shown to be associated with knee pain in OA patients.87 To overcome the major limitation of PET imaging, i.e. lack of high anatomical resolution, utilization of PET/CT and PET/MRI hybrid imaging has been explored and the feasibility was demonstrated for assessment of early metabolic and morphologic markers of knee OA across multiple tissues.88 Namely, all subchondral bone lesions (BML, osteophytes and sclerosis) show hypermetabolism compared to normal-appearing bone on MRI.88 In addition, increasing research efforts have been made to deploy SPECT/CT for imaging of OA.89–91 SUVmax of quantitative bone SPECT/CT was shown to be highly correlated with radiographic (KL grading, rho = 0.0703, p < 0.0001) and MRI parameters for medial compartment knee OA (rho = 0.0714–0.0808, p ≤ 0.0002).90 Also, bone tracer uptake on SPECT/CT imaging showed positive correlation with the degree and size of chondral lesions detected by conventional MRI.91 Currently, the use of PET/MRI or SPECT/CT in OA imaging is not routinely performed in a clinical setting and available literature evidence is limited to studies showing feasibility of these techniques in OA imaging research.
Figure 6.
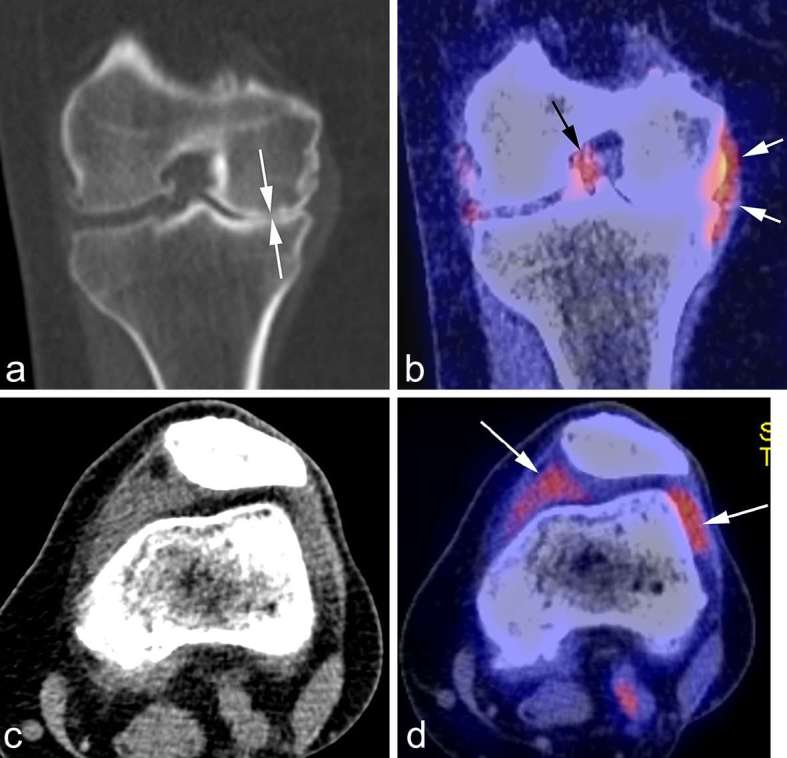
PET-CT. (a) Coronal CT reformatted image shows marked medial joint space narrowing reflecting advanced osteoarthritis (white arrows). (b) Corresponding fused PET image shows glucose accumulation around the medial meniscus reflecting focal synovitis (white arrows). Another focus of synovitis is shown around the PCL in the intercondylar notch (black arrow). (c) Axial CT image in soft tissue kernel shows distension of the joint capsule but is not able to differentiate between fluid and synovitis. Matched axial PET image shows marked glucose uptake in the medial and lateral peripatellar regions reflecting hypermetabolic active synovitis (arrows). PCL, posterior cruciate ligament; PET-CT, positron emission tomography CT.
Ultrasound evaluation of OA
In OA research, ultrasound has been most commonly utilized in imaging of hand OA and is less commonly used for imaging of larger joints such as the knee and hip. Ultrasound enables evaluation of synovial hypertrophy, increased vascularity and the presence of synovial fluid in OA joints. For examples, a recent study comparing erosive and non-erosive hand OA patients showed that Power Doppler activity demonstrating increased vascularity was found more frequently in erosive hand OA compared to non-erosive hand OA, and patients with erosive hand OA had more moderate-to-severe synovitis detected by greyscale ultrasound than those with non-erosive hand OA [OR = 2.02, 95% CI (1.25–3.26)].8 A longitudinal observational study showed ultrasound-detected greyscale synovitis and Power Doppler signals are significantly associated with radiographic progression of hand OA after 5 years.92 Using an ultrasound-based semiquantitative scoring system,93, 94 an epidemiological observational study showed that ultrasound can be used effectively in the assessment of femoral medial and lateral osteophytes and medial meniscal extrusion in subjects with and without symptomatic knee OA.95 Interestingly, another epidemiological study demonstrated that monthly alcohol intake was shown to be associated with ultrasound-detected synovitis in hand OA, although underlying reason for such an association remains to be determined.96 Thanks to ultrasound’s ability to perform dynamic imaging with patients in various positioning including standing/weight bearing, it may be able to offer better assessment of meniscal extrusion compared to MRI, in which weight-bearing imaging is not routinely performed.97
Overall, given its wide availability and relatively lower cost compared to MRI, as well as its ability to depict tissue-specific morphological changes that cannot be depicted by radiography, ultrasound may play a role as a useful imaging technique as an adjunct to radiography.95
Conclusion
Research efforts to uncover the disease process of OA as well as to discover a disease modifying OA drug continue. MRI continues to play a large role in these endeavors, while compositional MRI techniques will increasingly become important due to their ability to assess “premorphologic” biochemical changes of articular cartilage and other tissues in and around joints. Radiography remain the primary imaging modality for defining inclusion/exclusion criteria as well as an outcome measure in OA clinical trials, despite known limitations for visualization of OA features. Compositional MRI techniques show promise for predicting structural and clinical outcomes in OA research. Ultrasound can be a useful adjunct to radiography and MRI particularly for evaluation of hand OA. Newer imaging techniques such as hybrid PET/MRI enable evaluation of the joint as a whole organ with simultaneous assessment of morphological changes and metabolic activities in both osseous and non-osseous structures. Thus, there may be a potential for these hybrid systems to play an increasing role in OA research by allowing integration of biochemical and structural information on MRI with metabolic information obtained from PET related to OA disease pathogenesis.
Contributor Information
Daichi Hayashi, Email: dhayashi@bu.edu.
Frank W Roemer, Email: Frank.Roemer@uk-erlangen.de.
Ali Guermazi, Email: guermazi@bu.edu.
REFERENCES
- 1.Sakellariou G, Conaghan PG, Zhang W, Bijlsma JWJ, Boyesen P, D'Agostino MA, et al. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann Rheum Dis 2017; 76: 1484–94. doi: 10.1136/annrheumdis-2016-210815 [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Altman RD, Cicuttini F, Crema MD, Duryea J, Eckstein F, et al. OARSI clinical trials recommendations: knee imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 698–715. doi: 10.1016/j.joca.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 3.Gold GE, Cicuttini F, Crema MD, Eckstein F, Guermazi A, Kijowski R, et al. OARSI clinical trials recommendations: hip imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 716–31. doi: 10.1016/j.joca.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter DJ, Arden N, Cicuttini F, Crema MD, Dardzinski B, Duryea J, et al. OARSI clinical trials recommendations: hand imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 732–46. doi: 10.1016/j.joca.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther 2011; 13: 247. doi: 10.1186/ar3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011; 377: 2115–26. doi: 10.1016/S0140-6736(11)60243-2 [DOI] [PubMed] [Google Scholar]
- 7.Rathod T, Marshall M, Thomas MJ, Menz HB, Myers HL, Thomas E, et al. Investigations of potential phenotypes of foot osteoarthritis: cross-sectional analysis from the clinical assessment study of the foot. Arthritis Care Res 2016; 68: 217–27. doi: 10.1002/acr.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haugen IK, Mathiessen A, Slatkowsky-Christensen B, Magnusson K, Bøyesen P, Sesseng S, et al. Synovitis and radiographic progression in non-erosive and erosive hand osteoarthritis: is erosive hand osteoarthritis a separate inflammatory phenotype? Osteoarthritis Cartilage 2016; 24: 647–54. doi: 10.1016/j.joca.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 9.Haugen IK, Slatkowsky Christensen B, Bøyesen P, Sesseng S, van der Heijde D, Kvien TK. Increasing synovitis and bone marrow lesions are associated with incident joint tenderness in hand osteoarthritis. Ann Rheum Dis 2016; 75: 702–8. doi: 10.1136/annrheumdis-2014-206829 [DOI] [PubMed] [Google Scholar]
- 10.Sharma L, Nevitt M, Hochberg M, Guermazi A, Roemer FW, Crema M, et al. Clinical significance of worsening versus stable preradiographic MRI lesions in a cohort study of persons at higher risk for knee osteoarthritis. Ann Rheum Dis 2016; 75: 1630–6. doi: 10.1136/annrheumdis-2015-208129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacFarlane LA, Yang H, Collins JE, Guermazi A, Jones MH, Teeple E, et al. Associations among meniscal damage, meniscal symptoms and knee pain severity. Osteoarthritis Cartilage 2017; 25: 850–7. doi: 10.1016/j.joca.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957; 16: 494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study. BMJ 2012; 345: e5339. doi: 10.1136/bmj.e5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guermazi A, Hayashi D, Roemer F, Felson DT, Wang K, Lynch J, et al. Severe radiographic knee osteoarthritis--does Kellgren and Lawrence grade 4 represent end stage disease?--the MOST study. Osteoarthritis Cartilage 2015; 23: 1499–505. doi: 10.1016/j.joca.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 2007; 15(Suppl A): A1–A56. doi: 10.1016/j.joca.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 16.Culvenor AG, Felson DT, Niu J, Wirth W, Sattler M, Dannhauer T, et al. Thigh muscle specific-strength and the risk of incident knee osteoarthritis: the influence of sex and greater body mass index. Arthritis Care Res 2017; 69: 1266–70. doi: 10.1002/acr.23182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zikria B, Hafezi-Nejad N, Roemer FW, Guermazi A, Demehri S. Meniscal surgery: risk of radiographic joint space narrowing progression and subsequent knee replacement – data from the osteoarthritis initiative. Radiology 2017; 282: 807–16. doi: 10.1148/radiol.2016160092 [DOI] [PubMed] [Google Scholar]
- 18.Krasnokutsky S, Oshinsky C, Attur M, Ma S, Zhou H, Zheng F, et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol 2017; 69: 1213–20. doi: 10.1002/art.40069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arden NK, Cro S, Sheard S, Doré CJ, Bara A, Tebbs SA, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthritis Cartilage 2016; 24: 1858–66. doi: 10.1016/j.joca.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein F, Boudreau R, Wang Z, Hannon MJ, Duryea J, Wirth W, et al. Comparison of radiographic joint space width and magnetic resonance imaging for prediction of knee replacement: a longitudinal case-control study from the osteoarthritis initiative. Eur Radiol 2016; 26: 1942–51. doi: 10.1007/s00330-015-3977-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth M, Wirth W, Emmanuel K, Culvenor AG, Eckstein F. The contribution of 3D quantitative meniscal and cartilage measures to variation in normal radiographic joint space width-Data from the Osteoarthritis Initiative healthy reference cohort. Eur J Radiol 2017; 87: 90–8. doi: 10.1016/j.ejrad.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal NA, Frick E, Duryea J, Roemer F, Guermazi A, Nevitt MC, et al. Correlations of medial joint space width on fixed-flexed standing computed tomography and radiographs with cartilage and meniscal morphology on magnetic resonance imaging. Arthritis Care Res 2016; 68: 1410–6. doi: 10.1002/acr.22888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G, CS/GS Combined Therapy Study Group. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol 2017; 69: 77–85. doi: 10.1002/art.39819 [DOI] [PubMed] [Google Scholar]
- 24.Kwoh CK, Roemer FW, Hannon MJ, Moore CE, Jakicic JM, Guermazi A, et al. Effect of oral glucosamine on joint structure in individuals with chronic knee pain: a randomized, placebo-controlled clinical trial. Arthritis Rheumatol 2014; 66: 930–9. doi: 10.1002/art.38314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarraya M, Hayashi D, Guermazi A, Kwoh CK, Hannon MJ, Moore CE, et al. Susceptibility artifacts detected on 3T MRI of the knee: frequency, change over time and associations with radiographic findings: data from the joints on glucosamine study. Osteoarthritis Cartilage 2014; 22: 1499–503. doi: 10.1016/j.joca.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 26.Roemer FW, Aydemir A, Lohmander S, Crema MD, Marra MD, Muurahainen N, et al. Structural effects of sprifermin in knee osteoarthritis: a post-hoc analysis on cartilage and non-cartilaginous tissue alterations in a randomized controlled trial. BMC Musculoskelet Disord 2016; 17: 267. doi: 10.1186/s12891-016-1128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roubille C, Martel-Pelletier J, Raynauld JP, Abram F, Dorais M, Delorme P, et al. Meniscal extrusion promotes knee osteoarthritis structural progression: protective effect of strontium ranelate treatment in a phase III clinical trial. Arthritis Res Ther 2015; 17: 82. doi: 10.1186/s13075-015-0579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherian JJ, Parvizi J, Bramlet D, Lee KH, Romness DW, Mont MA. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage 2015; 23: 2109–18. doi: 10.1016/j.joca.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 29.Hayashi D, Xu L, Roemer FW, Hunter DJ, Li L, Katur AM, et al. Detection of osteophytes and subchondral cysts in the knee with use of tomosynthesis. Radiology 2012; 263: 206–15. doi: 10.1148/radiol.12111649 [DOI] [PubMed] [Google Scholar]
- 30.Martini K, Becker AS, Guggenberger R, Andreisek G, Frauenfelder T. Value of tomosynthesis for lesion evaluation of small joints in osteoarthritic hands using the OARSI score. Osteoarthritis Cartilage 2016; 24: 1167–71. doi: 10.1016/j.joca.2016.01.982 [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Driban JB, Price LL, Lo GH, McAlindon TE. Magnetic resonance image sequence influences the relationship between bone marrow lesions volume and pain: data from the osteoarthritis initiative. Biomed Res Int 2015; 2015: 1–5. doi: 10.1155/2015/731903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemer FW, Kwoh CK, Hannon MJ, Crema MD, Moore CE, Jakicic JM, et al. Semiquantitative assessment of focal cartilage damage at 3T MRI: a comparative study of dual echo at steady state (DESS) and intermediate-weighted (IW) fat suppressed fast spin echo sequences. Eur J Radiol 2011; 80: e126–e131. doi: 10.1016/j.ejrad.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 33.Hayashi D, Guermazi A, Kwoh CK, Hannon MJ, Moore C, Jakicic JM, et al. Semiquantitative assessment of subchondral bone marrow edema-like lesions and subchondral cysts of the knee at 3T MRI: a comparison between intermediate-weighted fat-suppressed spin echo and dual echo steady state sequences. BMC Musculoskelet Disord 2011; 12: 198. doi: 10.1186/1471-2474-12-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol 2013; 9: 236–51. doi: 10.1038/nrrheum.2012.223 [DOI] [PubMed] [Google Scholar]
- 35.Jarraya M, Hayashi D, Roemer FW, Guermazi A. MR imaging-based semi-quantitative methods for knee osteoarthritis. Magn Reson Med Sci 2016; 15: 153–64. doi: 10.2463/mrms.rev.2015-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004; 12: 177–90. doi: 10.1016/j.joca.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 37.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthritis Cartilage 2011; 19: 990–1002. doi: 10.1016/j.joca.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roemer FW, Hunter DJ, Winterstein A, Li L, Kim YJ, Cibere J, et al. Hip osteoarthritis MRI scoring system (HOAMS): reliability and associations with radiographic and clinical findings. Osteoarthritis Cartilage 2011; 19: 946–62. doi: 10.1016/j.joca.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 39.Haugen IK, Østergaard M, Eshed I, McQueen FM, Bird P, Gandjbakhch F, et al. Iterative development and reliability of the OMERACT hand osteoarthritis MRI scoring system. J Rheumatol 2014; 41: 386–91. doi: 10.3899/jrheum.131086 [DOI] [PubMed] [Google Scholar]
- 40.Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior cruciate ligament osteoarthritis score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage 2014; 22: 668–82. doi: 10.1016/j.joca.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Nardo L, Kumar D, Wyatt CR, Souza RB, Lynch J, et al. Scoring hip osteoarthritis with MRI (SHOMRI): A whole joint osteoarthritis evaluation system. J Magn Reson Imaging 2015; 41: 1549–57. doi: 10.1002/jmri.24722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaremko JL, Jeffery D, Buller M, Wichuk S, McDougall D, Lambert RG, et al. Preliminary validation of the knee inflammation MRI scoring system (KIMRISS) for grading bone marrow lesions in osteoarthritis of the knee: data from the Osteoarthritis Initiative. RMD Open 2017; 3: e000355. doi: 10.1136/rmdopen-2016-000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroon FPB, Conaghan PG, Foltz V, Gandjbakhch F, Peterfy C, Eshed I, et al. Development and reliability of the OMERACT thumb base osteoarthritis magnetic resonance imaging scoring system. J Rheumatol 2017; 44: 1694–8. doi: 10.3899/jrheum.161099 [DOI] [PubMed] [Google Scholar]
- 44.Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - methodologic aspects and definition of change. BMC Musculoskelet Disord 2016; 17: 466: 466. doi: 10.1186/s12891-016-1310-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the foundation for the national institute of health osteoarthritis biomarker consortium. Arthritis Rheumatol 2016; 68: 2422–31. doi: 10.1002/art.39731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Voet JA, Runhaar J, van der Plas P, Vroegindeweij D, Oei EH, Bierma-Zeinstra SMA. Baseline meniscal extrusion associated with incident knee osteoarthritis after 30 months in overweight and obese women. Osteoarthritis Cartilage 2017; 25: 1299–303. doi: 10.1016/j.joca.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 47.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Grago J, et al. Partial meniscectomy is associated with increased risk of incident radiographic osteoarthritis and worsening cartilage damage in the following year. Eur Radiol 2017; 27: 404–13. doi: 10.1007/s00330-016-4361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa's fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthritis Cartilage 2016; 24: 383–97. doi: 10.1016/j.joca.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 49.Kaukinen P, Podlipská J, Guermazi A, Niinimäki J, Lehenkari P, Roemer FW, et al. Associations between MRI-defined structural pathology and generalized and localized knee pain - the oulu knee osteoarthritis study. Osteoarthritis Cartilage 2016; 24: 1565–76. doi: 10.1016/j.joca.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 50.Crema MD, Roemer FW, Li L, Alexander RC, Chessell IP, Dudley AD, et al. Comparison between semiquantitative and quantitative methods for the assessment of knee synovitis in osteoarthritis using non-enhanced and gadolinium-enhanced MRI. Osteoarthritis Cartilage 2017; 25: 267–71. doi: 10.1016/j.joca.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 51.Hayashi D, Roemer FW, Katur A, Felson DT, Yang SO, Alomran F, et al. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum 2011; 41: 116–30. doi: 10.1016/j.semarthrit.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 52.Guermazi A, Hayashi D, Roemer FW, Zhu Y, Niu J, Crema MD, et al. Synovitis in knee osteoarthritis assessed by contrast-enhanced magnetic resonance imaging (MRI) is associated with radiographic tibiofemoral osteoarthritis and MRI-detected widespread cartilage damage: the MOST study. J Rheumatol 2014; 41: 501–8. doi: 10.3899/jrheum.130541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace G, Cro S, Doré C, King L, Kluzek S, Price A, et al. Associations between clinical evidence of inflammation and synovitis in symptomatic knee osteoarthritis: a substudy. Arthritis Care Res 2017; 69: 1340–8. doi: 10.1002/acr.23162 [DOI] [PubMed] [Google Scholar]
- 54.de Lange-Brokaar BJ, Ioan-Facsinay A, Yusuf E, Kroon HM, Zuurmond AM, Stojanovic-Susulic V, et al. Evolution of synovitis in osteoarthritic knees and its association with clinical features. Osteoarthritis Cartilage 2016; 24: 1867–74. doi: 10.1016/j.joca.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 55.Guermazi A, Hayashi D, Roemer FW, Niu J, Quinn EK, Crema MD, et al. Brief report: partial- and full-thickness focal cartilage defects contribute equally to development of new cartilage damage in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol 2017; 69: 560–4. doi: 10.1002/art.39970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang AH, Chmiel JS, Almagor O, Guermazi A, Prasad PV, Moisio KC, et al. Association of baseline knee sagittal dynamic joint stiffness during gait and 2-year patellofemoral cartilage damage worsening in knee osteoarthritis. Osteoarthritis Cartilage 2017; 25: 242–8. doi: 10.1016/j.joca.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldman LH, Tang K, Facchetti L, Heilmeier U, Joseph GB, Nevitt MC, et al. Role of thigh muscle cross-sectional area and strength in progression of knee cartilage degeneration over 48 months - data from the osteoarthritis initiative. Osteoarthritis Cartilage 2016; 24: 2082–91. doi: 10.1016/j.joca.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sayre EC, Guermazi A, Esdaile JM, Kopec JA, Singer J, Thorne A, et al. Associations between MRI features versus knee pain severity and progression: data from the vancouver longitudinal study of early knee osteoarthritis. PLoS One 2017; 12: e0176833. doi: 10.1371/journal.pone.0176833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Z, Otahal P, Wang B, Jin X, Laslett LL, Wluka AE, et al. Cross-sectional and longitudinal associations between serum inflammatory cytokines and knee bone marrow lesions in patients with knee osteoarthritis. Osteoarthritis Cartilage 2017; 25: 499–505. doi: 10.1016/j.joca.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 60.Ramonda R, Favero M, Vio S, Lacognata C, Frallonardo P, Belluzzi E, et al. A recently developed MRI scoring system for hand osteoarthritis: its application in a clinical setting. Clin Rheumatol 2016; 35: 2079–86. doi: 10.1007/s10067-016-3303-0 [DOI] [PubMed] [Google Scholar]
- 61.Damman W, Liu R, Bloem JL, Rosendaal FR, Reijnierse M, Kloppenburg M. Bone marrow lesions and synovitis on MRI associate with radiographic progression after 2 years in hand osteoarthritis. Ann Rheum Dis 2017; 76: 214–7. doi: 10.1136/annrheumdis-2015-209036 [DOI] [PubMed] [Google Scholar]
- 62.Eckstein F, Peterfy C. A 20 years of progress and future of quantitative magnetic resonance imaging (qMRI) of cartilage and articular tissues-personal perspective. Semin Arthritis Rheum 2016; 45: 639–47. doi: 10.1016/j.semarthrit.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 63.Eckstein F, Buck R, Wirth W. Location-independent analysis of structural progression of osteoarthritis-Taking it all apart, and putting the puzzle back together makes the difference. Semin Arthritis Rheum 2017; 46: 404–10. doi: 10.1016/j.semarthrit.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 64.Schaefer LF, Sury M, Yin M, Jamieson S, Donnell I, Smith SE, et al. Quantitative measurement of medial femoral knee cartilage volume - analysis of the OA biomarkers consortium FNIH study cohort. Osteoarthritis Cartilage 2017; 25: 1107–13. doi: 10.1016/j.joca.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hafezi-Nejad N, Guermazi A, Roemer FW, Hunter DJ, Dam EB, Zikria B, et al. Prediction of medial tibiofemoral compartment joint space loss progression using volumetric cartilage measurements: data from the FNIH OA biomarkers consortium. Eur Radiol 2017; 27: 464–73. doi: 10.1007/s00330-016-4393-4 [DOI] [PubMed] [Google Scholar]
- 66.Raynauld JP, Pelletier JP, Delorme P, Dodin P, Abram F, Martel-Pelletier J. Bone curvature changes can predict the impact of treatment on cartilage volume loss in knee osteoarthritis: data from a 2-year clinical trial. Rheumatology 2017; 56: 989–98. doi: 10.1093/rheumatology/kew504 [DOI] [PubMed] [Google Scholar]
- 67.Jin X, Wang BH, Wang X, Antony B, Zhu Z, Han W, et al. Associations between endogenous sex hormones and MRI structural changes in patients with symptomatic knee osteoarthritis. Osteoarthritis Cartilage 2017; 25: 1100–6. doi: 10.1016/j.joca.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 68.Ruhdorfer A, Haniel F, Petersohn T, Dörrenberg J, Wirth W, Dannhauer T, et al. Between-group differences in infra-patellar fat pad size and signal in symptomatic and radiographic progression of knee osteoarthritis vs non-progressive controls and healthy knees - data from the FNIH biomarkers consortium study and the osteoarthritis initiative. Osteoarthritis Cartilage 2017; 25: 1114–21. doi: 10.1016/j.joca.2017.02.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emmanuel K, Quinn E, Niu J, Guermazi A, Roemer F, Wirth W, et al. Quantitative measures of meniscus extrusion predict incident radiographic knee osteoarthritis--data from the osteoarthritis initiative. Osteoarthritis Cartilage 2016; 24: 262–9. doi: 10.1016/j.joca.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bloecker K, Wirth W, Guermazi A, Hitzl W, Hunter DJ, Eckstein F. Longitudinal change in quantitative meniscus measurements in knee osteoarthritis--data from the osteoarthritis initiative. Eur Radiol 2015; 25: 2960–8. doi: 10.1007/s00330-015-3710-7 [DOI] [PubMed] [Google Scholar]
- 71.Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthritis Cartilage 2015; 23: 1639–53. doi: 10.1016/j.joca.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 72.Link TM, Neumann J, Li X. Prestructural cartilage assessment using MRI. J Magn Reson Imaging 2017; 45: 949–65. doi: 10.1002/jmri.25554 [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Chang G, Bencardino J, Babb JS, Krasnokutsky S, Abramson S, et al. T1rho MRI of menisci in patients with osteoarthritis at 3 tesla: a preliminary study. J Magn Reson Imaging 2014; 40: 588–95. doi: 10.1002/jmri.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson KJ, Surowiec RK, Ho CP, Devitt BM, Fripp J, Smith WS, et al. Quantifiable imaging biomarkers for evaluation of the posterior cruciate ligament using 3-T magnetic resonance imaging: a feasibility study. Orthop J Sports Med 2016; 4: 232596711663904. doi: 10.1177/2325967116639044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng CC, Mei CS, Duryea J, Chung HW, Chao TC, Panych LP, et al. Dual-pathway multi-echo sequence for simultaneous frequency and T2 mapping. J Magn Reson 2016; 265: 177–87. doi: 10.1016/j.jmr.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sveinsson B, Chaudhari AS, Gold GE, Hargreaves BA. A simple analytic method for estimating T2 in the knee from DESS. Magn Reson Imaging 2017; 38: 63–70. doi: 10.1016/j.mri.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matzat SJ, McWalter EJ, Kogan F, Chen W, Gold GE. T2 relaxation time quantitation differs between pulse sequences in articular cartilage. J Magn Reson Imaging 2015; 42: 105–13. doi: 10.1002/jmri.24757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong H, Miller DJ, Urish KL. T2 map signal variation predicts symptomatic osteoarthritis progression: data from the Osteoarthritis Initiative. Skeletal Radiol 2016; 45: 909–13. doi: 10.1007/s00256-016-2360-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res 2017; 35: 699–706. doi: 10.1002/jor.23358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y, Yang R, Jia S, Li Z, Zhou Z, Lou T. Computer-aided diagnosis of early knee osteoarthritis based on MRI T2 mapping. Biomed Mater Eng 2014; 24: 3379–88. doi: 10.3233/BME-141161 [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Regatte RR. T₁ρ MRI of human musculoskeletal system. J Magn Reson Imaging 2015; 41: 586–600. doi: 10.1002/jmri.24677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madelin G, Poidevin F, Makrymallis A, Regatte RR. Classification of sodium MRI data of cartilage using machine learning. Magn Reson Med 2015; 74: 1435–48. doi: 10.1002/mrm.25515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Tiel J, Kotek G, Reijman M, Bos PK, Bron EE, Klein S, et al. Is T1ρ mapping an alternative to delayed gadolinium-enhanced MR imaging of cartilage in the assessment of sulphated glycosaminoglycan content in human osteoarthritic knees? An in vivo validation study. Radiology 2016; 279: 523–31. doi: 10.1148/radiol.2015150693 [DOI] [PubMed] [Google Scholar]
- 84.Kogan F, Hargreaves BA, Gold GE. Volumetric multislice gagCEST imaging of articular cartilage: optimization and comparison with T1rho. Magn Reson Med 2017; 77: 1134–41. doi: 10.1002/mrm.26200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krishnamoorthy G, Nanga RPR, Bagga P, Hariharan H, Reddy R. High quality three-dimensional gagCEST imaging of in vivo human knee cartilage at 7 Tesla. Magn Reson Med 2017; 77: 1866–73. doi: 10.1002/mrm.26265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guha A, Wyatt C, Karampinos DC, Nardo L, Link TM, Majumdar S. Spatial variations in magnetic resonance-based diffusion of articular cartilage in knee osteoarthritis. Magn Reson Imaging 2015; 33: 1051–8. doi: 10.1016/j.mri.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parsons MA, Moghbel M, Saboury B, Torigian DA, Werner TJ, Rubello D, et al. Increased 18F-FDG uptake suggests synovial inflammatory reaction with osteoarthritis: preliminary in-vivo results in humans. Nucl Med Commun 2015; 36: 1215–9. doi: 10.1097/MNM.0000000000000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kogan F, Fan AP, McWalter EJ, Oei EHG, Quon A, Gold GE. PET/MRI of metabolic activity in osteoarthritis: a feasibility study. J Magn Reson Imaging 2017; 45: 1736–45. doi: 10.1002/jmri.25529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maas O, Joseph GB, Sommer G, Wild D, Kretzschmar M. Association between cartilage degeneration and subchondral bone remodeling in patients with knee osteoarthritis comparing MRI and (99m)Tc-DPD-SPECT/CT. Osteoarthritis Cartilage 2015; 23: 1713–20. doi: 10.1016/j.joca.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 90.Kim J, Lee HH, Kang Y, Kim TK, Lee SW, So Y, et al. Maximum standardised uptake value of quantitative bone SPECT/CT in patients with medial compartment osteoarthritis of the knee. Clin Radiol 2017; 72: 580–9. doi: 10.1016/j.crad.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 91.Dordevic M, Hirschmann MT, Rechsteiner J, Falkowski A, Testa E, Hirschmann A. Do chondral lesions of the knee correlate with bone tracer uptake by using SPECT/CT? Radiology 2016; 278: 223–31. doi: 10.1148/radiol.2015141714 [DOI] [PubMed] [Google Scholar]
- 92.Mathiessen A, Slatkowsky-Christensen B, Kvien TK, Hammer HB, Haugen IK. Ultrasound-detected inflammation predicts radiographic progression in hand osteoarthritis after 5 years. Ann Rheum Dis 2016; 75: 825–30. doi: 10.1136/annrheumdis-2015-207241 [DOI] [PubMed] [Google Scholar]
- 93.Saarakkala S, Waris P, Waris V, Tarkiainen I, Karvanen E, Aarnio J, et al. Diagnostic performance of knee ultrasonography for detecting degenerative changes of articular cartilage. Osteoarthritis Cartilage 2012; 20: 376–81. doi: 10.1016/j.joca.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 94.Koski JM, Kamel A, Waris P, Waris V, Tarkiainen I, Karvanen E, et al. Atlas-based knee osteophyte assessment with ultrasonography and radiography: relationship to arthroscopic degeneration of articular cartilage. Scand J Rheumatol 2016; 45: 158–64. doi: 10.3109/03009742.2015.1055797 [DOI] [PubMed] [Google Scholar]
- 95.Podlipská J, Guermazi A, Lehenkari P, Niinimäki J, Roemer FW, Arokoski JP, et al. Comparison of diagnostic performance of semi-quantitative knee ultrasound and knee radiography with MRI: oulu knee osteoarthritis study. Sci Rep 2016; 6: 22365. doi: 10.1038/srep22365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magnusson K, Mathiessen A, Hammer HB, Kvien TK, Slatkowsky-Christensen B, Natvig B, et al. Smoking and alcohol use are associated with structural and inflammatory hand osteoarthritis features. Scand J Rheumatol 2017; 46: 388–95. doi: 10.1080/03009742.2016.1257736 [DOI] [PubMed] [Google Scholar]
- 97.Nogueira-Barbosa MH, Gregio-Junior E, Lorenzato MM, Guermazi A, Roemer FW, Chagas-Neto FA, et al. Ultrasound assessment of medial meniscal extrusion: a validation study using MRI as reference standard. AJR Am J Roentgenol 2015; 204: 584–8. doi: 10.2214/AJR.14.12522 [DOI] [PubMed] [Google Scholar]